Abstract
Animals and humans are chronically exposed to endocrine disrupting chemicals (EDCs) which are ubiquitous in the environment. There are strong circumstantial links between environmental EDC exposure and both declining human/wildlife reproductive health and the increasing incidence of reproductive system abnormalities. Verification of such links, however, is difficult and requires animal models exposed to 'real life', environmentally relevant concentrations/mixtures of environmental contaminants (ECs), particularly in- utero, when sensitivity to EC exposure is high.
The aim of this study was to determine whether the fetal sheep reproductive neuroendocrine axis, particularly GnRH and galaninergic systems were affected by maternal exposure to a complex mixture of chemicals, applied to pasture, in the form of sewage sludge. Sewage sludge contains high concentrations of a spectrum of EDCs and other pollutants, relative to environmental concentrations but is frequently recycled to land as a fertiliser. We found that foetuses exposed, to the EDC mixture in-utero through their mothers, had lower GnRH mRNA expression in the hypothalamus and lower GnRHR and galanin receptor (GALR) mRNA expression in the hypothalamus and pituitary gland. Strikingly, this, treatment had no significant effect on maternal GnRH or GnRHR mRNA expression although GALR mRNA expression within the maternal hypothalamus and pituitary gland was reduced. This study clearly demonstrates that the developing foetal neuroendocrine axis is sensitive to real-world mixtures of environmental chemicals. Given the important role of GnRH and GnRHR in the regulation of reproductive function, its known in-utero programming role, and the role of galanin in the regulation of many physiological/neuroendocrine systems, in-utero changes in the activity of these systems are likely to have long term consequences in adulthood and represent a novel pathway through which EC mixtures could perturb normal reproductive function.
Keywords: GnRH, galanin, hypothalamus, pituitary, sheep
Introduction
There is a wealth of evidence linking exposure to environmental chemicals (ECs) with harmful effects on physiological systems in both animals and humans (1–4). Exposure to such chemicals, is increasingly associated with disorders of male reproductive function (1–3), precocious puberty (4, 5) early menopause and breast cancer in females (4, 6). ECs are typically present as complex mixtures and include endocrine disrupting compounds (EDCs), such as phthalates, polychlorinated biphenyls (PCBs) and nonylphenol (7, 8), and heavy metals. Given that these diverse compounds can act via different mechanisms to perturb endocrine function (9), investigation of these complex interactions presents a major emerging issue for research and risk assessment.
Exposure to environmental pollutants can occur via inhalation, ingestion and direct contact but the health impacts are influenced by a variety of physiological parameters including rates of uptake, metabolism and excretion. Prenatal and early developmental life stages are particularly sensitive to EDCs (10) and exposure during these critical periods may programme processes influencing adult reproductive health and productivity (11). Such exposures can be via the placenta or, post-natally, via milk. Much of the evidence for detrimental effects of ECs has come from studies of acute supra-environmental doses of single chemicals (12) although these don’t take account of the fact that humans, domestic animals and wildlife are chronically exposed to mixtures of low levels of environmental pollutants which can also exert significant effects (6). Sewage sludge, is a by-product of waste water treatment that contains a variety of pollutants (13–15), at levels higher than those found within our immediate environment (16). At present, most sewage sludge is either incinerated or recycled onto pasture, as a fertiliser. The use of sewage sludge as a fertiliser provides an ideal model system in which to study the effects of exposure to environmentally relevant levels of EDCs (after the EDC burden is diluted by spreading on pasture) on physiological systems. Sewage sludge application to pasture results in only a slight increase in EDC concentrations in the soil, grazed herbage and in livers of animals reared on sludge-exposed pastures (14, 17, 18). On the basis of reported “no observable effect levels (NOELs)” the observed concentrations of individual chemicals would not be expected to pose a significant health risks. Nevertheless, domestic animals exposed to EDCs using this model have numerous physiological and neuroendocrine abnormalities, including altered foetal testis and ovary development (19, 20), foetal hypothalamic kisspeptin mRNA expression (21), behavioural changes in adult offspring (22) and maternal bone density (15).
Reproductive success depends on activity within the hypothalamic gonadotrophin-releasing hormone (GnRH) neurosecretory system which is dynamically regulated and highly sensitive to the organisational and activational effects of endogenous steroids (23, 24). Galanin, a 29-amino acid cellular messenger (neurotransmitter/modulator) widely distributed within the central (CNS) and peripheral nervous system (PNS) is involved in a wide range of regulatory functions in many species. Galanin has clearly been shown to be involved in the oestrogenic regulation of hypothalamic GnRH secretion (25) and LH secretion from the pituitary gland in laboratory rodents (26). Despite fewer functional studies in sheep, reports again support a role for galanin in the regulation of reproductive function in this species since galanin is expressed in appropriate areas of the hypothalamus (27), it is colocalised within GnRH neurones in both males and females (28) and the coexpression of galanin and ERα within the preoptic area is oestradiol responsive (29). Galanin acts via three receptor subtypes: GALR1, GALR2, and GALR3 all of which are expressed in the sheep hypothalamus (24, 30) and have been shown to be expressed on GnRH neurones (31). We therefore proposed that galanin and its receptors are potential EDC target systems that could affect the activity within the reproductive neuroendocrine system.
The primary aim of this study was to test the hypothesis that maternal exposure to sewage sludge would suppress GnRH mRNA expression and inhibit mRNA expression within galaninergic systems (either galanin or galanin receptor mRNA expression) in the foetal hypothalamus and pituitary gland. Our secondary aim was to determine whether exposure to a “real life” mixture of ECs perturbed expression of GnRH, galanin or GALR1-3 mRNA in the maternal hypothalamus and pituitary gland.
Materials and Methods
Animals
Control (C) and sludge-exposed (E) pregnant ewes were maintained on pasture treated with conventional inorganic fertilizers, or sewage sludge, respectively. Sewage sludge had been applied to these pastures for 4 years prior to the present study, according to protocols described in detail elsewhere (19). Briefly, for E animals sludge was applied twice annually to three 9-ha plots at a rate of 2.25 metric tons of dry matter per hectare to the whole of each plot until seven separate applications had been made. This rate of sludge application, which resulted in the application of about 225 kg nitrogen/ha/year, was consistent with normal United Kingdom management practice at the time. Animals were not allowed to graze the pasture for a minimum of 3 weeks after sludge application, as prescribed by legislation (Great Britain Parliament 1989). C animals were maintained on similar pasture to which 225 kg of nitrogen/ha/year was applied using conventional, inorganic fertilisers. At 110 days of gestation (GD110), maternal and foetal animals were euthanised (Schedule 1, U.K. Animals (Scientific Procedures) Act, 1986). Immediately after euthanasia, hypothalami and pituitary glands were collected from mothers (6 control and 6 exposed) and their foetuses (9 control and 9 exposed, 5:4 ratio of female to male per group) halved, immediately frozen on dry ice and stored at -80°C before later mRNA extraction and analysis. There was an approximately equal representation of foetuses from single, twin and triplet pregnancies in C and E groups and body weights at the time of tissue collection were not significantly different between groups (C:1305±233g vs E:1220±189g).
Tissue Preparation
While still frozen, maternal and foetal hypothalamic blocks were cut into coronal slices (~4mm in adult, ~2mm in foetal lambs), using external landmarks on the base of the brain, with the most rostral cut ~ 1mm in front of the optic chiasm such that the first slice encompassed the preoptic area and the second the mediobasal hypothalamus/median eminence as previously described (24). From each tissue block, approximately 100-200mg of tissue was harvested for RNA extraction, from an area close to the ventricle. Approximately 100-200mg of tissue was also harvested from the mid sagittal face of maternal and foetal pituitary glands for RNA extraction. Total RNA was extracted from each tissue using TRIzol® (Invitrogen, UK) as recommended by the manufacturers and mRNA (200-300ng) was reverse transcribed using Moloney-Murine Leukemia virus (M-MLV) reverse transcriptase (Invitrogen, UK), random hexamers (Promega UK) and Rnasin (Promega, UK) as described previously (32). Purity and quantity of mRNA and cDNA were assessed using an ND-1000 spectrophotometer (NanoDrop® Technologies).
Quantitative PCR (qPCR)
qPCR was conducted using oligonucleotide primers and Taqman probes for ovine β-actin, galanin, GALR1, GALR2 and GALR, GnRH and GnRHR as previously validated and described previously (24). The Taqman probes for galanin, GALR1, GALR2 and GALR3, β-actin GnRH and GnRHR were synthesized by Eurogentec and contained FAM as the 5’-reporter and Blackhole Quencher 1 as the 3’-quencher. Sequences for all primers and probes are shown in Table 1. All qPCR reactions were performed in duplicate and a reagent blank was included within each plate to detect contamination by genomic DNA.
Table 1.
qPCR probes and primer sequences
| Target | mRNA Primer Sequence 5’-3’ | GenBank Accession | |
|---|---|---|---|
| β-actin | β-actin-F: TCCTTCCTGGGCATGGAATC | ||
| β-actin-R: GGGCGCGATGATCTTGATCT | U39357 | ||
| β-actin-Probe (FAM labelled): CCTTCCTTCCTGGGCATGGAATCC | |||
| Galanin | GAL-F: GAGAGGCTGGACCCTGAACA | ||
| GAL-R: CGTGAAATGACCTGTGGTTGTC | EF192581 | ||
| GAL-Probe (FAM labelled): TGCCGGCTACCTTCTCGGACCA | |||
| GALR1 | GALR1-F: CACACCACGTAGGCCTTCTTG | ||
| GALR1-R: GACGTCAGCAACCAGACCTTCT | EF192582 | ||
| GALR1-Probe (FAM labelled): CGCTGGTTGGGCCACTGCTCC | |||
| GALR2 | GALR2-F: AGCCGTCCAGGGTGTAGATG | ||
| GALR2-R: CGACCTGTGTTTCATCGTGTG | EF192583 | ||
| GALR2-Probe (FAM labelled): CTTGGAAGGGCACGCAG | |||
| GALR3 | GALR3-F: ACGACGGATCTATTCATACCTCAAC | ||
| GALR3-R: GATGGCGGCCTGGAAAG | EF192584 | ||
| GALR3-Probe (FAM labelled): CTGGCGGCAGCTGACCTCTGCT | |||
| GnRH | GnRH-F: GGTCGATCAGCCAGTAGAACCT | ||
| GnRH-R: AGGTCCCTCAGAGGAGAATGG | U02517 | ||
| GnRH-Probe (FAM labelled): TGGTGAACAATGCACCCACAGCACT | |||
| GnRHR | GnRHR-F: TGTAACACACTGCAGTTTTCCACA | ||
| GnRHR-R: AAGAGGGATGATGAAGAGGCAG | L22215 | ||
| GnRHR-Probe (FAM labelled): TGGCATCAAGCCTTTTATAACTTTTTCACCTTCA | |||
qPCRs for ovine GnRH, GnRHR galanin, GALR1, GALR2, GALR3 and the endogenous reference gene β-actin (21, 24) were conducted using a Mx3000P™ Real-Time PCR System (Stratagene) according to the manufacturer’s instructions. Each qPCR reaction (25µl) contained PCR Buffer II (Applied Biosystems, 50 mM KCl, 10 mM Tris-HCl (pH8.3), 1.5 mM MgCl2, 0.001% (v/v) gelatin), 0.2 mM of each deoxynucleotide triphosphate, 0.625 U AmpliTaq Gold DNA polymerase (Applied Biosystems), template cDNA (2µl), and optimized quantities of the respective probe and primers for the genes of interest and molecular biology grade H2O (BDH, UK). Thermal cycling conditions were 10 min at 97°C (initial denaturation step) and 45 cycles of 45 sec at 95°C (denaturation step) and 1 min at 60°C (primer annealing and elongation). GnRH, galanin, GALR1, GALR2, GALR3 mRNA expression was quantified using the comparative CT (cycle threshold) method (33) and gene expression calculated relative to the reference gene (β-actin).
Statistical Analysis
All data are presented as mean ± S.E.M. All data was normalised by log transformation and analysed using either Student’s T-test or two-way ANOVA where appropriate. A P value of less than 0.05 was considered statistically significant.
In the foetal animals there was no significant effect of gender on the expression of any of the genes examined in either the hypothalamus or pituitary gland so data from both genders were combined. There was also no significant difference in mRNA expression for any of the genes measured in tissue taken from the two hypothalamic slices studied. Data from these two areas were therefore combined before further analysis.
Results
Maternal GnRH and GnRHR mRNA expression
Hypothalamic GnRH mRNA expression was not significantly different with exposure (C :0.40±0.26; E : 0.19±0.12). GnRHR mRNA expression was also similar in C and E ewes in both the hypothalamus (C: 0.27±0.23; E: 0.05±0.05) and pituitary gland (C: 0.35±0.12; E: 0.20±0.21).
Maternal galanin and galanin receptor mRNA expression
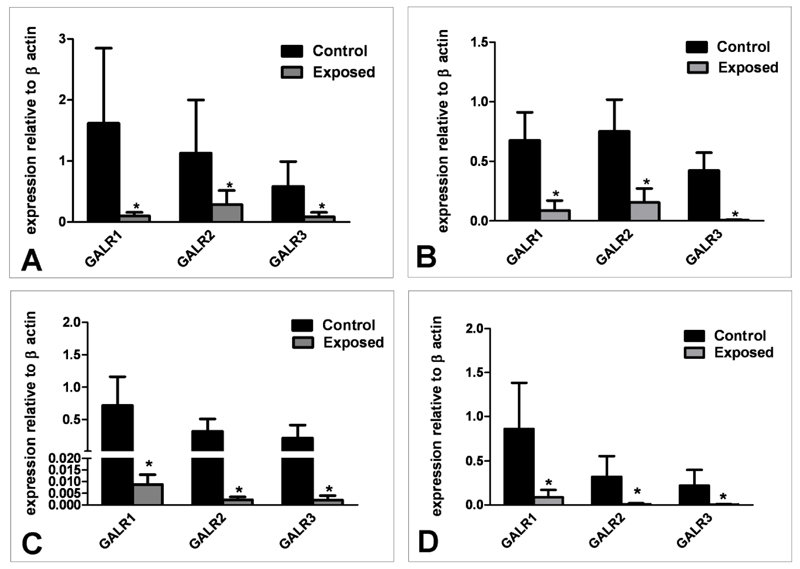
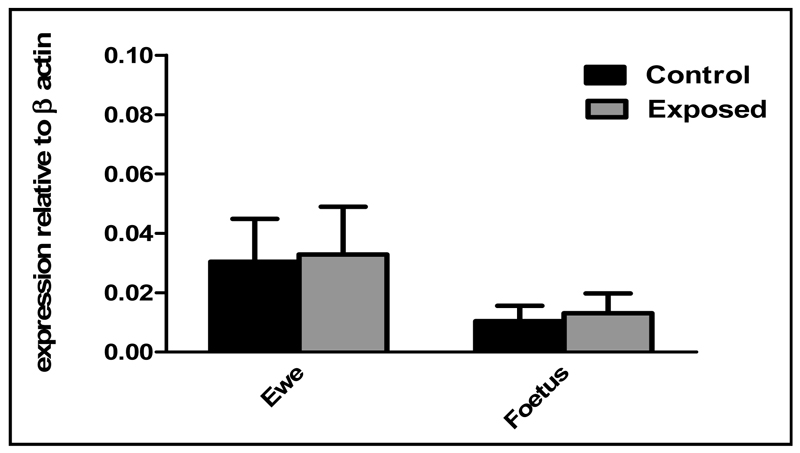
mRNA expression for all three galanin receptor subtypes (GALR1, GALR2 and GALR3) was significantly lower in E compared to C animals in both the hypothalamus (P<0.05, Figure 1A) and pituitary gland (P<0.05, Figure 1B). There was no difference in galanin receptor mRNA expression levels between the different tissues tested. Levels of galanin mRNA expression in the hypothalamus were not significantly different between E and C animals (Figure 2). Sewage sludge exposure also had no significant effect on galanin mRNA expression in the maternal pituitary gland (C: 0.008±0.0029 E: 0.01±0.009).
Fig. 1.
mRNA expression of galanin receptors 1, 2 and 3 relative to β-actin in maternal (A) hypothalamus and (B) pituitary gland and in foetal C) hypothalamus and D) pituitary gland following in-utero exposure to sewage sludge chemicals (Exposed). *P<0.05 in comparison with respective control value.
Fig. 2.
Maternal and foetal hypothalamic galanin mRNA expression following in-utero exposure to sewage sludge chemicals (Exposed).
Foetal GnRH and GnRHR mRNA expression
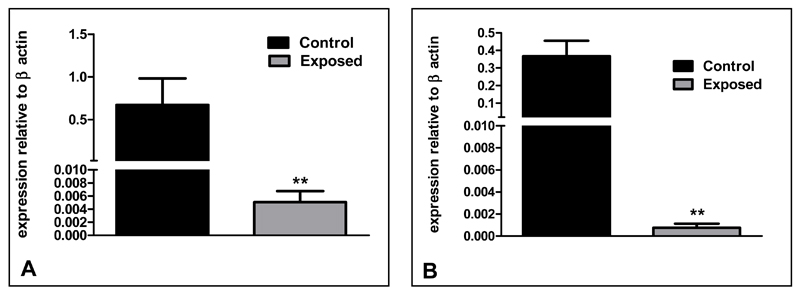
Unlike in the ewes, foetal hypothalamic GnRH (P<0.01, Figure 3A) and GnRHR mRNA expression (P<0.01, Figure 3B) were significantly lower in E than C foetuses. However, GnRHR mRNA expression in the foetal pituitary glands was not different between E and C animals (C: 0.34±0.13; E: 0.30±0.18).
Fig. 3.
mRNA expression of (A) GnRH and (B) GnRHR relative to β-actin in foetal hypothalamus following in-utero exposure to sewage sludge chemicals (Exposed). **P<0.01 in comparison with respective control value.
Foetal galanin and galanin receptor mRNA expression
As in the mothers, there was no difference in galanin receptor expression levels between the different tissues. Levels of hypothalamic galanin mRNA expression were similar for E and C foetuses (Figure 2) and galanin mRNA expression within the pituitary gland was also similar (C: 0.020±0.012 E: 0.0053±0.003). However, expression of GALR1, GALR2 and GALR3 mRNA was significantly lower in the hypothalami (P<0.05, Figure 1C) and pituitary glands (P<0.05, Figure 1D) of E compared to C animals.
Discussion
This study demonstrates that the expression of gene members of the GnRH and galaninergic systems within the foetal hypothalamus and pituitary gland are significantly affected by exposure of their dams to an environmentally relevant mixture of environmental pollutants, that contains known EDCs, by pasturing on sewage sludge treated pastures. Effects were most pronounced in the hypothalamus where expression of mRNAs for GnRH and its receptor, as well as the three galanin receptor isoforms, were suppressed. Gene expression in the mothers, who received the primary exposure, however, was not as profoundly affected as that in the foetuses they carried. As the affected systems stand at the apex of the reproductive axis, such effects could have major consequences for downstream reproductive processes and could contribute to the previously reported effects of this form of EDC exposure on development of the male (19) and female (20) gonadal systems.
The results of the present study have confirmed our hypothesis since hypothalamic GnRH mRNA expression was decreased in sewage sludge-exposed foetuses. Hypothalamic GnRH mRNA expression is suppressed by oestrogen (34), although it is also minimal during seasonal anoestrus in sheep (35) and in postmenopausal women (36). The sewage sludge used in this study contains many oestrogenic chemicals, such as diethylhexyl phthalate (DEHP), polychlorinated biphenyls (PCB), polybrominated diphenyl ethers (PBDE) congeners and polycyclic aromatic hydrocarbons (PAH) as previously published (14, 15); thus it is possible that the low levels of GnRH mRNA expression in exposed foetuses may be attributable to a suppressive action of the oestrogenic chemicals in sewage sludge. The precise nature of the “insult” to which foetuses are actually exposed, however, remains unclear since studies of foetal concentrations of EDCs in sheep exposed to these sludge treatments indicate that tissue burdens differ greatly between individuals, cannot be predicted from maternal tissue levels or environmental concentrations, and depend on EDC class (14). The results of the present study support and extend our previous neuroendocrine findings (21), and given our previous results, support the hypothesis that the effects of sewage sludge exposure may be exerted indirectly through reduced kisspeptinergic drive to the GnRH neurosecretory system.
In addition to a reduction in GnRH mRNA in the hypothalami of sewage sludge exposed animals, GnRHR mRNA expression was also reduced in E compared to C foetuses. GnRHR expression has been reported to be lower during anoestrous than in the luteal phase of the oestrous cycle (35) suggesting that it is sensitive to circulating steroids, although the precise role of changes in hypothalamic GnRHR expression in the regulation of reproductive function in the ewe remains to be determined. The reduced hypothalamic GnRHR mRNA expression following sewage sludge exposure is also consistent with our hypothesis that GnRHR are sensitive to exposure to exogenous oestrogenic, and possibly other, compounds. This possibility is supported by evidence that oestradiol and GnRH also interact to regulate GnRHR mRNA expression in the adult ovine pituitary gland (37, 38) however little is known about the regulation of hypothalamic GnRHR in foetal animals with a sexually immature neuroendocrine system. The reduced expression of hypothalamic GnRH and GnRHR mRNA could result in reduced LH secretion with adverse consequences for reproductive development and function in adulthood.
ECs may perturb the reproductive axis indirectly by acting on neurotransmitter systems that modulate GnRH output. Galanin can stimulate hypothalamic GnRH release (39, 40), pituitary LH secretion (39, 41) and can modulate the amplitude of the preovulatory LH surge (40, 42) in the rat. Although the role of galanin in the regulation of the GnRH /LH system in the sheep is less well characterised, galanin and its receptors are expressed in the ovine hypothalamus (24, 43), galanin is co-expressed in all ovine GnRH neurones (28) and GALR1 receptor immunoreactive neurones colocalise with GnRH (44). Interestingly our results show that mRNA expression of all three galanin receptors (GALR1, GALR2 and GALR3) was significantly reduced in both the hypothalamus and pituitary glands of sewage sludge-exposed foetuses. Additionally, while the treatment had no effect on maternal GnRH or GnRHR mRNA expression, hypothalamic and pituitary gland galanin receptor mRNA expression was significantly reduced in exposed mothers, as in the foetal animals, demonstrating that this system may be particularly sensitive to chemical exposure.
Galanin’s effects on the reproductive neuroendocrine systems are probably mediated predominantly via GALR1 (45). Little is known about the regulation of GALR1 gene expression but GnRH/GALR1 co-localisation changes during the ovine oestrous cycle, with the lowest levels of co-localisation during the late follicular phase (high oestrogen) and highest during the luteal phase (low oestrogen) (44) suggesting that it may be regulated by oestradiol. This would again be consistent with the putative effects of oestrogenic chemicals present in sewage sludge. The observed effects of sewage sludge exposure on mRNA expression of galanin, a neuroendocrine ligand and its receptors is similar to but the opposite of our previous findings in which kisspeptin mRNA was reduced in exposed animals without a change in mRNA expression of its receptor (21). These differential effects of ECs on neuroendocrine ligand and receptor mRNA expression indicates that GALR mRNA expression is more sensitive to endogenous oestradiol/oestrogen-like compounds and would suggest that alteration of receptor instead of ligand expression, might be used to regulate galanin’s actions.
The effects of sewage sludge exposure on the GnRH and galanin neurosecretory systems in this model occur as a result of chronic exposure during development, and were seen after the programming of many sexually dimorphic physiological characteristics. It is possible therefore that the effects of EC exposure could also alter programmed activities and affect long term reproductive function and fertility. Although this study is primarily focussed on the role of galanin and its receptors and their association with reproductive function, it should be noted that galanin has many other neuroendocrine roles and so alterations in galanin receptor expression may also impact on other physiological systems. Thus it is worth noting that animals raised in this model also exhibit alterations in male and female typical behaviours (46) which could occur either as a direct effect of EC on behavioural systems or an indirect effect of alterations in the reproductive neuroendocrine axis.
Given the known oestrogenic action of many of the chemicals present in the soil of sewage sludge treated pastures (47) and sensitivity of both GnRH and galanin to the regulatory effects of oestrogen, it is feasible that exposure to ECs in sludge may have led to the effects on down regulation seen in the GnRH and galaninergic systems observed in this study. Together with our previously published work (22), the data point to potential down-regulation of the positive drive to GnRH neurosecretion via decreased kisspeptin signalling and reduced receptivity to galanin signalling at the level of the hypothalamus. Since GnRH neurones lack classical oestrogen receptors (48, 49), this provides a potential mechanism through which exposure to oestrogenic chemicals might indirectly decrease GnRH neurosecretion and these changes might explain some of the additional physiological changes observed in the gonads of sewage sludge exposed foetuses, especially those in the male (19).
Interpretation and extrapolation of our findings to human health requires caution as human exposures to EDCs are ill-defined and may not be comparable to those of the current study. Nevertheless, since human waste is an important contributor to sewage sludge, it is highly likely that humans themselves are exposed to many of its constituents. The current findings suggest that low-level exposure to ‘safe’ levels of environmental chemicals can exert negative effects on the neuroendocrine axis which may impact on reproductive function and fertility.
Acknowledgements
We are grateful to Mrs. Carol Kyle (Macaulay) for excellent technical assistance and to the staff of the Hartwood Research Station for their assistance in the management of experimental animals. This work was funded by the Wellcome Trust.
Abbreviations
- EDCs
endocrine disrupting compounds
- ECs
environmental contaminants
- GALR
galanin receptor
- GnRHR
gonadotropin releasing hormone receptor
- qPCR
quantitative polymerase chain reaction
References
- 1.Fernandez MF, Olmos B, Granada A, Lopez-Espinosa MJ, Molina-Molina JM, Fernandez JM, Cruz M, Olea-Serrano F, Olea N. Human exposure to endocrine-disrupting chemicals and prenatal risk factors for cryptorchidism and hypospadias: a nested case-control study. Environ Health Perspect. 2007;115(Suppl 1):8–14. doi: 10.1289/ehp.9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8(2):143–59. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 4.Buck Louis GM, Gray LE, Jr, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, Sippell W, Abbott DH, Soto A, Tyl RW, Bourguignon JP, et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics. 2008;121(Suppl 3):S192–207. doi: 10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- 5.Cesario SK, Hughes LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs. 2007;36(3):263–74. doi: 10.1111/j.1552-6909.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110(9):917–21. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”--environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008;105(2):235–59. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor a cross-talk and mechanisms of action. Chemical Research in Toxicology. 2003;16(7):807–16. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 9.Swedenborg E, Ruegg J, Makela S, Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol. 2009;43(1):1–10. doi: 10.1677/JME-08-0132. [DOI] [PubMed] [Google Scholar]
- 10.Rhind SM. Are endocrine disrupting compounds a threat to farm animal health, welfare and productivity? Reprod Domest Anim. 2005;40(4):282–90. doi: 10.1111/j.1439-0531.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- 11.Rhind SM, Rae MT, Brooks AN. Environmental influences on the fetus and neonate--timing, mechanisms of action and effects on subsequent adult function. Domest Anim Endocrinol. 2003;25(1):3–11. doi: 10.1016/s0739-7240(03)00041-9. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe RM, Irvine DS. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? Bmj. 2004;328(7437):447–51. doi: 10.1136/bmj.328.7437.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webber MD, Lesage S. Organic Contaminants in Canadian Municipal Sludges. Waste Management Research. 1989;7(1):63–82. [Google Scholar]
- 14.Rhind SM, Kyle CE, Mackie C, McDonald L. Accumulation of endocrine disrupting compounds in sheep fetal and maternal liver tissue following exposure to pastures treated with sewage sludge. J Environ Monit. 2009;11(8):1469–76. doi: 10.1039/b902085c. [DOI] [PubMed] [Google Scholar]
- 15.Lind PM, Gustafsson M, Hermsen SAB, Larsson S, Kyle CE, Orberg J, Rhind SM. Exposure to pastures fertilised with sewage sludge disrupts bone tissue homeostasis in sheep. Science of the Total Environment. 2009;407(7):2200–8. doi: 10.1016/j.scitotenv.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Stevens JL, Northcott GL, Stern GA, Tomy GT, Jones KC. PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks, and polychlorinated n-alkanes in U.K. sewage sludge: survey results and implications. Environ Sci Technol. 2003;37(3):462–7. doi: 10.1021/es020161y. [DOI] [PubMed] [Google Scholar]
- 17.Rhind SM, Smith A, Kyle CE, Telfer G, Martin G, Duff E, Mayes RW. Phthalate and alkyl phenol concentrations in soil following applications of inorganic fertiliser or sewage sludge to pasture and potential rates of ingestion by grazing ruminants. J Environ Monit. 2002;4(1):142–8. doi: 10.1039/b107539j. [DOI] [PubMed] [Google Scholar]
- 18.Rhind SM, Kyle CE, Telfer G, Duff EI, Smith A. Alkyl phenols and diethylhexyl phthalate in tissues of sheep grazing pastures fertilized with sewage sludge or inorganic fertilizer. Environ Health Perspect. 2005;113(4):447–53. doi: 10.1289/ehp.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul C, Rhind SM, Kyle CE, Scott H, McKinnell C, Sharpe RM. Cellular and hormonal disruption of fetal testis development in sheep reared on pasture treated with sewage sludge. Environ Health Perspect. 2005;113(11):1580–7. doi: 10.1289/ehp.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler PA, Dora NJ, McFerran H, Amezaga MR, Miller DW, Lea RG, Cash P, McNeilly AS, Evans NP, Cotinot C, Sharpe RM, et al. In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol Hum Reprod. 2008;14(5):269–80. doi: 10.1093/molehr/gan020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellingham M, Fowler PA, Amezaga MR, Rhind SM, Cotinot C, Mandon-Pepin B, Sharpe RM, Evans NP. Exposure to a Complex Cocktail of Environmental Endocrine-Disrupting Compounds Disturbs the Kisspeptin/GPR54 System in Ovine Hypothalamus and Pituitary Gland. Environmental Health Perspectives. 2009;117(10):1556–62. doi: 10.1289/ehp.0900699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erhard HW, Boissy A, Rae MT, Rhind SM. Effects of prenatal undernutrition on emotional reactivity and cognitive flexibility in adult sheep. Behav Brain Res. 2004;151(1-2):25–35. doi: 10.1016/j.bbr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211(4488):1294–302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 24.Whitelaw CM, Robinson JE, Chambers GB, Hastie P, Padmanabhan V, Thompson RC, Evans NP. Expression of mRNA for galanin, galanin-like peptide and galanin receptors 1-3 in the ovine hypothalamus and pituitary gland: effects of age and gender. Reproduction. 2009;137(1):141–50. doi: 10.1530/REP-08-0266. [DOI] [PubMed] [Google Scholar]
- 25.Merchenthaler I. Estrogen stimulates galanin expression within luteinizing hormone-releasing hormone-immunoreactive (LHRH-i) neurons via estrogen receptor-beta (ER[beta]) in the female rat brain. Neuropeptides. 2005;39(3):341–3. doi: 10.1016/j.npep.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Steel JH, Gon G, Ohalloran DJ, Jones PM, Yanaihara N, Ishikawa H, Bloom SR, Polak JM. Galanin and Vasoactive Intestinal Polypeptide Are Colocalized with Classical Pituitary-Hormones and Show Plasticity of Expression. Histochemistry. 1989;93(2):183–9. doi: 10.1007/BF00315973. [DOI] [PubMed] [Google Scholar]
- 27.Chaillou E, Tramu G, Tillet Y. Distribution of galanin immunoreactivity in the sheep diencephalon. J Chem Neuroanat. 1999;17(3):129–46. doi: 10.1016/s0891-0618(99)00032-0. [DOI] [PubMed] [Google Scholar]
- 28.Dufourny L, Schofield N, Skinner DC. Immunoreactive galanin expression in ovine gonadotropin-releasing hormone neurones: No effects of gender or reproductive status. Journal of Neuroendocrinology. 2003;15(11):1062–9. doi: 10.1046/j.1365-2826.2003.01098.x. [DOI] [PubMed] [Google Scholar]
- 29.Tourlet S, Ziyazetdinova G, Caraty A, Tramu G, Delsol G, Tillet Y. Oestradiol effect on galanin-immunoreactive neurones in the diencephalon of the ewe. J Neuroendocrinol. 2005;17(3):145–51. doi: 10.1111/j.1365-2826.2005.01291.x. [DOI] [PubMed] [Google Scholar]
- 30.Chambers G, Whitelaw CM, Robinson JE, Evans NP. Distribution of galanin receptor-2 immunoreactive neurones in the ovine hypothalamus: no evidence for involvement in the control of gonadotrophin-releasing hormone secretion. J Neuroendocrinol. 2007;19(12):966–73. doi: 10.1111/j.1365-2826.2007.01609.x. [DOI] [PubMed] [Google Scholar]
- 31.Dufourny L, Skinner D. Abstract of the Society of Neuroscience. Washington DC: 2003. Gal-R3 Receptor Colocalisation in Ovine GnRH Neurons. [Google Scholar]
- 32.O'Shaughnessy PJ, Murphy L. Cytochrome P-450 17 alpha-hydroxylase protein and mRNA in the testis of the testicular feminized (Tfm) mouse. J Mol Endocrinol. 1993;11(1):77–82. doi: 10.1677/jme.0.0110077. [DOI] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C-T method. Nature Protocols. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 34.Petersen SL, McCrone S, Keller M, Shores S. Effects of estrogen and progesterone on luteinizing hormone-releasing hormone messenger ribonucleic acid levels: consideration of temporal and neuroanatomical variables. Endocrinology. 1995;136(8):3604–10. doi: 10.1210/endo.136.8.7628399. [DOI] [PubMed] [Google Scholar]
- 35.Ciechanowska M, Lapot M, Malewski T, Mateusiak K, Misztal T, Przekop F. Expression of the GnRH and GnRH receptor (GnRH-R) genes in the hypothalamus and of the GnRH-R gene in the anterior pituitary gland of anestrous and luteal phase ewes. Anim Reprod Sci. 2008;108(3-4):345–55. doi: 10.1016/j.anireprosci.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J Clin Endocrinol Metab. 1996;81(10):3540–6. doi: 10.1210/jcem.81.10.8855798. [DOI] [PubMed] [Google Scholar]
- 37.Adams BM, Sakurai H, Adams TE. Concentrations of gonadotropin-releasing hormone (GnRH) receptor messenger ribonucleic acid in pituitary tissue of orchidectomized sheep: effect of estradiol and GnRH. Biol Reprod. 1996;54(2):407–12. doi: 10.1095/biolreprod54.2.407. [DOI] [PubMed] [Google Scholar]
- 38.Turzillo AM, Nolan TE, Nett TM. Regulation of gonadotropin-releasing hormone (GnRH) receptor gene expression in sheep: interaction of GnRH and estradiol. Endocrinology. 1998;139(12):4890–4. doi: 10.1210/endo.139.12.6344. [DOI] [PubMed] [Google Scholar]
- 39.Lopez FJ, Merchenthaler I, Ching M, Wisniewski MG, Negrovilar A. Galanin - a Hypothalamic-Hypophysiotropic Hormone Modulating Reproductive Functions. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(10):4508–12. doi: 10.1073/pnas.88.10.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez FJ, Meade EH, Negrovilar A. Endogenous Galanin Modulates the Gonadotropin and Prolactin Proestrous Surges in the Rat. Endocrinology. 1993;132(2):795–800. doi: 10.1210/endo.132.2.7678800. [DOI] [PubMed] [Google Scholar]
- 41.Scheffen JR, Splett CL, Desotelle JA, Bauer-Dantoin AC. Testosterone-dependent effects of galanin on pituitary luteinizing hormone secretion in male rats. Biology of Reproduction. 2003;68(2):363–9. doi: 10.1095/biolreprod.102.005959. [DOI] [PubMed] [Google Scholar]
- 42.Sahu A, Xu B, Kalra SP. Role of Galanin in Stimulation of Pituitary Luteinizing-Hormone Secretion as Revealed by a Specific Receptor Antagonist, Galantide. Endocrinology. 1994;134(2):529–36. doi: 10.1210/endo.134.2.7507825. [DOI] [PubMed] [Google Scholar]
- 43.Chaillou E, Tramu G, Tillet Y. Distribution of galanin immunoreactivity in the sheep diencephalon. Journal of Chemical Neuroanatomy. 1999;17(3):129–46. doi: 10.1016/s0891-0618(99)00032-0. [DOI] [PubMed] [Google Scholar]
- 44.Dufourny L, Skinner DC. Distribution of galanin receptor 1-immunoreactive neurons in the ovine hypothalamus: Colocalization with GnRH. Brain Research. 2005;1054(1):73–81. doi: 10.1016/j.brainres.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 45.Jungnickel SR, Gundlach AL. [125I]-Galanin binding in brain of wildtype, and galanin- and GalR1-knockout mice: strain and species differences in GalR1 density and distribution. Neuroscience. 2005;131(2):407–21. doi: 10.1016/j.neuroscience.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Erhard HW, Rhind SM. Prenatal and postnatal exposure to environmental pollutants in sewage sludge alters emotional reactivity and exploratory behaviour in sheep. Sci Total Environ. 2004;332(13):101–8. doi: 10.1016/j.scitotenv.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Andreu V, Ferrer E, Rubio JL, Font G, Picó Y. Quantitative determination of octylphenol, nonylphenol, alkylphenol ethoxylates and alcohol ethoxylates by pressurized liquid extraction and liquid chromatography-mass spectrometry in soils treated with sewage sludges. Science of The Total Environment. 2007;378(1-2):124–9. doi: 10.1016/j.scitotenv.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 48.Herbison AE, Skynner MJ, Sim JA. Lack of detection of estrogen receptor-alpha transcripts in mouse gonadotropin-releasing hormone neurons (vol 140, pg 5195, 1999) Endocrinology. 2001;142(1):493. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- 49.Herbison AE, Robinson JE, Skinner DC. Distribution of Estrogen Receptor-Immunoreactive Cells in the Preoptic Area of the Ewe - Colocalization with Glutamic-Acid Decarboxylase but Not Luteinizing-Hormone-Releasing Hormone. Neuroendocrinology. 1993;57(4):751–9. doi: 10.1159/000126433. [DOI] [PubMed] [Google Scholar]