Abstract
Riboswitches are cis-encoded, cis-acting RNA elements that directly sense a physiological signal. Signal response results in a change in RNA structure that impacts gene expression. Elements of this type play an important role in bacteria, where they regulate a variety of fundamental cellular pathways. Riboswitch-mediated gene regulation most commonly occurs by effects on transcription attenuation, to control whether a full-length transcript is synthesized, or on translation initiation, in which case the transcript is constitutively synthesized but binding of the translation initiation complex is modulated. An overview of the role of riboswitch RNAs in bacterial gene expression will be provided, and a few examples are described in more detail to illustrate the types of mechanisms that have been uncovered.
Keywords: transcription attenuation, antitermination, translation control, RNA aptamer, regulation
Introduction
All cells must regulate gene expression in response to changes in growth conditions and cell physiology. A variety of regulatory mechanisms have evolved to permit measurement of key parameters and transmission of that information to the gene expression machinery. Recent studies have revealed an important regulatory role for cis-acting RNA elements. These elements can monitor physiological signals in a variety of ways, including effects on the dynamics of translation of short coding sequences, or by acting as binding sites for RNA binding proteins or complementary small RNAs. A subclass of these elements, called riboswitches, can directly measure physiological signals. In many of these cases, a key parameter in the signal response is an RNA structural shift that impacts expression of the adjacent coding sequences.
Riboswitches are cis-encoded, cis-acting RNA elements that regulate a broad range of genes in bacterial species, including those involved in metabolism or uptake of amino acids, cofactors, nucleotides and metal ions.1–4 Their function is distinct from that of small regulatory RNAs (sRNAs) that act in trans to regulate the activity of other RNA transcripts.5 Riboswitches have been identified that monitor specific uncharged tRNA species, a variety of small molecules, metal ions and temperature. RNA thermosensors are similar to other riboswitch classes in that they act in cis via RNA structural rearrangements; in these elements, the modulation of RNA structure occurs as a direct consequence of changes in temperature, without a requirement for binding of a ligand.6
Nearly all riboswitches identified to date are found in bacteria, where regulation generally occurs either at the level of transcription attenuation or translation initiation. All known riboswitch elements in bacteria are located upstream from the coding sequence(s) they regulate, usually in the 5' leader region of a transcript.
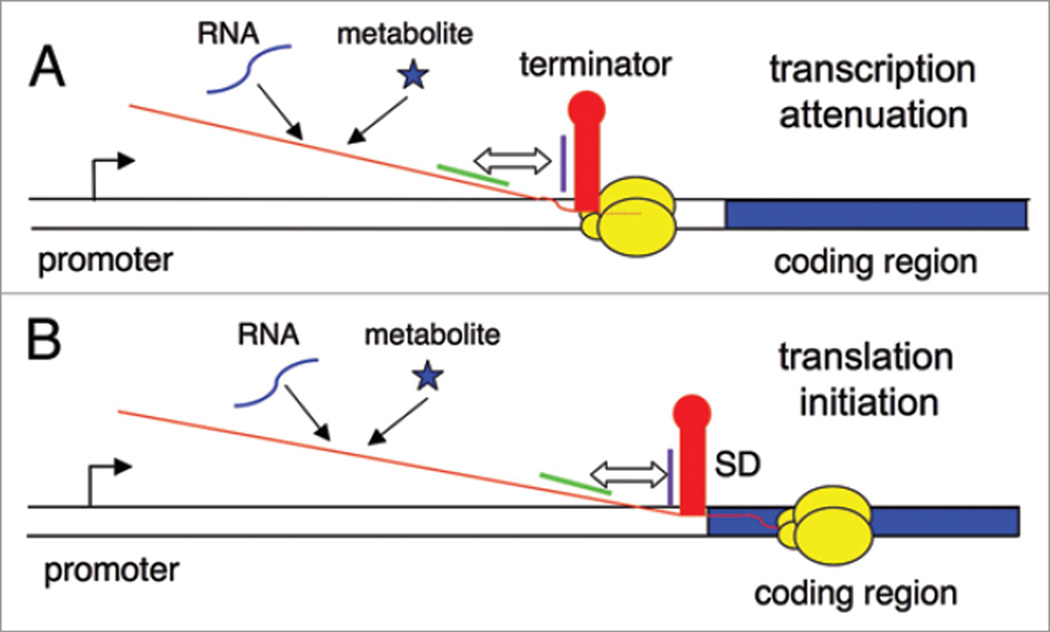
In genes regulated by transcription attenuation, the leader region includes an intrinsic transcriptional terminator, which is comprised of a G + C rich helix immediately followed by a series of U residues; formation of the terminator helix, or a competing antiterminator element, determines whether RNA polymerase (RNAP) will terminate or will continue transcription past the termination site, allowing expression of the downstream coding sequence (Fig. 1A). In contrast, genes regulated at the translational level are always transcribed, but include an RNA element that can sequester the ribosome binding site, usually by pairing of the Shine-Dalgarno (SD) sequence with a complementary anti-SD (ASD) sequence; formation of the ASD-SD helix prevents binding of the 30S ribosomal subunit, and prevention of formation of this helix by sequestration of the ASD sequence in an alternate structure is required for expression (Fig. 1B). A few metabolite-binding RNAs have been found in eukaryotic systems, where they regulate mRNA splicing and transcript stability using riboswitch elements at the 3' end of the transcript or overlapping an intron.7,8 The wide variety and large number of genes regulated by riboswitches in bacteria demonstrate their overall importance to cell physiology. This review will focus on the tRNA-sensing T box riboswitch, and how it compares to metabolite-sensing riboswitch elements.
Figure 1.
The riboswitch mechanism. DNA is shown as a double line, the promoter region is shown as a bent arrow, the regulated coding sequence is shown as a blue rectangle, RNAP is shown as yellow ovals, and the nascent transcript is a red line. (A) Regulation of transcription attenuation. Genes that are regulated at this level include an intrinsic terminator (red stem-loop) in the leader region, upstream of the regulated coding sequence. If this helix forms in the nascent RNA when RNAP is paused during synthesis of the U run (dotted red line), RNAP will terminate transcription. Pairing of sequences on the 5' side of the terminator (purple line) with complementary sequences that are further upstream (green line) results in formation of an alternate antiterminator structure. Binding of regulatory factors (RNA or metabolites) determines whether the RNA folds into the terminator or antiterminator structure. (B) Regulation of translation initiation. Genes that are regulated at this level include a structure (red stem-loop) that can sequester the SD sequence, which results in inhibition of binding of the 30S ribosomal subunit. Pairing of anti-SD (ASD) sequences (purple line) with complementary sequences that are further upstream (green line) results in formation of an alternate structure that sequesters the ASD sequence. Binding of regulatory factors (RNA or metabolites) determines whether the RNA folds into the ASD-SD structure or the competing structure that sequesters the ASD sequence.
RNA-Binding Riboswitches: The T Box System
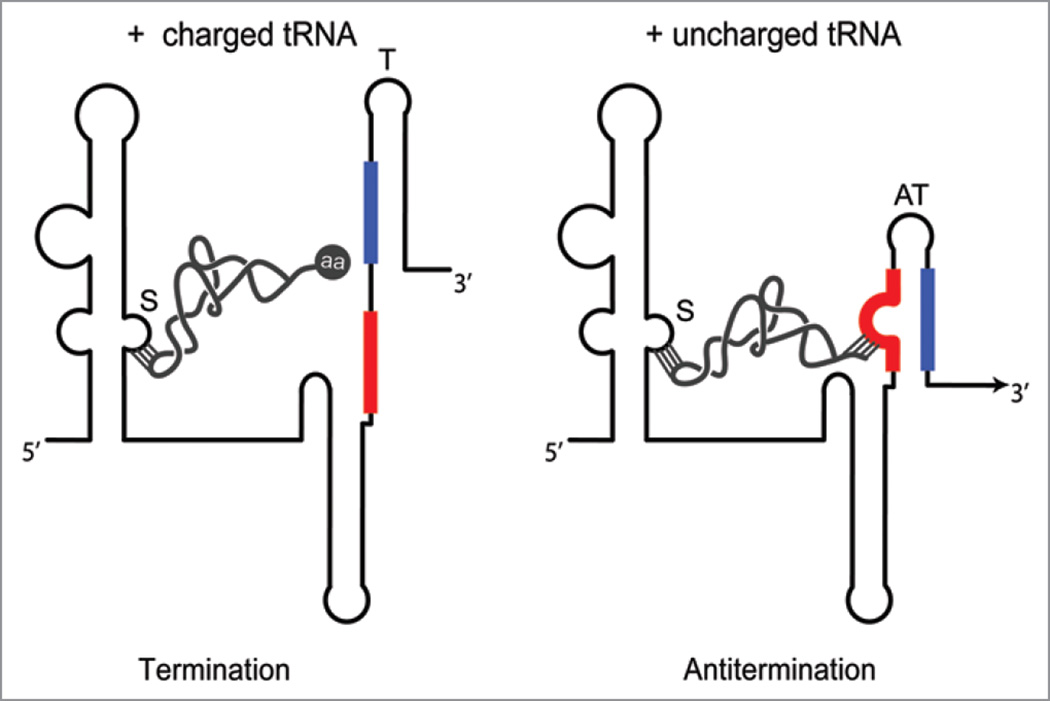
The T box regulatory mechanism is found primarily in Gram-positive bacteria, where it regulates a variety of genes involved in amino acid biosynthesis and uptake, and tRNA aminoacylation.9 Interaction of the mRNA leader region of the target gene with a specific uncharged tRNA promotes expression of the downstream coding sequence (Fig. 2).10,11 T box genes are readily identified by the presence of conserved sequence and structural features in the 5' leader regions of their mRNAs, and >1,000 have now been identified.9,12 The T box mechanism typically regulates at the level of transcription attenuation, although some T box RNAs in Gram-negative bacteria and members of the Actinomycetes are predicted to regulate at the level of translation initiation. In both cases, binding of the uncharged tRNA stabilizes a structural element (antiterminator or anti-ASD helix) that competes with the inhibitory element (terminator or ASD-SD helix). T box leader RNAs can specifically recognize and directly bind their effector molecule, and tRNA-directed antitermination can be reproduced in vitro, in the absence of other cellular factors.13,14
Figure 2.
The T box riboswitch. Regulation at the level of transcription attenuation, which is the most common mechanism, is shown. The riboswitch senses the relative amounts of uncharged and charged tRNA, for a specific tRNA species, which is recognized primarily by codon-anticodon pairing at the Specifier Sequence (S). Charged tRNA (left) can interact only at the Specifier Sequence; the presence of the amino acid (aa) at the 3' end of the tRNA blocks pairing of the acceptor end of the tRNA with the antiterminator bulge; the terminator helix (T, blue-black) forms, and transcription terminates before RNAP transcribes the downstream coding sequence. Uncharged tRNA (right) can interact at both the Specifier Sequence and the antiterminator (AT) bulge; pairing at the antiterminator stabilizes that structure (red-blue), which sequesters sequences (blue) that would otherwise participate in formation of the terminator helix. Transcription continues (arrow), and the downstream coding sequence is transcribed. In genes regulated at the level of translation initiation, transcription is predicted to be constitutive and the terminator helix is replaced by an ASD-SD helix that sequesters the SD sequence and prevents binding of the ribosome. tRNA binding stabilizes a competing structure analogous to the antiterminator that sequesters the ASD sequence, and frees the SD sequence to allow access of the ribosome.
T box leader RNA structure
Phylogenetic analyses revealed a general pattern for T box RNA secondary structure.10 This pattern includes three helical elements (Stems I, II and III) and a pseudoknot (Stem IIA/B), all of which precede the competing antiterminator/terminator (or SD sequestration control) elements. Whereas there is some variability in the conservation of particular subdomains, and considerable primary sequence variation, the core elements at the base of Stem I and in the antiterminator (or equivalent translational control structure) are always present. These elements make the primary contacts to the tRNA ligand. Specificity of tRNA binding is directed primarily by a triplet sequence (the Specifier Sequence) that is always found in an internal loop of Stem I, usually adjacent to an S-turn structural motif. The Specifier Sequence represents a codon that matches the amino acid specificity of the corresponding gene (e.g., tyrosyl genes contain a UAC tyrosine codon, tryptophanyl genes contain a UGG tryptophan codon, etc.,); this observation led to the hypothesis that the Specifier Sequence is responsible for specific tRNA recognition.
tRNA is the ligand for the T box riboswitch
Genetic and biochemical studies were used to test the hypothesis that the Specifier Sequence base-pairs with the anticodon of the corresponding tRNA. Initial studies showed that expression of the Bacillus subtilis tyrS gene was induced by conditions that resulted in reduced charging of tRNATyr.10,15 Mutation of the tyrS UAC Specifier Sequence to a UUC phenylalanine codon not only resulted in loss of response to tRNATyr, but also resulted in a switch to a response to tRNAPhe.10 Similar results were subsequently observed for a variety of genes in the T box family. Proof that tRNA is the true effector was provided by replacement of the tyrS Specifier Sequence with a nonsense codon, which resulted in very low expression that was restored by introduction of the corresponding nonsense suppressor tRNA with a matching anticodon.10 The low expression in the absence of a corresponding tRNA suggested that the default state of the T box riboswitch is “off,” and that the tRNA is required to promote antitermination. The role of uncharged tRNA as the effector was demonstrated by overproduction of an unchargable variant of tRNATyr, which allowed expression of tyrS under normal growth conditions.16
Discrimination between uncharged and charged tRNA is mediated by the antiterminator. This element includes a highly conserved 7 nt internal bulge (UGGNACC), and the first four residues of the bulge pair with the 4 unpaired nt (NCCA) at the 3' end of the tRNA; the N residues in the antiterminator and the tRNA covary, and charged tRNA is unable to make this interaction.16 Specifier Sequence-anticodon base pairing therefore provides the primary determinant for specific tRNA recognition, whereas antiterminator-acceptor end pairing allows discrimination between uncharged and charged tRNA, so that only a specific uncharged tRNA serves as a signal to promote expression of genes involved in aminoacylation of that tRNA. A role for competition between uncharged and charged tRNA was suggested by the observation that expression was higher when antitermination was driven by an unchargable tRNA for which no corresponding chargable tRNA was present in the cell (i.e., an unchargable nonsense suppressor tRNA, with a T box leader variant containing the corresponding nonsense codon at the Specifier Sequence).16 These studies suggested that, unlike most riboswitches that respond to a single signal, the T box monitors the ratio of two physiologically relevant forms of the same ligand, namely the charged and uncharged species of an individual tRNA.
The leader RNA-tRNA interaction can occur without assistance from cellular factors
tRNA-dependent antitermination was demonstrated in an in vitro transcription assay using purified RNAP and tRNAs generated in vitro by T7 RNAP transcription.13 These studies took advantage of the fact that the B. subtilis glyQS T box leader is a natural deletion variant missing Stem II and the Stem IIA/B pseudoknot. Transcription of a glyQS DNA template in the absence of tRNA resulted in nearly 100% termination, and addition of tRNAGly resulted in efficient readthrough of the termination site. Other tRNAs were unable to promote antitermination in vitro, indicating that the nascent transcript could effectively discriminate the correct tRNA from incorrect tRNAs. Addition of an extra residue at the 3' end of the tRNA, to generate a structural mimic of charged tRNA, resulted in termination; this charged tRNA mimic acted as a competitive inhibitor of the ability of uncharged tRNAGly to promote antitermination,14,17 supporting a model in which both charged and uncharged tRNA can interact with the leader RNA, but only uncharged tRNA can stabilize the antiterminator to allow readthrough. The ability of charged tRNA to compete with uncharged tRNA for access to the leader RNA is consistent with in vivo results that showed higher expression directed by an unchargable tRNA in the absence of the corresponding chargable tRNA.16
T7 RNAP-generated leader RNAs were also shown to be competent for specific tRNA binding, further supporting the model that the leader RNA alone is capable of specific recognition of the cognate uncharged tRNA.14 Structural mapping of the leader RNA in the presence and absence of tRNA demonstrated that the RNA folds into a structure consistent with the model, and that binding of uncharged tRNA results in protection of the Specifier Sequence and antiterminator bulge (regions known to participate in base pairing interactions) and also promotes changes throughout the RNA. Since the charged tRNA mimic protects only the Specifier Sequence, it appears that interaction of the acceptor end of uncharged tRNA with the antiterminator is necessary for the large-scale structural changes observed in the complex.14
The three-dimensional structure of an intact T box riboswitch has not yet been determined. Analysis of the antiterminator domain in isolation demonstrated that this domain can specifically bind the acceptor end of the tRNA.18 NMR analysis of the antiterminator domain showed that the residues that form the antiterminator bulge are positioned to display the UGGN residues for pairing with the tRNA acceptor end.19 The bulge is somewhat flexible, but a mutation that increases flexibility of the bulge negatively impacts tRNA binding and antitermination in vivo and in vitro, suggesting that the degree of flexibility is constrained to allow effective sampling of conformational variability.18 The NMR structure of the Specifier Loop region has also recently been obtained (Wang J, Henkin TM and Nikonowicz EP, unpublished). The overall structure in this region also matches the model, including confirmation of the S-turn motif adjacent to the Specifier Sequence. How these elements are positioned in the context of the rest of the T box leader RNA structure remains to be elucidated.
Metabolite-Binding Riboswitches
Most metabolite-binding riboswitches have two separate domains, an aptamer domain responsible for specific ligand binding, and a gene expression domain that is responsible for the effect on expression of the regulated coding sequence(s). These two domains usually communicate via a ligand-dependent structural rearrangement in the aptamer domain that sequesters (or releases) residues that interact with the gene expression domain. This differs from the T box riboswitch, in which the tRNA ligand interacts directly with the antiterminator domain. A few metabolite-binding riboswitches, such as the SMK box, resemble the T box system in that the gene expression domain is an intrinsic part of the ligand binding domain. In most cases, expression of the downstream gene is on in the absence of the effector molecule, and binding of the effector causes reduced expression. There are a few examples (analogous to the T box system) where binding of the effector promotes expression. While tRNA selectivity of the T box system is determined at least in part by codon-anticodon base pairing,10,13 the basis for the ability of each metabolite-binding RNA to specifically recognize its cognate effector, and discriminate against closely related compounds, appears to be different for each class of riboswitch molecules, as revealed by crystal structure analysis.2,20 Artificial systems have also been adapted to allow directed control of gene expression in response to addition of a regulatory molecule.21
A variety of ligands
Metabolite-binding riboswitches have been identified as genes involved in metabolism or uptake of amino acids, cofactors, nucleotides and metal ions.2 In each case, the RNA specifically binds and responds to a ligand that is involved in the corresponding pathway, usually a biologically relevant end-product for pathways involved in acquisition of a cellular building block, and more rarely a substrate for a pathway involved in degradation of that compound. Known metabolite-binding riboswitches include those responsive to thiamine pyrophosphate, flavin mononucleotide, adenosylcobalamin, S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), guanine, adenine, 2'-deoxyguanosine, cyclic di-GMP, 7-aminomethyl-7-deazaguanine, lysine, glycine and metal ions; the glucosamine-6-phosphate-responsive glmS ribozyme is a special case where binding of the ligand activates self-cleavage of the RNA rather than a structural rearrangement.1–3
Each riboswitch not only must bind the appropriate molecule specifically and discriminate against related molecules, but also must have an affinity for the ligand that matches the physiological concentration of the ligand in the cell. This is particularly important for feedback repression pathways, so that expression is induced when the cellular pools drop below the threshold necessary for optimal growth. An inappropriately high affinity could lead to repression of biosynthetic genes when the level of the relevant biosynthetic product is lower than that required for efficient utilization of that product in cellular pathways. Too low affinity for the ligand could result in expression of genes when their products are not required, which is wasteful of cell resources. In some cases, multiple copies of the same riboswitch can differentially regulate a series of different transcriptional units as a consequence of subtle variations in riboswitch sequence and/or structure. For example, the 11 SAM-responsive S box elements in B. subtilis exhibit a 100-fold range in sensitivity to SAM, and this variability in sensitivity can be correlated to the functional role of the relevant gene product.22 Furthermore, different classes of riboswitch elements have evolved to recognize the same effector. The SAM-binding riboswitches will be discussed to illustrate the diversity within a single group of metabolite-binding riboswitches.
SAM-binding riboswitches: S box, SMK box, SAM-II
Three different classes of riboswitches have been identified that respond to SAM. All of these RNAs discriminate effectively between SAM and SAH, the biproduct of utilization of SAM as a methyl donor. These riboswitches differ in structure, mechanism of action and ligand-binding properties, illustrating the ability of diverse RNAs to converge to a common regulatory effect while retaining important functional differences. Comparison of these three riboswitches to each other (and to the T box system) provides a snapshot of riboswitch variability.
The S box riboswitch
The S box riboswitch is a well-characterized riboswitch that fits the basic pattern for metabolite-binding riboswitches by having separate aptamer-binding and gene expression domains. This riboswitch is found primarily in Gram-positive bacteria, where it regulates genes involved in biosynthesis or uptake of methionine or SAM.23 Regulation most commonly occurs at the level of transcription attenuation, but there are also examples that are predicted to function at the level of translation initiation. The secondary structural model of the S box riboswitch is shown in Figure 3. This model was based on phylogenetic analysis of an initial set of 18 sequences,23 and has been corroborated by additional phylogenetic and mutational studies,23–25 and structural mapping in our lab and by others.25–28
Figure 3.
The S box riboswitch. Regulation at the level of transcription attenuation, which is the more common mechanism, is shown. The SAM-binding aptamer is comprised of helices P1–P4. When SAM levels are low, the more stable antiterminator structure (AT, red-blue) forms, and transcription continues into the downstream coding sequence (arrow head). When SAM levels are high, SAM (*) binds in a pocket formed by stacking of the P1 and P3 helices; this stabilizes the P1 helix, which serves as an anti-antiterminator (AAT, black-red) because it sequesters sequences (red) that would otherwise participate in formation of the antiterminator. Inhibition of antiterminator formation releases sequences from the 3' side of the antiterminator (blue) for formation of the terminator helix (blue-black), and transcription terminates. The SAM binding pocket is stabilized by tertiary interactions including the pseudoknot formed between the loop of P2 and the junction between helices P3 and P4 (dashed line). In genes regulated at the level of translation initiation, transcription is predicted to be constitutive and the terminator helix is replaced by an ASD-SD helix that sequesters the SD sequence and prevents binding of the ribosome. SAM binding stabilizes a structure analogous to the anti-antiterminator that sequesters sequences that would otherwise pair with the ASD sequence, and frees the ASD sequence to pair with the SD to block access of the ribosome.
The key features of the model (in terms of gene regulation) are the intrinsic terminator and the competing antiterminator. Sequences necessary for formation of the antiterminator can participate in another competing helix (P1) that was proposed to function as an anti-antiterminator.23 Initial genetic analysis supported a model in which formation of the anti-antiterminator helix is favored when cells are grown in the presence of methionine, and destabilized when methionine is limiting, resulting in formation of the antiterminator element and transcription of the downstream coding sequence. The structural model includes 4 helical elements (P1–P4) in the SAM-binding domain. Sequences in the P1–P4 region were proposed to be required for monitoring methionine, since mutations in these regions result in loss of repression during growth in methionine.23
In vivo studies suggested that SAM was a likely effector for S box-dependent regulation, as perturbations in SAM pools resulted in the expected effects on S box gene expression.26 A mutation in the SAM synthetase gene that results in decreased SAM synthetase activity and decreased SAM pools resulted in elevated S box gene expression,29 consistent with the model that SAM is the regulatory ligand in vivo. This was confirmed by in vitro transcription termination assays that demonstrated that antitermination occurs in the absence of SAM, and addition of SAM is sufficient to promote transcription termination of S box leader constructs.26 We also showed that RNAs containing the P1–P4 region can bind SAM in vitro, and that SAM binding results in stabilization of P1, as predicted by the model. Similar results were reported by the Breaker and Nudler labs.27,28
In the secondary structural model, the P1–P4 helices converge in a 4-way junction to form the SAM binding pocket. Tertiary interactions between the terminal loop of P2 and the junction region between helices P3 and P4 (J3/4) were proposed based on phylogenetic analyses23 and confirmed by mutational analysis and structural probing.25 A kink-turn motif in the P2 helix was also predicted to be important for facilitating the tertiary interaction.24 These predictions were validated by determination of the crystal structure of an S box riboswitch from Thermoanaerobacter tengcongensis, a thermophilic member of the Firmicute group,29 and by a similar structure for the B. subtilis yitJ RNA (Lu C, Pradhan V, Tomsic J, Ding F, Holmes WM, Henkin TM and Ke A, unpublished). The crystal structures showed that the riboswitch folds into two sets of coaxial stacked helices (P1/P4 and P2/P3), and that SAM is positioned between helices P1 and P3. The SAM molecule is enveloped by the RNA and assumes a compact conformation in which the methionine moiety stacks on top of the adenine. The methyl group of SAM is not directly recognized. However, the positive charge on the sulfur group of SAM makes favorable electrostatic interactions with the carbonyl oxygens of two conserved uridine residues in the RNA. This feature explains the preferential binding of SAM over SAH (>100-fold), and a similar mode of discrimination against SAH is used by the SMK box and SAM-II riboswitches.
The SAM-II riboswitch
The SAM-II element is found primarily in Proteobacteria.31 This RNA is much smaller than the S box riboswitch, and binding of SAM promotes formation of an H-type pseudoknot that results in occlusion of the SD sequence.32 Regulation is therefore predicted to occur at the level of translation initiation, although biochemical analyses to support this prediction have not yet been carried out. In contrast to what was observed for the S box riboswitch, the SAM molecule assumes an extended conformation in the SAM-SAM-II crystal structure. As noted above, the positive charge on the sulfur group forms electrostatic interactions with conserved uridines in the RNA, in a manner similar to what was observed for the S box RNA. All of the SAM functional groups are recognized by the SAM-II RNA, resulting in very stringent discrimination against SAM analogs.31,32 Unlike most riboswitch RNAs, the predicted regulatory domain of the riboswitch (i.e., the ASD-SD pairing) appears to be intrinsic to the ligand binding domain, although there are no obvious base-specific contacts that dictate specific interactions with the regulatory domain. This structural arrangement is also found in the SMK box riboswitch, despite major differences in the SAM binding pocket itself.
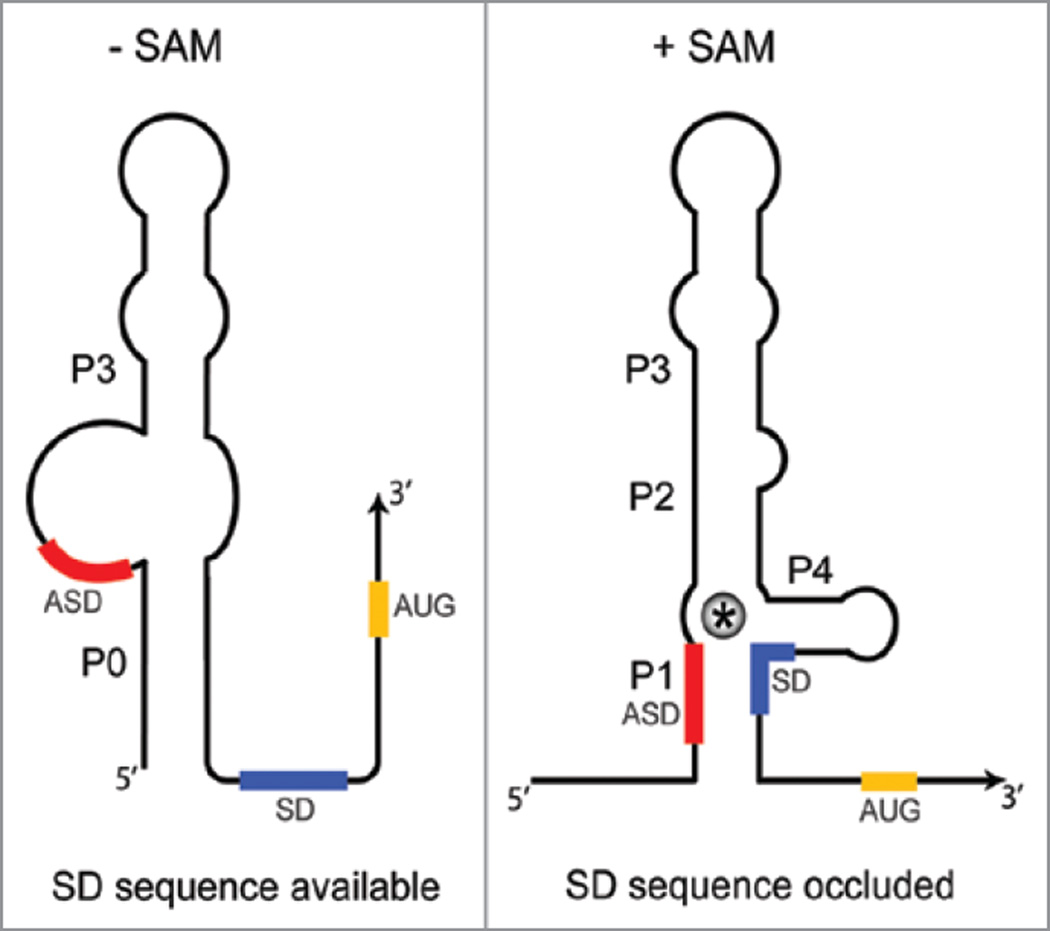
The SMK box riboswitch
A third class of SAM-binding riboswitch elements was identified upstream of metK (SAM synthetase) genes in lactic acid bacteria, including Enterococcus and Streptococcus sp.33 Binding of SAM to this element, designated the SMK box (Fig. 4), was predicted to result in sequestration of the SD sequence, and inhibition of translation initiation. The RNA was shown to bind SAM in vitro, and SAM-dependent structural rearrangements, including protection of the SD sequence, were demonstrated. We also showed that insertion of an SMK box element from Enterococcus faecalis into a reporter gene fusion resulted in SAM-dependent repression of translation in vivo using a B. subtilis heterologous host. No effect on transcription was detected, consistent with the model that regulation occurs at the level of translation. Mutations that were predicted to disrupt conserved elements within the structure resulted in loss of SAM binding in vitro, and loss of SAM-dependent repression in vivo. Surprisingly, mutations that disrupted the ASD-SD pairing resulted in loss of SAM binding in vitro, and certain compensatory mutations that restored base pairing also restored SAM binding and SAM-dependent repression. However, certain mutations in the SD region caused loss of binding even in combination with compensatory mutations in the ASD sequence.33
Figure 4.
The SMK box riboswitch. Regulation is at the level of translation initiation for all SMK box riboswitches uncovered to date. In the absence of SAM, the SD sequence is available for binding of the 30S ribosomal subunit, and sequences at the 5' end pair to form the P0 helix. Binding of SAM (*) results in disruption of the P0 helix and sequestration of the SD sequence in the ASD-SD helix (P1). SAM makes direct contacts with residues within the SD. Sequestration of the SD sequence prevents ribosome binding, resulting in repression of gene expression.
Filter binding assays and reverse transcriptase primer extension assays were used to characterize the effect of SAM on binding of 30S ribosomal subunits to the RNA. These studies showed that preincubation of the SMK box with SAM results in decreased binding of 30S subunits; in contrast, preincubation with SAH has no effect.34 The position of the 30S subunit on the RNA was identified by ribosomal toeprint assays, which confirmed the assignment of the AUG start codon; addition of SAM resulted in a major reduction in the extension product corresponding to the edge of the 30S subunit, as predicted, and also resulted in detection of a new product that corresponds to the ASD-SD pairing. Mutations previously shown to prevent SAM binding also prevented the SAM-dependent effect on 30S subunit binding and ASD-SD pairing. This study not only confirmed the model that binding of SAM is sufficient to prevent 30S subunit binding, but also provided direct evidence for SAM-dependent ASD-SD pairing.
In collaboration with the Ke lab, we solved the crystal structure of the RNA-SAM complex, which revealed that the mechanism of SAM recognition is completely different from that observed in the S box and SAM-II RNAs.35 SAM makes direct contacts with residues within the ASD-SD helix, and specifically recognizes the central nucleotide of the SD sequence. We had previously noted that all SMK box sequences include noncanonical SD sequences (GGGGG vs. the canonical GGAGG), and that mutation of the central G to the consensus A disrupted SAM binding and SAM-dependent repression, even in the presence of a compensatory ASD mutation.33 This observation was explained by the crystal structure results, which revealed that G90 is involved in the A73:G90-C25 base triple, formation of which is predicted to be lost when the G was replaced with an A.
The crystal structure also demonstrated that, in contrast to the S box and SAM-II riboswitches, the methionine moiety of SAM is not enveloped in the RNA but is instead accessible to solvent. This is consistent with the observation that the SMK box is less stringent in ligand recognition than the other SAM-binding riboswitches (Smith AM, Grundy FJ and Henkin TM, unpublished). Despite this difference, the discrimination against SAH, which is the biologically relevant SAM analog, utilizes a set of electrostatic interactions with the positive sulfonium ion of SAM similar to those utilized by the S box and SAM-II RNAs. As noted above, SAH is the byproduct of utilization of SAM as a methyl donor, and accidental repression by binding of SAH to the riboswitch would have serious consequences for growth as it would result in diminished SAM production under conditions when SAM pools are depleted. It therefore appears that despite very different structural arrangements, the three classes of SAM-binding riboswitches have evolved to take advantage of similar properties of SAM vs. SAH to mediate differential recognition of the true and potentially deleterious ligand.
The SMK box riboswitch differs from most riboswitches identified to date in that it is hypervariable in size. The hypervariable domains are located at the top of the P3 helix and within the P4 helix, resulting in a large extension in one or both of these helices.33 This variablility results in a size range of 80–400 nt in closely related organisms. The presence of large insertions within the riboswitch complicates identification of SMK box elements, as some of the conserved elements are very short, and the distances between conserved elements can vary by >300 nt. While elements resembling the SMK box have been identified only in metK genes in lactic acid bacteria, it is possible that related elements have remained undetected because of hypervariability. The extensive variation in size observed in this riboswitch may indicate that other riboswitch classes (or subclasses of known riboswitches) may have been missed because of similar issues.
Conclusions
Riboswitch RNAs have been characterized using a powerful mix of bioinformatic, genetic, biochemical and biophysical approaches. These combined tools have revealed an impressive diversity of structure, function and mechanism of action. Regulatory RNAs of this type play a crucial role in modulating bacterial cell growth. Different riboswitch elements are differentially represented in various phylogenetic groups of bacteria, which makes it likely that new types will be recognized as other branches of the bacterial tree are explored. Although only a handful of examples have been identified so far in archaea and in eukaryotic cells, it seems likely that similar regulatory mechanisms remain to be uncovered.
Acknowledgments
This work was supported by National Institutes of Health grants GM47823 and GM63615 to T.M.H.
References
- 1.Grundy FJ, Henkin TM. From ribosome to riboswitch: control of gene expression in bacteria by RNA structural rearrangements. Crit Rev Biochem Mol Biol. 2006;41:329–338. doi: 10.1080/10409230600914294. [DOI] [PubMed] [Google Scholar]
- 2.Henkin TM. Riboswitch RNAs: using RNA to sense cellular metabolism. Genes Dev. 2008;22:3383–3390. doi: 10.1101/gad.1747308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montange RK, Batey RT. Riboswitches: emerging themes in RNA structure and function. Annu Rev Biophys. 2008;37:117–133. doi: 10.1146/annurev.biophys.37.032807.130000. [DOI] [PubMed] [Google Scholar]
- 4.Dambach MD, Winkler WC. Expanding roles for metabolite-sensing regulatory RNAs. Curr Opin Microbiol. 2009;12:161–169. doi: 10.1016/j.mib.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storz G, Opdyke JA, Wassarman KM. Regulating bacterial transcription with small RNAs. Cold Spring Harbor Symp Quant Biol. 2006;71:269–273. doi: 10.1101/sqb.2006.71.033. [DOI] [PubMed] [Google Scholar]
- 6.Narberhaus F, Waldminghaus T, Chowdbury S. RNA thermometers. FEMS Microbiol Rev. 2006;30:3–16. doi: 10.1111/j.1574-6976.2005.004.x. [DOI] [PubMed] [Google Scholar]
- 7.Kubodera T, Watanabe M, Yoshiuchi K, Yamashita N, Nishimura A, Nakai S, et al. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5'-UTR. FEBS Lett. 2003;555:516–520. doi: 10.1016/s0014-5793(03)01335-8. [DOI] [PubMed] [Google Scholar]
- 8.Cheah MT, Wachter A, Sudarsan N, Breaker RR. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature. 2007;447:497–500. doi: 10.1038/nature05769. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez-Preciado A, Henkin TM, Grundy FJ, Yanofsky C, Merino E. Biochemical features and functional implications of the RNA-based T-box regulatory mechanism. Microbiol Mol Biol Rev. 2009;73:36–61. doi: 10.1128/MMBR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy FJ, Henkin TM. tRNA as a positive regulatory of transcripton antitermination in B. subtilis. Cell. 1993;74:475–482. doi: 10.1016/0092-8674(93)80049-k. [DOI] [PubMed] [Google Scholar]
- 11.Henkin TM, Grundy FJ. Sensing metabolic signals with nascent RNA transcripts: The T-box and S-box riboswitches as paradigms. Cold Spring Harbor Symp Quant Biol. 2006;71:231–237. doi: 10.1101/sqb.2006.71.020. [DOI] [PubMed] [Google Scholar]
- 12.Vitreschak AG, Mironov AM, Lyubetsky VA, Gelfand MS. Comparative genomic analysis of T-box regulatory systems in bacteria. RNA. 2008;14:717–735. doi: 10.1261/rna.819308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy FJ, Winkler WC, Henkin TM. tRNA-mediated transcription antitermination in vitro: codon-anticodon pairing independent of the ribosome. Proc Natl Acad Sci USA. 2002;99:11121–11126. doi: 10.1073/pnas.162366799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yousef MR, Grundy FJ, Henkin TM. Structural transitions induced by the interaction between tRNAGly and the Bacillus subtilis glyQS T box leader RNA. J Mol Biol. 2005;349:273–287. doi: 10.1016/j.jmb.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 15.Henkin TM, Glass BL, Grundy FJ. Analysis of the Bacillus subtilis tyrS gene: Conservation of a regulatory sequence in multiple tRNA synthease genes. J Bacteriol. 1992;174:1299–1306. doi: 10.1128/jb.174.4.1299-1306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy FJ, Rollins SM, Henkin TM. Interaction between the acceptor end of tRNA and the T box stimulates antitermination in the Bacillus subtilis tyrS gene: A new role for the discriminator base. J Bacteriol. 1994;176:4518–4526. doi: 10.1128/jb.176.15.4518-4526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy FJ, Yousef MR, Henkin TM. Monitoring uncharged tRNA during transcription of the Bacillus subtilis glyQS gene. J Mol Biol. 2005;346:73–81. doi: 10.1016/j.jmb.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 18.Gerdeman MS, Henkin TM, Hines JV. In vitro structure-function studies of the Bacillus subtilis tyrS antiterminator: Evidence for factor-independent tRNA acceptor stem binding specificity. Nucl Acids Res. 2002;30:1065–1072. doi: 10.1093/nar/30.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerdeman MS, Henkin TM, Hines JV. Solution structure of the B. subtilis T box antiterminator RNA: Seven-nucleotide bulge characterized by stacking and flexibility. J Mol Biol. 2003;326:189–201. doi: 10.1016/s0022-2836(02)01339-6. [DOI] [PubMed] [Google Scholar]
- 20.Montange RK, Batey RT. Riboswitches: emerging themes in RNA structure and function. Annu Rev Biophys. 2008;37:117–133. doi: 10.1146/annurev.biophys.37.032807.130000. [DOI] [PubMed] [Google Scholar]
- 21.Suess B, Weigand JE. Engineered riboswitches: overview, problems and trends. RNA Biol. 2008;5:24–29. doi: 10.4161/rna.5.1.5955. [DOI] [PubMed] [Google Scholar]
- 22.Tomsic J, McDaniel BA, Grundy FJ, Henkin TM. Natural variability in S-adenosylmethionine (SAM)-dependent riboswitches: S box elements in Bacillus subtilis exhibit differential sensitivity to SAM in vivo and in vitro. J Bacteriol. 2008;190:823–833. doi: 10.1128/JB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundy FJ, Henkin TM. The S box regulon: a new global transcripton termination control system for methionine and cysteine biosynthesis genes in Gram-positive bacteria. Mol Microbiol. 1998;30:737–749. doi: 10.1046/j.1365-2958.1998.01105.x. [DOI] [PubMed] [Google Scholar]
- 24.Winkler WC, Grundy FJ, Murphy BA, Henkin TM. The GA motif: An RNA element common to bacterial antitermination systems, rRNA and eukaryotic RNAs. RNA. 2001;7:1165–1172. doi: 10.1017/s1355838201002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDaniel BA, Grundy FJ, Henkin TM. A tertiary structural element in S box leader RNAs is required for SAM-directed transcription termination. Mol Microbiol. 2005;57:1008–1021. doi: 10.1111/j.1365-2958.2005.04740.x. [DOI] [PubMed] [Google Scholar]
- 26.McDaniel BAM, Grundy FJ, Artsimovitch I, Henkin TM. Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the leader RNA. Proc Natl Acad Sci USA. 2003;100:3083–8088. doi: 10.1073/pnas.0630422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epshtein V, Mironov AS, Nudler E. The riboswitch-mediated control of sulfur metabolism in bacteria. Proc Natl Acad Sci USA. 2003;100:5052–5056. doi: 10.1073/pnas.0531307100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler WC, Nahvi A, Sudarsan N, Barrick JE, Breaker RR. An mRNA structure that controls gene expression by binding S-adenosylmethionine. Nat Struct Biol. 2003;10:701–707. doi: 10.1038/nsb967. [DOI] [PubMed] [Google Scholar]
- 29.McDaniel BAM, Grundy FJ, Kurlekar V, Tomsic J, Henkin TM. Identification of a mutation in the Bacillus subtilis SAM synthetase gene that results in derepression of S box gene expression. J Bacteriol. 2006;188:3674–3681. doi: 10.1128/JB.188.10.3674-3681.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montange RK, Batey RT. Structure of the S-adeosylmethionine riboswitch regulatory mRNA element. Nature. 2006;441:1172–1175. doi: 10.1038/nature04819. [DOI] [PubMed] [Google Scholar]
- 31.Corbino KA, Barrick JE, Lim J, Welz R, Tucker BJ, Puskarz I, et al. Evidence for a second class of S-adenosylmethionine riboswitches and other regulatory RNA motifs in alpha-proteobacteria. Genome Biol. 2005;6:70. doi: 10.1186/gb-2005-6-8-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert SD, Rambo RP, Van Tyne D, Batey RT. Structure of the SAM-II riboswitch bound to S-adenosylmethionine. Nat Struct Mol Biol. 2008;15:761–772. doi: 10.1038/nsmb.1371. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs RT, Grundy FJ, Henkin TM. The SMK box is a new SAM-binding RNA for translational regulation of SAM synthetase. Nat Struct Mol Biol. 2006;13:226–233. doi: 10.1038/nsmb1059. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs RT, Grundy FJ, Henkin TM. S-adenosylmethionine directly inhibits binding of 30S ribosomal subunits to the SMK box translational riboswitch RNA. Proc Natl Acad Sci USA. 2007;104:4876–4880. doi: 10.1073/pnas.0609956104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu C, Smith AM, Fuchs RT, Ding F, Rajashankar K, Henkin TM, Ke A. Crystal structures of the SAM-III/SMK riboswitch reveal the SAM-dependent translation inhibition mechanism. Nat Struct Mol Biol. 2008;15:1076–1083. doi: 10.1038/nsmb.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]