Abstract
Amelogenin and ameloblastin are 2 extracellular matrix proteins that are essential for the proper development of enamel. We recently reported that amelogenin and ameloblastin colocalized during the secretory stage of enamel formation when nucleation of enamel crystallites occurs. Direct interactions between the 2 proteins have been also demonstrated in our in vitro studies. Here, we explore interactions between their fragments during enamel maturation. We applied in vivo immunofluorescence imaging, quantitative co-localization analysis, and a new FRET (fluorescence resonance energy transfer) technique to demonstrate ameloblastin and amelogenin interaction in the maturing mouse enamel. Using immunochemical analysis of protein samples extracted from 8-d-old (P8) first molars from mice as a model for maturation-stage enamel, we identified the ~17-kDa ameloblastin (Ambn-N) and the TRAP (tyrosine-rich amelogenin peptide) fragments. We used Ambn-N18 and Ambn-M300 antibodies raised against the N-terminal and C-terminal segments of ameloblastin, as well as Amel-FL and Amel-C19 antibodies against full-length recombinant mouse amelogenin (rM179) and C-terminal amelogenin, respectively. In transverse sections, co-localization images of N-terminal fragments of amelogenin and ameloblastin around the prism boundary revealed the “fish net” pattern of the enamel matrix. Using in vivo FRET microscopy, we further demonstrated spatial interactions between amelogenin and ameloblastin N-terminal fragments. In the maturing mouse enamel, the association of these residual protein fragments created a discontinuity between enamel rods, which we suggest is important for support and maintenance of enamel rods and eventual contribution to unique enamel mechanical properties. We present data that support cooperative functions of enamel matrix proteins in mediating the structural hierarchy of enamel and that contribute to our efforts to design and develop enamel biomimetic material.
Keywords: dental enamel, amelogenesis, fluorescence resonance energy transfer, confocal microscopy, photobleaching, prismatic structure
Introduction
Regulation of organized growth of dental enamel crystals and their assembly into rods, creating their morphology and hierarchical structure, are dependent on the proper functions of enamel extracellular matrix proteins. The 3 major “structural” proteins in the enamel matrix of developing teeth are amelogenin, enamelin, and ameloblastin (Fincham et al. 1999). Using in vivo co-localization and in vitro biophysical techniques, we have shown evidence that amelogenin forms complexes with both ameloblastin (Mazumder et al. 2014) and enamelin (Fan et al. 2009; Gallon et al. 2013) at the mineralization front.
Enamel proteins are short-lived and eventually degraded into smaller functional components by proteinases such as matrix metalloproteinase 20 and kallikrein 4, which are secreted by ameloblasts (Bartlett and Simmer 1999). It has been suggested that in the maturing enamel, these principal residual proteins are concentrated in the organic matrix that accumulates to form the rod sheath (Hu et al. 1997). This matrix creates connections in between the rods and between rods and interrods; it also creates and maintains discontinuities. In humans, rod sheaths are prominent in mature teeth (Orams 1966). These proteinaceous remnants also have very important mechanical functions (Boyde 1997; Haines 1968; White et al. 2001). An in vivo study showed that the N-terminal fragments of ameloblastin immunolocalized to the sheath space during the maturation stage in porcine enamel (Uchida et al. 1997). Using a colloidal gold immunocytochemical labeling of maturing rat enamel, the presence of amelogenin in the organic matrix that forms the rod sheath has been also documented (Nanci 2013). Amelogenin was also found in the organic matrix around most of the periphery of the rod in mature mouse enamel (Nanci 2013). While there is solid evidence for the presence of specific protein fragments in maturing enamel, it is not known whether these fragments interact or act individually during the maturation stage.
Here, we performed an immunochemical analysis of matrix proteins, extracted from first molars of mice at postnatal days 1, 5, and 8 (P1, P5, and P8), to identify amelogenin and ameloblastin protein fragments that were generated during amelogenesis with a focus on maturation stage enamel. P8 mandibular molars representing the maturation stage of amelogenesis were used for in vivo protein-protein interaction studies (Simmer et al. 2009). We performed an immunochemical analysis of first molar enamel matrix samples, as well as immunofluorescent staining of demineralized mandibular molar sections, to identify protein fragments and their localization in the mature enamel matrix. Several synthetic antipeptide antibodies, which can recognize the N- and C-terminal segments of amelogenin and ameloblastin, were used. We applied quantitative co-localization analysis, a well-established confocal imaging technique, to show co-localization between the fragments of amelogenin and ameloblastin in the rod periphery. We also developed a new fluorescence resonance energy transfer (FRET) imaging tool with mouse tooth sections to show interaction of proteins at the molecular level.
Materials and Methods
Immunochemical Analysis
All mouse experiments were conducted according to the guidelines of the Institutional Animal Care and Use Committee at the University of Southern California. Total protein was extracted from mouse first molars (maxillary and mandibular) at P1, P5, and P8 with 0.5M acetic acid (Mazumder et al. 2014) and were separated with 16% Tris-glycine and 16% Tris-tricine polyacrylamide gels by SDS (sodium dodecyl sulphate) gel electrophoresis and transferred to polyvinylidene fluoride (0.20 µm) membranes. Membranes were immunostained with primary antibodies, followed by secondary antibodies conjugated with horseradish peroxide (Pierce Antibody; Thermo Scientific), and then developed after exposure to enhanced chemiluminescence substrate (GE Healthcare). The primary antibodies used were as follows: chicken anti-amelogenin (Amel-FL [amelogenin full-length antibody]: a gift from Professor M. Snead, University of Southern California), goat anti-amelogenin (Amel-C19 [amelogenin C-terminal antibody]: SC-33109; Santa Cruz Biotechnology), goat anti-amelogenin-Nt (Amel-Nt [amelogenin N-terminal antibody] ab54507; Abcam), rabbit anti-ameloblastin (Ambn-M300 [ameloblastin C-terminal antibody]: SC-50534; Santa Cruz Biotechnology), and goat anti-ameloblastin (Ambn-N18 [ameloblastin N-terminal antibody]: SC-33100, Santa Cruz Biotechnology). Detailed descriptions and dilutions of antibodies are listed in the Appendix Table.
Tissue Section Preparation
The detailed protocol for tissue preparation is described in our previous reports (Gallon et al. 2013; Mazumder et al. 2014). Briefly, mandibles were dissected from mice on the day after their delivery, P1, and P8 and fixed. The P8 samples were demineralized in 0.2% paraformaldehyde, 0.05% glutaraldehyde, and 10% ethylenediaminetetraacetic acid (EDTA) for ~1 to 3 wk (pH 8.0) at 4 °C with agitation. After dehydration, all the samples were embedded in paraffin with 2 different orientations to obtain transverse and sagittal sections of 7-µm thickness and the sections were placed on glass slides.
Confocal Imaging of Mandibular Molars
Tissue sections were immunolabeled to visualize the (co-)localization of amelogenin and ameloblastin under a Leica TCS SP5 confocal microscope (Appendix Scheme 1), as described previously (Mazumder et al. 2014). The primary antibodies used for staining amelogenin and its fragments were 1:1000 chicken Amel-FL, 1:500 goat Amel-C19, and 1:100 rabbit Amel-Nt (ab54507; Abcam). The corresponding secondary antibodies were 1:100 bovine anti-chicken–fluorescein isothiocyanate (FITC), bovine anti-goat-FITC (Santa Cruz Biotechnology), and goat anti-rabbit-FITC (Vector), respectively. For ameloblastin, 1:250 Ambn-N18 primary antibody and 1:100 rabbit anti-goat-Texas Red secondary antibody (Santa Cruz Biotechnology) were used.
In Vivo Quantitative Co-localization Analysis
A biologically meaningful set of co-localization coefficients (Manders et al. 1993) was calculated. These coefficients quantified the colocalized fraction of each molecular species in the same spatial regions of a microscopic image, as previously described (Gallon et al. 2013; Mazumder et al. 2014).
Acceptor Photobleaching FRET
The doubly labeled P8 tissue sections (Appendix Scheme 2; Mills et al. 2003) were examined under a 63× 1.4-NA oil immersion objective and 7× zoom. FRET was measured with the acceptor photobleaching method in Leica Microsystems LASAF FRET AB Wizard (Bastiaens and Jovin 1996; Wouters et al. 1998; Day et al. 2001; Kenworthy 2001; Zal et al. 2002). Once activated, the experiment ran automatically in the following sequence. First images of the donor and acceptor were taken before bleaching. The bleaching then began while the system zoomed in on the regions of interest (ROIs) for the chosen number of frames. Finally, postbleaching images of the donor and acceptor were taken. The donor (fluorescein isothiocyanate [FITC]) and acceptor (tetramethylrhodamine [TRITC]) fluorophores were excited with 488- and 543-nm laser lines at 7% and 24% intensity, respectively. The acceptor channel was then photobleached by defining 9 ROIs and scanning repeatedly (100 times) with a 543-nm laser line at 100% intensity until fluorescence signals were at background levels. All the settings—such as image resolution, image format (1,024 × 1,024 pixels), scan speed (400 Hz), and gain and offset for each channel—were kept similar throughout 1 FRET experiment. The energy transfer efficiency (EFRET) and the distance between donor and acceptor molecule (R) were calculated through the following equations:
for all ID post > ID pre, where ID post and ID pre are the fluorescence intensities of the donor after and before photobleaching, respectively. The efficiency of energy transfer also varies inversely with the sixth power of the distance separating the donor and acceptor fluorophores:
The Förster radius (R0) is the distance between 2 fluorophores when EFRET will be 0.5. For the FITC-TRITC (donor-acceptor) pair, the R0 is 5.5 nm. From equations 1 and 2 and R0, we can measure distance between these 2 fluorophores:
Here, we consider a minimum cutoff of FRET efficiency >0.25 for distance measurement (Haugland 1996).
Results
Protein Fragments in the Maturing Enamel Matrix
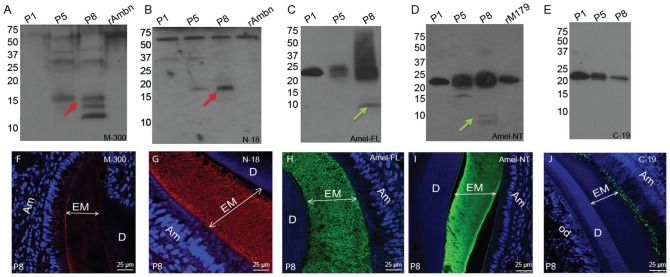
A Tris-glycine SDS-PAGE (sodium dodecyl sulphate–polyacrylamide gel electrophoresis) showing the total proteins present in the mouse first molar extracts from P1, P5, and P8 is shown in Appendix Figure 1. For the identification of mouse ameloblastin proteolytic products extracted from P1, P5, and P8, Ambn-M300 and Ambn-N18 were used (Fig. 1A, B). Western blot analysis of extracted protein samples revealed that at P1 (representing secretory stage), parental ameloblastin protein (~62 kDa) was predominant (best seen in Fig. 1B). Immunostaining of samples from P5 and P8 with Ambn-M300 showed protein bands near ~62, 40, 33, 29, 17, 15, and 13 kDa, which presumably correspond to C-terminal processing products of ameloblastin (Murakami et al. 1997; Uchida et al. 1997; Fig. 1A). At P8, the lower molecular weight fragments were predominant (Fig. 1A). The N-terminal antibody, Ambn-N18, recognized a protein band near ~17 kDa (red arrows in Fig. 1A, B) at P5 and P8 but did not react with the bands at around 15 and 13 kDa.
Figure 1.
Detection of proteolytic products of ameloblastin and amelogenin during amelogenesis and their localization in the enamel matrix at postnatal day 8. Western blots of ameloblastin with (A) Ambn-M300 (ameloblastin C-terminal antibody) and (B) Ambn-N18 (ameloblastin N-terminal antibody). Western blots of amelogenin with (C) Amel-FL (amelogenin full-length antibody), (D) Amel-Nt (amelogenin N-terminal antibody), and (E) Amel-C19 (amelogenin C-terminal antibody). Samples were prepared from first molars of mice at postnatal days 1, 5, and 8 (P1, P5, and P8). Red and green arrows represent the N-terminal 17-kDa ameloblastin and TRAP fragments of amelogenin respectively. Immunofluorescence of mouse molar enamel matrix stained with the (F) Ambn-M300, (G) Ambn-N18, (H) Amel-FL, (I) Amel-Nt, and (J) Amel-C19 antibodies. DAPI stains the nuclei in blue. Am, ameloblast; EM, enamel matrix; D, dentine; Od, odontoblast; TRAP, tyrosine-rich amelogenin peptide.
For the identification of amelogenin fragments in the enamel matrix, Amel-FL, Amel-C19, and Amel-Nt were used (Fig. 1C–E). At P1, immunostaining with all the 3 amelogenin antibodies showed intact amelogenin protein near 25 kDa (Fig. 1C–E). At P8, Amel-FL immunostained protein bands near 25, 20, 13, and ~5 to 6 kDa (green arrows in Fig. 1C, D), while Amel-C19 detected bands at 25 and 20 kDa. The Amel-Nt detected 2 bands around 5 to 6 kDa at P8 (Fig. 1D). These are attributed to the 5-kDa (Fincham et al. 1981) and 7-kDa (Robinson et al. 1995; Sun et al. 2008) tyrosine-rich amelogenin peptide (TRAP) described previously for bovine and porcine amelogenin.
Immunofluorescence study of P8 molar enamel sections immunostained with Ambn-M300 revealed that the cleaved fragments generated from major C-terminal processing leave the enamel layer (Fig. 1F), while immunostaining with Ambn-N18 showed that the N-terminal portions of ameloblastin persist for longer periods in the enamel layer (Fig. 1G). The localization of amelogenin fragments in the mouse molar at P8 is represented in Figure 1H–J. Amel-FL and Amel-Nt immunostained the entire thickness of enamel matrix (Fig. 1H, I), while Amel-C19 stained only the matrix adjacent to the secretory face of ameloblasts (Fig. 1J).
Co-localization of N-terminal Ameloblastin and Amelogenin Fragments at P8 around Enamel Rods
From the immunochemical and immunofluorescence localization results in Figure 1, we determined that at P8 N-terminal proteolytic products of ameloblastin and amelogenin remain in the maturing mouse enamel matrix along the entire thickness of the enamel. To visualize their co-localization pattern and for further detailed observations of the distribution of these protein fragments, we acquired high-resolution confocal images of molar tissue sections and performed quantitative co-localization analysis (Fig. 2; Appendix Figs. 2, 3).
Figure 2.
Quantitative co-localization of ameloblastin and amelogenin at postnatal day 8 (P8). (A, B) Confocal image of mouse mandibular molar in transverse section. Co-localization of amelogenin (green; Appendix Fig. 2B, B1) and ameloblastin (red; Appendix Fig. 2C, C1) is revealed by overlapping signals resulting in yellow staining. (C) Co-localization pattern of amelogenin and ameloblastin in sagittal section (Appendix Fig. 3A–C). (D–F) White pixels exhibiting actual co-localization in the confocal images after background correction, which are used for generation of co-localization coefficients of the 2 proteins in different configurations in both transverse and sagittal sections. (G) Average co-localization coefficient of each region of interest (ROI) obtained from 3 different configurations (along the ameloblasts secretory face, along enamel thickness, and around enamel rods; Appendix Fig. 2F–H) in transverse section and (H) average co-localization coefficient of each ROI obtained from 2 different configurations in sagittal section (Appendix Fig. 3E, F). Am, ameloblast; D, dentine.
The yellow areas in the confocal images of the transverse section (Fig. 2A, B; Appendix Fig. 2A–C) indicate that the N-terminal amelogenin (stained in green, Appendix Fig. 2B) and N-terminal ameloblastin (stained in red, Appendix Fig. 2C) were present in the same spatial region. The magnified image revealed that they colocalized around enamel rods, forming a “fish net” pattern of the matrix throughout the enamel thickness (Fig. 2B). In the sagittal section, ribbons of protein remnants were observed to make linear demarcations between enamel rods; they colocalized across the enamel layer in the plane almost perpendicular to the dentin surface (Fig. 2C, Appendix Fig. 3A–C).
To quantify the percentage of co-localization in the confocal images, quantitative co-localization analysis was performed to obtain the co-localization coefficients of 2 antigens, after a threshold value was set for both channels (Fig. 2D–F). To generate coefficients in both transverse and sagittal sections, ROIs (10 × 10 µm2) were defined along the secretory faces of the ameloblasts (cyan arrow and circles) and along the thickness of enamel (purple arrow and circles; Fig. 2D, F). Around the rod periphery, coefficients were calculated within 1 × 0.3–µm2 ROIs (Fig. 2E). The detailed calculations of co-localization coefficient values for each area of interest are presented in Appendix Figures 2F–H and 3E, F. As can be seen from comparative average co-localization coefficients in a transverse section in Figure 2G, the coefficients for amelogenin and ameloblastin are >0.5. In the transverse sections, both antigens showed equal co-localization contribution within ~0.80 to 0.85 coefficient values in all 3 configurations (Fig. 2G). In the sagittal section, along the secretory face of the ameloblasts, lower coefficient values for ameloblastin (~0.4) were observed as compared with amelogenin (~0.6; Fig. 2H, Appendix Fig. 3E). This suggests that in the outermost layer of maturing enamel (i.e., in the newly secreted matrix), the proportion of free ameloblastin is higher than free amelogenin (Torres-Quintana et al. 2005), whereas in a mature enamel layer (enamel thickness configuration), the protein fragments colocalize in the same proportions (both have coefficient values of ~0.6; Appendix Fig. 3F1, F2).
Ameloblastin-Amelogenin Interaction at a Molecular Level: In Situ FRET Analysis
FRET microscopy was used to study amelogenin-ameloblastin interaction at the molecular level. We developed a new protocol to study protein-protein interactions in fixed EDTA-treated (demineralized) mature dental enamel tissue sections from mice. Control fluorescent immunohistochemical studies were performed to ensure that cross-reactivity did not occur between the secondary antibodies and the tissue and that the species-specific secondary antibodies did not label the other primary antibody epitopes (Appendix Fig. 4). An acceptor photobleaching method was used to perform FRET analysis following immunohistochemical processing of transverse sections with chicken anti-Amel-FL labeled with anti-chicken-FITC antibody (donor) and goat anti-Ambn-N18 labeled with anti-goat-TRITC (acceptor) (Bastiaens and Jovin 1996). Images of the donor and acceptor and their overlap before and after acceptor photobleaching were acquired separately for FRET analysis (Fig. 3A–C). Energy transfer efficiencies were then quantified according to the release of quenching of donor fluorescence due to FRET, which was measured by comparing the intensity of donor fluorescence before and after photobleaching of the acceptor (Fig. 3A1, A2). Measurements of FRET efficiencies in the selected region of enamel matrix (green ROI in Fig. 3) after acceptor photobleaching were represented as a pseudo-color image (Fig. 4A). FRET efficiency is displayed as an absolute range from high (red, 0.89) to low (purple, 0.0) values on this image. The difference in fluorescence intensity of the donor before (ID pre) and after (ID post) photobleaching gives a direct indication of positive FRET efficiency (see equation 1; Fig. 4B). Since the donor-acceptor (FITC-TRITC) pair has an R0 value (a distance at 0.5 FRET efficiency) of approximately 5.5 nm (Haugland 1996), detection of a FRET signal between these fluorophores would indicate that the molecules to which they are attached are located at distances compatible with a direct molecular interaction. We selected small areas around the enamel rod boundary to obtain the FRET efficiencies and then used equation 3 to calculate the corresponding interfluorophore distances. Here, we consider only a specific range of interfluorophore distances, namely 5.75 to 6.75 nm (Fig. 4C), which were generated from ROIs (areas drawn in white lines in Fig. 4A) having FRET efficiencies >0.25. Hence, our in vivo FRET results show that N-terminal ameloblastin segments have direct molecular interactions with amelogenin fragments in the maturing enamel matrix around the rods.
Figure 3.
Acceptor photobleaching fluorescence resonance energy transfer images of transverse section of mouse mandibular molar at postnatal day 8 (P8). (A) Donor emission channel, amelogenin (Amel) stained with anti-chicken-FITC secondary antibody. (B) Acceptor emission channel, ameloblastin (Ambn) stained with anti-goat-TRITC secondary antibody. (C) Overlay of donor and acceptor channels. (A1, B1, C1) Magnified images of donor and acceptor channels and their overlay, which were acquired sequentially before acceptor photobleaching. (A2, B2, C2) Magnified images after acceptor photobleaching. The regions chosen for photobleaching are represented by white boxes in B1 and B2. The height and width of the boxes range from 7 to 12 μm and 10 to 14 μm, respectively. Am, ameloblast; D, dentine; EM, enamel matrix; FITC, fluorescein isothiocyanate; ROI, region of interest; TRITC, tetramethylrhodamine.
Figure 4.
Ameloblastin-amelogenin interactions at P8 in situ. (A) Fluorescence resonance energy transfer (FRET) efficiency image produced by FRET AB Wizard. FRET efficiency is displayed as an absolute range from highest (red, 0.89) to lowest (purple, 0.0). We choose small regions of interest (ROIs) to obtain the FRET efficiency value around the rod sheath (areas drawn with white lines). (B) Donor (fluorescein isothiocyanate) emission intensities before and after acceptor (tetramethylrhodamine) photobleaching. (C) Plot of FRET efficiency against calculated distance between the donor and acceptor from each ROI, based on equation 3. ROI labeled with green shows distances between fluorophores with FRET efficiency <0.2.
Discussion
To obtain further support for ameloblastin-amelogenin spatial interactions in vivo and provide insight into their postulated cooperative function, we applied in vivo quantitative co-localization analysis and FRET techniques to 2 fluorescently labeled proteins in the mandibular first molars of mice. We developed a method by which one can reliably detect protein-protein interactions within fixed teeth sections through conventional immunohistochemical approaches (Mills et al. 2003). FRET detects the proximity of fluorescently labeled molecules over distances <10 nm and is known to be a reliable technique for in situ protein-target interactions (Wouters et al. 1998; Zal et al. 2002).
Ameloblastin rapidly undergoes C-terminal processing at the secretory stage of amelogenesis (Murakami et al. 1997; Uchida et al. 1997). The C-terminal cleaved fragments leave the enamel layer, while the N-terminal portions persist for longer periods (Uchida et al. 1997; Nanci et al. 1998). Amelogenin also undergoes immediate processing at the C-terminal upon secretion. As maturation progresses, subsequent cleavage of amelogenin produces a 5-kDa N-terminal TRAP that persists in the enamel matrix during the transition and maturation stages (Fincham et al. 1981; Robinson et al. 1995; Brookes et al. 2001; Chen et al. 2000).
Our current co-localization and immunochemical analysis with our previous report shows that at P1 (secretory stage) the full-length 62-kDa ameloblastin interacts with the full-length 25-kDa amelogenin at the mineralization initiation sites of enamel, supporting their function as promoter of crystallization during appositional growth (Appendix Fig. 5) (Nanci et al. 1998; Mazumder et al. 2014). Our recent in vitro CD study showed that full-length amelogenin can induce structural elements to disordered ameloblastin (Mazumder et al. 2014).
Here, we further focused on elucidating protein-protein interactions between residual fragments of amelogenin and ameloblastin that are present in the maturing enamel matrix. During the maturation stage (first molar, P8), the ~17-kDa N-terminal ameloblastin and N-terminal amelogenin were identified as residual fragments, and they were found to colocalize around the periphery of the enamel rods. This remnant protein complex was concentrated in the organic matrix in a “fish net” pattern that created discontinuities among enamel rods (Snead 1996; Uchida et al. 1997). The in vivo FRET results clearly show that in mouse tooth sections, N-terminal ameloblastin and amelogenin form a heteromolecular complex around most of the periphery of the rods.
In a parallel in vitro study, using ameloblastin-derived peptides and a series of recombinant amelogenins, as well as a synthetic TRAP peptide, we reported direct amelogenin-ameloblastin interactions. Utilizing intrinsic fluorescence spectroscopy and circular dichroism, we demonstrated that the interaction occurs via the N-terminal 45 residues of amelogenin (TRAP) and ameloblastin peptide encoded by exon 5 (Su et al., 2016).
The ~17-kDa N-terminal ameloblastin fragment contains an important functional domain that is encoded by exon 5 and 6. When exons 5 and 6 were deleted from the Ambn gene in mice, the result was a complete lack of enamel layer (Fukumoto et al. 2004; Smith et al. 2009; Wazen et al. 2009). A very recent study showed that deletion of ameloblastin exon 6 causes nonsyndromic human amelogenesis imperfecta (Poulter et al. 2014).
The loss of the amelogenin N-terminal domain consisting of 1 to 42 residues in transgenic mice led to structural defects in the forming enamel and disorganized rod-interrod boundaries (Paine et al. 2000). We speculate that the formation of a heteroassembly of amelogenin with ameloblastin is disturbed in the absence of the TRAP domain. We suggest that the N-terminal functional domain of ameloblastin with N-terminal proteolytic fragments of amelogenin (most likely TRAP) has an influence on enamel rod organization. Therefore, any deletion or mutation of these domains may affect their interactions and consequently disrupt enamel prismatic microstructure. We further postulate that the residual ameloblastin and amelogenin complex is one of the favorable components of enamel matrix in maturing enamel and may contribute to the unique mechanical properties of mature enamel (Haines 1968; Boyde 1997; White et al. 2001; He and Swain 2008; Castiblanco et al 2015).
Recent studies using double-mutant animal models suggested that amelogenin and ameloblastin function synergistically to preserve the prismatic structure of enamel. It was observed that the dental enamel of Amel X-/-/Ambn-/- double-mutant mice was thinner and lacked a prismatic pattern than that of Amel X-/- or Ambn-/- mice (Hatakeyama et al. 2009). It has been observed that during appositional growth of the enamel layer, ameloblastin co-distributes with amelogenin in the majority of secretory granules, which suggests that a large portion of these proteins may be secreted at the same time and may intermix as they are released (Zalzal et al. 2008).
In summary, our current co-localization and newly developed FRET experiments, combined with immunochemical analysis, clearly demonstrate that amelogenin and ameloblastin fragments interact around the prism boundaries during the maturation stage of amelogenesis. We speculate that these fragments assemble to serve as an organic mesh that supports the integrity of enamel rods, maintaining the hierarchical microstructure of enamel. Residues of these fragments would later contribute to the unique mechanical behavior (elastic moduli) of mature enamel (He and Swain 2008).
Author Contributions
P. Mazumder, contributed to conception design, data acquisition, analysis, or interpretation, drafted and critically revised the manuscript; S. Prajapati, contributed to data acquisition and analysis and drafted and critically revised the manuscript; R. Bapat, contributed to data acquisition and analysis; J. Moradian-Oldak, contributed to design and data analysis and interpretation. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Prof. Malcolm Snead for kindly providing us with the antibody against amelogenin.
Footnotes
This research was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research (R01 grants DE-013414 and DE-020099) to J.M.O.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Bartlett JD, Simmer JP. 1999. Proteinases in developing dental enamel. Crit Rev Oral Biol Med. 10(4):425–441. [DOI] [PubMed] [Google Scholar]
- Bastiaens PI, Jovin TM. 1996. Microspectroscopic imaging tracks the intracellular processing of a signal transduction protein: Fluorescent-labeled protein kinase C beta I. Proc Natl Acad Sci U S A. 93(16):8407–8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyde A. 1997. Microstructure of enamel. In: Dental enamel: Ciba Foundation symposium 205. New York (NY): John Wiley & Sons, Ltd; p. 18–31. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Kirkham J, Shore RC, Wood SR, Slaby I, Robinson C. 2001. Amelin extracellular processing and aggregation during rat incisor amelogenesis. Arch Oral Biol. 46(3):201–208. [DOI] [PubMed] [Google Scholar]
- Castiblanco GA, Rutishauser D, Ilag LL, Martignon S, Castellanos JE, Mejía W. 2015. Identification of proteins from human permanent erupted enamel. Eur J Oral Sci. 123(6):390–395. [DOI] [PubMed] [Google Scholar]
- Chen WY, Bell AW, Simmer JP, Smith CE. 2000. Mass spectrometry of native rat amelogenins: primary transcripts, secretory isoforms, and C-terminal degradation. J Dent Res. 79(3):840–849. [DOI] [PubMed] [Google Scholar]
- Day RN, Periasamy A, Schaufele F. 2001. Fluorescence resonance energy transfer microscopy of localized protein interactions in the living cell nucleus. Methods. 25(1):4–18. [DOI] [PubMed] [Google Scholar]
- Fan D, Du C, Sun Z, Lakshminarayanan R, Moradian-Oldak J. 2009. In vitro study on the interaction between the 32 kda enamelin and amelogenin. J Struct Biol. 166(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham AG, Belcourt AB, Termine JD, Butler WT, Cothran WC. 1981. Dental enamel matrix: sequences of two amelogenin polypeptides. Biosci Rep. 1(10):771–778. [DOI] [PubMed] [Google Scholar]
- Fincham AG, Moradian-Oldak J, Simmer JP. 1999. The structural biology of the developing dental enamel matrix. J Struct Biol. 126(3):270–299. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, Krebsbach PH, Nanci A, Kulkarni AB, Yamada Y. 2004. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 167(5):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallon V, Chen L, Yang X, Moradian-Oldak J. 2013. Localization and quantitative co-localization of enamelin with amelogenin. J Struct Biol. 183(2):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines DJ. 1968. Physical properties of human tooth enamel and enamel sheath material under load. J Biomech. 1(2):117–125. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Fukumoto S, Nakamura T, Haruyama N, Suzuki S, Hatakeyama Y, Shum L, Gibson CW, Yamada Y, Kulkarni AB. 2009. Synergistic roles of amelogenin and ameloblastin. J Dent Res. 88(4):318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland RP. 1996. Handbook of fluorescent probes and research chemicals. Eugene (OR): Molecular Probes. [Google Scholar]
- He LH, Swain MV. 2008. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J Mech Behav Biomed Mater. 1(1):18–29. [DOI] [PubMed] [Google Scholar]
- Hu CC, Fukae M, Uchida T, Qian Q, Zhang CH, Ryu OH, Tanabe T, Yamakoshi Y, Murakami C, Dohi N, et al. 1997. Sheathlin: cloning, cDNA/polypeptide sequences, and immunolocalization of porcine enamel sheath proteins. J Dent Res. 76(2):648–657. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK. 2001. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods. 24(3):289–296. [DOI] [PubMed] [Google Scholar]
- Manders EM, Verbeek FJ, Aten JA. 1993. Measurement of colocalization of objects in dual-color confocal images. J Microsc. 169(Pt 3):375–382. [DOI] [PubMed] [Google Scholar]
- Mazumder P, Prajapati S, Lokappa S, Gallon V, Moradian-Oldak J. 2014. Analysis of co-assembly and co-localization of ameloblastin and amelogenin. Front Physiol. 5:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JD, Stone JR, Rubin DG, Melon DE, Okonkwo DO, Periasamy A, Helm GA. 2003. Illuminating protein interactions in tissue using confocal and two-photon excitation fluorescent resonance energy transfer microscopy. J Biomed Opt. 8(3):347–356. [DOI] [PubMed] [Google Scholar]
- Murakami C, Dohi N, Fukae M, Tanabe T, Yamakoshi Y, Wakida K, Satoda T, Takahashi O, Shimizu M, Ryu OH, et al. 1997. Immunochemical and immunohistochemical study of the 27- and 29-kDa calcium-binding proteins and related proteins in the porcine tooth germ. Histochem Cell Biol. 107(6):485–494. [DOI] [PubMed] [Google Scholar]
- Nanci A. 2013. Enamel: composition, formation, and structure. In: Ten Cate’s oral histology development, structure, and function. 8th ed. St. Louis (MO): Elsevier Mosby; p. 122–164. [Google Scholar]
- Nanci A, Zalzal S, Lavoie P, Kunikata M, Chen WY, Krebsbach PH, Yamada Y, Hammarstrom L, Simmer JP, Fincham AG, et al. 1998. Comparative immunochemical analyses of the developmental expression and distribution of ameloblastin and amelogenin in rat incisors. J Histochem Cytochem. 46(8):911–934. [DOI] [PubMed] [Google Scholar]
- Orams HJ. 1966. An examination of the prism core, prism sheath and interprismatic substance using the electon microscope. Aust Dent J. 11(2):93–104. [DOI] [PubMed] [Google Scholar]
- Paine ML, Zhu DH, Luo W, Bringas P, Goldberg M, White SN, Lei YP, Sarikaya M, Fong HK, Snead ML. 2000. Enamel biomineralization defects result from alterations to amelogenin self-assembly. J Struct Biol. 132(3):191–200. [DOI] [PubMed] [Google Scholar]
- Poulter JA, Murillo G, Brookes SJ, Smith CEL, Parry DA, Silva S, Kirkham J, Inglehearn CF, Mighell AJ. 2014. Deletion of ameloblastin exon 6 is associated with amelogenesis imperfecta. Hum Mol Genet. 23(20):5317–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Kirkham J, Bonass WA, Shore R, Brookes S. 1995. Role of the extracellular matrix in enamel development. In Robinson C, Kirkham J, Shore R, eds. Dental enamel: formation to destruction. Boca Raton (FL): CRC Press; p. 105–133. [Google Scholar]
- Simmer JP, Hu Y, Lertlam R, Yamakoshi Y, Hu JC. 2009. Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J Biol Chem. 284(28):19110–19121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Wazen R, Hu YY, Zalzal SF, Nanci A, Simmer JP, Hu JC. 2009. Consequences for enamel development and mineralization resulting from loss of function of ameloblastin or enamelin. Eur J Oral Sci. 117(5):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead ML. 1996. Enamel biology logodaedaly: getting to the root of the problem, or “who’s on first.” J Bone Miner Res. 11(7):899–904. [DOI] [PubMed] [Google Scholar]
- Su J, Balakrishna Chandrababu K, Moradian-Oldak J. 2016. Ameloblastin peptide encoded by exon 5 interacts with amelogenin N-terminus. Biochemistry and Biophysics Reports. 7:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Fan D, Fan Y, Du C, Moradian-Oldak J. 2008. Enamel proteases reduce amelogenin-apatite binding. J Dent Res. 87(12):1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Quintana MA, Gaete M, Hernandez M, Farias M, Lobos N. 2005. Ameloblastin and amelogenin expression in postnatal developing mouse molars. J Oral Sci. 47(1):27–34. [DOI] [PubMed] [Google Scholar]
- Uchida T, Murakami C, Dohi N, Wakida K, Satoda T, Takahashi O. 1997. Synthesis, secretion, degradation, and fate of ameloblastin during the matrix formation stage of the rat incisor as shown by immunocytochemistry and immunochemistry using region-specific antibodies. J Histochem Cytochem. 45(10):1329–1340. [DOI] [PubMed] [Google Scholar]
- Wazen RM, Moffatt P, Zalzal SF, Yamada Y, Nanci A. 2009. A mouse model expressing a truncated form of ameloblastin exhibits dental and junctional epithelium defects. Matrix Biol. 28(5):292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SN, Luo W, Paine ML, Fong H, Sarikaya M, Snead ML. 2001. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anisotropy in human enamel. J Dent Res. 80(1):321–326. [DOI] [PubMed] [Google Scholar]
- Wouters FS, Bastiaens PI, Wirtz KW, Jovin TM. 1998. FRET microscopy demonstrates molecular association of non-specific lipid transfer protein (nsL-TP) with fatty acid oxidation enzymes in peroxisomes. EMBO J. 17(24):7179–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zal T, Zal MA, Gascoigne NR. 2002. Inhibition of T cell receptor-coreceptor interactions by antagonist ligands visualized by live FRET imaging of the T-hybridoma immunological synapse. Immunity. 16(4):521–534. [DOI] [PubMed] [Google Scholar]
- Zalzal SF, Smith CE, Nanci A. 2008. Ameloblastin and amelogenin share a common secretory pathway and are co-secreted during enamel formation. Matrix Biol. 27(4):352–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.