Abstract
The host immune response plays a key role in bacteria-induced alveolar bone resorption. Endogenous control of the magnitude and duration of inflammatory signaling is considered an important determinant of the extent of periodontal pathology. Suppressor of cytokine signaling (SOCS) proteins are inhibitors of cytokine signaling pathways and may play a role in restraining periodontal inflammation. We hypothesized that SOCS-3 regulates alveolar bone loss in experimental periodontitis. Periodontal bone loss was induced in 16-wk-old myeloid-specific SOCS-3-knockout and wild-type (WT) C57Bl6-B.129 mice by oral inoculation 9 times with 109 colony-forming units of Porphyromonas gingivalis A7436 through an oral gavage model for periodontitis. Sham controls for both types of mice received vehicle without bacteria. The mice were euthanized 6 wk after the last oral inoculation. Increased bone loss was demonstrated in P. gingivalis–infected SOCS-3-knockout mice as compared with P. gingivalis–infected WT mice by direct morphologic measurements, micro–computed tomography analyses, and quantitative histology. Loss of SOCS-3 function resulted in an increased number of alveolar bone osteoclasts and increased RANKL expression after P. gingivalis infection. SOCS-3 deficiency in myeloid cells also promotes a higher P. gingivalis lipopolysaccharide-induced inflammatory response with higher secretion of IL-1β, IL-6, and KC (IL-8) by peritoneal macrophages as compared with WT controls. Our data implicate SOCS-3 as a critical negative regulator of alveolar bone loss in periodontitis.
Keywords: inflammation, Porphyromonas gingivalis, animal model, osteoclasts, macrophages, cytokines
Introduction
Periodontitis is the sixth-most prevalent disease in the world and the primary cause for tooth loss in adults (Kassebaum et al. 2014). It is estimated that in the United States, at least 47% of adults aged ≥30 y have periodontitis (Eke et al. 2012). A dysregulated inflammatory response and a failure of endogenous resolution pathways lead to a sustained microbial challenge that amplifies periodontal tissue destruction (Van Dyke 2011). The regulation of inflammatory mediators by endogenous mechanisms and the balance between pro- and anti-inflammatory mediators determine the severity and extent of tissue destruction in the periodontium (Kantarci et al. 2006). Endogenous control of the magnitude and duration of inflammatory signaling is thought to be an important determinant of the extent of periodontal pathology.
Suppressor of cytokine signaling (SOCS) proteins are a family of intracellular signaling molecules that are produced in response to a diverse range of microbial and immunologic stimuli, including TNF-α, IFN-γ, IL-6, and lipopolysaccharide (LPS; Morita et al. 2000; Kinjyo et al. 2002; Rakesh and Agrawal 2005). SOCS proteins have been shown to be key negative regulators of cytokine signaling in several tissues, inhibiting the JAK/STAT signal transduction pathway (Alexander 2002). The activation of cytokine signaling via the Janus kinase (JAK) and activators of transcription (STAT) pathways may represent a critical mechanism by which inflammatory cytokines contribute to osteoclast activation and bone loss (Menezes et al. 2008). SOCS proteins form a negative-feedback loop to inhibit cytokine signal transduction (Alexander 2002; Yoshimura et al. 2012) by binding to JAK/STAT or cytokine receptors. Among the SOCS family members, SOCS-1 and SOCS-3 are the most potent inhibitors of cytokine-induced signals (Rakesh and Agrawal 2005). Injections of Escherichia coli LPS in the palatal gingival tissues of first molars of rats increased inflammation; induced an upregulation of SOCS-3 expression paralleled by an increase of the proinflammatory cytokines IL-1β, IL-6, and TNF-α; and increased phosphorylation of STAT3 (Chaves de Souza et al. 2013). SOCS-3 downregulates additional proinflammatory mediators induced by LPS in osteoblasts, thereby playing a critical role in osteoblast-mediated immune signaling (Yan et al. 2010; Gao et al. 2013). The analysis of mice lacking genes for SOCS proteins has shed additional light on their critical importance in restraining inflammation and allowing optimal levels of protective immune responses in several inflammatory disorders, including arthritis, atherosclerosis, and cancer (Wong et al. 2006; Recio et al. 2015; Yu et al. 2015).
SOCS proteins are potentially involved in the control of LPS and cytokine signaling pathways in periodontal inflammation. The mechanisms of cytokine signaling control in periodontal disease remain elusive. The increased expression of SOCS-1, SOCS-2, and SOCS-3 mRNA in diseased periodontal tissues from patients with gingivitis and periodontitis has suggested that SOCS may act as a stop signal for periodontal disease progression (Garlet et al. 2006). In addition, an experimental model of ligature-induced periodontitis in rats suggests that SOCS-1 and SOCS-3 are directly correlated with regulatory mechanisms involved in periodontal disease destruction (de Souza et al. 2011). To investigate further the role of SOCS-3 in periodontal disease, we studied experimental periodontitis in SOCS-3-deficient mice.
Materials and Methods
Animals and Experimental Periodontitis
Sixteen-week-old male myeloid-specific SOCS-3-knockout (SOCS-3-KO) C57Bl6-B.129 mice and C57Bl6-B.129 wild-type (WT) mice were engineered as previously described (Yan et al. 2015). Mice were genotyped by real-time polymerase chain reaction (Transnetyx). All animals were maintained under pathogen-free conditions and matched for age. All animals were kept in separate cages to avoid cross-infection and fed a standard pellet diet and tap water ad libitum before experiments. Once weekly, the animals were weighed to ensure proper growth and nutrition. To minimize any potential effects of estrogen in the experiment, only male animals were used. All animal procedures described in this study were approved by Institutional Animal Care and Use Committee of the Forsyth Institute. This study conformed with ARRIVE guidelines for preclinical studies.
Periodontal bone loss was induced by oral inoculation with Porphyromonas gingivalis A7436, as originally described by Baker et al. (1994), with slight modifications (see Appendix). In WT mice, 1 group was infected (n = 5; experimental) and 1 group was sham treated (n = 6; controls), while in SOCS-3-KO mice, 1 group was infected (n = 5; experimental) and 1 group was sham treated (n = 5; controls). WT mice and SOCS-3-KO mice were randomly assigned to be infected (experimental) or sham treated (controls). Experimental groups were orally inoculated 9 times at 2-d intervals (Monday, Wednesday, Friday for 3 wk) with 109 colony-forming units of P. gingivalis, spectrophotometrically determined at 600 nm and suspended in 300 µL of phosphate-buffered saline (PBS) with 2% carboxymethylcellulose vehicle (Sigma-Aldrich) to form a thick slurry. Sham controls received PBS with carboxymethylcellulose alone. The mice were euthanized at 6 wk with CO2 overdose after the last oral inoculation; the maxilla was dissected free of muscles and the soft tissues; and the attached gingiva was kept intact with the bone. The maxilla was split into 2 halves from the midline between the central incisors. The right half was taken for morphometric and micro–computed tomography (μCT) analysis of bone loss, while the left half was used for histologic and immunohistochemical analyses.
Morphometric Characterization of Periodontal Disease
To evaluate the extent of alveolar bone loss, right hemisected maxillae were defleshed in a dermestid beetle colony, immersed in 10% hydrogen peroxide careful with removal of remaining soft tissue, washed with distilled water, air-dried, and stained with 0.5% methylene blue for visual distinction between the tooth and bone. Images of 3 maxillary molar teeth and alveolar bone were captured with a digital microscope (Zeiss Axio Observer A1) equipped with a digital camera (AxioCam HRc r1.6) and saved as JPEG files. An image of a precise ruler was captured at the same magnification and used for calibration. Linear and area measurements were performed with Olympus Microsuite 3.2 imaging software. The distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured at 6 predetermined points on the buccal surfaces of maxillary molars and results expressed in millimeters. The polygonal area enclosed by the CEJ, the lateral margins of the exposed tooth root, and the ABC were also measured, and results were expressed in millimeters squared.
μCT Analysis of Alveolar Bone
For quantitative 3-dimensional analysis of the alveolar bone loss, samples of right hemimaxilla were placed in standardized cylindrical sample holders filled with 1× PBS and scanned with high-resolution μCT 40 (Scanco Medical). The specimens were scanned in all 3 spatial planes at a resolution of 12 mm. The 2-dimensional image data were stored in DICOM format (Digital Imaging and Communications in Medicine) and transferred to a computer for 3-dimensional reconstruction and analyses. Each scan was reconstructed at a mesh size of 10 × 10 × 10 µm (10 voxel), and 3-dimensional digitized images were generated for each specimen.
All images were converted into TIFF stack images for further processing with IMARIS 8.1 software (Bitplane) to rotate the samples into a standard orientation and segment the teeth from the alveolar bone (see Appendix). The teeth were automatically segmented with IMARIS 8.1 software by creating new masked surfaces for teeth and bone, followed by automatic segmentation of the alveolar bone. The volume of alveolar bone included in the region of interest (bone volume / total volume) (ROI [BV/TV]) was measured for each sample, and comparisons were made among different groups.
Histologic Analysis of Periodontal Tissues and Number of Osteoclasts
For quantification of changes in the alveolar bone, the mean value (±SEM) of the linear distance between the line connecting the CEJ of 2 adjacent teeth and ABC for first-second and second-third molars was calculated. Osteoclastogenesis was examined in TRAP (tartrate-resistant acid phosphatase)–stained sections. An area of interest (AOI) with standardized dimensions was generated. The coronal surface of this box was applied at the level of the CEJ of the mesial molar of each slide, to allow standardized/equal comparisons of the quantified alveolar bone. Active osteoclasts were defined as multinucleated (≥2) TRAP-positive cells in contact with the bone surface (Li and Amar 2007). The total number of osteoclasts at the surface of the bone included in the AOI and the number of osteoclasts per square millimeter of alveolar bone were quantified. Detailed methods are found in the Appendix.
RANKL and OPG Expression in Periodontal Tissues
Immunohistochemistry was performed on sections (5 μm) that were deparaffinized and rehydrated. Antigen retrieval was performed with 10mM Tris, 1mM EDTA buffer, pH 9.0, in a microwave oven at 50% power (1 min) and 20% power (10 min; details appear at the Appendix). RANKL+ and OPG+ cells lining the alveolar bone surface were enumerated.
Peritoneal Macrophage Cell Culture and P. gingivalis LPS Stimulation
Two milliliters (2.0 mL) of 4% thioglycollate broth (Sigma) was injected intraperitoneally in WT and SOCS-3-KO mice. Three days later, the mice were sacrificed. Peritoneal macrophages were isolated by lavage of the peritoneal cavity with cold PBS and collected by centrifugation. Cells were then cultured in Dulbecco’s Modified Eagle Medium (Thermo Fisher Scientific Inc.) supplemented with 5% fetal bovine serum and 100 IU/mL of penicillin-streptomycin at a concentration of 1.5 × 106 cells/well in separate 6-well plates. After 2 h, nonadherent cells were discarded, and the remaining adherent cells were treated with either culture medium alone (control) or with 100 ng/mL of P. gingivalis A7436 LPS (n = 6 wells per group; Shapira et al. 1998). Supernatants were collected after 12 h and stored at −80 °C for cytokine analysis. Cytokine levels (IL-1β, IL-6, and KC [IL-8]) in the culture supernatants were determined with commercially available multiplex assay kits (EMD Millipore Corporation) according to the manufacturer’s instructions.
Statistical Analysis
Results are presented as mean ± SEM for the in vivo data and mean ± SD for in vitro. Data were analyzed with 1-way analysis of variance and Fisher’s least significant difference post hoc test correcting for multiple comparisons (SPSS 22; IBM Corporation). P < 0.05 was considered statistically significant.
Results
Alveolar Bone Loss Is Increased in P. gingivalis–Infected SOCS-3-KO Mice
To examine the impact of myeloid SOCS-3 deficiency on periodontal bone resorption, we inoculated P. gingivalis A7436 into the oral cavity in WT and myeloid-specific SOCS-3-KO mice. Morphometric, histomorphometric, and µCT analyses were performed (Figs. 1–3).
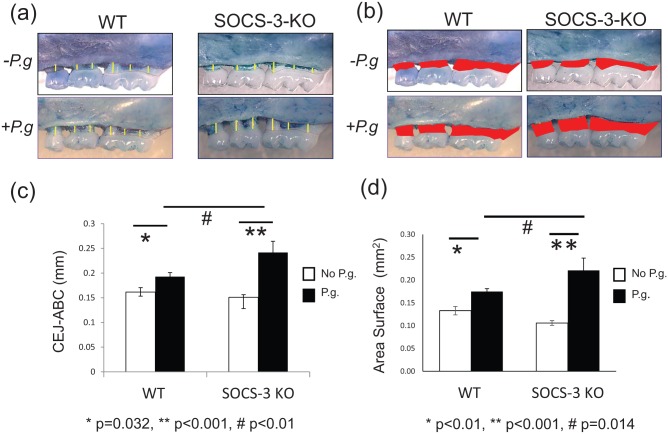
Figure 1.
Alveolar bone loss caused by oral infection with Porphyromonas gingivalis A7436. Measurement of bone levels was made by comparing the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) at 6 buccal sites (a) and the area of exposed roots (b) of the 3 molars on the right side of the maxilla on sham-infected and P. gingivalis–infected wild-type (WT) and myeloid suppressor of cytokine signaling 3–knockout (SOCS-3-KO) mice. P. gingivalis–infected SOCS-3-KO mice presented significantly more bone loss than P. gingivalis–infected WT mice (P < 0.01) and sham-infected SOCS-3-KO mice (P < 0.001). No significant difference in bone levels was seen between sham-infected WT and SOCS-3-KO mice. Bars represent mean ± SEM (n = 5 or 6 mice/group) (c, d). Statistically significant at P < 0.05. P.g, P. gingivalis.
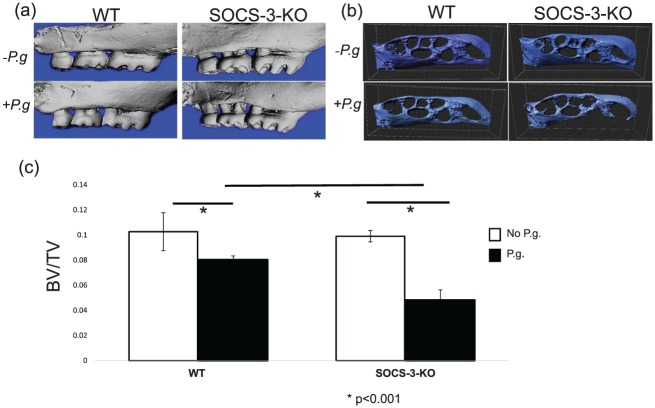
Figure 2.
Buccal view of right hemimaxilla of sham-infected and Porphyromonas gingivalis–infected wild-type (WT) and myeloid suppressor of cytokine signaling 3–knockout (SOCS-3-KO) mice, as reconstructed by the micro–computed tomography (a). Occlusal-apical view of right hemimaxilla of sham-infected and P. gingivalis–infected WT and myeloid SOCS-3-KO mice, as reconstructed by micro–computed tomography after automatic segmentation of the teeth from the alveolar bone with IMARIS 8.1 software (b). The residual volume of alveolar bone in P. gingivalis–infected SOCS-3-KO mice was significantly less than in P. gingivalis–infected WT and sham-infected mice. Bars represent mean ± SD of bone volume / total volume (BV/TV; n = 5 or 6 mice/group) (c). Statistically significant at P < 0.05. P.g, P. gingivalis.
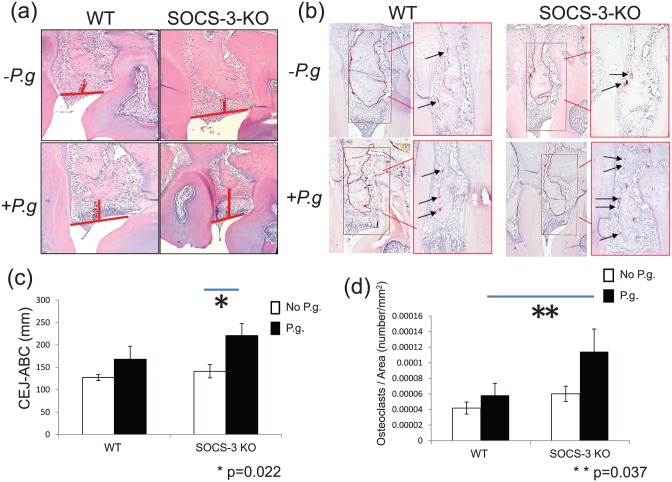
Figure 3.
Alveolar bone loss was assessed from images of hematoxylin and eosin–stained sections captured at ×400 magnification (a). The distance from the cementoenamel junction to the alveolar bone crest was measured between first-second and second-third molars of the left side of the maxilla of sham-infected and Porphyromonas gingivalis–infected wild-type (WT) and myeloid suppressor of cytokine signaling 3–knockout (SOCS-3-KO) mice (a). Histomorphometric analysis indicates that P. gingivalis–infected SOCS-3-KO mice presented significantly more bone loss when compared with other groups of mice (c). Bars represent mean ± SEM (n = 5 or 6 mice/group) (c). Statistically significant at P < 0.05. Number of osteoclasts was assessed from images of TRAP (tartrate-resistant acid phosphatase)–stained sections captured at ×200 and ×400 magnification (b). TRAP-positive multinucleated cells were visually enumerated (arrows in panel b) between maxillary first-second molar and second-third molars in a specific area of interest with standardized dimensions and surface. P. gingivalis–infected SOCS-3-KO mice presented a significantly higher number of osteoclasts per square millimeter of alveolar bone (d). Bars represent mean ± SEM (n = 5 or 6 mice/group; b, c). Statistically significant at P < 0.05. P.g, P. gingivalis.
Linear analysis of bone loss (Fig. 1a, c) revealed that P. gingivalis–infected SOCS-3-KO mice exhibited significantly more bone loss than P. gingivalis–infected WT mice (P < 0.01). P. gingivalis–infected SOCS-3-KO mice showed significantly more bone loss than sham-infected SOCS-3-KO mice (P < 0.001), and P. gingivalis–infected WT mice lost significantly more bone than sham infected WT mice (P = 0.032). No significant difference in bone levels was seen between sham-infected WT and SOCS-3-KO mice. Analysis of the area of exposed roots (Fig. 1b, d) confirmed the linear observations. P. gingivalis–infected SOCS-3-KO mice presented significantly more bone loss than P. gingivalis–infected WT mice (P = 0.014). P. gingivalis–infected SOCS-3-KO mice showed significantly greater area of exposed roots than sham-infected SOCS-3-KO mice (P < 0.001), and P. gingivalis–infected WT mice lost significantly more bone than sham infected WT mice (P < 0.01). No significant difference in area of exposed roots was observed between sham-infected WT and SOCS-3-KO mice.
A volumetric quantitative analysis of alveolar bone loss with μCT verified the results obtained with macroscopic analysis (Fig. 2a). P. gingivalis–infected SOCS-3-KO mice presented significantly less residual volume (BV/TV) of alveolar bone than P. gingivalis–infected WT mice (P < 0.001; Fig. 2b, c).
Histomorphometric measurements of sections stained with hematoxylin and eosin showed that the distance from the CEJ to ABC was significantly greater after infection with P. gingivalis in SOCS-3-KO mice compared with WT mice (Fig. 3a). The distance from the CEJ to ABC was significantly higher in P. gingivalis–infected SOCS-3-KO mice when compared with sham-infected SOCS-3-KO mice (P = 0.022; Fig. 3c). No significant difference in the distance from the CEJ to ABC was observed between sham-infected SOCS-3-KO mice and sham-infected WT mice.
Osteoclast Numbers Are Increased after P. gingivalis Infection in SOCS-3-KO Mice
The number of osteoclasts in tissue sections was determined by TRAP staining. TRAP-positive multinucleated cells lining the alveolar bone surface were visually enumerated (arrows in Fig. 3b) between maxillary first-second molar and second-third molar in a specific AOI with standardized dimensions and surface (Fig. 3b). P. gingivalis–infected SOCS-3- KO mice presented significantly higher number of osteoclasts per square millimeter of alveolar bone (P = 0.037; Fig. 3d). No significant difference was seen between sham-infected WT and SOCS-3-KO mice.
RANKL and OPG Expression in Periodontal Tissues
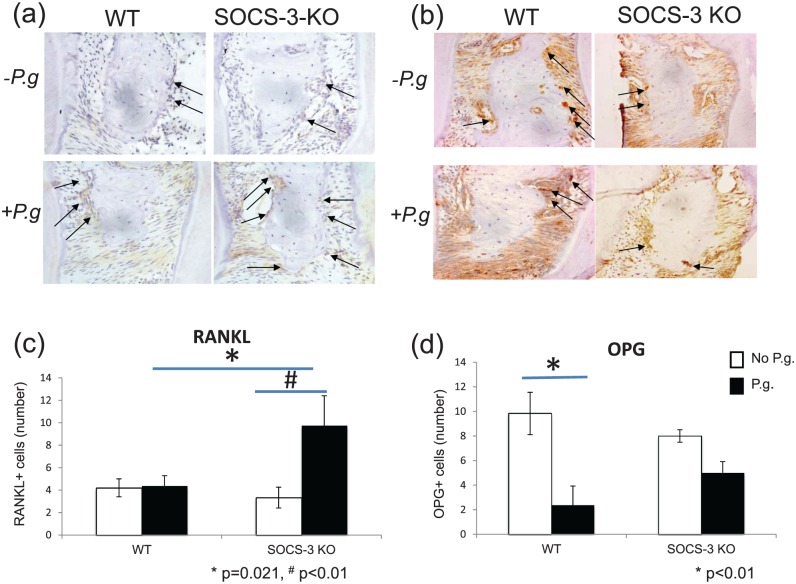
Infection with P. gingivalis resulted in significantly higher numbers of RANKL-positive cells lining the alveolar bone surface in SOCS-3-KO mice as compared with WT (P = 0.021; Fig. 4a, c). There was a reduction of OPG-positive cells lining the alveolar bone surface in both groups infected with P. gingivalis when compared with sham infected. No differences were observed in OPG-positive cells between P. gingivalis–infected SOCS-3-KO and P. gingivalis–infected WT (P = 0.262; Fig. 4b, d). No differences were noted between sham-infected WT and SOCS-3-KO mice (P = 0.622).
Figure 4.
Representative images of paraffin sections of periodontal tissues from wild-type (WT) and myeloid suppressor of cytokine signaling 3–knockout (SOCS-3-KO) mice immunostained with RANKL (a) and OPG (b). The images have been captured at ×400 magnification. RANKL- and OPG-positive cells are indicated with arrows. Porphyromonas gingivalis–infected SOCS-3-KO mice presented a significantly higher number of RANKL-positive cells lining the alveolar bone surface as compared with P. gingivalis–infected WT mice (c). P. gingivalis infection resulted in a reduction of OPG-positive cells lining the alveolar bone surface in WT and SOCS-3-KO mice (d). Statistically significant at P < 0.05. P.g, P. gingivalis.
SOCS-3 Regulates P. gingivalis LPS-Induced Cytokine Secretion by Macrophages In Vitro
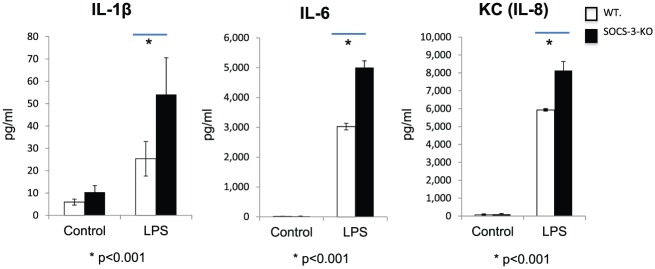
Culture supernatants of peritoneal macrophages from WT and myeloid-specific SOCS-3-KO mice incubated with or without 100 ng/mL of P. gingivalis LPS were analyzed with Luminex multiplex bead immunoassay. Twelve hours after incubation, P. gingivalis LPS stimulation resulted in a significant increase in the secretion of IL-1β, IL-6, and KC (IL-8) from SOCS-3-KO mice as compared with macrophages from WT ones (P < 0.001). Without any P. gingivalis LPS stimulation, no significant difference in the secretion of IL-1β, IL-6, and KC (IL-8) was observed between WT and SOCS-3-KO peritoneal macrophages (P > 0.05; Fig. 5).
Figure 5.
Suppressor of cytokine signaling 3–knockout (SOCS-3-KO) regulates cytokine production by peritoneal macrophages stimulated with Porphyromonas gingivalis lipopolysaccharide (LPS). Peritoneal macrophages isolated from wild-type (WT) and myeloid lineage–specific SOCS-3-KO mice were incubated with either culture medium alone (control) or 100 ng/mL of P. gingivalis LPS for 12 h. The levels of IL-1β, IL-6, and KC (IL-8) in the culture supernatants were measured with Luminex multiplex bead immunoassay. A significantly higher secretion of IL-1β, IL-6, and KC (IL-8) by macrophages from myeloid SOCS-3-KO mice was observed when compared with macrophages from WT ones after P. gingivalis LPS stimulation (P < 0.001). No significant differences in the cytokine levels between control samples of different mouse genotype groups were observed (P > 0.05). The values presented are the mean ± SD (n = 6 wells per group).
Discussion
This report identifies a critical role for SOCS-3 signaling in regulation of inflammatory periodontal bone loss. We show that myeloid-specific SOCS-3-KO mice are more susceptible to P. gingivalis–induced periodontitis in the oral gavage model. Increased bone loss was demonstrated by direct morphologic measurements, µCT analyses, and quantitative histology. Loss of SOCS-3 function resulted in increased numbers of alveolar bone osteoclasts and increased RANKL expression, supporting the hypothesis that SOCS-3 is a negative regulator of inflammatory bone loss. SOCS-3 deficiency in myeloid cells also promotes a higher P. gingivalis LPS-induced inflammatory response with a higher secretion of IL-1β, IL-6, and KC (IL-8) by peritoneal macrophages from SOCS-3-KO mice compared with WT ones.
A dysregulated inflammatory response leads to dysbiosis of periodontal pathogens, and a failure of endogenous resolution pathways may amplify the destruction of periodontal tissues. SOCS proteins form a negative-feedback loop to inhibit cytokine signal transduction and attenuate the expression of inflammatory mediators by binding to JAK/STAT or cytokine receptors (Alexander 2002; Yoshimura et al. 2012). Previous in vivo studies suggest the involvement of SOCS-3 in the negative regulation of periodontal inflammatory networks by demonstrating increased levels of SOCS-3 in inflamed tissues (Garlet et al. 2006; de Souza et al. 2011). However, the exact role of SOCS-3 in periodontal disease remains unclear. Tissue-specific deletion of SOCS-3 allowed better definition of relevance to the inflammatory/immune response. Since studies of the genetic basis of susceptibility and resistance to periodontal disease are difficult to perform in humans, mouse models play a prominent role.
Several protocols have been proposed for inoculating periodontal pathogens into the oral cavity of experimental animals, including diet, ligature, and oral infection by gavage (Graves et al. 2012). Infection of the oral cavity by topical administration of P. gingivalis is a well-established model to test the impact of immune deficiencies on periodontal pathogenesis (Graves et al. 2012). Recognizing that the C57Bl6 is one of the most resistant mouse strains in the oral gavage model for periodontitis and that different strains of P. gingivalis are associated with varied extent of bone loss (Baker et al. 2000), increasing the inoculations of P. gingivalis from 3 to 9, combined with the selection of an aggressive strain of P. gingivalis (A7436), resulted in a successful establishment of bone loss in all P. gingivalis–infected mice that allowed us to explore the role of SOCS-3 in periodontal bone loss.
Here, we demonstrate increased alveolar bone loss in P. gingivalis–infected SOCS-3-KO mice with direct morphologic measurements, µCT analyses, and quantitative histology. Macroscopic and histomorphometric analyses are most often reported. The limitations of these methods include linear or 2-dimensional information by measuring the horizontal bone loss that does not take into consideration infrabony defects (Wilensky et al. 2005). Verifying the 2-dimensional morphometric analysis of bone loss with μCT analysis provided us additional 3-dimensional assessment of the bone in our experimental animals. Indeed, as shown in Figure 2, P. gingivalis–infected SOCS-3-KO mice exhibited significantly less residual bone volume than that of P. gingivalis–infected WT, with significant destruction of alveolar bone all around the teeth, a finding that would have been missed if only macroscopic and histomorphometric analysis were performed. μCT analysis focuses on the difference in changes of the ABC, as opposed to studies that analyze up to the apices of the teeth. Clearly, alveolar bone density decreases more at the gingival margin in the infected animals (Zhang et al. 2014).
SOCS-3 is a critical negative regulator of bone loss in other osteolytic inflammatory diseases. Inflammatory arthritis is particularly severe in SOCS-3-KO mice, characterized by increased osteoclast generation and bone destruction, suggesting that SOCS-3 regulates osteoclastogenesis and bone turnover (Wong et al. 2006). SOCS-3 expression in CD11c+ DC–T cell–Aggregatibacter actinomycetemcomitans co-cultures was strongly associated with enhanced osteoclastogenesis in vitro (Zhang et al. 2009). The increased osteoclasts in the periodontal microenvironment observed in our P. gingivalis–infected SOCS-3-KO mice support loss of SOCS-3 regulation of inflammatory cell cytokine secretion, increasing the overall severity of bone loss.
Deletion of SOCS-3 and infection with P. gingivalis resulted in significantly greater numbers of RANKL-positive cells lining the alveolar bone surface in SOCS-3-KO mice. Similar data were reported in an apical periodontitis mouse model, where an inverse correlation between mRNA levels of SOCS proteins and RANKL was found in periapical granulomas (Menezes et al. 2008). The loss of SOCS-3 regulation leads to a milieu rich in inflammatory cytokines that could disrupt the bone homeostasis and lead to bone loss (Zhang et al. 2009). This scenario could also explain the increased number of OPG-positive cells in P. gingivalis–infected SOCS-3-KO mice, indicating that the molecular mechanisms of bone remodeling are still active during establishment of periodontal lesions (Belibasakis and Bostanci 2012; de Araujo Junior et al. 2013) and prolonged in P. gingivalis–infected SOCS-3-KO mice. Here we show that SOCS-3 deficiency in myeloid cells also promotes a higher P. gingivalis LPS-induced inflammatory response by inducing a higher secretion of IL-1β, IL-6, and KC (IL-8) by peritoneal macrophages. This could be another possible mechanism underlying the negative regulation of alveolar bone loss by SOCS-3. The role of SOCS-3 in bone inflammation is complex; more details of the SOCS-3 pathway in multiple other cell types orchestrating inflammatory bone loss are necessary to understand the mechanisms of bone inflammatory diseases including periodontitis.
In summary, our data implicate SOCS-3 as a critical negative regulator of alveolar bone loss in periodontitis. Our findings raise the possibility that SOCS-3 may be a promising therapeutic target for the management of periodontitis.
Author Contributions
E. Papathanasiou, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; A. Kantarci, A. Konstantinidis, H. Gao, T.E. Van Dyke contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
The authors of this study thank the veterinary and technical personnel of the Forsyth Institute Animal Center for assistance during animal handling and experimental procedures. The authors also thank Justine Dobeck for assisting in the process of histologic samples, Daniel Nguyen for technical support, and Steven Wilbert and Jordan Briscoe for assisting in micro–computed tomography analysis.
Footnotes
This study was partly supported by grants from the National Institute of Dental and Craniofacial Research, U.S. Public Health Service (DE25020 to T.E. Van Dyke; DE020906 to A. Kantarci).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Alexander WS. 2002. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2(6):410–416. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Dixon M, Roopenian DC. 2000. Genetic control of susceptibility to porphyromonas gingivalis-induced alveolar bone loss in mice. Infect Immun. 68(10):5864–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PJ, Evans RT, Roopenian DC. 1994. Oral infection with porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 39(12):1035–1040. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Bostanci N. 2012. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 39(3):239–248. [DOI] [PubMed] [Google Scholar]
- Chaves de Souza JA, Nogueira AV, Chaves de Souza PP, Kim YJ, Silva Lobo C, Pimentel Lopes de Oliveira GJ, Cirelli JA, Garlet GP, Rossa C., Jr. 2013. Socs3 expression correlates with severity of inflammation, expression of proinflammatory cytokines, and activation of stat3 and p38 mapk in lps-induced inflammation in vivo. Mediators Inflamm. 2013:650812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo Junior RF, Souza TO, de Moura LM, Torres KP, de Souza LB, Alves Mdo S, Rocha HO, de Araujo AA. 2013. Atorvastatin decreases bone loss, inflammation and oxidative stress in experimental periodontitis. PloS one. 8(10):e75322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza JA, Nogueira AV, de Souza PP, Cirelli JA, Garlet GP, Rossa C., Jr. 2011. Expression of suppressor of cytokine signaling 1 and 3 in ligature-induced periodontitis in rats. Arch Oral Biol. 56(10):1120–1128. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance Workgroup. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 91(10):914–920. [DOI] [PubMed] [Google Scholar]
- Gao A, Kantarci A, Herrera BS, Gao H, Van Dyke TE. 2013. A critical role for suppressors of cytokine signaling 3 in regulating LPS-induced transcriptional activation of matrix metalloproteinase-13 in osteoblasts. PeerJ. 1:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet GP, Cardoso CR, Campanelli AP, Martins W, Jr, Silva JS. 2006. Expression of suppressors of cytokine signaling in diseased periodontal tissues: a stop signal for disease progression? J Periodontal Res. 41(6):580–584. [DOI] [PubMed] [Google Scholar]
- Graves DT, Kang J, Andriankaja O, Wada K, Rossa C., Jr. 2012. Animal models to study host-bacteria interactions involved in periodontitis. Front Oral Biol. 15:117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci A, Hasturk H, Van Dyke TE. 2006. Host-mediated resolution of inflammation in periodontal diseases. Periodontol 2000. 40:144–163. [DOI] [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. 2014. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 93(11):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. 2002. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 17(5):583–591. [DOI] [PubMed] [Google Scholar]
- Li CH, Amar S. 2007. Morphometric, histomorphometric, and microcomputed tomographic analysis of periodontal inflammatory lesions in a murine model. J Periodontol. 78(6):1120–1128. [DOI] [PubMed] [Google Scholar]
- Menezes R, Garlet TP, Trombone AP, Repeke CE, Letra A, Granjeiro JM, Campanelli AP, Garlet GP. 2008. The potential role of suppressors of cytokine signaling in the attenuation of inflammatory reaction and alveolar bone loss associated with apical periodontitis. J Endod. 34(12):1480–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Naka T, Kawazoe Y, Fujimoto M, Narazaki M, Nakagawa R, Fukuyama H, Nagata S, Kishimoto T. 2000. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor alpha-induced cell death in fibroblasts. Proc Natl Acad Sci U S A. 97(10):5405–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh K, Agrawal DK. 2005. Controlling cytokine signaling by constitutive inhibitors. Biochem Pharmacol. 70(5):649–657. [DOI] [PubMed] [Google Scholar]
- Recio C, Oguiza A, Mallavia B, Lazaro I, Ortiz-Muñoz G, Lopez-Franco O, Egido J, Gomez-Guerrero C. 2015. Gene delivery of suppressors of cytokine signaling (socs) inhibits inflammation and atherosclerosis development in mice. Basic Res Cardiol. 110(2):8. [DOI] [PubMed] [Google Scholar]
- Shapira L, Champagne C, Van Dyke TE, Amar S. 1998. Strain-dependent activation of monocytes and inflammatory macrophages by lipopolysaccharide of Porphyromonas gingivalis. Infect Immun. 66(6):2736–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke TE. 2011. Proresolving lipid mediators: potential for prevention and treatment of periodontitis. J Clin Periodontol. 38 Suppl 11:119–125. [DOI] [PubMed] [Google Scholar]
- Wilensky A, Gabet Y, Yumoto H, Houri-Haddad Y, Shapira L. 2005. Three-dimensional quantification of alveolar bone loss in porphyromonas gingivalis-infected mice using micro-computed tomography. J Periodontol. 76(8):1282–1286. [DOI] [PubMed] [Google Scholar]
- Wong PK, Egan PJ, Croker BA, O’Donnell K, Sims NA, Drake S, Kiu H, McManus EJ, Alexander WS, Roberts AW, et al. 2006. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest. 116(6):1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Cao J, Wu M, Zhang W, Jiang T, Yoshimura A, Gao H. 2010. Suppressor of cytokine signaling 3 inhibits LPS-induced IL-6 expression in osteoblasts by suppressing CCAAT/enhancer-binding protein {beta} activity. J Biol Chem. 285(48):37227–37239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Liu Y, Gao H, Wang X. 2015. Suppressors of cytokine signaling 3 is essential for fcgammar-mediated inflammatory response via enhancing ccaat/enhancer-binding protein delta transcriptional activity in macrophages. Exp Cell Res. 337(1):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. 2012. SOCS, inflammation, and autoimmunity. Front Immunol. 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Liu Y, McFarland BC, Deshane JS, Hurst DR, Ponnazhagan S, Benveniste EN, Qin H. 2015. SOCS3 deficiency in myeloid cells promotes tumor development: involvement of STAT3 activation and myeloid-derived suppressor cells. Cancer Immunol Res. 3(7):727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ju J, Rigney T, Tribble G. 2014. Porphyromonas gingivalis infection increases osteoclastic bone resorption and osteoblastic bone formation in a periodontitis mouse model. BMC Oral Health. 14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Alnaeeli M, Singh B, Teng YT. 2009. Involvement of SOCS3 in regulation of CD11C+ dendritic cell-derived osteoclastogenesis and severe alveolar bone loss. Infect Immun. 77(5):2000–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.