Abstract
Organic Anion Transporting Polypeptides (OATPs), encoded by genes of the Solute Carrier Organic Anion (SLCO) family, are transmembrane proteins involved in the uptake of various compounds of endogenous or exogenous origin. In addition to their physiological roles, OATPs influence the pharmacokinetics and drug-drug interactions of several clinically relevant compounds. To examine the function and molecular interactions of human OATPs, including several poorly characterized family members, we expressed all 11 human OATPs at high levels in the baculovirus-Sf9 cell system. We measured the temperature- and inhibitor-sensitive cellular accumulation of sodium fluorescein and fluorescein-methotrexate, two fluorescent substrates of the OATPs, OATP1B1 and 1B3. OATP1B1 and 1B3 were functional in Sf9 cells, showing rapid uptake (t1/2(fluorescein-methotrexate) 2.64 and 4.16 min, and t1/2(fluorescein) 6.71 and 5.58 min for OATP1B1 and 1B3, respectively) and high-affinity transport (Km(fluorescein-methotrexate) 0.23 and 0.53 μM, and Km(fluorescein) 25.73 and 38.55 μM for OATP1B1 and 1B3, respectively) of both substrates. We found that sodium fluorescein is a general substrate of all human OATPs: 1A2, 1B1, 1B3, 1C1, 2A1, 2B1, 3A1, 4A1, 4C1, 5A1 and 6A1, while fluorescein-methotrexate is only transported by 1B1, 1B3, 1A2 and 2B1. Acidic extracellular pH greatly facilitated fluorescein uptake by all OATPs, and new molecular interactions were detected (between OATP2B1 and Imatinib, OATP3A1, 5A1 and 6A1 and estradiol 17-β–D-glucuronide, and OATP1C1 and 4C1 and prostaglandin E2). These studies demonstrate for the first time the applicability of the insect cell system for the functional expression of the entire human OATP family, and for drug-OATP interaction screening.
Keywords: Organic Anion Transporting Polypeptide, fluorescent assay, new substrates, drug screening
1. INTRODUCTION
The role of uptake transporters in drug pharmacokinetics is being increasingly recognized. One group of uptake transporters gaining recognition for their relevance to the absorption and toxicity of clinically relevant drugs are the Organic Anion Transporting Polypeptides (OATPs) [1, 2]. OATPs are plasma membrane proteins that mediate sodium and ATP-independent transport of large (over 300 Da), charged or amphipathic compounds into cells [3-5]. The 11 known OATPs are encoded by genes of the Solute Carrier Organic Anion (SLCO) family.
Based on their substrate specificity, OATPs can be divided into two groups. OATP1A2, 1B1, 1B3 and 2B1 transport a wide range of compounds, from bile acids and hormones, to various therapeutics including statins, antiviral agents and antibiotics. Conversely, OATP3A1, 1C1, 2A1, 4A1, 4C1 and 5A1 have been documented to transport only a few compounds [4, 6, 7]. Currently, there is no published information on the function or substrate specificity of OATP6A1, primarily due to the lack of convenient expression systems and functional assays.
OATP1B1 and 1B3 are important in the hepatic re-uptake of bile acids, bilirubin and in the hepatic clearance of several drugs [2]. Mutations in SLCO1B1 and 1B3 result in Rotor syndrome, a benign disorder characterized by increased serum bilirubin levels. The role of the 1A/1B family of OATPs in the hepatic disposition of bile salts, bilirubin and therapeutic drugs was demonstrated via knock out [8] and transgenic mouse models [9]. Deletion of the mouse orthologue of OATP1C1 results in mild, brain-specific hypothyroidism confirming the function of this protein in thyroid transport [10]. The physiological relevance of the remaining OATPs remains to be elucidated.
Several single nucleotide polymorphisms (SNPs) in OATP-encoding genes have been linked to altered drug pharmacokinetics, suggesting that these transporters play an important role in in the uptake and elimination of drugs [11]. Population studies and in vitro experiments demonstrate that SNPs in OATP1A2, 1B1, 1B3 and 2B1 affect the uptake of hormones and antihistamines, as well as cholesterol-lowering and anticancer drugs [11]. OATPs also show altered expression in various cancer cells. Liver-specific OATP1B1 and 1B3 were down-regulated in liver cancers, and highly upregulated in tumors of the ovarian gland (1B1), colon (1B1 and 1B3), breast (1B3), prostate (1B3) and lung (1B3) [1]. Similarly, OATP6A1, normally restricted to the testes, was found in cancerous tissues of the bladder, brain and lung [1]. The potential diagnostic and therapeutic value of the cancer-specific localization of OATPs is currently under intense investigation.
Despite the clear clinical relevance, the substrate recognition patterns of only four members of the OATP family, 1A2, 1B1, 1B3 and 2B1 have been thoroughly investigated. The bottleneck in establishing the substrate/inhibitor profile of OATPs is the scarcity and high cost of radioactively labeled substrates. Mass spectrometry (MS) may be an alternative to assays based on radiolabeled substrates [12]. However, MS is more time-consuming and requires high protein expression levels to obtain an acceptable signal to noise ratio. Contrary to these methods, the measurement of fluorescent substrates is sensitive, safe, relatively inexpensive and amenable to automatized detection.
Sodium fluorescein (Na-Fluo) and fluorescein-methotrexate (Fl-MTX) have been shown to be suitable for the screening of potential OATP1B1/1B3-interacting compounds [13, 14]. Additionally, Fluo-3, indocyanine green and fluorescent bile acids have been shown to be readily transported by OATP1B1 and 1B3 [15-17]. However, fluorescent assays for the remaining members of the OATP family have not yet been described, and for 6A1, there are no known substrates.
In this study, our aim was to develop a comprehensive, fluorescence-based functional assay to analyze the function of all known human OATPs. We expressed these proteins in the baculovirus-insect cell expression system and developed a functional assay measuring the cellular uptake of Na-Fluo and Fl-MTX. We show that Na-Fluo is transported by all 11 human OATPs, and, under optimal assay conditions, 1A2 and 2B1 are able to mediate the uptake of fluorescein-methotrexate. Our insect cell-based method provides the first fluorescent assay for the functional investigation of all human OATPs, which will facilitate further screening of potential OATP substrates and structure-function relationship analyses.
2. Materials and Methods
2.1. Materials
Cholic acid (CA), glycocholic acid (GC), propidium iodide, prostaglandinE2 (PGE2), rifampicin (Rif), sodium fluorescein salt (Na-Fluo), taurocholic acid (TC) and ursolic acid (UA) were purchased from Sigma-Aldrich (Budapest, Hungary). Fluorescein-methotrexate (Fl-MTX) was obtained from Biotium, Inc. (Hayward, CA, US). Restriction endonucleases were from New England Biolabs, Ltd. (Ipswitch, MA, US).
2.2. Plasmid constructs
To generate the expression plasmids for human OATPs, we first engineered unique restriction sites that allowed for cloning of all human OATPs into the baculovirus transfer vector pAcUW21 (BD Biosciences, San Jose, CA, US). The modified plasmid, termed pAcUW-L2, was constructed by inserting the oligonucleotide linkers, 5’ GGCCGTGAATTCGGTACCTCGAGCTCGCGGCCGCT-3’ and 5’-GATCAGCGGCCGCGAGCTCGAGGTACCGAATTCAC-3’, between the BamHI and NotI sites of pAcUW21-L/ABCG2, a vector generated previously in our lab [18]. Full-length cDNA sequences encoding human OATPs were then introduced into pAcUW-L2 using the appropriate restriction sites.
OATP1A2, 1B3, 1C1, 2A1, 2B1, 4A1, 5A1 and 6A1 were obtained from the Harvard PlasmID Repository (Harvard Medical School, Boston, MA, US), and the cloning of OATP1B1 (Gene ID: AB026257), 3A1 variant 1 (AB031050) and 4C1 (353189) was performed using the previously constructed vector, pSPORT1 [19, 20]. OATP1B1 and 3A1 transfer vectors were constructed by isolating the corresponding full-length OATP cDNA from pSPORT1, and then subcloning into the pAcUW21-L2 plasmid between the KpnI and NotI restriction endonuclease sites. Sequence analysis revealed that the OATP1B1 cDNA encodes a polymorphism (N130D, rs2306283), therefore, we reverted the sequence to wild-type (Q9Y6L6.2) using site-directed mutagenesis. The primers used in the mutagenesis reaction were: 1B1-N130N for 5’-ACTAATATCAATTCATCAGAAAATTCAACA-3’, 1B1-N130N rev 5’-TGTTGAATTTTCTGATGAATTGATATTAGT-3’. To generate the OATP6A1 construct, the corresponding cDNA was removed from a pBluescriptR vector (BC034976, HsCD00333181) using NotI and BamHI restriction enzymes, and subcloned into pAcUW21-L/ABCG2. Sequencing revealed that the vector HsCD00333181 contains a shorter isoform of 6A1, which is missing amino acids 206-267, compared to the canonical sequence (Q86UG4-1). The cDNA corresponding to the missing region was synthesized by ShineGene Molecular Bio-Technologies, Inc. (Shanghai, China), and subcloned into the pAcUW-21-L/OATP6A1 isoform 2 vector between the NdeI and SpeI sites. The open reading frames of 1A2 (BC042452, HsCD00333163), 1B3 (BC141525, HsCD00348132), 1C1 (BC022461, HsCD00332885), 2A1 (BC041140, HsCD00338568), 2B1 (BC041095.1, HsCD00378878), 4A1 (BC015727, HsCD00334491), 4C1 and 5A1 (BC137424, HsCD00342690) were amplified by HF PCR (Phusion® High-Fidelity PCR Kit, NEB, Ipswitch, MA, US) following the manufacturer’s instructions and using the following primers:
| 1A2 | for | GGAAGATCTGCGGCCGCGCCACCATGGGAGAAACTGAGAA |
| rev | ATTGAGCTCCTGCAGTTACAATTTAGTTTTCAAT | |
| 1B3 | for | GTAAATGCGGCCGCAACTCGAGGCCACCATGGACCAACATCAACAT |
| rev | GTACATGCGGCCGCACTGCAGTTAGTTGGCAGCAGCATT | |
| 1C1 | for | TGTTTAAACTCTAGAGCCACCATGGACACTTCATCCAAAGAA |
| rev | TAACCTGCAGGCGGCCGCGTTTCTAAAGTTGAGTTTCCTTG | |
| 2A1 | for | GTAAATGCGGCCGCAAGAATTCGCCACCATGGGGCTCCTGCCCA |
| rev | GTACATGCGGCCGCTAAGCTTTCAGATGAGGCCTGCCGC | |
| 2B1 | for | GTAAATGCGGCCGCAAGAATTCGCCACCATGGGACCCAGGATAGG |
| rev | GTACATGCGGCCGCTAAGCTTTCACACTCGGGAATCCTC | |
| 4A1 | for | GGAAGATCTGATATCGCCACCATGCCCCTGCATCAGCTG |
| rev | ATTGAGCTCAAGCTTTCAGACGCTGCTCTGGAG | |
| 4C1 | for | GGAAGATCTGCGGCCGCGCCACATGAAGAGCGCCAAAGGT |
| rev | ATTGAGCTCAAGCTTTCACCCTTCTTTTACTAT | |
| 5A1 | for | TGTTTAAACTCTAGAGCCACCATGGACGAAGGCACTGGACTGC |
| rev | TAACCTGCAGGAGCGGCCGCCTTCTTCCATTTTCAAGCTTCAGGAGG |
The amplified fragments were digested using the appropriate restriction enzymes (1A2, 4A1 and 4C1: BglII and SacI, 1B3: XhoI and NotI, 2A1 and 2B1: EcoRI and NotI, and 1C1 and 5A1: PmeI and NotI) and subcloned into the pAcUW21-L2 plasmid. Because sequencing of the OATP4A1 construct revealed that the purchased cDNA contains a known SNP of OATP4A1 (232G>A (V78I), rs1047099), the canonical sequence (Q96BD0-1) was generated via QuickChange mutagenesis using the following primers: 5’AGGTGCGGTACGTCTCGG 3’ (4A1 for and 5’CCGAGACGTACCGCACCT 3’ (4A1 reverse). The DNA sequences for all constructs were verified by sequencing analyses.
2.3. Maintenance of Sf9 cell cultures and generation of recombinant baculoviruses
Sf9 (Spodoptera frugiperda) cells were grown in suspension culture using TNM-FH insect medium (Sigma Aldrich, Budapest, Hungary) supplemented with 10% fetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin, at 27°C. Recombinant baculoviruses, carrying the different human OATP cDNA sequences, were generated using the BaculoGold Transfection Kit (BD Biosciences, San Jose, CA, US) according to the manufacturer’s instructions. After several rounds of amplification, the virus stocks were stored at 4°C. Transport experiments were performed using the virus stocks that resulted in the highest OATP expression levels (as determined by Western blot).
2.4. Immunodetection of OATPs
Whole cell lysates of Sf9 cells (5-10 μg) were separated on 7.5% Laemmli SDS-PAGE gels and transferred onto PVDF membranes. Immunoblotting was performed as described [18]. Membranes were incubated overnight with polyclonal antibodies. The antibodies used for the detection of OATP1A2, 1B1, 1B3, 1C1, 2B1, 3A1_v1 and 4A1 were previously described [19]. Antibodies against OATP2A1 (HPA013742), 4C1 (HPA036516), 5A1 (HPA025062) and 6A1 (HPA054126) were purchased from Atlas Antibodies (Stockholm, Sweden). The following antibody dilutions were used: 1/250 for 5A1, 1/500 for 1A2, 1B1, 2A1, 4C1 and 6A1, and 1/1000 for 1B3, 1C1, 2B1, 3A1_v1 and 4A1. After washing, PVDF membranes were incubated with 10,000-20,000× diluted, HRP-conjugated anti-rabbit secondary antibodies (Jackson ImmunoResearch, Suffolk, UK). Luminescence was detected using the Luminor Enhancer Solution kit by Thermo Scientific (Waltham, MA, US).
2.5. Cellular Dye Uptake and Calculation of OATP Transport Activity
The accumulation of fluorescent molecules was measured as previously described [18], with some minor modifications. Specifically, OATP-transduced Sf9 cells were collected 40 hours post infection, washed once and suspended to 2–5 × 105cells/reaction in one of the following buffers: Buffer pH 7.4-8.4: 125 mM NaCl, 4.8 mM KCl, 1.2 mM CaCl2, 1.2 mM KH2PO4, 12 mM MgSO4, 25 mM HEPES, and 5.6 mM glucose, with the pH adjusted to 8.4 or 7.4 using 10 N NaOH or 10 N HCl, respectively. Buffer pH 4.5-7.4: 125 mM NaCl, 4.8 mM KCl, 1.2 mM CaCl2, 1.2 mM KH2PO4, 12 mM MgSO4, 25 mM MES, and 5.6 mM glucose, with the pH adjusted to 7.4, 6.5, 5.5 or 4.5 using 10 N NaOH or 1M HEPES.
Cells (2–5 × 105/reaction) were pre-incubated in the presence or absence of inhibitors for 5 min at 37°C. The reaction was initiated with the addition of two times concentrated Fl-MTX or Na-Fluo to a final concentration of 1 μM except for the measurement of concentration dependence. Uptake was stopped after 10 min (unless stated otherwise) of incubation at 37°C by adding 1 ml of ice-cold phosphate-buffered saline. The cells were kept on ice until flow cytometry analysis. The cellular fluorescence of 10,000 live cells was determined using an Attune® Acoustic Focusing Cytometer (Applied Biosystems, Life Technologies, Carlsbad, CA, US) at an excitation wavelength of 488 nm and an emission wavelength of 530/30 nm. Dead cells labeled with propidium iodide (1 μg/ml) were excluded. In control experiments, the levels of fluorescent substrates were analyzed in Sf9 cells expressing a Drosophila melanogaster telomerase subunit (the plasmid encoding this nuclear protein was a generous gift from Dr. Imre Boros at the Biological Research Center in Szeged, Hungary). Activity data were calculated by subtracting the background levels of fluorescence as measured in control cells. Functional data for each OATP represent the mean of at least 3 independent experiments performed on different days.
2.6. Data analysis
Kinetic parameters of dye uptake were calculated using non-linear curve fitting (Hill fit) and Origin 8.6 software. A Student’s t-test was used to calculate any statistical significance. The p value for statistical significance was set at 0.05 (*), 0.01 (**) or 0.001 (***).
3. RESULTS
3.1. Transient expression of human OATP1B1 and OATP1B3 results in increased fluorescein-methotrexate and Na-fluorescein uptake in Sf9 cells
The baculovirus Sf9 (Spodoptera frugiperda) insect cell expression system is a well-established tool to express membrane proteins. This system has two main advantages over other expression systems. Protein expression levels in Sf9 cells are much higher than those achieved in mammalian cell lines, and, unlike in yeast or bacterial systems, the proper routing of heterologous proteins is unaffected [21-23]. Interestingly, with the exception of OATP2B1, the applicability of this system for the analysis of human OATPs has not yet been described [24].
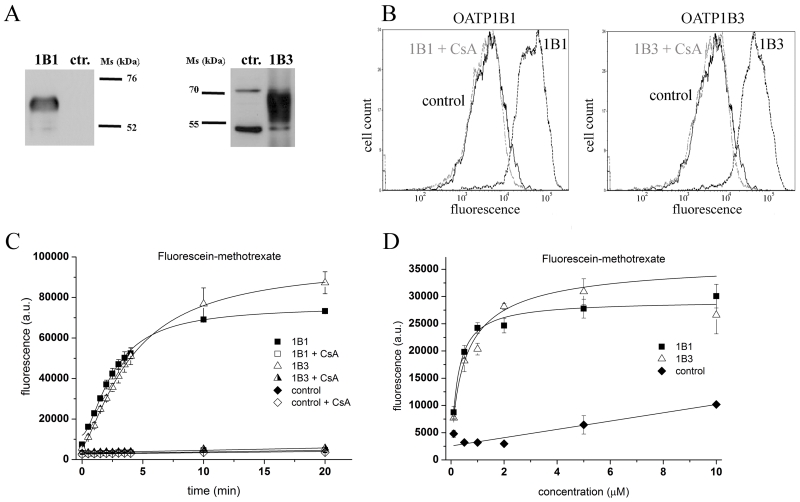
First, we expressed the two best characterized members of the OATP family, OATP1B1 and 1B3, in Sf9 cells. The immunoblot in Figure 1A shows that the heterologous proteins were efficiently expressed in the baculovirus-infected insect cells. The functionality of OATP1B1 and 1B3 in this system was tested by measuring the cellular accumulation of two well-described substrates. The baculovirus-insect cell system is a transient expression system, where virus infection results in the lysis of cells. However, we have previously demonstrated that at 36-40 hours post-infection, the levels of the heterologously-expressed protein are at near maximum, while 25-30 % of the cells still have intact plasma membranes [18]. Dead cells may be readily excluded from the analysis based on propidium-iodide positivity, and the uptake of Fl-MTX or Na-Fluo can be selectively measured within intact cells that express high levels of the heterologously-expressed protein.
Figure 1. Overexpression of human OATP1B1 and 1B3 results in increased Fl-MTX accumulation within insect cells.
A: Western blot detection of human OATP1B1 and 1B3 expressed in Sf9 insect cells. Total cell lysates (5 μg) were separated on 7.5% SDS gels and the proteins were transferred to PVDF membranes. Anti-OATP1B1 and anti-1B3 polyclonal antibodies [19] (500× and 1000× dilutions, respectively) and 10,000× diluted HRP-conjugated anti-rabbit polyclonal antibodies were used to detect protein expression. Control (ctr.) refers to Sf9 cells overexpressing an unrelated protein (see Methods). The figure shows a representative experiment.
B: Flow cytometry detection of Fl-MTX accumulation in Sf9 cells overexpressing an unrelated protein (control), OATP1B1 or 1B3. Cells were incubated with 1 μM Fl-MTX in the presence (gray line) or absence (dashed line) of 20 μM cyclosporin A for 10 minutes at 37°C, pH 7.4 (see Methods). The figure shows a representative experiment.
C 1 μM Fl-MTX uptake determined by flow cytometry at different time points in the presence or absence of 20 μM cyclosporin A (CsA). The data points represent the average geomean +/− SD fluorescence values obtained in three independent experiments.
D Accumulation (2 minutes) of various concentrations of Fl-MTX in insect cells was determined by flow cytometry. Average geomean +/− SD values of three independent experiments are shown.
As shown in Figure 1B, Sf9 cells transduced with OATP1B1 and 1B3 cDNA exhibit increased accumulation of Fl-MTX compared to control cells infected with a recombinant virus containing the cDNA of an unrelated protein. The observed transport was rapid, showing saturation kinetics with the following t1/2 values: 2.64 +/− 0.53 and 4.16 +/− 0.28 min for 1B1 and 1B3, respectively. OATP1B1- and 1B3-mediated uptake was absent at 4°C (not shown) and in the presence of a known inhibitor, cyclosporin A [25]. The Km values of 0.23 +/− 0.04 μM and 0.53 +/− 0.40 μM for 1B1 and 1B3, respectively, were lower than those observed in mammalian cells [14] (Figure 1, Panels C and D).
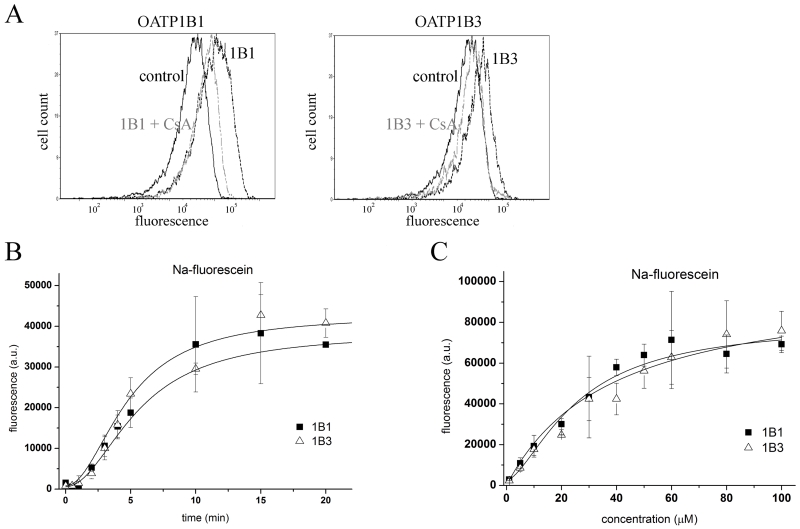
The transport of another well-known fluorescent substrate, Na-Fluo, in OATP1B1 or 1B3 overexpressing Sf9 cells (Figure 2A) was similar to that observed for Fl-MTX, showing a rapid uptake (t1/2 6.71 +/− 2.33 and 5.58 +/− 0.11 for 1B1 and 1B3, respectively) and Km values of 25.73 +/− 4.68 μM and 38.55 +/− 15.00 μM for 1B1 and 1B3, respectively (Figure 2B and C). These results correspond to those obtained in mammalian cells [13].
Figure 2. Na-fluorescein uptake in Sf9 cells overexpressing human OATP1B1 or 1B3.
A Histograms show the fluorescence of 1 μM Na-Fluo in Sf9 cells expressing an unrelated protein (control), OATP1B1 or OATP1B3 in the presence or absence of 20 μM cyclosporin A (CsA). The result of 1 representative experiment is shown.
B 1 μM Na-Fluo accumulation was measured for 0.5-20 minutes at 37°C and pH 7.4. Geomean fluorescence in 1B1 or 1B3-overexpressing insect cells (minus background fluorescence measured in unrelated virus-infected cells) was determined by flow cytometry. Data points represent the average of three independent experiments +/− SD values.
C Accumulation of various concentrations (see axis x) of Na-Fluo after incubation for 4 minutes at 37°C and pH 7.4 as measured by flow cytometry. Geomean values of three independent experiments +/− SD values are shown.
To further characterize OATP1B1/1B3-mediated transport, we examined the effect of estradiol 17–β–D-glucuronide, various bile acids and other previously identified OATP1B1/1B3-interacting compounds on the uptake of Fl-MTX or Na-Fluo (Figure 3). We observed a significant inhibitory effect on OATP1B1/1B3-mediated transport of the majority of these compounds (but not in control cells), confirming that the observed uptake in insect cells was due to the activity of the overexpressed human proteins. However, the inhibitory potential of the tested compounds differed for the two substrates used, which may be due to potential differences in compound-specific binding sites.
Figure 3. Effect of various OATP-interacting compounds on the uptake of Fl-MTX or Na-Fluo.
Accumulation of 1 μM Fl-MTX or 1 μM Na-Fluo at 37°C for 10 minutes at pH 7.4 was measured in the absence (nt) or presence of various compounds. Activity is determined by comparing the percentage of fluorescence compared to control, non-treated cells. Bars represent the average values of at least three independent experiments (+/− SD). nt: non-treated control, CsA: 20 μM cyclosporin A, UA: 20 μM ursolic acid, CA: 150 μM cholic acid, GC: 150 μM glycocholate, TC: 150 μM taurocholate, EG: 50 μM estradiol 17-β–D-glucuronide, PGE2: 5 μM prostaglandin E2, Rif: 10 μM rifampicin, and MTX: 10 μM methotrexate. Significantly different form the non-treated control: * p< 0.05, ** p<0.01, *** p<0.001.
3.2. Functional expression of the entire human OATP family in Sf9 insect cells reveals that fluorescein is a pan-OATP substrate
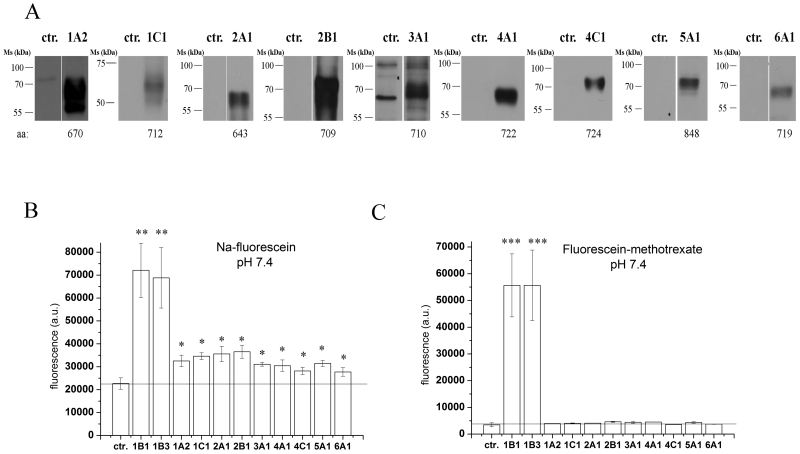
After confirming that the insect cell system is suitable for the functional expression of OATP1B1 and 1B3, we generated Sf9 cells overexpressing the remaining 9 human OATPs: 1A2, 1C1, 2A1, 2B1, 3A1, 4A1, 4C1, 5A1 and 6A1. The expression levels of all proteins were determined via Western blotting (Figure 4A). Specific bands corresponding to the core-glycosylated proteins were detected in each case. [26].
Figure 4. Overexpression and functional characterization of the entire human OATP panel in insect cells.
A Western blot detection of various human OATPs overexpressed in insect cells. Total cell lysates (5 μg for 1C1, 2B1, 4A1, 4C1 and 10 μg for 1A2, 1B1, 1B3, 2A1, 3A1, 5A1 and 6A1) were loaded on 7.5% Laemmli gels. After transferring onto PVDF membranes, the proteins were labeled with the specific antibodies (see the Methods). Control (ctr.) represents Sf9 cells expressing an unrelated protein.
B and C Sf9 cells overexpressing the given OATP (see axis x) or an unrelated protein (ctr.) were incubated with 1 μM Na-Fluo (B) or 1 μM Fl-MTX (C) at 37°C for 10 minutes at pH 7.4. Intracellular fluorescence was determined by flow cytometry. Graphs show geomean fluorescence, bars represent the average of three independent experiments +/− SD values. Significantly different from the control: * p< 0.05, ** p<0.01, *** p<0.001.
Using transport conditions that were optimized for OATP1B1 and 1B3 (10 min incubation at 37°C, pH 7.4, using 1 μM of substrate), we measured Na-Fluo and Fl-MTX uptake in insect cells that expressed individual members of the entire human OATP family. As shown in Figure 4B, all members of the human OATP family are able to transport fluorescein. In contrast, Fl-MTX was only transported by OATP1B1 and 1B3 (Figure 4C).
3.3. Effect of pH on Na-fluorescein and Fl-MTX transport
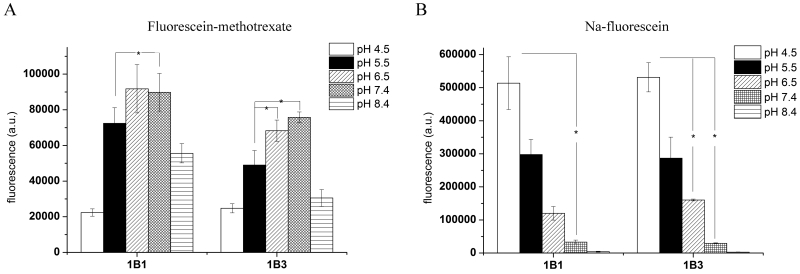
The above described experiments were performed at pH 7.4. However, previous studies demonstrated that acidic extracellular pH increases the transport of various compounds by the majority of OATPs [27]. As Sf9 cells are grown at pH 6.2, it was possible that the use of a neutral transport buffer precluded the formation of the inwardly directed H+ gradient necessary for optimal transport by pH-sensitive OATPs. To address this issue and to investigate how the extracellular milieu may influence OATP transport in insect cells, we tested the effect of various buffers, ranging from pH 4.5 to pH 8.5, on OATP1B1/1B3-mediated uptake. Using pH-adjusted buffers, we demonstrate that OATP1B1 and 1B3 are able to transport Fl-MTX over a wide pH range, although maximal activity is observed at a neutral pH (Figure 5A). Interestingly, however, Na-Fluo transport was significantly enhanced in an acidic extracellular environment, suggesting that the transport of Na-Fluo may be triggered by a proton gradient (Figure 5B). The emission intensity of fluorescein decreases at acidic pH values ([28], and data not shown). Therefore, the elevated Na-Fluo signal observed under acidic pH conditions likely reflects increased Na-Fluo uptake and not a non-specific increase in substrate fluorescence.
Figure 5. Uptake of Fl-MTX and Na-Fluo using different buffers.
Insect cells overexpressing OATP1B1 or 1B3 were incubated with 1 μM Fl-MTX (panel A) or 1 μM Na-Fluo (panel B) in various buffers with a pH range from 4.5 to 8.5 (see Methods) for 10 minutes at 37°C. Values represent geomean fluorescence minus the background fluorescence detected in control cells. Data were obtained from three independent flow cytometry experiments. Bars show +/− SD values. Student’s t-test, significant difference: * p< 0.05.
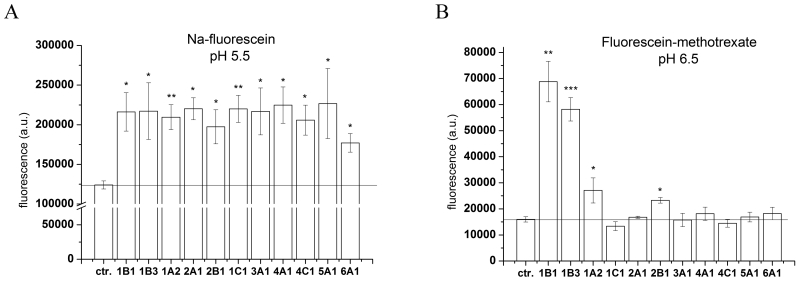
Because the transport of Na-Fluo by OATP1B1 and 1B3 was significantly enhanced under acidic assay conditions, we examined the effect of acidic pH on the transport activity of the entire human OATP panel. Investigation of OATP1B1/1B3-mediated transport revealed that the optimal pH range for Fl-MTX transport in Sf9 cells is 6.5-7.4, while that of Na-Fluo is 4.5-5.5. To achieve maximal activity under physiologically relevant conditions, we tested Fl-MTX and Na-Fluo uptake by all human OATPs at pH 6.5 and 5.5, respectively. Consistent with the results observed for OATP1B1 and 1B3, acidification of the extracellular milieu resulted in increased Na-fluorescein transport by all human OATPs despite the increased background fluorescence (Figure 6A). In contrast, an acidic environment only activated Fl-MTX transport for OATP1A2 and 2B1, as the remaining OATPs were inactive (Figure 6B).
Figure 6. Na-Fluo or Fl-MTX uptake at acidic pH in Sf9 cells overexpressing the entire human OATP panel.
Sf9 cells overexpressing OATPs (see axis x) were incubated with 1 μM Na-Fluo (A) or 1 μM Fl-MTX (B) for 10 minutes at 37°C in buffer pH 5.5 (panel A) or pH 6.5 (panel B). Columns show geomean fluorescence. Bars represent an average of three independent experiments +/− SD values. Significantly different from the control: * p< 0.05, ** p<0.01, *** p<0.001.
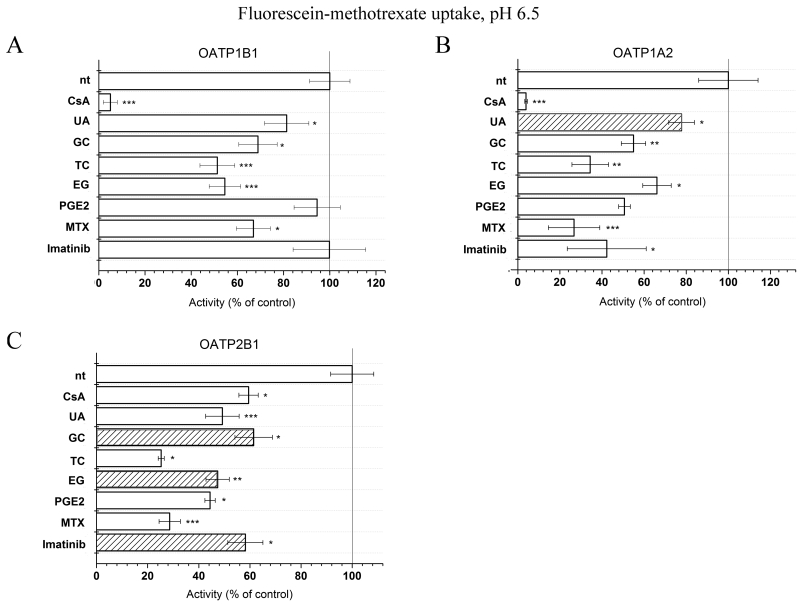
3.4. Effect of inhibitors
To determine whether the insect cell-based Na-Fluo or Fl-MTX assay is suitable for testing substrates/inhibitors of all human OATPs, we repeated the assays in the presence of several previously described OATP-interacting compounds. Figure 7 shows that despite lower baseline transport rates, the transport of Fl-MTX by OATP1A2 and 2B1 was inhibited by CsA, ursolic acid, bile acids, PGE2, MTX and Imatinib. The optimized assay conditions allowed for the identification of several novel OATP-interacting compounds. We demonstrate that ursolic acid, a pentacyclic triterpenoid found in fruit rind influences the activity of OATP1A2. Further, we find that estradiol 17-β-D-glucuronide, glycocholate and Imatinib modulate the function of OATP2B1.
Figure 7. Effect of various compounds on the accumulation of Fl-MTX in insect cells overexpressing OATP1B1, 1A2 or 2B1.
Cells were incubated with 1 μM Fl-MTX at pH 6.5 for 10 minutes in the presence or absence of various compounds. Columns represent activity as a percentage of non-treated (control) fluorescence measured without the added inhibitors/substrates. Measurements were repeated at least four times, columns show average +/− SD values. Striped bars indicate newly identified interactions. * p< 0.05, ** p<0.01 and *** p<0.001 indicate significant difference from the non-treated control.
Similar results were obtained using the Na-Fluo uptake assay (Figure 8). We found that the transport activity for each human OATP was influenced by at least one test compound. Cyclosporin A inhibited the uptake mediated by OATP1B3, 1A2, 2A1 and 2B1, prostaglandin E2 decreased the transport by OATP1A2, 1C1, 2A1, 2B1 and 4C1, and ursolic acid by OATP2A1. Interestingly, estradiol 17-β-D-glucuronide and ursolic acid induced the Na-Fluo transport activity of several OATPs, suggesting that Na-Fluo transport may be allosterically activated by these compounds.
Figure 8. Effect of various compounds on the accumulation of Na-Fluo in insect cells overexpressing the entire OATP panel (A-D).
Cells were incubated with 1 μM Na-Fluo at pH 5.5 for 10 minutes in the presence of 20 μM cyclosporin A, 50 μM estradiol 17-β–D-glucuronide, 5 μM prostaglandin E2 or 20 μM ursolic acid. Columns represent activity as a percentage of the non-treated (control) fluorescence measured without the added inhibitors/substrates. Columns show average +/− SD values, n=4. Striped columns indicate new interactions. Significance was determined by Student’s t-test. * p< 0.05, ** p<0.01, *** p<0.001.
4. Discussion
Members of the OATP family are major determinants of the uptake and elimination of drugs [2, 29]. Administration of drugs or food components that are either substrates or inhibitors of OATPs may result in adverse drug toxicity [29]. Therefore, the investigation of a potential interaction between OATPs with known pharmacological relevance (OATP1B1 and 1B3) and a new molecular entity is recommended by the International Transporter Consortium [30], the US Food and Drug Administration and by the European Medicines Agency. In addition to OATP1B1 and 1B3, OATP1A2 and 2B1 are also known to transport a wide variety of substrates. Since these transporters are expressed at high levels in the intestine (2B1), liver (2B1) and the blood-brain barrier (1A2, 2B1), they should also be considered as important determinants of drug pharmacokinetics. Additionally, several OATPs show cancer-specific expression and therefore may be exploited in targeted drug delivery [1, 31]. Clearly, information on the substrate specificity and the role in ADME-T (Absorption, Distribution, Metabolism, Excretion and Toxicity) for all of the human OATPs is needed.
Given the absence of suitable functional assays, most members of the human OATP family remain poorly characterized. The major impediment to the investigation of OATP function is the limited availability of radioactively- or fluorescently-labeled substrates and OATP-specific inhibitors. Functional studies are also hindered by the limited number of cellular models with well-characterized transporter expression. To address these issues, we established an insect cell-based fluorescent transport assay for the investigation of the entire human OATP family. The main advantage of this approach compared to mammalian expression systems is the high level of protein expression, and therefore, the lack of a significant background transport activity. However, to date, only OATP2B1 was studied in insect cells. Tschantz and colleagues demonstrated functional expression of OATP2B1 in Sf9 insect cells, with saturable uptake of [3H]estrone-3-sulphate [24]. In this study, we focused on the narrow window of time when the cells express high levels of the transporter, but are still viable. This setup enabled the measurement of OATP-mediated intracellular accumulation of fluorescent substrates in intact cells. Assays using fluorescent compounds are exceptionally suited for the measurement of transport activity given the relative low cost of test substrates, the sensitivity of the transport assay and the potential for application in a high-throughput setup. However, fluorescent substrate-based transport assays were available previously only for OATP1B1 and 1B3 [13-17, 32].
Proteins overexpressed in the insect cell system are core-glycosylated [26] and several studies indicated that the lack of N-glycosylation impairs OATP function [24, 33-35]. To assess the functionality of OATPs expressed in Sf9 cells, we first tested two well-characterized OATPs, OATP1B1 and 1B3, for their ability to transport known substrates. We found that OATP1B1 and 1B3 mediate a rapid, saturable, temperature- and inhibitor-sensitive uptake of Na-Fluo and Fl-MTX (Figures 1 and 2) similar to that observed in mammalian cells [13, 14]. Moreover, we demonstrated that known OATP1B-interacting compounds inhibit the uptake activity of both transporters, although the degree of inhibition differed for each substrate (Figure 3). The molecular mechanism underlying this substrate-dependent inhibition, a well-documented characteristic of OATP1B transporters, is likely due to the difference in affinity of the binding site for the individual substrates [14]. Our data suggest that several assays, using a variety of substrates, will be required to evaluate the pharmacological relevance of OATP-drug interactions.
Several laboratories have found that the acidic extracellular milieu stimulates OATP-mediated transport [27, 36-42]. In the human body, acidic environments may be found under both physiological (in the intestine and kidney) and pathological conditions (in tumors, during inflammation or liver disease). Therefore, a pH-dependent activation of OATP function may provide tissue-specific or local regulation of these proteins [43]. When we investigated the pH dependence of OATP1B1/1B3-mediated transport (Figure 5), we found that Fl-MTX transport is maximal at near neutral pH, while Na-Fluo uptake is significantly increased in an acidic environment. The other OATPs had low level Na-Fluo transport at pH 7.4 (Figure 4B) even when higher substrate concentrations (5-10 μM) and a longer incubation time (30 minutes) were used (data not shown). Lowering the pH to 5.5, however, resulted in a significant increase in Na-Fluo transport by all OATPs (Figure 6A), demonstrating that Na-Fluo is a general, pH-induced OATP substrate. The precise mechanism by which the acidic milieu triggers OATP transport is not fully understood. A low extracellular pH (low [H]+EC) may result in the protonation of the transporter, i.e. on a pH-sensitive histidine (His) residue located in the third transmembrane domain region [27], or it may alter the charge of the transported molecule. Leuthold and colleagues demonstrated that OATP1C1, lacking the pH sensor His, is not activated by an acidic extracellular milieu [27]. Although we found that OATP1C1-mediated transport was activated by low [H]+EC, this discrepancy may be due to the different substrates that were used.
The Na-Fluo assay is the first transport assay suitable for the characterization of the entire human OATP family. Using a slightly acidified transport buffer (pH 6.5), we identified Fl-MTX as a new substrate of OATP1A2 and 2B1 (Figure 6B), suggesting that Fl-MTX can also be used to test substrate interactions for these transporters (Figure 7). The identification of physiological substrates is of particular importance for poorly characterized members of the OATP family, including OATP5A1, which has only been documented to transport quercetin [6], and OATP6A1, whose function and physiological substrates remain unknown. The relevance of a functional assay encompassing the entire human OATP family is that it may serve as a tool for the identification of new molecular interactions. To test if our assay is able to identify new substrates and inhibitors, we tested and confirmed previously documented interactions of OATP1A2, 1B1, 1B3 and 2B1 with bile acids, cyclosporin A, PGE2 and methotrexate (Figures 3, 7 and 8). Furthermore, we identified novel interacting compounds: UA for 1A2, 2A1, 3A1, 4A1, 4C1 and 6A1, cyclosporin A for 2A1, EG for 3A1, 5A1 and 6A1, and PGE2 for 1C1 and 4C1 (Figures 7 and 8). These data indicate a potential role for new members of the family (OATP3A1, 5A1 and 6A1) in hormone transport. It has to be noted that our results do not reveal the mechanism of inhibition, as compounds interacting with the transport of the fluorescent test substrate may be competitive or noncompetitive inhibitors. Given that OATP5A1 and 6A1 expression levels are elevated in various cancers [1, 44], these findings may have significant implications regarding the survival of hormone-dependent tumors. The results from our fluorescent assay analysis revealed novel interacting compounds (estradiol 17-β–D-glucuronide, glycocholate and Imatinib), even for the relatively well-characterized 2B1 (Figure 7). The interaction between OATP2B1 and Imatinib, a Bcr-Abl inhibitor used in the treatment of chronic myeloid leukemia, supports the notion that SNPs in SLCO2B1 may influence Imatinib pharmacokinetics resulting in inter-individual differences in therapy response [45].
Our assay also revealed unexpected interactions. In contrast to De Bruyn et al., we found significant Na-Fluo transport mediated by OATP2B1 at pH 7.4 [13]. In the case of OATP1B1, Na-Fluo transport at pH 5.5 was activated, rather than inhibited, by cyclosporin A, EG, PGE2 and UA. This activating effect appeared to be pH-specific, as these compounds inhibited Na-Fluo uptake at pH 7.4 (Figure 3). In experiments not documented here, we found a similar, pH-dependent activation of Fl-MTX transport by several known interacting compounds. A similar activating effect was found by EG and UA, which enhanced the Na-Fluo transport activity of 3A1, 5A1 6A1, and 3A1, 4A1, 4C1 and 6A1, respectively (Figure 8). These data suggest that transport activity is allosterically modulated, a mechanism previously documented for OATP1B3 and 2B1 [14, 46, 47]. Alternatively, a co-transport mechanism, with one substrate stimulating the uptake of the other, is also feasible, although, this would need to be confirmed by measuring the transport of labeled EG, PGE2 or UA, in the presence of Na-Fluo.
In summary, we established a novel functional assay for the investigation of the entire human OATP family. We demonstrate that the insect cell-based assay provides a useful tool for the systemic, comparative study of the function of each OATP. Functional characterization of the OATPs offers a chance for the identification of physiological substrates, the investigation of the role of SNPs in substrate recognition, and paves the way for a vesicle-based high-throughput screening assay.
Acknowledgements
We are grateful to Nóra Kucsma for her technical help and to Balázs Sarkadi and András Váradi for scientific discussions.
Funding: This research has been supported by the Hungarian Research Fund (OTKA 109423), the Momentum Program of the Hungarian Academy of Sciences, the TTK_All grant of the Research Centre for Natural Sciences, Hungarian Academy of Sciences (0611-15405) and by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (Cs. Ö-L.). Csilla Özvegy-Laczka is a recipient of a fellowship by the MedInProt program. BS is supported by grant # 310030_144195 from the Swiss National Science foundation. BH is funded by National Institutes of Health grant GM077336.
Abbreviations
- CA
cholic acid
- CsA
cyclosporin A
- EG
estradiol 17-β–D-glucuronide
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MES
4-Morpholineethanesulfonic acid
- Na-Fluo
Na-fluorescein
- Fl-MTX
fluorescein-methotrexate
- GC
glycocholate
- MTX
methotrexate
- PGE2
prostaglandin E2
- PVDF
Polyvinylidene fluoride
- Rif
rifampicin
- Sf9
Spodoptera frugiperda insect cells
- TC
taurocholate
- UA
ursolic acid
Footnotes
Chemical compounds studied in this article
Sodium fluorescein (PubChem CID: 16850); fluorescein-methotrexate (PubChem CID: 86761749); Imatinib (PubChem CID: 5291); estradiol 17-β-D-glucuronide (PubChem CID: 66424); ursolic acid (PubChem CID: 64945); cyclosporin A (PubChem CID: 5284373); prostaglandin E2 (PubChem CID: 5280360); glycocholate (PubChem CID: 5460316); taurocholate (PubChem CID: 6675)
Authorship Contributions:
Participated in research design and data analysis: I. P., D. K., Cs. Ö-L.
Performed experiments: I. P., D. K., O. N., Gy. V., M. G., Cs. Ö-L.
Cloned the cDNA constructs for OATP1B1, 3A1 and 4C1 and developed the antibodies against OATP1A2, 1B1, 1B3, 1C1, 2B1, 3A1_v1 and 4A1: B. S., B. H.
Wrote the manuscript: D. K., I. P., G. Sz. and Cs. Ö-L.
Conflict of interest: The authors declare that they have no conflict of interest.
References
- [1].Buxhofer-Ausch V, Secky L, Wlcek K, Svoboda M, Kounnis V, Briasoulis E, et al. Tumor-specific expression of organic anion-transporting polypeptides: transporters as novel targets for cancer therapy. J Drug Deliv. 2013;2013:863539. doi: 10.1155/2013/863539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang Y, Hays A, Noblett A, Thapa M, Hua DH, Hagenbuch B. Transport by OATP1B1 and OATP1B3 enhances the cytotoxicity of epigallocatechin 3-O-gallate and several quercetin derivatives. J Nat Prod. 2013;76:368–73. doi: 10.1021/np3007292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hagenbuch B, Stieger B. The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med. 2013;34:396–412. doi: 10.1016/j.mam.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–87. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bossuyt X, Muller M, Hagenbuch B, Meier PJ. Polyspecific drug and steroid clearance by an organic anion transporter of mammalian liver. The Journal of pharmacology and experimental therapeutics. 1996;276:891–6. [PubMed] [Google Scholar]
- [6].Glaeser H, Bujok K, Schmidt I, Fromm MF, Mandery K. Organic anion transporting polypeptides and organic cation transporter 1 contribute to the cellular uptake of the flavonoid quercetin. Naunyn-Schmiedeberg’s archives of pharmacology. 2014;387:883–91. doi: 10.1007/s00210-014-1000-6. [DOI] [PubMed] [Google Scholar]
- [7].Olszewski-Hamilton U, Svoboda M, Thalhammer T, Buxhofer-Ausch V, Geissler K, Hamilton G. Organic Anion Transporting Polypeptide 5A1 (OATP5A1) in Small Cell Lung Cancer (SCLC) Cells: Possible Involvement in Chemoresistance to Satraplatin. Biomarkers in cancer. 2011;3:31–40. doi: 10.4137/BIC.S7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, et al. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest. 2010;120:2942–52. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van de Steeg E, van der Kruijssen CM, Wagenaar E, Burggraaff JE, Mesman E, Kenworthy KE, et al. Methotrexate pharmacokinetics in transgenic mice with liver-specific expression of human organic anion-transporting polypeptide 1B1 (SLCO1B1) Drug Metab Dispos. 2009;37:277–81. doi: 10.1124/dmd.108.024315. [DOI] [PubMed] [Google Scholar]
- [10].Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 2012;153:1528–37. doi: 10.1210/en.2011-1633. [DOI] [PubMed] [Google Scholar]
- [11].Clarke JD, Cherrington NJ. Genetics or environment in drug transport: the case of organic anion transporting polypeptides and adverse drug reactions. Expert Opin Drug Metab Toxicol. 2012;8:349–60. doi: 10.1517/17425255.2012.656087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gozalpour E, Greupink R, Wortelboer HM, Bilos A, Schreurs M, Russel FG, et al. Interaction of digitalis-like compounds with liver uptake transporters NTCP, OATP1B1, and OATP1B3. Molecular pharmaceutics. 2014;11:1844–55. doi: 10.1021/mp400699p. [DOI] [PubMed] [Google Scholar]
- [13].De Bruyn T, Fattah S, Stieger B, Augustijns P, Annaert P. Sodium fluorescein is a probe substrate for hepatic drug transport mediated by OATP1B1 and OATP1B3. J Pharm Sci. 2011;100:5018–30. doi: 10.1002/jps.22694. [DOI] [PubMed] [Google Scholar]
- [14].Gui C, Obaidat A, Chaguturu R, Hagenbuch B. Development of a cell-based high-throughput assay to screen for inhibitors of organic anion transporting polypeptides 1B1 and 1B3. Curr Chem Genomics. 2010;4:1–8. doi: 10.2174/1875397301004010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baldes C, Koenig P, Neumann D, Lenhof HP, Kohlbacher O, Lehr CM. Development of a fluorescence-based assay for screening of modulators of human organic anion transporter 1B3 (OATP1B3) European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2006;62:39–43. doi: 10.1016/j.ejpb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- [16].de Graaf W, Hausler S, Heger M, van Ginhoven TM, van Cappellen G, Bennink RJ, et al. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol. 2011;54:738–45. doi: 10.1016/j.jhep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- [17].Yamaguchi H, Okada M, Akitaya S, Ohara H, Mikkaichi T, Ishikawa H, et al. Transport of fluorescent chenodeoxycholic acid via the human organic anion transporters OATP1B1 and OATP1B3. Journal of lipid research. 2006;47:1196–202. doi: 10.1194/jlr.M500532-JLR200. [DOI] [PubMed] [Google Scholar]
- [18].Ozvegy C, Varadi A, Sarkadi B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. J Biol Chem. 2002;277:47980–90. doi: 10.1074/jbc.M207857200. [DOI] [PubMed] [Google Scholar]
- [19].Huber RD, Gao B, Sidler Pfandler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. American journal of physiology Cell physiology. 2007;292:C795–806. doi: 10.1152/ajpcell.00597.2005. [DOI] [PubMed] [Google Scholar]
- [20].Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–33. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- [21].Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992;267:4854–8. [PubMed] [Google Scholar]
- [22].Bakos E, Hegedus T, Hollo Z, Welker E, Tusnady GE, Zaman GJ, et al. Membrane topology and glycosylation of the human multidrug resistance-associated protein. J Biol Chem. 1996;271:12322–6. doi: 10.1074/jbc.271.21.12322. [DOI] [PubMed] [Google Scholar]
- [23].Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, et al. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun. 2001;285:111–7. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- [24].Tschantz WR, Pfeifer ND, Meade CL, Wang L, Lanzetti A, Kamath AV, et al. Expression, purification and characterization of the human membrane transporter protein OATP2B1 from Sf9 insect cells. Protein Expr Purif. 2008;57:163–71. doi: 10.1016/j.pep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- [25].Shitara Y, Itoh T, Sato H, Li AP, Sugiyama Y. Inhibition of transporter-mediated hepatic uptake as a mechanism for drug-drug interaction between cerivastatin and cyclosporin A. The Journal of pharmacology and experimental therapeutics. 2003;304:610–6. doi: 10.1124/jpet.102.041921. [DOI] [PubMed] [Google Scholar]
- [26].Contreras-Gomez A, Sanchez-Miron A, Garcia-Camacho F, Molina-Grima E, Chisti Y. Protein production using the baculovirus-insect cell expression system. Biotechnol Prog. 2014;30:1–18. doi: 10.1002/btpr.1842. [DOI] [PubMed] [Google Scholar]
- [27].Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. American journal of physiology Cell physiology. 2009;296:C570–82. doi: 10.1152/ajpcell.00436.2008. [DOI] [PubMed] [Google Scholar]
- [28].Sjöback R, Nygren J, Kubista M. Absorption and fluorescence properties of fluorescein. Spectrochimica Acta Part A. 1995;51:L7–L21. [Google Scholar]
- [29].Maeda K. Organic anion transporting polypeptide (OATP)1B1 and OATP1B3 as important regulators of the pharmacokinetics of substrate drugs. Biological & pharmaceutical bulletin. 2015;38:155–68. doi: 10.1248/bpb.b14-00767. [DOI] [PubMed] [Google Scholar]
- [30].Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nature reviews Drug discovery. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu T, Li Q. Organic anion-transporting polypeptides: a novel approach for cancer therapy. Journal of drug targeting. 2014;22:14–22. doi: 10.3109/1061186X.2013.832767. [DOI] [PubMed] [Google Scholar]
- [32].Yamaguchi H, Kobayashi M, Okada M, Takeuchi T, Unno M, Abe T, et al. Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer letters. 2008;260:163–9. doi: 10.1016/j.canlet.2007.10.040. [DOI] [PubMed] [Google Scholar]
- [33].Lee TK, Koh AS, Cui Z, Pierce RH, Ballatori N. N-glycosylation controls functional activity of Oatp1, an organic anion transporter. American journal of physiology Gastrointestinal and liver physiology. 2003;285:G371–81. doi: 10.1152/ajpgi.00358.2002. [DOI] [PubMed] [Google Scholar]
- [34].Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. The Journal of biological chemistry. 2005;280:9610–7. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- [35].Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. American journal of physiology Gastrointestinal and liver physiology. 2008;294:G1052–9. doi: 10.1152/ajpgi.00584.2007. [DOI] [PubMed] [Google Scholar]
- [36].Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. The Journal of pharmacology and experimental therapeutics. 2003;306:703–8. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- [37].Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. The Journal of pharmacology and experimental therapeutics. 2004;308:438–45. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- [38].Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, et al. Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug Metab Dispos. 2006;34:1423–31. doi: 10.1124/dmd.106.009530. [DOI] [PubMed] [Google Scholar]
- [39].Satlin LM, Amin V, Wolkoff AW. Organic anion transporting polypeptide mediates organic anion/HCO3- exchange. J Biol Chem. 1997;272:26340–5. doi: 10.1074/jbc.272.42.26340. [DOI] [PubMed] [Google Scholar]
- [40].Kanai N, Lu R, Bao Y, Wolkoff AW, Schuster VL. Transient expression of oatp organic anion transporter in mammalian cells: identification of candidate substrates. The American journal of physiology. 1996;270:F319–25. doi: 10.1152/ajprenal.1996.270.2.F319. [DOI] [PubMed] [Google Scholar]
- [41].Marin JJ, Mangas D, Martinez-Diez MC, El-Mir MY, Briz O, Serrano MA. Sensitivity of bile acid transport by organic anion-transporting polypeptides to intracellular pH. Biochimica et biophysica acta. 2003;1611:249–57. doi: 10.1016/s0005-2736(03)00080-4. [DOI] [PubMed] [Google Scholar]
- [42].Nishimura T, Kubo Y, Kato Y, Sai Y, Ogihara T, Tsuji A. Characterization of the uptake mechanism for a novel loop diuretic, M17055, in Caco-2 cells: involvement of organic anion transporting polypeptide (OATP)-B. Pharmaceutical research. 2007;24:90–8. doi: 10.1007/s11095-006-9127-x. [DOI] [PubMed] [Google Scholar]
- [43].Martinez-Becerra P, Briz O, Romero MR, Macias RI, Perez MJ, Sancho-Mateo C, et al. Further characterization of the electrogenicity and pH sensitivity of the human organic anion-transporting polypeptides OATP1B1 and OATP1B3. Molecular pharmacology. 2011;79:596–607. doi: 10.1124/mol.110.069971. [DOI] [PubMed] [Google Scholar]
- [44].Brenner S, Klameth L, Riha J, Scholm M, Hamilton G, Bajna E, et al. Specific expression of OATPs in primary small cell lung cancer (SCLC) cells as novel biomarkers for diagnosis and therapy. Cancer Lett. 2015;356:517–24. doi: 10.1016/j.canlet.2014.09.025. [DOI] [PubMed] [Google Scholar]
- [45].Gong IY, Kim RB. Impact of genetic variation in OATP transporters to drug disposition and response. Drug metabolism and pharmacokinetics. 2013;28:4–18. doi: 10.2133/dmpk.dmpk-12-rv-099. [DOI] [PubMed] [Google Scholar]
- [46].Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. European journal of pharmacology. 2008;584:57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Grube M, Kock K, Karner S, Reuther S, Ritter CA, Jedlitschky G, et al. Modification of OATP2B1-mediated transport by steroid hormones. Molecular pharmacology. 2006;70:1735–41. doi: 10.1124/mol.106.026450. [DOI] [PubMed] [Google Scholar]