Abstract
Background:
The antipsychotic, haloperidol, is extremely efficient in the treatment of schizophrenia but its application is constrained because of irreversible adverse drug reactions. Hence, in this study, we investigate the differential effects of black seed oil on cholinesterase [acetylcholinesterase (AChE) and butrylcholinesterase (BuChE), ectonucleotidase (5′-nucleotidase), lactate dehydrogenase (LDH) and monoamine oxidase (MAO)] activities and relevant markers of oxidative stress in the cerebrum of haloperidol-induced neuronal-damaged rats.
Methods:
The animals were divided into six groups (n = 10): normal control rats; haloperidol-induced rats: induced rats were pre-, co- and post-treated with black-seed oil respectively, while the last group was treated with extract oil only. The treatment was performed via oral administration and the experiment lasted 14 days.
Results:
The results revealed an increase in 5I nucleotidase, a marker of adenosine triphosphate (ATP) and adenosine monophosphate (AMP) hydrolysis, as well as AChE, BuChE and MAO activities, with concomitant decrease in LDH activity of cerebrum in induced rats when compared with controls. Also, administration of haloperidol caused systemic oxidative damage and adverse histopathological changes in neuronal cells, indications of mental disorder. The differential treatments with black-seed oil prevented these alterations by increasing LDH and decreasing 5I nucleotidase, AChE, BuChE and MAO activities in the cerebrum. Essential oil post-treatment is most efficacious in reversing haloperidol-induced neuronal damage in rat; followed by pre- and cotreatment, respectively.
Conclusions:
We concluded that essential black-seed oil enhanced the wellness of aminergic, purinergic and cholinergic neurotransmissions of haloperidol-induced neuronal damage in rats.
Keywords: bioenergetics, black-seed oil, cerebral damage, haloperidol, neurotransmitters, reversal
Introduction
Currently, the World Health Organization estimates that neurological disorders and their direct consequences affect one billion people worldwide [Volpe, 2008; Cieza et al. 2015]. Recent studies also demonstrate that neurological disorders such as Alzheimer’s disease (AD), dementia and Parkinson’s disease (PD) are found among all age groups and in all geological regions [Ross and Tabrizi, 2011; Johnson et al. 2013]. Unfortunately, many developing countries have the double burden of these diseases [Olesen et al. 2012]. It has been estimated that the number of people with neurological disorders will increase considerably in years to come [Raggi and Leonardi, 2015]. The number of people affected by dementia and mental disorder is likely to double every 20 years [Gustavsson et al. 2011]. This endemic might be linked to the prescribed antipsychotic drugs which alter the physiological activity of the central nervous system (CNS) [Yohko et al. 2012; Birkhofer et al. 2013]. Antipsychotic medications eventually disrupt or damage neurons and neural cells that transmit and process signals in the brain [Valenti et al. 2011].
Haloperidol (HAL) was approved for the treatment of schizophrenia [Brayfield, 2013] and is commonly known to generate selective effects on the CNS by competitive blockade of postsynaptic dopamine (D2) receptors in the mesolimbic dopaminergic system [Valenti et al. 2011; Goikolea et al. 2013; Toshi et al. 2014]. And as a typical neuroleptic, it causes extrapyramidal symptoms, AD, including tardive dyskinesia (TD) [Na-Ri and Moon-Doo, 2011; Levine et al. 2011; Levine and Ruha, 2012; Samad, 2015]. It occurred via an oxidative-stress mechanism following the production of inhibitors in the mitochondria [Gupta et al. 2007; Citrome, 2011; Valenti et al. 2011]. A recent study showed that a leading antipsychotic drug (HAL) temporarily reduces the size of a brain region that controls movement and coordination, causing distressing side effects such as shaking, drooling and restless leg syndrome [Tost et al. 2010; Todd, 2012]. It was further established that after 2 hours of injection with HAL, there was manifestation of impaired motor abilities that coincided with diminished grey-matter volume in the striatum [Tost et al. 2010; Lyons and Pahwa, 2011] of the brain region.
Countless health-promoting plant foods are linked to the presence of various collections of essential phenolic phytochemicals, including phenolic acids and flavonoids [Wang et al. 2010; Oboh et al. 2012a, 2012b]. Phenolic compounds are derived metabolites of plants and are present in the human diet [Oboh and Ogunruku, 2010]. They are strong antioxidant molecules and play a crucial role in health improvement and disease aversion [Akinyemi et al. 2015]. Oil from black seeds has been recognized for possessing medicinal properties and their uses in traditional medicine have been on record for years. They are consumed as whole grain or ground into a powder and mixed with diets. Therefore, polyphenol-rich seeds could act as potential chemotherapeutic intervention in the management of neuronal damage and brain-associated dysfunctions. Scant studies have examined their neurological actions on antipsychotic-induced damaged drugs, particularly when differential treatments (pre, co and post) are employed. Also, the specific underlying biochemical mechanisms in targeting neuronal disorders are poorly expounded. Thus, this study was designed to examine the differential protection of pre-, co- and post-treatment with essential black-seed (Nigella sativa) oil in HAL-induced neural-cell damage using rat model; and to possibly elucidate the biomechanisms that promote neuronal wellness.
Material and methods
Chemicals and reagents
HAL, and substrates adenosine monophosphate (AMP), acetylcholine iodide, butyrylcholine iodide, benzylamine-5, 5-dithio-bis-2-nitrobenzoic acid (DTNB), glutathione (GSH), hydrogen peroxide, trichloroacetic acid (TCA) and thiobarbituric acid (TBA) were purchased from Sigma (St Louis, MO, USA). All other reagents were of analytical grade and obtained from the British Drug Houses (Poole, Dorset, UK). Kits for lactate dehydrogenase (LDH) activity were purchased from Randox Laboratory Limited, UK.
Sample collection
The fresh seeds of black seeds (N. sativa) were obtained from Al-Medinat Ventures, Kwara State, Ilorin, Nigeria. Authentication of the plants was confirmed at the Department of Botany, University of Ilorin, Nigeria. The seeds were sorted, to remove all possible stones and dirty material and grounded into a powder to enhance efficiency of extracting the active component(s).
Quantification of compounds by HPLC-DAD
Analysis of phenolic compounds by HPLC-DAD reverse-phase chromatographic analyses was carried out under gradient conditions. Black seeds, at a concentration of 15 mg/ml, were injected by means of a model SIL-20A Shimadzu auto sampler. The separations were carried out using a Phenomenex C18 column (4.6 mm x 250 mm x 5 µm particle size). The mobile phases were 0.5% (v/v) aqueous acetic acid (solvent A) and 1% (v/v) formic acid in acetonitrile (solvent B). The binary-elution system was as follows: 2% B at initial 5 minutes to wash the column, a linear gradient was 8% B (25 minutes), 12% B (45 minutes), and 24% B (60 minutes). After 80 minutes, the organic-phase concentration was brought back to 2% (B) and lasted 6 minutes for column equilibration. A flow rate of 0.6 ml/minute and an injection volume of 50 μl were used [Boligon et al. 2015]. The sample and mobile phases were filtered through a 0.45 μm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use. Stock solutions of standard references were prepared in the HPLC mobile phase at a concentration range of 0.030–0.500 mg/ml. Quantifications were carried out by integration of the peaks using the external-standard method, at 270 nm for gallic acid; 280 nm for catechin; 325 nm for chlorogenic and caffeic acids, and 366 nm for orientin, quercetin, quercitrin, rutin and luteolin. The chromatography peaks were confirmed by comparing its retention time with those of reference standards and by DAD spectra (200–600 nm). The calibration-curve calculation used for gallic acid was y = 12783x + 1194.7 (r = 0.9999); for chlorogenic acid was y = 13508x + 1175.0 (r = 0.9997); for caffeic acid was y = 13536x + 1368.9 (r = 0.9995); for orientin was y = 12671x + 1358.6 (r = 0.9998); for catechin was y = 11947x + 1259.3 (r = 0.9998); for quercetin was y = 11307x + 1183.5 (r = 0.9996); for quercitrin was y = 13856x + 1327.4 (r = 0.9997); for rutin was y = 12743x + 1328.9 (r = 0.9999); and for luteolin was y = 11734x + 1176.8 (r = 0.9993). All chromatography operations were carried out at ambient temperature and in triplicate. The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the responses and the slope using three independent analytical curves. LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve [Reis et al. 2014].
Animals
Adult male Wistar rats (150–200 g) from the Animal House of the University of Ibadan, Nigeria were used in this experiment. They were housed in the metallic cage at the central Animal House of Kwara State University, Malete. The animals were maintained at a constant temperature (30–32°C) on a 12-hour light/dark cycle with free access to food and water. The animals were used according to the institutional guidelines of Nigeria Academy and are in accordance with international guidelines. The research adhered to the ethics of the Institutional Animal Ethical Committee.
Experimental protocol
The rats were acclimatized for 2 weeks and randomly divided into six groups of 10 animals each (n = 10). Group 1 was given distilled water only via oral route. Group 2 was given a therapeutic dose (15 mg/kg body weight) of haloperidol. Group 3 was given 150 mg/kg body weight of black-seed oil (BSO before) for 7 days plus a therapeutic dose (15 mg/kg body weight) of haloperidol (HAL after) for 7 days. Group 4 was given a therapeutic dose (15 mg/kg body weight) of haloperidol (HAL) plus 150 mg/kg body weight of black-seed oil (BSO). Group 5 was given a therapeutic dose (15 mg/kg body weight) of haloperidol for 7 days (HAL before) plus 150 mg/kg body weight of black-seed oil for 7 days (BSO after). Group 6 was given 150 mg/kg body weight of black-seed oil (BSO only). The rats were fed with standard food and had free access to drinking water throughout the entire experiment. The experiment lasted 2 weeks (14 days). After the treatment, the animals were submitted to euthanasia being previously anesthetized with ethyl acetate.
Homogenate preparation
The cerebrum was removed for brain homogenate preparation. The cerebrum was homogenized in 10 volumes of an ice-cold medium, consisting of 1.15% potassium chloride and 50 mmol Tris-HCl buffer with a pH of 7.4 in a motor-driven Teflon-glass homogenizer. The supernatants were isolated at 4°C and used for biochemistry and enzymatic assays.
Biochemical enzymatic-antioxidants assay
The brain supernatant collected was used for the estimation of catalase (CAT) activity using hydrogen peroxide as the substrate according to the method of Clairborne [Clairborne, 1995]. Superoxide dismutase (SOD) activity was determined by measuring the inhibition of auto-oxidation of epinephrine pH 10.2, at 30 ± 1°C according to Misra and Fridovich [Misra and Fridovich, 1989]. Protein concentration was determined by the method of Lowry and colleagues [Lowry et al. 1951]. Glutathione-S- transferase (GST) was assayed using 1-chloro-2, 4-dinitrobenzene as the substrate according to the method of Habig [Habig, 1974] and expressed as moles of CDNB-GSH complex formed per minute per milligram of protein (mol/min/mg protein).
Glutathione assay
Reduced GSH was determined at 412 nm using the method described by Jollow and colleagues [Jollow et al. 1974].
Lipid peroxidation assay
Lipid peroxidation was quantified as malondialdehyde (MDA) according to the method described by Ohkawa and colleagues [Ohkawa et al. 1979] and expressed as μmol/mg protein.
Lactate dehydrogenase (LDH) assay
The cerebellum homogenate was assayed for LDH activity using a commercially available kit (Randox Laboratories, UK). The assay was made according to the manufacturer’s instructions [Weisshaar et al. 1975].
Neuronal 5I-nucleotidase assay
The 5I-nucleotidase activity was determined essentially by the method of Heymann and colleagues [Heymann et al. 1984] in a reaction medium containing 100 μl of 10 mmol MgCI2 and 100 μl of 5 mmol Tris–KCl buffer, pH 7.6, to final volume of 500 μl. The reaction was initiated by the addition of 150 μl 10 mmol AMP. 150 μl of the homogenate was added to the reaction mixture and incubated at 37°C for 20 minutes. In all cases, reaction was stopped by the addition of 500 μl of 10% trichloroacetic acid (TCA) and the protein precipitated was removed by centrifugation for 10 minutes. 500 μl of the supernatant was added to 500 μl of 1.6% ammonium molybdate, then 200 μl of sodium acetate and 800 μl of 10% ferrous sulphate. The released inorganic phosphate (Pi) was assayed at 700nm using colorimetric reagent and KH2PO4 as standard. Controls were made by adding brain-homogenate fraction after TCA addition, to correct for nonenzymatic nucleotide hydrolysis. Enzyme activities are reported as mmol Pi released/minute/mg of protein.
Acetylcholinesterase and butyrylcholinesterase activities assays
The acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) enzymatic assays were determined by modification of the spectrophotometric method of Ellman [Perry et al. 2000]. The reaction mixture (2 ml final volume) contained 100 mmol K+-phosphate buffer, pH 7.5 and 1 mmol 5,50-dithiobisnitrobenzoic acid (DTNB). The method is based on the formation of the yellow anion, 5,50-dithio-bis-acid-nitrobenzoic, measured by absorbance at 412 nm after 2 minutes.
Monoamine oxidase activity assay
Monoamine oxidase (MAO) activity was estimated using benzylamine as the MAO substrate according to the method described by Kettler and colleagues [Kettler et al. 1990] with slight modification. Reaction mixture contained 100 mmol phosphate buffer of pH 7.4, 200 µm benzylamine and 0.4 mg/ml of homogenate. The final volume of the reaction mixture was 250 µl. Mixtures were incubated at 37°C for 1 hour and cooled on ice. 500 µl of distilled water, 250 µl of 10% ZnSO4 and 50µl of 1 mol NaOH were heated for 2 minutes, cooled on ice and centrifuged (1000g for 10 minutes). The supernatant was diluted (by 5×) with 1 mol NaOH, while the absorbance was read at 450nm.
Histopathological examination
After the treatment, the cerebra were separated and placed in 4% para-formaldehyde at 4°C for 48 hours. After dehydration, transparency, paraffin immersion and paraffin embedding, the cerebrum was sliced along the median anteroposterior axes at a thickness of 6 mm. The section was stained with hematoxylin and eosin for morphological observation and defining positions. Sections were read and images were captured using an Olympus microscope (BX51).
Statistical analysis
The data in each group were expressed as mean ± standard deviation. A one-way analysis of variance (ANOVA) was used to analyze the results and the Duncan multiple test was used for the post hoc analysis [Zar, 1984]. The Statistical Package for Social Science (SPSS) 17.0 for Windows was used for the analysis and the least significance difference (LSD) was accepted at p < 0.05.
Results
HPLC analysis
The HPLC profile of black seed is acquired as shown in Figure 1. The sample contains other minor compounds in addition to gallic acid (retention time: tR = 10.03 minutes, peak 1), catechin (tR = 12.37 minutes, peak 2), chlorogenic acid (tR = 20.15 minutes, peak 3), caffeic acid (tR = 23.76 minutes, peak 4), orientin (tR = 30.58 minutes, peak 5), rutin (tR = 36.81 minutes, peak 6), quercitrin (tR = 43.97 minutes, peak 7), quercetin (tR = 47.19 minutes, peak 8) and luteolin (tR = 51.06 minutes, peak 9).
Figure 1.
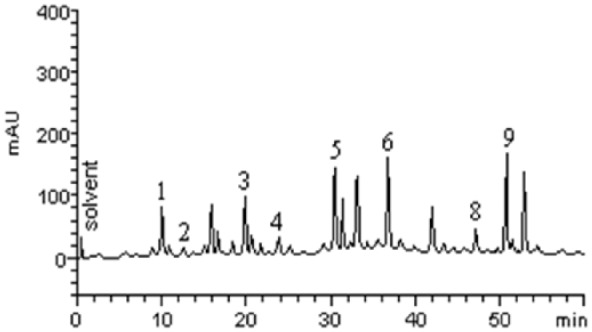
Representative high-performance liquid chromatography profile of black-seed aqueous extract. Gallic acid (peak 1), catechin (peak 2), chlorogenic acid (peak 3), caffeic acid (peak 4), orientin (peak 5), rutin (peak 6), quercitrin (peak 7), quercetin (peak 8) and luteolin (peak 9).
mAU (SI unit of Recorder response).
Marker of oxidative damage
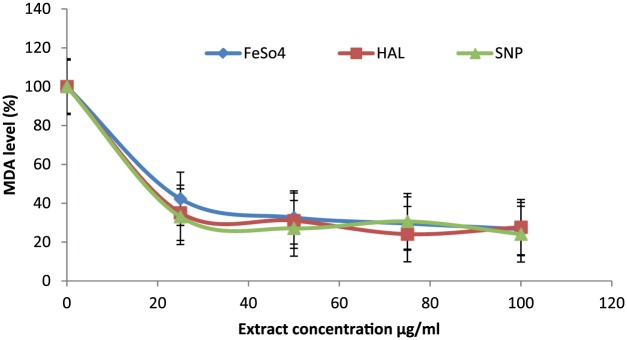
The levels of MDA, a marker of lipid peroxidation, decreased significantly (p < 0.05) in rat brain treated with BSO in vitro, in a dose-dependent manner (Figure 2), that is, the MDA levels decreased from 100% to 20% with 100 μg/ml of extract. Also, the level of MDA was increased in the group of animals subacutely administered with HAL in vivo (Figure 3) by 49%, when compared with control. Whereas increased MDA levels were markedly (p < 0.05) prevented on pre- and coadministration with BSO (Figure 3) by 49% and 45%, respectively. Also, the MDA content of the post-treated group was significantly (p < 0.05) reversed by 33% in relation to the HAL-only group. In addition, the group of animals treated with BSO only significantly (p < 0.05) reduced the level of MDA by 5.3% when compared with the control group (Figure 3).
Figure 2.
Lipid peroxidation: in vitro; oil from black seed (BSO) inhibits sulphur nitroprusside (SNP), iron sulphate (FeS04) and haloperidol (HAL) These pro-oxidants induced lipid peroxidation dose dependently in rat’s cerebrum. The percentage inhibition of MDA (malondialdehyde) production was expressed in 100%. Significance was accepted at p < 0.05.
Figure 3.

Malondialdehyde (MDA) level from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation.
Antioxidant status in the cerebrum
The activity of CAT in the postmitochondrial fraction of rat cerebrum was not significantly decreased in the animals treated with HAL compared with the control animals (Figure 4). Pre- and cotreated groups increased CAT activity, not significantly, by 42% and 37%, respectively, relative to the HAL-only group. The post-treated group was significantly (p < 0.05) increased by 67% in relation to HAL-only group. For the activity of SOD (Figure 5), the HAL only, pretreated and cotreated groups showed values significantly higher than the control group, whereas the groups post-treated and extract only didn’t show any significant difference when compared with the control or HAL-only groups. In addition, subacute treatment of HAL in rats caused a significant decrease (p < 0.05) in neuronal GSH and antioxidant protein by 33%, when compared with the corresponding group of control animals (Figure 6). Pre-, co- and post-treatment did not significantly (p > 0.05) reverse the effect when compared with HAL only (Figure 6). Furthermore, there was no significant difference between experimental animals treated with HAL when compared with the control group (Figure 7). Group of animals pretreated prevented the decrease by 66% but not significantly. However, animals co- and post-treated resulted in significant (p < 0.05) increase in GST activity by 89% and 109% with respect to HAL only. Correspondingly, animals administered with BSO only increased the activity of GST in relation to HAL only (Figure 7).
Figure 4.

Catalase (CAT) activity from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation.
Figure 5.
Superoxide dismutase (SOD) activity from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically.
SD, standard deviation.
Figure 6.
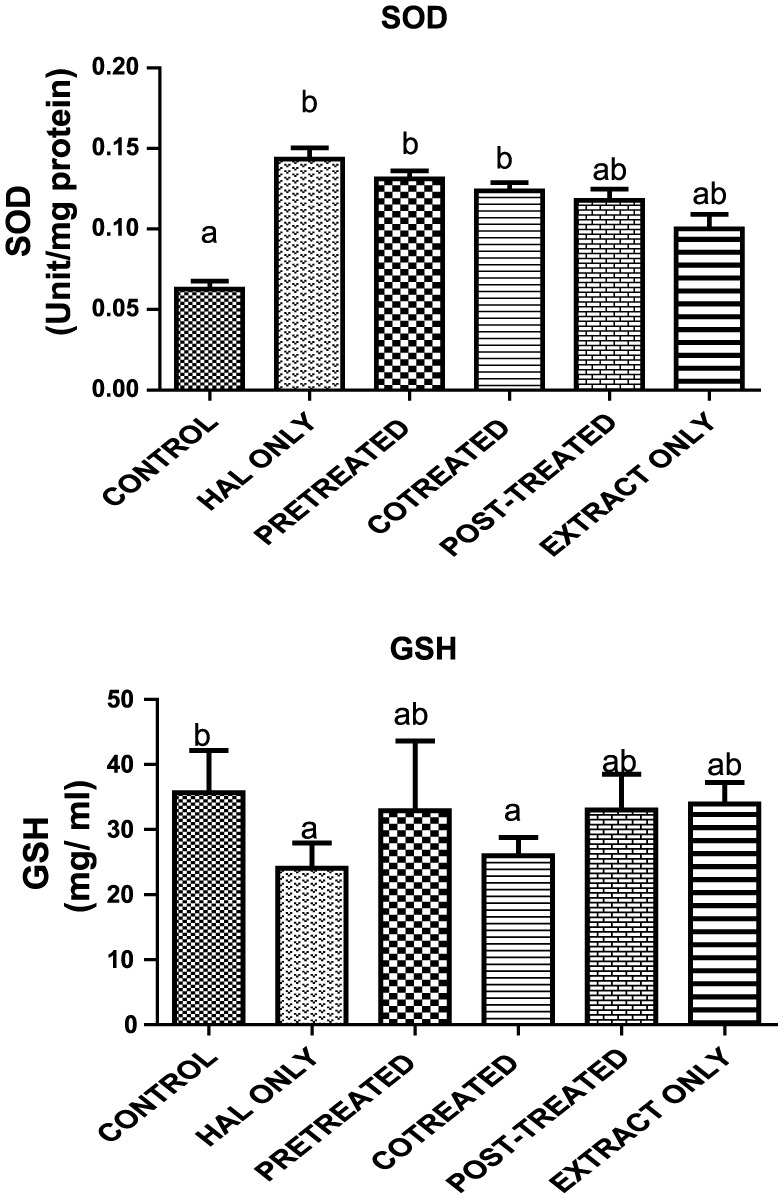
Reduced glutathione (GSH) level from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation; SOD, superoxide dismutase.
Figure 7.

Glutathione-S-transferase (GST) activity from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation; SOD, superoxide dismutase; CDNB, 1-Chloro-2,4-dinitrobenzene; GSH, glutathione.
Activities of lactate dehydrogenase and neuronal 5I nucleotidase
The HAL-only group had significantly decreased (p < 0.05) activity of LDH, a key marker linked to the production of adenosine triphosphate (ATP) in the cerebral cortex, by 36%, in relation to the control group (Figure 8). The treated groups were able to reverse this effect. Conversely, HAL-treated animals nonsignificantly (p > 0.05) increased the activity of neuronal 5I nucleotidase, a relevant marker of AMP and ATP hydrolysis, by 8% when compared with the control group (Figure 9). Pre- and cotreatment significantly (p < 0.05) prevented the elevation by 12% and 22%, respectively; while post-treatment significantly (p < 0.05) reversed the increase by 16%. Also, BSO only caused a significant decrease by 18% in ATP hydrolysis when compared with the control group.
Figure 8.

Lactate dehydrogenase (LDH) activity from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation.
Figure 9.

5I-nucleotidase activity from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation.
Biomarkers of neurological failure
The activity of AChE, a marker of neuronal loss, was significantly increased (p < 0.05) by 250% when compared with the control group after subacute treatment with HAL (Figure 10). Pre-, co- and post-treatment caused a nonsignificant decrease (p > 0.05) by 33%, 21% and 38%, respectively, against the HAL-induced group. Similarly, the activity of BuChE was elevated by 20% when compared with the control group after subacute administration with HAL (Figure 11). The considerable high activity of BuChE was remarkably downregulated (p < 0.05) on pre-, co-, and post-treatment with the oil by 58%, 31% and 38%, respectively, against the HAL-induced group. In the same way, the activity of MAO between control and HAL groups was similar: not statistically significant (Figure 12). The MAO activity was significantly lower (p < 0.05) in the pretreated group than in the HAL-only group. Generally, the study observed that extract oil post treatment is most efficacious in reversing HAL-induced neuronal damage in rat, followed by pre- and cotreatment, respectively.
Figure 10.

Acetylcholineesterase (AChE) activity from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation.
Figure 11.

Butrylcholineesterase (BuChE) activity from cerebral cortex in haloperidol-induced neuronal damage rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation.
Figure 12.

Monoamine oxidase (MAO) activity from cerebral cortex in haloperidol-induced neuronal-damaged rats treated with oil from black seeds. Data are presented as mean ± SD (n = 10). Bars with different letters are statistically different.
SD, standard deviation.
Histopathology examination on the neuronal cells
As shown in Figures 13, the control group, under light microscope, showed no visible lesions to the neurons or normal neuronal cells (NRCs). HAL-administered rats showed congestion of meningeal blood vessels (CMB). Also, few foci of mild vacuolation (spongiosis) of neurons (SPNs) were observed. The pretreated group showed no visible lesions to the neurons or NRCs. Similarly, the cotreated group revealed no visible lesions to the neurons or NRCs. Post-treated animals showed multiple foci of moderate vacuolation of neurons (MVN). Finally, the extract (oil) only depicted grey matter containing neurons, which were unremarkable, and moderate congestion of meningeal blood vessels (MCMB) were observed.
Figure 13.

Neuronal histopathology changes on rat administered with BSO in HAL-induced damage (original magnification × 400).
Control: there were no visible lesions to the neurons or normal neuronal cells (NRC); HAL only: showed congestion of meningeal blood vessels (CMB) and a few foci of mild vacuolation (spongiosis) of neurons (SPNs) were observed; Pretreated: there were no visible lesions to the neurons or NRC; Cotreated: there were no visible lesions to the neurons or NRC; Post-treated: showed multiple foci of moderate vacuolation of neurons (MVN); Extract only: the grey matter contained neurons which were unremarkable and moderate congestion of meningeal blood vessels (MCMB) were observed.
Discussion
The present study evaluates the differential effects of essential oil from black seed on markers of neuronal lesions (ectonucleotidase, MAOs and cholinesterase activities) from cerebrum of HAL-induced neuronal-damaged rats. As shown in Table 1, recent analysis of the oil revealed luteolin, gallic acid, catechin, caffeic acid, quercetin, chlorogenic acid, rutin, and orientin [Cuneyt et al. 2012; Wu-Yang et al. 2012]. Latest characterization also revealed that the major active components of the seed oil were thymoquinone and dithymoquinone, as shown in Figure 14 [Omar et al. 1999; Hala et al. 2006]. Phenolics and thymoquinone were reported to avert alterations in ectonucleotidase and cholinesterase activities in experimental rats in vivo [Anwar et al. 2013; Akinyemi et al. 2015]. As observed, there was a significant rise in MDA content after treatment with HAL. This result is in agreement with previous investigations, where administration of HAL elicited direct toxic effects on mammalian neurons and the striatum of the brain [Tost et al. 2010]. It was also reported that HAL downsized synapses, the junctions through which neurons communicate, and diminished in response to antipsychotic treatments in rat [Tost et al. 2010]. This outcome occurs due to the ability of HAL to produce neural reactive oxygen species (ROS), activated by myeloperoxidase at the site of inflammation [Taka et al. 2015]. The ROS damages the postsynaptic membrane and physical structure of the cerebrum, which is antecedent to cerebrovascular disease, cognitive impairment and vascular dementia [White et al. 2007; John and Oller, 2010]. However, differential psychotherapy of pre-, co- and postadministration with black-seed oil caused a significant reduction in HAL-induced rats. This is in agreement with Sanjeev and colleagues [Sanjeev et al. 2010], where they reported no visible cell membrane lesions in a group of animals treated with thymoquinone. The reversal effect is in line with the use of thymoquinone as antitumor and antipancreatic cancer [Yusufi et al. 2013; Mu et al. 2015].
Table 1.
Composition of black seed (Nigella sativa) aqueous extracts.
| Compounds | Nigella sativa (mg/g) | LOD (µg/ml) | LOQ (µg/ml) |
|---|---|---|---|
| Gallic acid | 2.46 ± 0.03a | 0.025 | 0.082 |
| Catechin | 0.27 ± 0.01b | 0.018 | 0.059 |
| Chorogenic acid | 2.81 ± 0.01c | 0.013 | 0.044 |
| Caffeic acid | 0.54 ± 0.01d | 0.009 | 0.030 |
| Orientin | 4.75 ± 0.02e | 0.011 | 0.037 |
| Rutin | 4.79 ± 0.01e | 0.024 | 0.081 |
| Quercitrin | ND | 0.028 | 0.093 |
| Quercetin | 0.86 ± 0.01f | 0.009 | 0.030 |
| Luteolin | 4.83 ± 0.01e | 0.026 | 0.085 |
LOD, limit of detection; LOQ, limit of quantification; ND, not detected.
The results are expressed as mean ± SEM of three determinations. Comparing various groups, different letters indicate statistically significant findings. a-f = P<0.05.
Figures 14(a) and 14(b).

Chemical structure of active ingredients of Nigella sativa essential oil, Hala et al. [2006].
Neurodegenerative diseases are CNS disorders characterized by the progressive loss of neuronal tissues. Studies have observed that the brain is sensitive to oxidative damage because of its high oxygen utilization, comparatively low levels of antioxidant-defence proteins [Muriach et al. 2014; Akintunde and Oboh, 2015], and extremely high content of polyunsaturated lipids that are easily oxidized [Lobo et al. 2010; Bente and Rune, 2011]. However, examining cerebral antioxidant molecules has been one of the tremendous discoveries in diagnosing neuropathy of AD, which could be helpful to slow down the progression [Birkhofer et al. 2013]. As discovered in this study, only the antioxidant enzyme SOD was altered in the cerebral rats followed by depleted, reduced GSH level after administration with a neuroleptic drug (HAL). This could be attributed to the loss of dopaminergic neurons that precede the GSH depletion and linked to pathophysiological mechanisms of PDs [Murata et al. 2008; Gu et al. 2013]. Reduced alteration of HAL on both enzymatic and nonenzymatic antioxidants reflected results from other studies that reported free radicals were directly or indirectly implicated in the pathogenesis of several neurodegenerative diseases such as AD, PD, and amyotrophic lateral sclerosis [Kong and Lin, 2010; Butterfield et al. 2012]. The ameliorating effects of BSO on the pre-, co- and post-treated groups were as a result of its free-radical prophylactic action. Significantly, the effect was connected to the synergy of phenolic compounds and thymoquinone from black-seed oil that had been recognized as a mediator against free radicals and antioxidants, safeguarding neuronal loss [Yusufi et al. 2013]. So, this investigation clearly demonstrates that phenolic–rich oil reverses the brain-related dysfunctions via the various techniques described and perpetrates potent antioxidative effects in the cerebrum.
The proteomics approach identified that numbers of proteins are excessively nitrated in the brain of AD which includes alpha- and gamma-enolase, LDH, neuropolypeptide h3 and triose phosphate isomerase [Sultana et al. 2013]. Studies have also reported that nitration of proteins was connected to loss of energy-metabolism maintenance, an indicator of AD [Poon et al. 2005; Chinta and Andersen, 2011]. This clearly indicates that HAL possesses the ability to facilitate nitration of LDH with endogenous amines. This is in agreement with Barber and Shaw, when they reported lactate dehydrogenase 2 (LDH2) as carbonylated proteins with reduced activity in the brainstem of symptomatic mice [Barber and Shaw, 2010]. The observed fall in HAL-induced rats was linked to the low expression of the LDHA gene [Sekido and Lovell-Badge, 2013]. Therefore, this could lead to a reduction of extracellular ATP in the synaptic cleft, which may impair the purinergic signaling, since it reduces the availability of extracellular ATP. On the contrary, pre-, co- and post-administration of black-seed oil notably elevated LDH activity. Similarly, the phenolic compounds present in the extract oil could be responsible for the increased LDH activity. Phenolic compounds such as curcumin, quercetin, tannic acid and others have been reported to exhibit spontaneous deamination of nitrated proteins in rats [Cuneyt et al. 2012; Wu-Yang et al. 2012].
Furthermore, modulation of the enzyme ectonucleotidase from the purinergic system and cholinesterases from the cholinergic system could be a useful approach in the management or prevention of cerebrovascular diseases. ATP is an endogenous cellular component of the energy pathway involved in various groups of functions in the CNS [Burnstock and Pelleg, 2015]. Also, ATP acts as a fast neurotransmitter, cotransmitter in autonomic and sensory nerves and as a presynaptic modulator [Burnstock and Pelleg, 2015]. Moreover, our results showed a nonsignificant increase in 5I-nucleotidase activity in the cerebrum of HAL-induced damaged rats. Nonsignificant difference may be linked to the fact that HAL administration was short-lived. This observation corroborated recent studies that demonstrated the potential toxic effects of drugs were linked to prolonged typical neuroleptic administration [Samad, 2015; Levine et al. 2011; Levine and Ruha, 2012]. This still suggests that the activity of 5I-nucleotidase essentially causes the production of extracellular adenosine [Burnstock, 2006; Burnstock and Pelleg, 2015]. The 5I-nuleotidase is one of the major enzymes in the regulation of extracellular levels of adenosine in the synaptic cleft. An increase of 5I-nucleotidase activity increases the adenosine formation by hydrolysing AMP to adenosine. Thus, the effect of administering HAL on this enzyme, though not statistically different, may lead to an increased removal of extracellular adenosine, decreasing ATP levels, which may lead to impairment of the adenosinergic neurotransmission. However, pre-, co- and post-treatment with black-seed oil prevented this alteration, thus, decreasing extracellular level of adenosine in the synaptic cleft. A recent study reported that adenosine in the brain modulates neuronal and synaptic activity, as well as regulating mental disorder [Burnstock and Pelleg, 2015]. Hence, the reduction on the ATP levels can weaken the neuronal Long-Term Synaptic Potentiation (LTP), thereby leading to memory loss [Lovinger, 2010].
The cholinergic system is responsible for normal functioning of both the CNS and peripheral nervous system [Baldissarelli et al. 2016]. Acetylcholine (ACh) is the key neurotransmitter of the cholinergic system, playing vital roles such as learning, memory, movement control, and modulation of cerebral blood circulation [Lovinger, 2010]. The levels of AChs are controlled by cholinesterases capable of hydrolyzing the neurotransmitters in several tissues. Our results demonstrated an increase in the activity of this enzyme in the cerebrum in HAL-induced rats. It is predicted that the increased AChE activity in synapses of HAL-induced rats could lead to sudden degradation of AChs; and subsequent reduced stimulation of ACh receptors would cause an undesirable effect on cholinergic neurotransmission, as well as progressive cognitive impairment and other neurological dysfunctions in prescribed patients [Lovinger, 2010]. However, pre-, co- and post-treatment with black-seed oil nonsignificantly prevented the increase in AChE activity of HAL-induced rats. Nonsignificant difference may be linked to the fact that the oil dose used was not enough to cause the significant protection. This, however, suggests that AchE-receptor expression was still regulated after BSO treatment. It also indicates that ACh levels in the synaptic cleft were increased, thereby enhancing the cognitive functions, such as learning and memory [Lovinger, 2010]. This finding suggests an endogenous interaction between phytochemicals of oil from black seed and the cholinergic system. Clinically, the action of the black-seed oil provides a possible underlying mechanism in combating neurological disorders and brain tumors.
BuChE was reported as the principal enzyme in the hydrolysis of butyrylcholine (BuCh) [Akintunde and Oboh, 2015]. A recent study showed that the marked reduction of this neurotransmitter in the cerebrum was implicated in AD [Akintunde and Oboh, 2015]. As observed in the study, there was a considerable high activity of BuChE in rat cerebrum induced by HAL. This corroborates the finding of Eubanks and colleagues [Eubanks et al. 2006] who reported that high activity of BuChE was discovered among patients suffering from AD. Nerve impulses are transmitted from nerve cell to nerve cell or through involuntary muscles by BuCh. At the cholinergic synapses, BuChE breaks down BuCh into choline and butyrate. BuChE therefore regulates nerve-impulse transmission across cholinergic synapses [Pecic et al. 2011]. Inhibition of BuChE was one of the hopeful strategies for the treatment of neurological disorders such as AD, senile dementia, ataxia and myasthenia gravis [Anand et al. 2012]. Our study showed significant prevention of increased BuChE following pre-, co- and post-treatment with black-seed oil. The decrease could be traced to the phytochemicals present in the oil, especially thymoquinone and dithymoquinone, which have the potential to restore and decline cognitive-function loss among individuals with progression of AD. Also, several essential oils and their monoterpenes, as well as eugenols, were potent BuChE inhibitors [Cuneyt et al. 2012; Wu-Yang et al. 2012].
MAOs from the dopaminergic system belong to the protein family of flavin-containing amine oxidoreductases that catalyze the oxidation of monoamines. They are abundant enzymes in the brain and effective in the treatment of patients with depression, panic disorder, and other anxiety disorders [Stahl and Felker, 2008]. Also, their inhibition depicts the pharmacological basis for neuropathy prevention [Shuanglin, 2013]. As we observed in this study, oral administration of HAL did not cause the removal of the neurotransmitters (norepinephrine, serotonin and dopamine). This may be linked to the period of (subacute) exposure. This investigation was in line with previous studies showing that high activity of MAO was responsible for psychiatric and neurological disorders, particularly during a prolonged exposure to pro-oxidants [Thase, 2012; Shulman et al. 2013; Akintunde and Oboh, 2015]. In addition, the highest activity of MAO was found in people with high loss of memory and those with a brain tumor [Shulman et al. 2013]. In our study, results revealed that only pretreatment with black-seed oil caused a significant reduction in cerebral MAO activity while co- and post-treatments were not effectual. The fall in MAO activity was linked to the active component, thymoquinone, and the synergistic effect of other phenolic compounds such as caffeic acid, gallic acid, quercetin, curcumin and others present in the oil [Abukhader, 2013; Forouzanfar et al. 2014; Darakhshan et al. 2015].
Pathologically, animal groups that were therapeutically administered with HAL displayed MCMB with few foci of mild vacuolation SPNs. These abnormities were linked to incidence of necrosis of the Purkinje cells [Mouchira and Mohi, 2010]. This discovery is line with recent advances reporting that damage to neuronal cells has an essential function in pathogenesis of the brain [Puente et al. 2010; Akintunde and Oboh, 2015]. Also, another author reported that considerable damage to the neurons impairs cognitive behavior and memory dysfunction [Rubinstein et al. 2015]. This also suggests that expression of gene-encoding neurotransmitters is mutated [Kurian et al. 2011; Hansen et al. 2014]. The group of animal pre- and cotreated with black-seed oil showed no visible lesions to the neurons. The failure of post-treatment to wholly reverse the toxic effects of HAL indicates its prolonged lesions to neuronal cells. Generally, essential oil post-treatment is the most efficacious in preventing HAL-induced neuronal damage in rat; followed by pre- and cotreatment, respectively.
Conclusion
The results obtained in the study demonstrate alterations in both enzymatic and nonenzymatic antioxidants, LDH, 51-nucleotidase, BuChE, AChE and MAO activities with concomitant increase in lipid peroxidation from the cerebrum of HAL-induced damaged rats. This indicates that dopaminergic, purinergic and cholinergic neurotransmissions are remarkably compromised in HAL-induced rats. Conversely, differential treatments with black-seed oil prevented and reversed these alterations by decreasing BuChE, AChE and MAO activities, and AMP hydrolysis with increased ATP in the synaptic cleft of rat cerebrum. Since pre-, co- and post-treatment prevented the alterations induced by HAL, it can be assumed that essential oil from black seed exerts neuroprotective potential, which was attributed to the phenolic compounds, particularly the active molecules known as thymoquinone and dithymoquinone. We then recommend from our laboratory that pre-, co- and post-treatment from black-seed oil provides possible care for people suffering from cerebrovascular dysfunctions and neurological diseases associated with antipsychotic drugs.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Jacob K. Akintunde, Toxicology and Safety Unit, Department of Environmental Health Sciences, Faculty of Public Health, College of Medicine, University of Ibadan, Ibadan, 200284-23402, Nigeria.
C. Abigail Irechukwu, Biochemistry Unit, Department of Biosciences and Biotechnology, College of Pure and Applied Sciences, Kwara State University, P.M.B 1530, Malete, Nigeria.
References
- Abukhader M. (2013) Thymoquinone in the clinical treatment of cancer: Fact or fiction? Pharmacogn Rev 7: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akintunde J., Oboh G. (2015) Sub-chronic exposure to leachate activates key markers linked with neurological disorder in Wistar male rat. Environ Sci Pollut Res 22: 18541–18553. [DOI] [PubMed] [Google Scholar]
- Akinyemi J., Gustavo R., Vera M., Naiara S, Pauline da C, Andréia C., et al. (2015) Effect of dietary supplementation of ginger and turmeric rhizomes on ectonucleotidases, adenosine deaminase and acetylcholinesterase activities in synaptosomes from the cerebral cortex of hypertensive rats. J Appl Biomed 6 May 2016. [Epub ahead of print] [Google Scholar]
- Anand P., Singh B., Singh N. (2012) A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg Med Chem 20: 1175–1180. [DOI] [PubMed] [Google Scholar]
- Anwar J., Spanevello R., Pimentel V., Gutierres J., Thomé G., Cardoso A., et al. (2013) Caffeic acid treatment alters the extracellular adenine nucleotide hydrolysis in platelets and lymphocytes of adult rats. Food Chem Toxicol 56: 459–466. [DOI] [PubMed] [Google Scholar]
- Baldissarelli J., Santi A., Schmatz R., Abdalla F., Cardoso A., Martins C., et al. (2016) Hypothyroidism enhanced ectonucleotidases and acetylcholinesterase activities in rat synaptosomes can be prevented by the naturally occurring polyphenol quercetin. Cell Mol Neurobiol 16 February 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber S., Shaw P. (2010) Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med 48: 629–641. [DOI] [PubMed] [Google Scholar]
- Bente L., Rune B. (2011) Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr Res 55 DOI: 10.3402/fnr.v55i0.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhofer A., Geissendoerfer J., Alger P., Mueller A., Rentrop M., Strubel T., et al. (2013) The deceleration capacity-a new measure of heart rate variability evaluated in patients with schizophrenia and anti-psychotic treatment Eur Psychia 28: 81–86. [DOI] [PubMed] [Google Scholar]
- Boligon A., Piana M., Kubiça T., Mario D., Dalmolin T., Bonez P., et al. (2015) HPLC analysis and antimicrobial, antimycobacterial and antiviral activities of Tabernaemontana catharinensis A. DC. J Appl Biomed 13: 7–18. [Google Scholar]
- Brayfield A. (2013) Haloperidol Martindale: The Complete Drug Reference. London: Pharmaceutical Press, pp; 229–230. [Google Scholar]
- Burnstock G. (2006) Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 27: 166–176. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Pelleg A. (2015) Cardiac purinergic signalling in health and disease. Puriner Signal 11: 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield D., Perluigi M., Reed T., Muharib T., Hughes C., Robinson R., et al. (2012) Redox proteomics in selected neurodegenerative disorders: From its infancy to future applications. Antioxid Redox Signal 17: 1610–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta S., Andersen J. (2011) Nitrosylation and nitration of mitochondrial complex I in Parkinson’s disease. Free Radic Res 45: 53–58. [DOI] [PubMed] [Google Scholar]
- Cieza A., Anczewska M., Ayuso-Mateos J., Baker M., Bickenbach J., Chatterji S. (2015) Understanding the impact of brain disorders: towards a ‘horizontal epidemiology’ of psychosocial difficulties and their determinants. PLoS One 10: e0136271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citrome L. (2011) Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation anti-psychotic. Int J Clin Pract 65: 189–210. [DOI] [PubMed] [Google Scholar]
- Clairborne A. (1995) Catalase activity. In: Greewald A. (ed), Handbook of methods for oxygen radical research. Florida: CRC Press, pp; 237–242. [Google Scholar]
- Cuneyt D., Ayhan T., Hilal S., Kubra S., Ihsan B., Ismail T., et al. (2012). A comparative study on phenolic composition, antioxidant activity and essential oil content of wild and cultivated sage (Salvia fruticosa Miller) as influenced by storage. Industr Crops and Prod 39: 170–176. [Google Scholar]
- Darakhshan S., Bidmeshki P., Hosseinzadeh C., Sisakhtnezhad S. (2015) Thymoquinone and its therapeutic potentials. Pharmacol Res 95–96: 138–158. [DOI] [PubMed] [Google Scholar]
- Eubanks L., Rogers C., Berscher A., Koob G., Olson A., et al. (2006). A molecular link between the active component of marijuana and Alzheimer’s disease. Pathology Mol Pharm 3: 773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar F., Bazzaz B., Hosseinzadeh H. (2014) Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects. Iran J Basic Med Sci 17: 929–38. [PMC free article] [PubMed] [Google Scholar]
- Goikolea J., Colom F., Torres I., Capapey J., Valentí M., Undurraga J., et al. (2013) Lower rate of depressive switch following antimanic treatment with second-generation anti-psychotics versus haloperidol. J Affect Disor 144: 191–198. [DOI] [PubMed] [Google Scholar]
- Gu F., Chauhan V., Chauhan A. (2013). Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: alterations in the activities and protein expression of glutathione-related enzymes. Free Radic Biol Med 65: 488. [DOI] [PubMed] [Google Scholar]
- Gupta A., Lawrence A., Krishnan K. (2007). Current concepts in the mechanisms and management of drug-induced QT prolongation and torsades de pointes. Am Heart J 153: 891–899. [DOI] [PubMed] [Google Scholar]
- Habig W., Pabst M., Jacoby W. (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biochem 249: 7130–7139. [PubMed] [Google Scholar]
- Hala G., Nahed E., Regine S. (2006) The medicinal potential of black seed (Nigella sativa) and its components. Recent Advan Phytochem 2: 133–153. [Google Scholar]
- Hansen F., Skjorringe T., Yasmeen S., Arends N., Sahai M., Erreger K. (2014) Missense dopamine transporter mutations associate with adult Parkinsonism and ADHD. See comment in PubMed Commons belowJ Clin Invest 124: 3107–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D., Reddington M., Kreutzberg G. (1984) Sub-cellular localization of 5I-nucleotidase in rat brain. J Neurochem 43: 971–978. [DOI] [PubMed] [Google Scholar]
- John W., Oller J. (2010) The antithesis of entropy: biosemiotic communication from genetics to human language with special emphasis on the immune systems entropy. Entropy (Basel) 12: 631–705. [Google Scholar]
- Johnson K., Minoshima S., Bohnen N. (2013) Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J Nucl Med 54: 476–490. [DOI] [PubMed] [Google Scholar]
- Jollow D., Mitchell J., Zampaglione N., Gillette J. (1974). Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacol 11: 151–169. [DOI] [PubMed] [Google Scholar]
- Kettler R., Da Prada M., Burkard W. (1990) Comparison of mono-amine oxidase-A inhibition by moclobemide in vitro and ex vivo in rats. Acta Psychiatr Scand 82: 101–102. [DOI] [PubMed] [Google Scholar]
- Kong Q., Lin C. (2010) Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci 67: 1817–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian M., Li Y., Zhen J., Meyer E., Hai N., Christen H. (2011) Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol 10: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., LoVecchio F., Tafoya P. (2011) Paliperidone overdose with delayed onset of toxicity. Ann Emerg Med 58: 80–92. [DOI] [PubMed] [Google Scholar]
- Levine M., Ruha A. (2012) Overdose of atypical anti-psychotics clinical presentation, mechanisms of toxicity and management. CNS Drugs 26: 601–611. [DOI] [PubMed] [Google Scholar]
- Lobo V., Patil A., Phatak A., Chandra N. (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger D. (2010) Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharm 58: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O., Rosenbrough N., Farr A., Randall R. (1951) Protein measurement with folin phenol reagent. Biol Chem 193: 265. [PubMed] [Google Scholar]
- Lyons K., Pahwa R. (2011) The impact and management of nonmotor symptoms of Parkinson’s disease. Am J Manag Care 17: S308–314. [PubMed] [Google Scholar]
- Misra H., Fridovich I. (1989) The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay of superoxide dismutase. Toxicol Biol Chem 2417: 3170. [PubMed] [Google Scholar]
- See comment in PubMed Commons belowMouchira M., Mohi E. (2010) The significance subarachnoid cerebrosipinal fluids (CSF) in the development of metacestode of cereralis in sheep with references to its pathological effect. Global Veter 4: 343–348. [Google Scholar]
- Mu G., Zhang L., Li H., Liao Y., Yu H. (2015) Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig Dis Sci 60: 1067–1080. See comment in PubMed Commons below [DOI] [PubMed] [Google Scholar]
- Murata T., Ohtsuka C., Terayama Y. (2008). Increased mitochondrial oxidative damage and oxidative DNA damage contributes to the neurodegenerative process in sporadic amyotrophic lateral sclerosis. Free Radic Res 42: 221–225. [DOI] [PubMed] [Google Scholar]
- Muriach M., Flores-Belver M., Romero F., Barcia J. (2014) Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev 2014: 102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na-Ri K., Moon-Doo K. (2011) Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboh G, Ademiluyi A., Akinyemi A. (2012a) Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale). Exp Toxicol Pathol 64: 315–319. [DOI] [PubMed] [Google Scholar]
- Oboh G., Ademiluyi A., Akinyemi A., Saliu J., Henle T., Schwarzenbolz U. (2012b) Inhibitory effect of polyphenol-rich extracts of Jute leaf (Corchorus olitorius) on key enzyme linked to type-2 diabetes (α-amylase and α-glucosidase) and hypertension (Angiotensin I- converting) in vitro. J Funct Foods 4: 450–458. [Google Scholar]
- Oboh G., Ogunruku O. (2010) Cyclophosphamide induced oxidative stress in brain: protective effect of hot short pepper (Capsicum frutescens L Var. abbreviatum). Exp Toxicol Pathol 62: 227–233. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358. [DOI] [PubMed] [Google Scholar]
- Olesen J., Gustavsson A., Svensson M., Wittchen H., Jönsson B. (2012) The economic cost of brain disorders in Europe. Eur J Neurol 19: 155–162. [DOI] [PubMed] [Google Scholar]
- Omar A., Abdulghani A., Peter A. (1999) High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa). J Pharm Biomed Anal 19: 757–762. [DOI] [PubMed] [Google Scholar]
- Poon H., Frasier M., Shreve N., Calabrese V., Wolozin B., Butterfield D. (2005). Mitochondrial associated metabolic proteins are selectively oxidized in A30P alpha-synuclein transgenic mice: a model of familial Parkinson’s disease. Neurobiol Dis 18: 492–498. [DOI] [PubMed] [Google Scholar]
- See comment in PubMed Commons below Pecic S., McAnuff M., Harding W. (2011) Nantenine as an acetylcholinesterase inhibitor: SAR, enzyme kinetics and molecular modeling investigations. J Enzyme Inhib Med Chem 26: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry N., Houghton P., Theobal D., Jenner P., Perry E. (2000) In vitro activity of S. lavan-dulaefolia (Spanish sage) relevant to treatment of Alzheimer’s disease. J Pharm Pharmacol 52: 895–902. [DOI] [PubMed] [Google Scholar]
- Puente E., Silverstein J., Bree A., Musikantow D., Wozniak D., Maloney S., et al. (2010) Recurrent moderate hypoglycemia ameliorates brain damage and cognitive dysfunction induced by severe hypoglycaemia. Diabetes 59: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi A., Leonardi M. (2015) Burden and cost of neurological diseases: a European North-South comparison. Acta Neurol Scand 132: 16–22. [DOI] [PubMed] [Google Scholar]
- Reis E., Neto F., Cattani V., Peroza L., Busanello A., Leal C., et al. (2014) Antidepressant-like effect of Ilex paraguariensis in rats. Biomed Res Inter 2014: Article ID 958209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C., Tabrizi S. (2011) Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 10: 83–98. [DOI] [PubMed] [Google Scholar]
- Rubinstein M., Westenbroek R., Yu F., Jones C., Scheuer T., Catterall W. (2015) Genetic background modulates impaired excitability of inhibitory neurons in a mouse model of Dravet syndrome. Neurobiol Dis 73: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad N. (2015) Rice bran oil prevents neuroleptic-induced extrapyramidal symptoms in rats: possible antioxidant mechanisms. J Food and Drug Anal 23: 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjeev B., Asfar S., Subhash P., Marjit W., Jubaraj B., Philip A., et al. (2010) Structure-activity studies on therapeutic potential of thymoquinone analogs in pancreatic cancer. Pharm Res 27: 1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R., Lovell-Badge R. (2013). Genetic control of testis development. Sex Dev 7: 21–32. [DOI] [PubMed] [Google Scholar]
- Shuanglin H. (2013) The molecular and pharmacological mechanisms of HIV-related neuropathic pain. Curr Neuropharmacol 11: 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman K., Herrmann N., Walker S. (2013) Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 27: 789–797. [DOI] [PubMed] [Google Scholar]
- Stahl S., Felker A. (2008) Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr 13: 855–870. [DOI] [PubMed] [Google Scholar]
- Sultana R., Cenini G., Butterfield A. (2013) Biomarkers of oxidative stress in neurodegenerative diseases. In: Villamena F. (ed.), Molecular Basis of Oxidative Stress: Chemistry, Mechanisms, and Disease Pathogenesis. Online publication: John Wiley & Sons, Inc; 28 June 2013. [Google Scholar]
- Taka E., Mazzio E., Goodman C., Redmon N., Flores-Rozas H., Reams R., et al. (2015) Anti-inflammatory effects of thymoquinone in activated BV-2 microglial cells. J Neuroimmunol 286: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase M. (2012) MAOIs and depression treatment guidelines. J Clin Psychiatry 73: e24. [DOI] [PubMed] [Google Scholar]
- Todd J. (2012) Parkinson’s disease and sleep/wake disturbances. Parkinsons Dis 15: 8. [Google Scholar]
- Toshi A., Stephen Z., Shiro T., Yair G., Myrto S., John M., et al. (2014) Initial severity of schizophrenia and efficacy of anti-psychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA Psychiat 72: 14. [DOI] [PubMed] [Google Scholar]
- Tost H., Maxmen A., Wehtje J. (2010) Anti-psychotic deflates the brain: Nature news. Natur Neurosc 13: 920–922. [Google Scholar]
- Valenti O., Cifelli P., Gill K., Grace A. (2011) Anti-psychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosc 31: 12330–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. (2008) Neurology of the Newborn (5th ed). Saunders, vol. 1, pp. 53–54. [Google Scholar]
- Wang Y., Bei Z., Yong X., Zhao-Jie L., Jing-Feng W., Chang-Hu X. (2010) The mechanism of dietary cholesterol effects on lipids metabolism in rats. Lipids Health Dis 9: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar H., Gossrau E., Faderl B. (1975) [Normal ranges of alpha-HBDH, LDH, AP, and LAP as measured with substrate-optimated test charges]. Med Welt 26: 387–392. [PubMed] [Google Scholar]
- White L., Cory-Slechta D., Gilbert M., Tiffany-Castiglioni E., Zawia N., Virgolini M. (2007) New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharm 225: 1–27. [DOI] [PubMed] [Google Scholar]
- Wu-Yang H., Hong-Cheng Z., Wen-Xu L., Chun-Yang L. (2012) Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J Zhejiang Univ Sci B 13: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohko I., Chiaki K., Ikuko K., Taku F., Mami F., Chie I., et al. (2012) Dose-dependent effect of anti-psychotic drugs on autonomic nervous system activity in schizophrenia. BMC Psychiat 12: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufi M., Banerjee S., Mohammad M., Khatal S., Venkateswara S., Khan E., et al. (2013) Synthesis, characterization and anti-tumor activity of novel thymoquinone analogs against pancreatic cancer. Bio Org Med Chem Lett 23: 3101–3104. [DOI] [PubMed] [Google Scholar]
- Zar J. (1984) Biostatistical analysis. Englewood Cliffs, NJ: Prentice-Hall International. [Google Scholar]