abstract
New neuron addition via continued neurogenesis in the postnatal/adult mammalian brain presents a distinct form of nervous system plasticity. During embryonic development, precise temporal and spatial patterns of neurogenesis are necessary to create the nervous system architecture. Similar between embryonic and postnatal stages, neurogenic proliferation is regulated by neural stem cell (NSC)-intrinsic mechanisms layered upon cues from their local microenvironmental niche. Following developmental assembly, it remains relatively unclear what may be the key driving forces that sustain continued production of neurons in the postnatal/adult brain. Recent experimental evidence suggests that patterned activity from specific neural circuits can also directly govern postnatal/adult neurogenesis. Here, we review experimental findings that revealed cholinergic modulation, and how patterns of neuronal activity and acetylcholine release may differentially or synergistically activate downstream signaling in NSCs. Higher-order excitatory and inhibitory inputs regulating cholinergic neuron firing, and their implications in neurogenesis control are also considered.
KEYWORDS: acetylcholine, ACh, adult neurogenesis, cholinergic circuit, ChAT neuron, neural stem cells, neurogenic niche, SVZ, SGZ, SEZ
Introduction
Resident neural stem cells (NSCs) in the brain present exciting possibilities for tissue regeneration and remodeling.1,2 During embryonic development, neurogenesis proceeds like clockwork, generating a full range of neurons in correct spatial and temporal sequences, enabling proper assembly of functional neural circuits. In the postnatal and adult mammalian brain, it is now well-accepted that NSCs are retained in discrete anatomical regions including the hippocampus and the walls of the lateral ventricles. Their continuous production of newborn neurons in the rodent lateral ventricular (LV) subventricular/subependymal zone (SVZ/SEZ) niche and in the hippocampal dentate gyrus subgranular zone (SGZ) (as well as potentially in the human striatum) offers endogenous sources for tissue regeneration and neural circuit plasticity.3-6 Maintaining a tissue stem cell population requires extra energy and resources, and when they acquire oncogenic mutations these proliferative cells can become a source for tumor formation,7,8 contributing harmful sequelae to the host tissue.9 While the need for neurogenesis during development to build the nervous system is rather clear, it remains relatively unclear what biological processes may be driving and sustaining new neuron production in specific regions in the postnatal/adult mammalian brain.
Postnatal neurogenesis in rodents provides a tractable experimental model to tackle molecular/cellular-level mechanisms regulating addition of new interneurons into established neural circuits.10-14 In the SGZ, astrocyte-like type 1 NSCs give rise to transient type 2 proliferative progenitors, which then produce DCX+ cells that mature into local dentate granule neurons.15,16 Ongoing SGZ neurogenesis in rodents contributes importantly to memory processing17 and neurological disease modeling,18 as well as having significant parallels in the human brain.19 LV neurogenesis is mediated by CSF-contacting GFAP+ glia functioning as NSCs, producing Mash1+ transiently amplifying progenitors which in turn differentiate into DCX+ neuroblasts that migrate to and become interneurons in the olfactory bulb.20,21 Generation of new neurons and glia from the LV niche contributes to experience-dependent plasticity in the postnatal brain,22,23 tissue remodeling after injury,24,25 and plays a critical role in olfactory-based rodent social behaviors.26-28 In the postnatal human brain, while some groups have reported potential olfactory bulb neurogenesis into adulthood,29,30 there is strong evidence that LV neurogenesis generates migrating interneurons for up to 2 y after birth.31 It has become increasingly clear that neurodevelopmental defects can be significant contributors to various brain pathologies later in life. Thus the analogous process in rodents can help us understand experimentally how NSC production of new neurons may be influenced by sensory/neural-circuit inputs during early postnatal human brain development.
It has been well-demonstrated that self-renewal of postnatal NSCs and their differentiation into neurons are controlled by conserved, cell-intrinsic molecular pathways.32-34 Extracellular factors and cell-cell interactions within the neurogenic microenvironmental niche also play critical roles regulating neurogenesis.35-38 Likewise, neurotransmitters such as GABA, glutamate, dopamine, and serotonin also contribute important modulatory roles during postnatal neurogenesis39-44: it has been generally assumed that neurotransmitters function through bulk release/non-synaptic mechanisms in the postnatal/adult neurogenic niches. In this fashion they are cytokine or growth factor-like, controlling NSC properties as neurotransmitters are leaked from nearby neuronal synaptic contacts following activity-dependent release. GABA spill-over from local parvalbumin+ interneuron regulating SGZ NSC proliferation/differentiation is an excellent example.45-47
Postnatal/adult NSCs often proliferate and differentiate in close proximity to neurons firing action potentials: the LV niche is anatomically adjacent to the striatum; and the SGZ niche is an integral part of the hippocampus. Thus neuronal activity patterns are attractive modulators for NSC proliferation and differentiation. Conceptually, if NSC fate choices can be directly linked to specific instructions from neuronal activity patterns, this will have important impact on circuit-level plasticity through new neuron production, as well as in nervous system diseases. This review will focus on cholinergic circuit control of postnatal neurogenesis: due to the complexity of cholinergic receptor physiology/function, the exact roles for acetylcholine (ACh) in this context have been difficult to define. Classical approaches including lesioning of cholinergic fibers, as well as pharmacological modulation of nicotinic and muscarinic receptors, first revealed the importance of cholinergic signaling in postnatal neurogenesis control. Recent findings incorporating optogenetic manipulation of cholinergic circuit have uncovered a direct link between neuronal activity patterns and neurogenic proliferation. In this mini-review, we will summarize these anatomical, pharmacological, and functional experimental results, and speculate on local cholinergic circuit wiring diagram and its possibilities for higher-level brain inputs that connect to behavioral paradigms/disease states.
Anatomical lesions and their effects on postnatal neurogenesis
Cholinergic neurons in the mammalian brain can be generally categorized into local interneurons such as those in the central cortex, hippocampus, and striatum; or long-range projection neurons such as those in the magnocellular basal nucleus, pontomesencephalic tegmentum, cranial nerve motor nuclei, and motor neurons in the spinal cord.48-52 For the projection neuron subtype, motor neurons in the spinal cord and cranial nerves, parasympathethic cholinergic neurons in the spinal cord, and cholinergic neurons within the sympathethic nervous system have been well-described.53 Within the brain, projecting cholinergic neurons have been organized into subgroups depending on their anatomical locations and projections (Fig. 1A).54 The nucleus basalis group includes: the nucleus basalis of Meynert and magnocellularis (B), substantia innominate (SI), and horizontal diagonal band of Broca (HDB). The medial septal group includes the medial septal nucleus (MS) and vertical diagonal band (VDB). And the pontine cholinergic group in the upper brain stem includes cholinergic neurons in the pedunculopontine tegmental nuclei (PPT) and laterodorsal tegmental nuclei (LDT). Cholinergic neurons within the local interneuron subtype are rather diverse, and include ACh-synthesising neurons in the caudate-putamen; nucleus accumbens; striatum; main and accessory olfactory bulbs; anterior olfactory nucleus; olfactory tubercule; hippocampus; cerebral cortex; basolateral hypothalamus; and spinal cord.53 Despite this anatomical diversity among cholinergic neurons, there are relatively few genetic strategies to specifically target distinct subpopulations of cholinergic neurons. In the context of postnatal neurogenic niches, cholinergic neurons from the medial septum and the diagonal band of Broca are believed to provide most of the cholinergic innervation to the SGZ niche (Fig. 1B).55,56 A newly identified cholinergic neuron population residing subependymally has recently been shown to modulate LV niche neurogenesis (Fig. 1C).57
Figure 1.
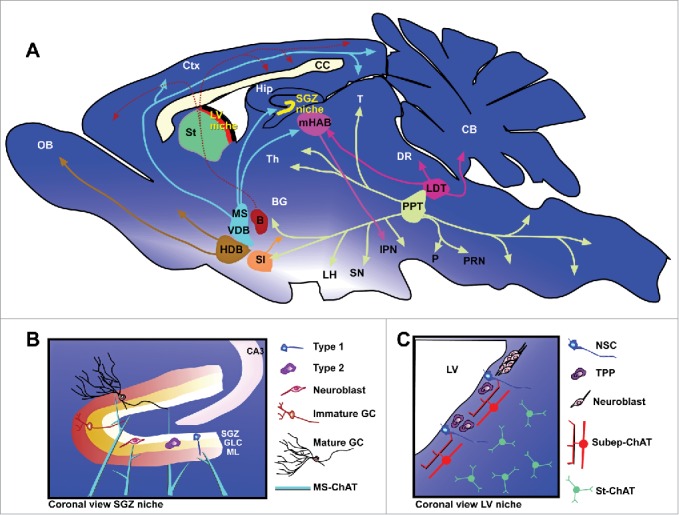
Cholinergic projections and neurogenic niches in the postnatal mouse brain. (A) Sagital section view showing major cholinergic nuclei and their known projections. Nuclei of the nucleus basalis group include: nucleus basalis of Meynert and magnocellularis (B); horizontal diagonal band of Broca (HDB); substantia innominate (SI). Nuclei of the medial septal group include: medial septal nucleus (MS) and vertical diagonal band (VDB). Nuclei of the pontine cholinergic group include: laterodorsal tegmental nuclei (LDT) and pedunculopontine tegmental nuclei (PPT). Other notable cholinergic neuron groups are found in: medial habenular nucleus (mHAB); striatum (St); and subependymal zone (SEZ). Major cholinergic neuron/nuclei projection targets include: basal ganglia (BG); cerebellum (CB); cortex (Ctx); dorsal raphae nucleus (DR); hippocampus (Hip); interpeduncular nucleus (IPN); lateral hypothalamus (LH); olfactory bulb (OB); pons (P); pontine reticular nucleus (PRN); substancia nigra (SN); thalamus (Th); and tectum (T). Neurogenic niches (LV and SGZ) are expanded in panels below. (B) Coronal section view of the SGZ neurogenic niche in the dentate gyrus (DG). Blue fibers indicate innervating projections from medial septal cholinergic neurons. Neurogenic cell types: astrocyte-like precursor (Type 1), transiently proliferating progenitor (Type 2), neuroblast, immature granule cell (GC), and mature GC. GLC = granule cell layer; ML = molecular layer of the DG. (C) Coronal view of the LV neurogenic niche, showing subep-ChAT neurons as well as neighboring striatal cholinergic neurons (St-ChAT). Neurogenic cell types include: (NSC) neural stem cell, Mash1+ transiently proliferating progenitor (TPP), and neuroblasts.
A robust and efficient method to label all cholinergic neurons is to drive fluorescence reporter via Cre recombinase expression from choline acetyltransferase (ChAT, required for acetylcholine synthesis) gene regulatory elements,58 although this approach does not distinguish cholinergic neuron subtypes. Primary anatomic lesion studies, while less elegant due to non-specific side effects, do allow for regional targeting of cholinergic neurons to access their potential functions during postnatal neurogenesis. Transection of the fimbria-fornix, which disrupts basal forebrain cholinergic projections to the hippocampus,59 has been reported to result in a concurrent decrease in dentate gyrus BrDU incorporation,60 suggesting decreased SGZ neurogenesis. Similarly, direct injection of N-methyl-d-aspartate (NMDA) to the cholinergic nuclei in the medial septal region to create excitotoxic lesions also reduced SGZ neurogenesis.61 Cholinergic neurons in the basal forebrain, medial septum, nucleus basalis of Meynert, and diagonal band of Broca all express high levels of p75 neurotrophin receptor (p75NTR). This exposes them to specific cellular elimination by precise stereotaxic injection of 192-IgG-SAP (192-Saporin), a chemical conjugate of p75NTR mouse clonal antibody to the ribosome-inactivating protein saporin. 192-Saporin-mediated removal of medial septal cholinergic neurons resulted in decreased SGZ neurogenesis,56,62 as well as decreased cellular proliferation in the LV niche.63 Together, these anatomical studies suggested that cholinergic neurons in the brain can play important roles to control postnatal neurogenesis.
Muscarinic and nicotinic cholinergic receptor activation and signaling
In addition to anatomical lesion studies, pharmacological approaches have also implicated cholinergic signaling as an important pathway controlling postnatal neurogenesis. ACh signals through both nicotinic and muscarinic acetylcholine receptors (nAChR and mAChR, respectively), which can be specifically targeted by pharmacological agents. Muscarinic agonists such as bethanechol, pilocarpine, and oxotremorine enhanced cellular proliferation when added to NSC cultures,64 hippocampal slices,62 or in vivo,65,66, while muscarinic antagonist had the opposite effect.67 Proliferation was also enhanced in cortical precursors following mAChR activation.68 While the effects are not as clear cut, nicotinic stimulation also appears to increase neurogenesis, as direct nicotine application in vivo increased LV Nestin+ cellular proliferation, resulting in subsequent BrdU-labeled NeuN+ granule neurons in the olfactory bulb.69 In the SGZ, pharmacological activation of the α7-subunit containing nAChRs have been shown to increase cellular proliferation.70 However, high doses of nicotine delivered chronically in vivo have an opposite effect in decreasing SGZ neurogenesis.71 Even though characterization of ACh receptor expression in neurogenic niches has not been extensive, LV NSCs have been reported to express α3- and α4- subunit containing nAChRs,57 similar to those residing in the rostral migratory stream which displayed α3β4 nAChR activity.72 In contrast to NSCs and DCX+ neuroblasts, Mash1+ transiently amplifying progenitors in the LV niche did not appear to express functional ACh receptors.57 In the SGZ, IHC and functional analyses have revealed the presence of M1 and M4 subunit mAChR expressions, as well as α7 and β2 nAChR subunit expressions in immature hippocampal neurons.73,74 M1 and M4 mAChRs also co-label with proliferating SGZ cells shortly following BrdU administration.75
These results suggest that adult neural stem/progenitor cell populations are sensitive to levels and timing of acetylcholine released, and that cholinergic receptor subtypes may mediate differential effects on cellular proliferation. mAChRs are metabotropic transmembrane proteins, coupled to G proteins, and activate various intracellular signaling pathways to provide sustained cellular responses.76,77 Meanwhile, nAChRs are pentameric, ionotropic channels consisting of several subunits: one alpha + 4 other subunits named beta, gamma, delta, and epsilon. nAChRs mediate fast cholinergic transmission in the peripheral and central nervous system.78 The subunit compositions of the various nAChRs determine their ionic permeability (e.g. Na+, K+, Ca2+), affinity for ACh, channel current kinetics, and channel desensitization.79-82 In the brain, mAChR and nAChR types are present on neurons at both synaptic and extra-synaptic sites,83-85, as well as on glial cells.86,87
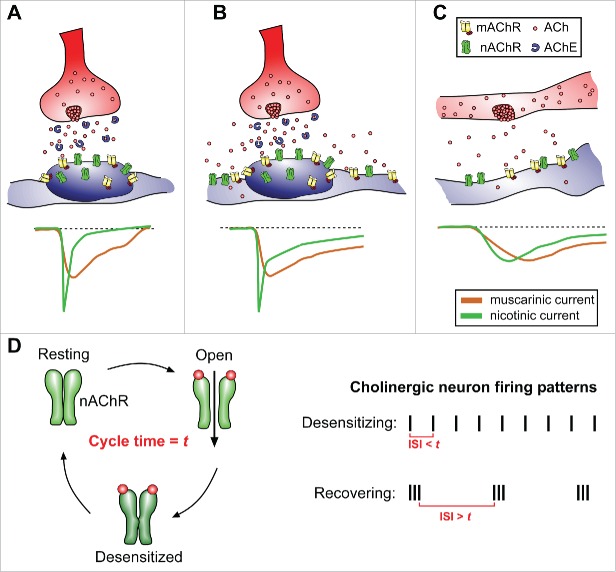
Low-level, sustained neuronal activity patterns that result in consistently low concentrations of neurotransmitter released are termed tonic firing patterns. On the other hand, more robust, synaptic, and temporally salient neuronal activities are referred to as phasic activity, occurring on much finer time-scales.88 Neuronal activity patterns of specific cholinergic populations can range from spontaneous low frequency spiking (tonic)89 to those that fire very irregularly or respond strongly to specific salient stimulation (phasic).90-92 The types of release will influence both the concentration and temporal profiles of ACh signaling, as well as the speed of ACh breakdown by acetylcholinesterases, which are particularly effective in synaptic clefts.92 One notable feature regarding nAChRs is their rapid desensitization following ACh-induced activation.80,81 This results in short bursts of ACh release having a qualitatively different effect on the receiving cell than ACh continuously present or absent (Fig. 2). Anatomically speaking, activity-dependent ACh released from synaptic sites facilitate fast, high concentration neurotransmitter access to receptors (Fig. 2A), while bulk/volume ACh release that occurs at non-synaptic sites or from nearby synaptic spillover provide low level neurotransmitter via diffusion over larger areas (Fig. 2B, C).93 These principles of ACh differentially signaling through 2 receptors, with temporal dynamics on nAChR activation/desensitization, underlie key features of ACh's important functions during neural circuit modulation (Fig. 2D). In neurons, nicotinic and muscarinic signaling can often be antagonistic, inducing differential currents or polarizations, divergent calcium signaling profiles,94 or distinct regulations of LTP.95 However, there are also examples where mAChR and nAChR signaling can be cooperative, producing complimentary depolarizing currents and convergence onto common biological intracellular cascades.96-98 This context-dependent complexity in signaling capacity may provide a palette richness for NSCs to functionally read-out subtle changes in local ACh availability.
Figure 2.
ACh release and receptor activation dynamics to convey neuronal activity patterns. (A) Schematic representation of ACh released directly onto a receptive zone, with a high density of nAChR and mAChR receptors. In such specialized contacts (neuronal synapse as an example), ACh upon release is quickly degraded by extracellular acetylcholinesterase. Nicotinic currents are typically rapid and fast-inactivating, while muscarinic currents are longer lasting. (B) Multiple neuronal activations can cause released ACh to spillover and activate nAChRs/mAChRs away from the immediate receptive zone. This leads to prolonged nicotinic and muscarinic currents in the responding cell. (C) Volume release of ACh stimulates larger fields of receptors at low concentrations. Cholinergic currents evoked by volume release may be small and prolonged. (D) Diagram of nAChR resting, activation, and desensitization cycle (cycle time = t, receptor subtype specific). Depending on the timing of cholinergic neuron inter-stimulus intervals (ISI), the resulting patterns of ACh release will enhance nAChR desensitization when ISI< t, or promote nAChR recovery for reactivation when ISI > t, resulting in distinct nicotinic activation dynamics in the receiving cell. AChE = acetylcholinesterase.
Identification of resident cholinergic neurons in subependymal niche
While cholinergic pharmacology has an effect on adult NSC proliferation in vivo,69 it remained unclear if these putative actions attributed to ACh are due to neuronal activity or are in fact indirect. To determine whether activity patterns from cholinergic neurons can directly control neurogenesis, one approach is to examine outcomes upon altering intrinsic excitability of these neurons. We conditionally deleted Ank3, a large adapter protein known to localize to neuronal axon initial segment, specifically in cholinergic neurons (ChATIRES-Cre/+; ank3flox/flox). Similar to previous observations in cerebellar Purkinje neurons,99 Ank3-mutant ChAT+ neurons showed an inability to precisely initiate and scale action potentials to electrical stimulation in ChATIRES-Cre/+; ank3flox/flox mutant mice.57 There was a marked reduction in DCX+ neuroblast chains along the LV niche, becoming progressively worse in adult mice. Ki67 and Mash1 IHC staining revealed a corresponding decrease in LV niche cellular proliferation, while caspase 3 staining showed no obvious increase in apoptosis. These results revealed that cholinergic circuit activity and precision are required to sustain the robustness of adult LV neurogenesis.
As cholinergic innervation is widespread in the brain, disrupting its activity will likely contribute to many effects, including modulation of non-cholinergic circuits. The observed neurogenesis defects in ChATIRES-Cre/+; ank3flox/flox mutant mice can also be caused by decreased ACh release in the SEZ niche via direct cholinergic innervation. To examine this possibility closer, we looked for cholinergic processes along the LV wall, and detected large ChAT+ neuronal cell bodies residing subependymally within the LV niche.57 DiI-filling of these subependymal ChAT+ (subep-ChAT) neurons revealed complex neuronal processes that were largely aspiny, and projected their axonal processes locally in the subependymal space (Fig. 3). A morphological feature for these subep-ChAT neurons, which can be located in both young and adult mice (examined up to 6 months of age), is their planar appearance paralleling the ependymal surface above. More importantly, unlike neighboring striatal cholinergic neurons which are spontaneously active, the subep-ChAT neurons did not exhibit basal-level spontaneous activity in acute brain slice preparation. In vivo optogenetic stimulation of subep-ChAT neurons in P30 ChATIRES-Cre/+; Rosa26R-ChR2EYFP mice significantly increased the numbers of Ki67+ proliferating cells and neurogenic progenitors in the LV niche. Conversely, in vivo optogenetic suppression of subep-ChAT neurons in P30 ChATIRES-Cre/+; Rosa26R-ArchaerhodopsinGFP mice decreased the numbers of Ki67+, Mash1+, and DCX+ cells in the LV niche.
Figure 3.
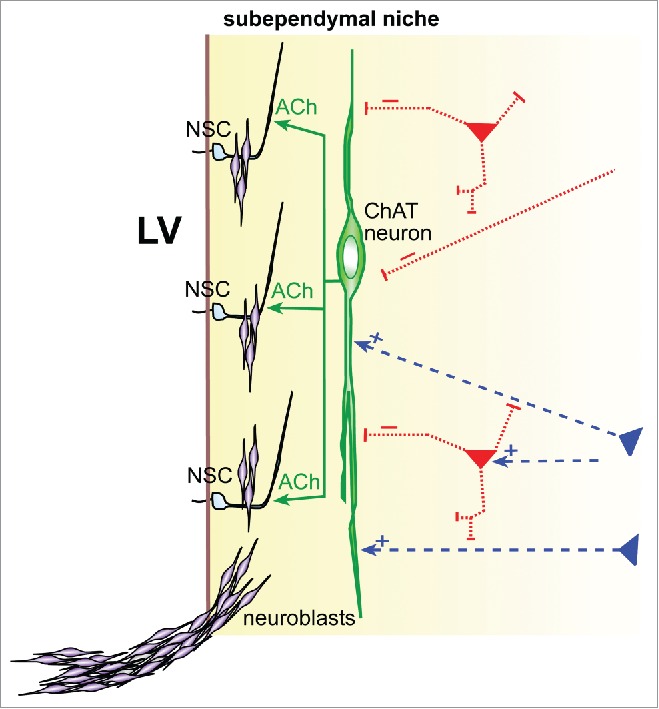
Subependymal cholinergic neuron bridging SEZ niche/neurogenesis to neural circuit-level control. Schematic representation of subep-ChAT neuron (green) providing ACh to modulate adult SEZ neural stem cells (NSC) production of new neuroblasts, which then migrate and assemble into neuroblast chains. Dashed lines represent putative excitatory (+, blue) or inhibitory (−, red) inputs onto subep-ChAT neuron dendrites. LV = lateral ventricle.
To determine whether LV NSCs can directly detect ACh release via cholinergic neuron activity, we performed whole-cell patch recording in NSCs, while simultaneously activating ChR2-expressing cholinergic inputs locally via 473 nm laser. This resulted in consistent frequency-dependent inward currents in NSCs, sensitive to both nicotinic and muscarinic blockade (Fig. 3).57 These neuronal activity-dependent responses from postnatal LV NSCs appeared distinct from synaptic “spill-over” mechanisms.46 Mechanistically, α3β4 nAChR as well as α7-subunit containing nAChRs have been reported to function during postnatal LV neurogenesis.70,72 Our IHC antibody staining and electrophysiological experiments revealed α3- and α4-subunit containing nAChR, as well as mAChR expression in nestin-CreER lineage-traced LV NSCs.57 Consistent with these results: α4β2 nAChR and M1/M4 mAChR agonists have been shown to control SGZ neurogenic proliferation and differentiation;100-102 and β2-subunit nAChR mutant mice have reduced SGZ proliferation over the life of the animal.103
These findings revealed subep-ChAT neurons as integral components of the cholinergic circuit controlling postnatal LV neurogenesis. Beyond these subep-ChAT neurons, it remains possible that there are other cholinergic neurons whose activity contributes to LV neurogenesis control. Anatomically, the nearest such population is located in the striatum: the well-studied tonically-active striatal cholinergic neurons. While genetic deletion of ChAT (removing ACh producing ability) in the striatal cholinergic population via Nkx2.1-Cre; ChATflox/flox mutant mice revealed no obvious LV neurogenesis defects,57 striatal cholinergic neurons may still play a role under physiological conditions that can be compensated for as needed by subep-ChAT neurons.
Circuit level control of cholinergic neuron activity
It is of great interest to understand, at the circuit level, how cholinergic neuron activity is regulated, resulting in postnatal neurogenesis control. Ank3 deletion from ChAT+ neurons showed that precise cholinergic circuit activity is required to sustain the robustness of adult LV neurogenesis. While IHC staining for p-rpS6, an activity-dependent marker for cholinergic neurons,104 revealed that subep-ChAT neurons are normally active in vivo, they lacked spontaneous activity in acute brain slice preparation, indicating their activity is contextually controlled by higher-level inputs. The sources for excitatory/inhibitory inputs onto subep-ChAT neurons are currently unclear, although CNS cholinergic neurons such as those found in the striatum, basal forebrain nuclei, hypothalamus, medial habenula, pontomesencephalic tegmentum, and medullary tegmentum, tend to have highly stereotyped patterns of afferent connectivity,105 serving as potential blueprints for subep-ChAT neuron connectivity. First, there is rich inter-connectivity between cholinergic cell groups, which form a contiguous plexus of overlapping dendritic arbors, collectively allowing each subsystem (e.g., striatum, basal forebrain, and pontomesencephalon) to receive and integrate information from various sensory modalities.106 Second, forebrain cholinergic neurons generally receive excitatory cortical inputs (Fig. 4).105 This pattern of innervation has been hypothesized to provide a means for global integration of ongoing neural activity, as cholinergic cell groups are frequently implicated in the modulation of attention and arousal associated with the reticular activating system.107 Furthermore, striatal cholinergic neurons adjacent to the LV niche, as well as those in the nucleus basilis, receive inputs from the intralaminar thalamus, as part of the reticular activating system (Fig. 4).105 All cholinergic neuron groups also receive noradrenergic input from the locus ceruleus and subceruleus.108 The basal forebrain, striatal, diencephalic, and pontomesencephalic cholinergic groups also receive sparse nigral or ventral tegmental dopaminergic inputs (Fig. 3).105
Figure 4.
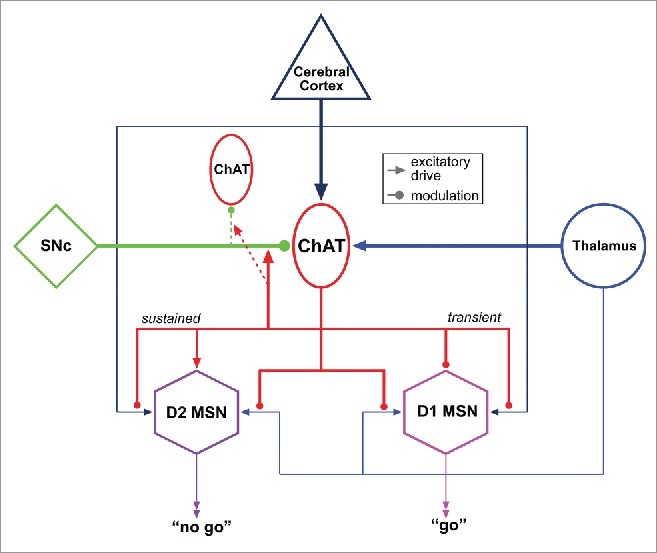
Example functional connectivity of a subgroup of CNS cholinergic neurons. Striatal cholinergic neurons (TANs) receive glutamatergic inputs from both cortex and intralaminar thalamus, as well as dopaminergic modulation from the substantia nigra pars compacta (SNc). Medium spiny neurons (MSNs), projection neurons in the striatum, express either type 1 or type 2 dopamine receptors (D1 or D2, respectively). Following thalamic stimulation, TANs generate a burst-pause pattern of activity that transiently and presynaptically inhibits thalamic and cortical excitation of D1 and D2 striatal MSNs through muscarinic receptor subtype M2 signaling. It also initiates a sustained, muscarinic receptor subtype M1-mediated facilitation of dendritic responsiveness in D2 MSNs: resulting in a bias of cortical and thalamic excitation toward D2 expressing, striatopallidal MSNs for the duration of the pause in TAN activity. The pause is dependent on dopaminergic signaling onto TANs. Functionally, thalamic excitation of TANs is thought to provide a window in which excitation of D2-expressing MSNs is enhanced, allowing for preferential recruitment of the striatopallidal pathway. Such wiring diagrams may serve as useful models to study subep-ChAT neuron connectivity. Distinct neuronal cell types and projection patterns are represented in different colors for clarity.
Within the striatum as a specific example, cholinergic neuron activity patterns are dynamically controlled via distal excitation modulating intrinsic neuronal membrane properties.109,110 The large, aspiny cholinergic interneurons of the striatum, referred to as tonically active neurons (TANs), represent a particularly well-studied population of spontaneously firing cholinergic neuron. In TANs, spontaneous activity is mediated by intrinsic membrane properties, specifically a sodium current and hyperpolarization-activated cation current which together drive tonic firing.89 Salient stimuli produce a characteristic pause in TAN firing, and TANs have long been viewed as important substrates for striatal associative and motor learning.111,112 In contextual recognition of salient stimuli driving action selection, the temporal, spatial, and motivational context of salient stimuli have all been shown to play a role in regulating spontaneous striatal cholinergic neuron activity.113 Similar pauses in cholinergic TAN activity are generated following stimulation of nigrostriatal afferents,110 and a burst-pause firing pattern is generated in response to stimulation of the intralaminar thalamus (Fig. 4).109
Although subep-ChAT cells are silent under resting conditions, this is relatively rare among other cholinergic cell groups.90,114 It remains to be seen whether the distinct activity profiles of subep-ChAT neurons vs. neighboring striatal TANs in brain slice preparations are due to differences in intrinsic membrane properties or their inhibitory tone. Within the local microcircuitry, GABAergic inhibitory interneurons can also provide dynamic control to alter cholinergic neuron activity states.114 While it has been well demonstrated that GABA is an important protein for several cell types during postnatal LV neurogenesis,115,116 its sources in the niche remains largely unclear. Conceptually, since cholinergic neurons groups are broadly interconnected, it is an intriguing possibility that subep-ChAT neurons participate in and sample the cholinergic plexus to transform global cascades of activity within the cholinergic system into functional neurogenesis. Furthermore, if subep-ChAT neurons are involved in associative learning tasks, believed to be an important function for striatal cholinergic TANs, a similar configuration of circuit-level connectivity may allow subep-ChAT neurons to respond to environmentally salient stimuli to direct neurogenesis. The highly stereotyped connectivity patterns of other cholinergic groups provide a template and substrate for investigating the circuit wiring diagram of subep-ChAT neurons.
Conclusion
Like a computer needing hardware upgrades to run increasingly sophisticated software, it is tempting to speculate that postnatal neurogenesis endow particular neural circuits with this capacity. The fact that neuronal firing patterns can direct NSC proliferation takes this concept one step further, and proposes that perhaps certain critical neural circuits may functionally instruct NSCs for their own neuronal additions over time. There are parallels in the glial biology field: pioneering study by Barres and Raff in 1993 showed that oligodendrocyte precursor proliferation can be dependent on neurons generating action potentials.117 Neuronal activity-dependent increases in myelination is now a well-accepted observation, and recent studies extended this concept to show the lack of inhibitory post-synaptic currents on oligodendrocyte precursors contributing to white matter defects following hypoxic injury,118 as well as glioma cellular proliferation profiting from neuronal activity.119 It is likely that future studies will find additional examples, perhaps in the process revealing that the control of postnatal neurogenesis by neuronal activity may be the norm, rather than an exception for cells proliferating in the brain in health and disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank members of the Kuo laboratory, Jerry Yakel (N.I.E.H.S), and Juan Song (U.N.C. Chapel Hill) for helpful discussions.
Funding
This work was supported by N.I.H. grant R01MH105416, R01NS078192, The March of Dimes, and George & Jean Brumley Endowment (C.T.K.).
References
- [1].Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta stone. Neuron 2011; 70:597-613; PMID:21609819; http://dx.doi.org/ 10.1016/j.neuron.2011.05.007 [DOI] [PubMed] [Google Scholar]
- [2].Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 2011; 12:88-104; PMID:21248788; http://dx.doi.org/ 10.1038/nrn2978 [DOI] [PubMed] [Google Scholar]
- [3].Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008; 132:645-60; PMID:18295581; http://dx.doi.org/ 10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]
- [4].Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 2011; 70:674-86; PMID:21609824; http://dx.doi.org/ 10.1016/j.neuron.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, et al.. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013; 153:1219-27; PMID:23746839; http://dx.doi.org/ 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. Neurogenesis in the striatum of the adult human brain. Cell 2014; 156:1072-83; PMID:24561062; http://dx.doi.org/ 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- [7].Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 2009; 15:45-56; PMID:19111880; http://dx.doi.org/ 10.1016/j.ccr.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev 2015; 29:1203-17; PMID:26109046; http://dx.doi.org/ 10.1101/gad.261982.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015; 347:78-81; PMID:25554788; http://dx.doi.org/ 10.1126/science.1260825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kelsch W, Sim S, Lois C. Watching synaptogenesis in the adult brain. Annu Rev Neurosci 2010; 33:131-49; PMID:20572770; http://dx.doi.org/ 10.1146/annurev-neuro-060909-153252 [DOI] [PubMed] [Google Scholar]
- [11].Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends Neurosci 2011; 34:20-30; PMID:20980064; http://dx.doi.org/ 10.1016/j.tins.2010.09.006 [DOI] [PubMed] [Google Scholar]
- [12].Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci 2012; 15:1613-20; PMID:23187693; http://dx.doi.org/ 10.1038/nn.3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Song J, Christian KM, Ming GL, Song H. Modification of hippocampal circuitry by adult neurogenesis. Dev Neurobiol 2012; 72:1032-43; PMID:22354697; http://dx.doi.org/ 10.1002/dneu.22014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crowther AJ, Song J. Activity-dependent signaling mechanisms regulating adult hippocampal neural stem cells and their progeny. Neurosci Bull 2014; 30:542-56; PMID:25082534; http://dx.doi.org/ 10.1007/s12264-014-1453-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yu DX, Marchetto MC, Gage FH. How to make a hippocampal dentate gyrus granule neuron. Development 2014; 141:2366-75; PMID:24917496; http://dx.doi.org/ 10.1242/dev.096776 [DOI] [PubMed] [Google Scholar]
- [16].Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Med 2015; 5:a018812; PMID:26351719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kropff E, Yang SM, Schinder AF. Dynamic role of adult-born dentate granule cells in memory processing. Curr Opin Neurobiol 2015; 35:21-26; PMID:26100379; http://dx.doi.org/ 10.1016/j.conb.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Braun SM, Jessberger S. Adult neurogenesis: mechanisms and functional significance. Development 2014; 141:1983-6; PMID:24803647; http://dx.doi.org/ 10.1242/dev.104596 [DOI] [PubMed] [Google Scholar]
- [19].Bergmann O, Spalding KL, Frisen J. Adult Neurogenesis in Humans. Cold Spring Harb Perspect Biol 2015; 7:a018994; PMID:26134318; http://dx.doi.org/ 10.1101/cshperspect.a018994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97:703-16; PMID:10380923 [DOI] [PubMed] [Google Scholar]
- [21].Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci 2008; 31:392-400; PMID:18603310; http://dx.doi.org/ 10.1016/j.tins.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Livneh Y, Adam Y, Mizrahi A. Odor processing by adult-born neurons. Neuron 2014; 81:1097-110; PMID:24508384; http://dx.doi.org/ 10.1016/j.neuron.2014.01.007 [DOI] [PubMed] [Google Scholar]
- [23].Sakamoto M, Ieki N, Miyoshi G, Mochimaru D, Miyachi H, Imura T, Yamaguchi M, Fishell G, Mori K, Kageyama R, et al.. Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning. J Neurosci 2014; 34:5788-99; PMID:24760839; http://dx.doi.org/ 10.1523/JNEUROSCI.0674-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature 2013; 497:369-73; PMID:23615612; http://dx.doi.org/ 10.1038/nature12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Faiz M, Sachewsky N, Gascon S, Bang KW, Morshead CM, Nagy A. Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell Stem Cell 2015; 17:624-34; PMID:26456685; http://dx.doi.org/ 10.1016/j.stem.2015.08.002 [DOI] [PubMed] [Google Scholar]
- [26].Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 2008; 11:1153-61; PMID:18758458; http://dx.doi.org/ 10.1038/nn.2185 [DOI] [PubMed] [Google Scholar]
- [27].Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci 2010; 13:753-8; PMID:20453850; http://dx.doi.org/ 10.1038/nn.2550 [DOI] [PubMed] [Google Scholar]
- [28].Sakamoto M, Kageyama R, Imayoshi I. The functional significance of newly born neurons integrated into olfactory bulb circuits. Front Neurosci 2014; 8:121; PMID:24904263; http://dx.doi.org/ 10.3389/fnins.2014.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bedard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Dev Brain Res 2004; 151:159-68; PMID:15246702; http://dx.doi.org/ 10.1016/j.devbrainres.2004.03.021 [DOI] [PubMed] [Google Scholar]
- [30].Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtås S, van Roon-Mom WM, Björk-Eriksson T, Nordborg C, et al.. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 2007; 315:1243-9; PMID:17303719; http://dx.doi.org/ 10.1126/science.1136281 [DOI] [PubMed] [Google Scholar]
- [31].Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al.. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011; 478:382-6; PMID:21964341; http://dx.doi.org/ 10.1038/nature10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009; 32:149-84; PMID:19555289; http://dx.doi.org/ 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol 2009; 25:253-75; PMID:19575663; http://dx.doi.org/ 10.1146/annurev.cellbio.042308.113256 [DOI] [PubMed] [Google Scholar]
- [34].Urban N, Guillemot F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci 2014; 8:396; PMID:25505873; http://dx.doi.org/ 10.3389/fncel.2014.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008; 3:279-88; PMID:18786415; http://dx.doi.org/ 10.1016/j.stem.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, Rawlins E, Bennett V, Garcia-Verdugo JM, Kuo CT. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron 2011; 71:61-75; PMID:21745638; http://dx.doi.org/ 10.1016/j.neuron.2011.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH, Parrinello S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol 2014; 16:1045-56; PMID:25283993; http://dx.doi.org/ 10.1038/ncb3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bjornsson CS, Apostolopoulou M, Tian Y, Temple S. It takes a village: constructing the neurogenic niche. Dev Cell 2015; 32:435-46; PMID:25710530; http://dx.doi.org/ 10.1016/j.devcel.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lennington JB, Pope S, Goodheart AE, Drozdowicz L, Daniels SB, Salamone JD, Conover JC. Midbrain dopamine neurons associated with reward processing innervate the neurogenic subventricular zone. J Neurosci 2011; 31:13078-87; PMID:21917791; http://dx.doi.org/ 10.1523/JNEUROSCI.1197-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Young SZ, Taylor MM, Bordey A. Neurotransmitters couple brain activity to subventricular zone neurogenesis. Eur J Neurosci 2011; 33:1123-32; PMID:21395856; http://dx.doi.org/ 10.1111/j.1460-9568.2011.07611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Crowther AJ, Song J. Activity-dependent signaling mechanisms regulating adult hippocampal neural stem cells and their progeny. Neurosci Bull 2014; 30:542-56; PMID:25082534; http://dx.doi.org/ 10.1007/s12264-014-1453-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pallotto M, Deprez F. Regulation of adult neurogenesis by GABAergic transmission: signaling beyond GABAA-receptors. Front Cell Neurosci 2014; 8:166; PMID:24999317; http://dx.doi.org/ 10.3389/fncel.2014.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tong CK, Chen J, Cebrian-Silla A, Mirzadeh Z, Obernier K, Guinto CD, Tecott LH, García-Verdugo JM, Kriegstein A, Alvarez-Buylla A. Axonal control of the adult neural stem cell niche. Cell Stem Cell 2014; 14:500-11; PMID:24561083; http://dx.doi.org/ 10.1016/j.stem.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Alenina N, Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res 2015; 277:49-57; PMID:25125239; http://dx.doi.org/ 10.1016/j.bbr.2014.07.038 [DOI] [PubMed] [Google Scholar]
- [45].Markwardt SJ, Dieni CV, Wadiche JI, Overstreet-Wadiche L. Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat Neurosci 2011; 14:1407-9; PMID:21983681; http://dx.doi.org/ 10.1038/nn.2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al.. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012; 489:150-4; PMID:22842902; http://dx.doi.org/ 10.1038/nature11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, et al.. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci 2013; 16:1728-30; PMID:24212671; http://dx.doi.org/ 10.1038/nn.3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 1982; 8:727-49; PMID:6182962 [DOI] [PubMed] [Google Scholar]
- [49].Woolf NJ, Eckenstein F, Butcher LL. Cholinergic projections from the basal forebrain to the frontal cortex: a combined fluorescent tracer and immunohistochemical analysis in the rat. Neurosci Lett 1983; 40:93-8; PMID:6633976 [DOI] [PubMed] [Google Scholar]
- [50].Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience 1984; 12:669-86; PMID:6382047 [DOI] [PubMed] [Google Scholar]
- [51].Nicoll RA. The septo-hippocampal projection: a model cholinergic pathway. Trends Neurosci 1985; 8:533-36; PMIDhttp://dx.doi.org/ 10.1016/0166-2236(85)90190-0 [DOI] [Google Scholar]
- [52].Smiley JF, Mesulam MM. Cholinergic neurons of the nucleus basalis of Meynert receive cholinergic, catecholaminergic and GABAergic synapses: an electron microscopic investigation in the monkey. Neuroscience 1999; 88:241-55; PMID:10051204 [DOI] [PubMed] [Google Scholar]
- [53].Kasa P. The cholinergic systems in brain and spinal cord. Prog Neurobiol 1986; 26:211-72; PMID:3523620 [DOI] [PubMed] [Google Scholar]
- [54].Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 1983; 10:1185-201; PMID:6320048 [DOI] [PubMed] [Google Scholar]
- [55].Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev 1995; 75:393-427; PMID:7724668 [DOI] [PubMed] [Google Scholar]
- [56].Frechette M, Rennie K, Pappas BA. Developmental forebrain cholinergic lesion and environmental enrichment: behaviour, CA1 cytoarchitecture and neurogenesis. Brain Res 2009; 1252:172-82; PMID:19084506; http://dx.doi.org/ 10.1016/j.brainres.2008.11.082 [DOI] [PubMed] [Google Scholar]
- [57].Paez-Gonzalez P, Asrican B, Rodriguez E, Kuo CT. Identification of distinct ChAT(+) neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat Neurosci 2014; 17:934-42; PMID:24880216; http://dx.doi.org/ 10.1038/nn.3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 2011; 13:195-204; PMID:21284986; http://dx.doi.org/ 10.1016/j.cmet.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rosenberg MB, Friedmann T, Robertson RC, Tuszynski M, Wolff JA, Breakefield XO, Gage FH. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science 1988; 242:1575-8; PMID:3201248 [DOI] [PubMed] [Google Scholar]
- [60].Fontana X, Nacher J, Soriano E, del Rio JA. Cell proliferation in the adult hippocampal formation of rodents and its modulation by entorhinal and fimbria-fornix afferents. Cereb Cortex 2006; 16:301-12; PMID:15958781; http://dx.doi.org/ 10.1093/cercor/bhi120 [DOI] [PubMed] [Google Scholar]
- [61].Van der Borght K, Mulder J, Keijser JN, Eggen BJ, Luiten PG, Van der Zee EA. Input from the medial septum regulates adult hippocampal neurogenesis. Brain Res Bull 2005; 67:117-25; PMID:16140170; http://dx.doi.org/ 10.1016/j.brainresbull.2005.06.018 [DOI] [PubMed] [Google Scholar]
- [62].Itou Y, Nochi R, Kuribayashi H, Saito Y, Hisatsune T. Cholinergic activation of hippocampal neural stem cells in aged dentate gyrus. Hippocampus 2011; 21:446-59; PMID:20054812; http://dx.doi.org/ 10.1002/hipo.20761 [DOI] [PubMed] [Google Scholar]
- [63].Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res 2004; 77:155-65; PMID:15211583; http://dx.doi.org/ 10.1002/jnr.20116 [DOI] [PubMed] [Google Scholar]
- [64].Calza L, Giuliani A, Fernandez M, Pirondi S, D'Intino G, Aloe L, Giardino L. Neural stem cells and cholinergic neurons: regulation by immunolesion and treatment with mitogens, retinoic acid, and nerve growth factor. Proc Natl Acad Sci USA 2003; 100:7325-30; PMID:12777625; http://dx.doi.org/ 10.1073/pnas.1132092100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ma W, Li BS, Zhang L, Pant HC. Signaling cascades implicated in muscarinic regulation of proliferation of neural stem and progenitor cells. Drug News Perspect 2004; 17:258-66; PMID:15334175 [DOI] [PubMed] [Google Scholar]
- [66].Veena J, Srikumar BN, Mahati K, Raju TR, Shankaranarayana Rao BS. Oxotremorine treatment restores hippocampal neurogenesis and ameliorates depression-like behaviour in chronically stressed rats. Psychopharm 2011; 217:239-53; PMID:21494789; http://dx.doi.org/ 10.1007/s00213-011-2279-3 [DOI] [PubMed] [Google Scholar]
- [67].Kotani S, Yamauchi T, Teramoto T, Ogura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience 2006; 142:505-14; PMID:16889901; http://dx.doi.org/ 10.1016/j.neuroscience.2006.06.035 [DOI] [PubMed] [Google Scholar]
- [68].Ma W, Maric D, Li BS, Hu Q, Andreadis JD, Grant GM, Liu QY, Shaffer KM, Chang YH, Zhang L, et al.. Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation. Eur J Neurosci 2000; 12:1227-40; PMID:10762352 [DOI] [PubMed] [Google Scholar]
- [69].Mudo G, Belluardo N, Mauro A, Fuxe K. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precursor cell proliferation. Neuroscience 2007; 145:470-83; PMID:17241745; http://dx.doi.org/ 10.1016/j.neuroscience.2006.12.012 [DOI] [PubMed] [Google Scholar]
- [70].Narla S, Klejbor I, Birkaya B, Lee YW, Morys J, Stachowiak EK, Terranova C, Bencherif M, Stachowiak MK. alpha7 nicotinic receptor agonist reactivates neurogenesis in adult brain. Biochem Pharmacol 2013; 86:1099-104; PMID:23933384; http://dx.doi.org/ 10.1016/j.bcp.2013.07.028 [DOI] [PubMed] [Google Scholar]
- [71].Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, Piazza PV. Nicotine self-administration impairs hippocampal plasticity. J Neurosci 2002; 22:3656-62; PMID:11978841; http://dx.doi.org/20026324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sharma G. The dominant functional nicotinic receptor in progenitor cells in the rostral migratory stream is the alpha3beta4 subtype. J Neurophysiol 2013; 109:867-72; PMID:23136348; http://dx.doi.org/ 10.1152/jn.00886.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 2006; 11:1145-59; PMID:16999735; http://dx.doi.org/ 10.1111/j.1365-2443.2006.01010.x [DOI] [PubMed] [Google Scholar]
- [74].John D, Shelukhina I, Yanagawa Y, Deuchars J, Henderson Z. Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus. Brain Res 2015; 1601:15-30; PMID:25553616; http://dx.doi.org/ 10.1016/j.brainres.2014.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging 2005; 26:939-46; PMID:15718053; http://dx.doi.org/ 10.1016/j.neurobiolaging.2004.07.015 [DOI] [PubMed] [Google Scholar]
- [76].Dutar P, Nicoll RA. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci 1988; 8:4214-24; PMID:3141594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Caulfield MP. Muscarinic receptors–characterization, coupling and function. Pharmacol Ther 1993; 58:319-79; PMID:7504306 [DOI] [PubMed] [Google Scholar]
- [78].Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry 2001; 49:166-74; PMID:11230867 [DOI] [PubMed] [Google Scholar]
- [79].Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 2002; 53:447-56; PMID:12436412; http://dx.doi.org/ 10.1002/neu.10153 [DOI] [PubMed] [Google Scholar]
- [80].Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol 2002; 53:457-78; PMID:12436413; http://dx.doi.org/ 10.1002/neu.10109 [DOI] [PubMed] [Google Scholar]
- [81].Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci 2005; 28:371-8; PMID:15979501; http://dx.doi.org/ 10.1016/j.tins.2005.04.009 [DOI] [PubMed] [Google Scholar]
- [82].Smit AB, Celie PH, Kasheverov IE, Mordvintsev DY, van Nierop P, Bertrand D, Tsetlin V, Sixma TK. Acetylcholine-binding proteins: functional and structural homologs of nicotinic acetylcholine receptors. J Mol Neurosci 2006; 30:9-10; PMID:17192605; http://dx.doi.org/ 10.1385/JMN:30:1:9 [DOI] [PubMed] [Google Scholar]
- [83].Mrzljak L, Levey AI, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci USA 1993; 90:5194-8; PMID:8389473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res Rev 1999; 30:219-35; PMID:10567725 [DOI] [PubMed] [Google Scholar]
- [85].Huh KH, Fuhrer C. Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses. Mol Neurobiol 2002; 25:79-112; PMID:11890459; http://dx.doi.org/ 10.1385/MN:25:1:079 [DOI] [PubMed] [Google Scholar]
- [86].Loreti S, Vilaro MT, Visentin S, Rees H, Levey AI, Tata AM. Rat Schwann cells express M1-M4 muscarinic receptor subtypes. J Neurosci Res 2006; 84:97-105; PMID:16634060; http://dx.doi.org/ 10.1002/jnr.20874 [DOI] [PubMed] [Google Scholar]
- [87].De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev Neurobiol 2012; 72:713-28; PMID:21913336; http://dx.doi.org/ 10.1002/dneu.20976 [DOI] [PubMed] [Google Scholar]
- [88].Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 2007; 56:141-54; PMID:17920021; http://dx.doi.org/ 10.1016/j.neuron.2007.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci 2000; 20:8493-503; PMID:11069957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci 2000; 20:1505-18; PMID:10662840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann NY Acad Sci 2008; 1129:225-35; PMID:18591483; http://dx.doi.org/ 10.1196/annals.1417.021 [DOI] [PubMed] [Google Scholar]
- [92].Unal CT, Golowasch JP, Zaborszky L. Adult mouse basal forebrain harbors two distinct cholinergic populations defined by their electrophysiology. Front Behav Neurosci 2012; 6:21; PMID:22586380; http://dx.doi.org/ 10.3389/fnbeh.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol 1997; 53:603-25; PMID:9421837 [DOI] [PubMed] [Google Scholar]
- [94].Rathouz MM, Vijayaraghavan S, Berg DK. Acetylcholine differentially affects intracellular calcium via nicotinic and muscarinic receptors on the same population of neurons. J Biol Chem 1995; 270:14366-75; PMID:7782297 [DOI] [PubMed] [Google Scholar]
- [95].Zhang W, Tan YF, Atwood HL, Martin Wojtowicz J. Biphasic effects of the cholinergic agonist carbachol on long-term potentiation in the dentate gyrus of the mammalian hippocampus. Neurosci Lett 2010; 479:157-60; PMID:20510338; http://dx.doi.org/ 10.1016/j.neulet.2010.05.056 [DOI] [PubMed] [Google Scholar]
- [96].Furukawa K, Abe Y, Sorimachi M, Akaike N. Nicotinic and muscarinic acetylcholine responses in the embryo chick ciliary ganglion cells. Brain Res 1994; 657:185-90; PMID:7820617 [DOI] [PubMed] [Google Scholar]
- [97].Kanemoto Y, Ishibashi H, Doi A, Akaike N, Ito Y. An electrophysiological study of muscarinic and nicotinic receptors of rat paratracheal ganglion neurons and their inhibition by Z-338. Br J Pharmacol 2002; 135:1403-14; PMID:11906953; http://dx.doi.org/ 10.1038/sj.bjp.0704610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ishibashi M, Yamazaki Y, Miledi R, Sumikawa K. Nicotinic and muscarinic agonists and acetylcholinesterase inhibitors stimulate a common pathway to enhance GluN2B-NMDAR responses. Proc Natl Acad Sci USA 2014; 111:12538-43; PMID:25114227; http://dx.doi.org/ 10.1073/pnas.1408805111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 1998; 143:1295-304; PMID:9832557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Van Kampen JM, Eckman CB. Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharm 2010; 58:921-9; PMID:20026137; http://dx.doi.org/ 10.1016/j.neuropharm.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Takarada T, Nakamichi N, Kitajima S, Fukumori R, Nakazato R, Le NQ, Kim YH, Fujikawa K, Kou M, Yoneda Y. Promoted neuronal differentiation after activation of alpha4/beta2 nicotinic acetylcholine receptors in undifferentiated neural progenitors. PLoS One 2012; 7:e46177; PMID:23056257; http://dx.doi.org/ 10.1371/journal.pone.0046177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].He N, Wang Z, Wang Y, Shen H, Yin M. ZY-1, a novel nicotinic analog, promotes proliferation and migration of adult hippocampal neural stem/progenitor cells. Cell Mol Neurobiol 2013; 33:1149-57; PMID:24057433; http://dx.doi.org/ 10.1007/s10571-013-9981-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Harrist A, Beech RD, King SL, Zanardi A, Cleary MA, Caldarone BJ, Eisch A, Zoli M, Picciotto MR. Alteration of hippocampal cell proliferation in mice lacking the β 2 subunit of the neuronal nicotinic acetylcholine receptor. Synapse 2004; 54:200-6; PMID:15472930; http://dx.doi.org/ 10.1002/syn.20081 [DOI] [PubMed] [Google Scholar]
- [104].Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW, Emiliano A, Ekstrand MI, Friedman JM. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 2012; 151:1126-37; PMID:23178128; http://dx.doi.org/ 10.1016/j.cell.2012.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 1991; 37:475-524; PMID:1763188 [DOI] [PubMed] [Google Scholar]
- [106].Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. I. Central nervous system. Neuroscience 1998; 84:331-59; PMID:9539209 [DOI] [PubMed] [Google Scholar]
- [107].Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol 1997; 48:649-84; PMID:9046571; http://dx.doi.org/ 10.1146/annurev.psych.48.1.649 [DOI] [PubMed] [Google Scholar]
- [108].Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience 2000; 95:933-52; PMID:10682701 [DOI] [PubMed] [Google Scholar]
- [109].Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 2010; 67:294-307; PMID:20670836; http://dx.doi.org/ 10.1016/j.neuron.2010.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Straub C, Tritsch NX, Hagan NA, Gu C, Sabatini BL. Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J Neurosci 2014; 34:8557-69; PMID:24948810; http://dx.doi.org/ 10.1523/JNEUROSCI.0589-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kimura M, Rajkowski J, Evarts E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci USA 1984; 81:4998-5001; PMID:6589643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol 2002; 53:590-605; PMID:12436423; http://dx.doi.org/ 10.1002/neu.10150 [DOI] [PubMed] [Google Scholar]
- [113].Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci 2007; 30:299-306; PMID:17420057; http://dx.doi.org/ 10.1016/j.tins.2007.03.011 [DOI] [PubMed] [Google Scholar]
- [114].Boucetta S, Jones BE. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane-anesthetized rats. J Neurosci 2009; 29:4664-74; PMID:19357291; http://dx.doi.org/ 10.1523/JNEUROSCI.5502-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci 2005; 8:1179-87; PMID:16116450; http://dx.doi.org/ 10.1038/nn1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Young SZ, Lafourcade CA, Platel JC, Lin TV, Bordey A. GABAergic striatal neurons project dendrites and axons into the postnatal subventricular zone leading to calcium activity. Front Cell Neurosci 2014; 8:10; PMID:24478632; http://dx.doi.org/ 10.3389/fncel.2014.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 1993; 361:258-60; PMID:8093806; http://dx.doi.org/ 10.1038/361258a0 [DOI] [PubMed] [Google Scholar]
- [118].Zonouzi M, Scafidi J, Li P, McEllin B, Edwards J, Dupree JL, Harvey L, Sun D, Hübner CA, Cull-Candy SG, et al.. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat Neurosci 2015; 18:674-82; PMID:25821912; http://dx.doi.org/ 10.1038/nn.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Venkatesh HS, Johung TB, Caretti V, Gibson EM, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, et al.. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015; 161:803-16; PMID:25913192; http://dx.doi.org/ 10.1016/j.cell.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]