Abstract
Neoadjuvant therapy is a well-established approach for the treatment of locally advanced or inflammatory breast cancer (BC) and has been increasingly used in recent years not only as a management strategy but also as a research tool. Recently, nanoparticle albumin-bound paclitaxel (nab-paclitaxel)/trastuzumab combinations have been associated with promising activity in different clinical settings. In the present case, we report a complete pathological response after neoadjuvant treatment with the trastuzumab/nab-paclitaxel combination in a locally advanced human epidermal growth factor receptor 2 (HER2)-positive BC patient, with a good toxicity profile. This combination may represent a valid therapeutic option in the neoadjuvant therapy for HER2-positive locally advanced BC.
Keywords: breast cancer, HER2-positive, nab-paclitaxel, trastuzumab, neoadjuvant therapy, pathologic complete response
Introduction
Neoadjuvant therapy (NAT), also known as primary systemic treatment, has been increasingly used in recent years as first-line treatment for operable breast cancer (BC), not only as a management strategy but also as a research tool. The advantages of this approach are the early use of a systemic treatment, with potential tumor downstaging, higher rates of breast-conserving surgery, and in vivo testing of tumor response to a chosen chemotherapy regimen.
The pathologic complete response (pCR) is considered a surrogate marker of efficacy for NAT, providing an approximate measure that correlates with long-term outcomes.1
A recent pooled analysis by Cortazar et al2 established the association between pCR and long-term outcomes in terms of event-free survival and overall survival (OS). In this pooled analysis, the association between pCR and long-term outcomes was strongest in patients with more aggressive tumor subtypes, namely, hormone receptor-negative/human epidermal growth factor receptor 2 (HER2)-positive and triple-negative breast cancers (TNBC).2 HER2 is overexpressed and/or amplified in 15–20% of BC, and it is associated with an aggressive clinical course of the disease.3,4 Trastuzumab has represented a major breakthrough in the treatment of metastatic and adjuvant HER2-positive BC. In the neoadjuvant setting, large clinical trials were conducted to evaluate the association of trastuzumab with different cytotoxic chemotherapeutic agents.5–7 Taxanes are a standard component of adjuvant chemotherapy regimens for patients with both node-positive or high-risk node-negative BC,8–10 and are commonly used as sequential regimens. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a solvent-free formulation of paclitaxel that avoids the risk of hypersensitivity reactions commonly observed with solvent-based taxanes (ie, paclitaxel and docetaxel) and the need of premedication. The dose of 260 mg/m2 administered intravenously over 30 minutes every 3 weeks is approved for the treatment of BC after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. The use of nab-paclitaxel in NAT has been preliminary evaluated in phase II studies with different regimens.11–13 Recently, the phase III GeparSepto trial showed that nab-paclitaxel in the neoadjuvant setting significantly increased pCR rates compared with solvent-based paclitaxel in all patient subgroups, in particular, in TNBC and in HER2-positive BC, combined with trastuzumab and pertuzumab.14
Herein, we report a pCR after neoadjuvant treatment with the trastuzumab/nab-paclitaxel combination in a locally advanced HER2-positive BC patient.
Case presentation
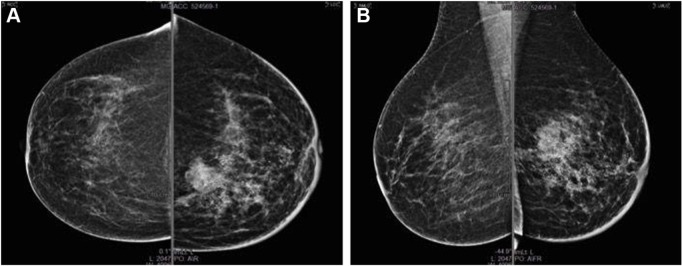
A 65-year-old Caucasian woman, with a family history of BC, referred to our institution in May 2015 after casual checking of a lump in the left breast. Written informed consent of the patient and approval of Comitato Etico Interaziendale of the Province of Messina were obtained for the present case. The mammography scan revealed a radiopaque solid infiltrative lesion in the upper-inner quadrant of 35 mm diameter, with irregular margins and multiple microcalcification clusters. Two additional deep located lesions of ~17 and 12 mm were also found in the upper-inner quadrant and mid-upper quadrant, respectively (Figure 1). Ultrasound imaging confirmed the presence of a 30 mm hypoechoic lesion with irregular margins and microcalcifications in the context, with additional two lesions (15 and 10 mm) peripherally and evidence of multiple pathological axillary lymph nodes with a maximum diameter of 22 mm.
Figure 1.
May 2015, baseline mammography.
Note: (A) craniocaudal and (B) mediolateral oblique projections.
Ultrasound-guided breast biopsy revealed an invasive carcinoma of no special type with the following biological characteristics: estrogen receptor negative, progesterone receptor negative, Mib-1>50%, and HER2 immunohistochemistry 3+. A computed tomography scan and a bone scintigraphy excluded the presence of distant metastases: stage IIIB (cT4N2M0). Serum tumor markers were within normal range of local laboratory: carcinoembryonic antigen (CEA) 5.1 ng/mL (0–4 ng/mL), cancer antigen 125 (CA 125) 8 U/mL (1.5–35 U/mL), cancer antigen 15-3 (CA 15-3) 29 U/mL (0–38 U/mL).
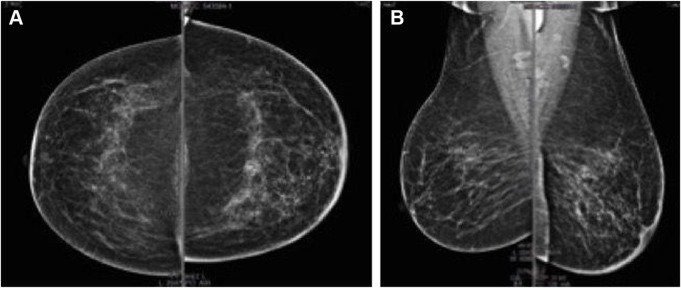
According to the disease stage, biomolecular characteristics of the tumor, and comorbidities (insulin-dependent diabetes mellitus type 2), the patient started neoadjuvant treatment with weekly trastuzumab (4 mg/kg loading dose followed by 2 mg/kg maintenance dose) and weekly nab-paclitaxel 125 mg/m2 for 12 weeks, with no relevant hematological and non-hematological toxicities. In September 2015, after the completion of neoadjuvant treatment, the radiological workup with mammography (Figure 2) and breast ultrasound documented a complete response with persistence of only microcalcifications and small reactive axillary lymph nodes. Serum tumor markers were within normal range of local laboratory: CEA 3.8 ng/mL, CA 125 7 U/L, CA 15–3 27 U/L.
Figure 2.
September 2015, mammography after neoadjuvant therapy.
Note: (A) craniocaudal and (B) mediolateral oblique projections.
Then in October 2015, she underwent conservative surgery plus homolateral axillary lymphadenectomy. The pathology review showed normal mammary gland tissues, without evidence of BC cells, and absence of metastases in lymph nodes, consistent with response to treatment and indicating pCR. After breast-conserving surgery, the patient underwent radiotherapy in the left residual breast and then was started on therapy with only adjuvant trastuzumab. The last serum tumor markers were within normal range of local laboratory: CEA 4.3 ng/mL, CA 125 7 U/L, CA 15–3 25 U/L.
Discussion
Neoadjuvant therapy is a well-established approach for the treatment of locally advanced or inflammatory BC.
Recently, the neoadjuvant setting has been emerging as a promising research tool in BC, providing an excellent platform for clinical development of novel anticancer agents, and an extraordinary source of tumor tissues for the study of tumor heterogeneity and the identification of novel predictive biomarkers and mechanisms of resistance to anticancer agents.
pCR has been adopted in neoadjuvant trials as a major endpoint, and correlates with both disease-free survival (DFS) and OS. An increasing body of evidence suggests the prognostic importance of pCR after neoadjuvant chemotherapy in TNBC and hormone receptor-negative/HER2-positive subsets. pCR is considered a potential surrogate marker of response to NAT and for long-term outcomes. Clinical studies in the neoadjuvant setting have shown that patients achieving pCR after neoadjuvant therapy have longer OS and DFS compared with women with residual disease.2
HER2 is overexpressed and/or amplified in 20% of BC and confers a more aggressive behavior, with a poor clinical outcome.3,4
Trastuzumab is a humanized recombinant monoclonal antibody directed against the extracellular domain of HER2, and trastuzumab-based therapy is firmly established as the standard of care for patients with HER2-positive BC, demonstrating survival benefit in both metastatic15 and early BC.16–19
Several randomized phase III trials with chemotherapy and trastuzumab have been reported in the neoadjuvant setting, showing that the addition of anti-HER2 inhibition to primary chemotherapy is associated with a higher pCR rate compared with chemotherapy alone (38–65% vs 19–26%).5–7
Recently, several trials have investigated the role of dual HER2 blockade in combination with chemotherapy in the neoadjuvant setting, showing that the combination of trastuzumab with other anti-HER2 agents is associated with a more complete HER2 signaling pathway inhibition. The combination of trastuzumab and lapatinib, an oral dual tyrosine kinase inhibitor, has been associated with a higher pCR rate compared with trastuzumab or lapatinib alone (47–60% vs 25–49%).20–22
Moreover, the combined administration of trastuzumab and pertuzumab, a recombinant humanized monoclonal antibody that binds with the extracellular dimerization domain II of HER2, prevents the heterodimerization of HER2 with other HER family members and increases the pCR rate (46–66% vs 29%) compared with trastuzumab alone in two phase 2 trials.23,24
More recently, in the prospective, randomized, phase 3 GeparSepto trial, nab-paclitaxel, a solvent-free, human albumin-stabilized formulation of paclitaxel, significantly increased pCR rate compared with solvent-based paclitaxel followed by epirubicin plus cyclophosphamide in primary early BC in all patient subgroups, with a major advantage among TNBC and HER2-positive BC (in the subtype HER2 positive in combination with trastuzumab and pertuzumab). The subgroup analysis of this study showed that patients with high Ki67 benefit more from the nab-paclitaxel treatment, underlying the particular chemosensitivity of these tumors.14 These data are in line with the activity seen in the present case. However, long-term follow-up of this study is needed to confirm whether the higher pCR rate will translate into longer DFS and OS.
The nab-paclitaxel plus trastuzumab combination has been evaluated in first-line metastatic BC in two phase II studies, reporting an interesting antitumor activity in HER2-overexpressing tumors.25,26
Recently, we reported the efficacy of the trastuzumab/nab-paclitaxel combination in a heavily pretreated HER2-positive BC patient with brain metastases.27
The pCR reported in the present case with the Trastuzumab and nab-Paclitaxel combination in a locally advanced HER2-positive patient underlines the efficacy and good safety profile of this combination in the neoadjuvant treatment HER2-positive disease. The activity seen in the present case might be related to the different mechanisms of action of these two agents and likely to a synergistic effect, even though in vitro studies might help to better clarify the biological consequences of this combination in BC cell lines.
The improved clinical activity seen with this combination does not significantly affect the safety profile of NAT, as demonstrated in the present case, with no significant early and late toxicities, in terms of hypersensitivity reactions, cardiotoxicity, and neurotoxicity. Moreover, the replacement of solvent-based taxanes and the subsequent avoidance of premedication have inherited advantages for patients with comorbidities, such as insulin-dependent diabetes mellitus type 2, as with our patient.
In conclusion, the nab-paclitaxel plus trastuzumab combination may be a valid option in the neoadjuvant treatment of HER2-positive breast cancer.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zardavas D, Piccart M. Neoadjuvant therapy for breast cancer. Annu Rev Med. 2015;66:31–48. doi: 10.1146/annurev-med-051413-024741. [DOI] [PubMed] [Google Scholar]
- 2.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 3.Zelnak AB, Wisinski KB. Management of patients with HER2-positive metastatic breast cancer: is there an optimal sequence of HER2-directed approaches? Cancer. 2015;121(1):17–24. doi: 10.1002/cncr.28815. [DOI] [PubMed] [Google Scholar]
- 4.Moasser MM, Krop IE. The evolving landscape of HER2 targeting in breast cancer. JAMA Oncol. 2015;1(8):1154–1161. doi: 10.1001/jamaoncol.2015.2286. [DOI] [PubMed] [Google Scholar]
- 5.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 7.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 8.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23(16):3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 9.Martín M, Rodríguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100(11):805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 10.Martín M, Seguí MA, Antón A, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363(23):2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 11.Robidoux A, Buzdar AU, Quinaux E, et al. A phase II neoadjuvant trial of sequential nanoparticle albumin-bound paclitaxel followed by 5-fluorouracil/epirubicin/cyclophosphamide in locally advanced breast cancer. Clin Breast Cancer. 2010;10(1):81–86. doi: 10.3816/CBC.2010.n.011. [DOI] [PubMed] [Google Scholar]
- 12.Yardley DA, Raefsky E, Castillo R, et al. Phase II study of neoadjuvant weekly nab-paclitaxel and carboplatin, with bevacizumab and trastuzumab, as treatment for women with locally advanced HER2+ breast cancer. Clin Breast Cancer. 2011;11(5):297–305. doi: 10.1016/j.clbc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Kaklamani VG, Siziopikou K, Scholtens D, et al. Pilot neoadjuvant trial in HER2 positive breast cancer with combination of nab-paclitaxel and lapatinib. Breast Cancer Res Treat. 2012;132(3):833–842. doi: 10.1007/s10549-011-1411-8. [DOI] [PubMed] [Google Scholar]
- 14.Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345–356. doi: 10.1016/S1470-2045(15)00542-2. [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 16.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 17.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 18.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 20.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30(16):1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 22.Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(12):1183–1192. doi: 10.1016/S1470-2045(13)70411-X. [DOI] [PubMed] [Google Scholar]
- 23.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 24.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 25.Conlin AK, Seidman AD, Bach A, et al. Phase II trial of weekly nanoparticle albumin-bound paclitaxel with carboplatin and trastuzumab as first-line therapy for women with HER2-overexpressing metastatic breast cancer. Clin Breast Cancer. 2010;10(4):281–287. doi: 10.3816/CBC.2010.n.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirtsching B, Cosgriff T, Harker G, Keaton M, Chidiac T, Min M. A phase II study of weekly nanoparticle albumin-bound paclitaxel with or without trastuzumab in metastatic breast cancer. Clin Breast Cancer. 2011;11(2):121–128. doi: 10.1016/j.clbc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Ricciardi GR, Russo A, Franchina T, Ferraro G, Adamo V. Efficacy of nab-paclitaxel plus trastuzumab in a long-surviving heavily pretreated HER2-positive breast cancer patient with brain metastases. Onco Targets Ther. 2015;8:289–294. doi: 10.2147/OTT.S74110. [DOI] [PMC free article] [PubMed] [Google Scholar]