Abstract
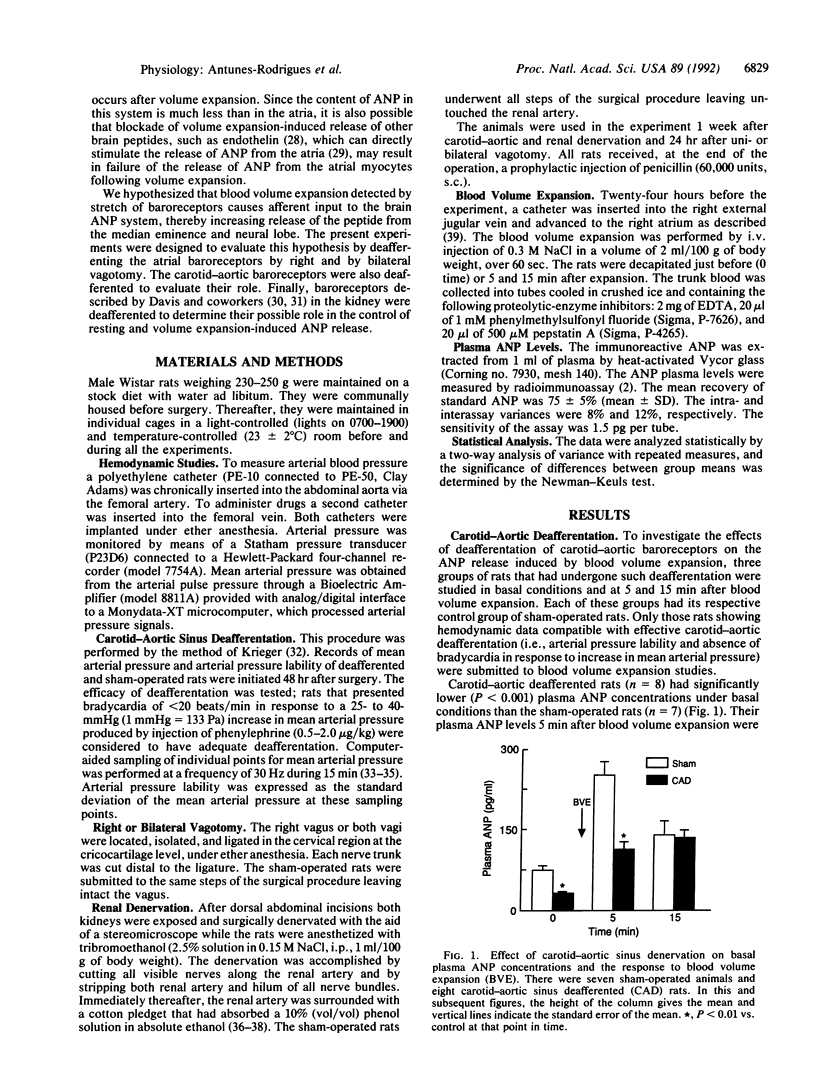
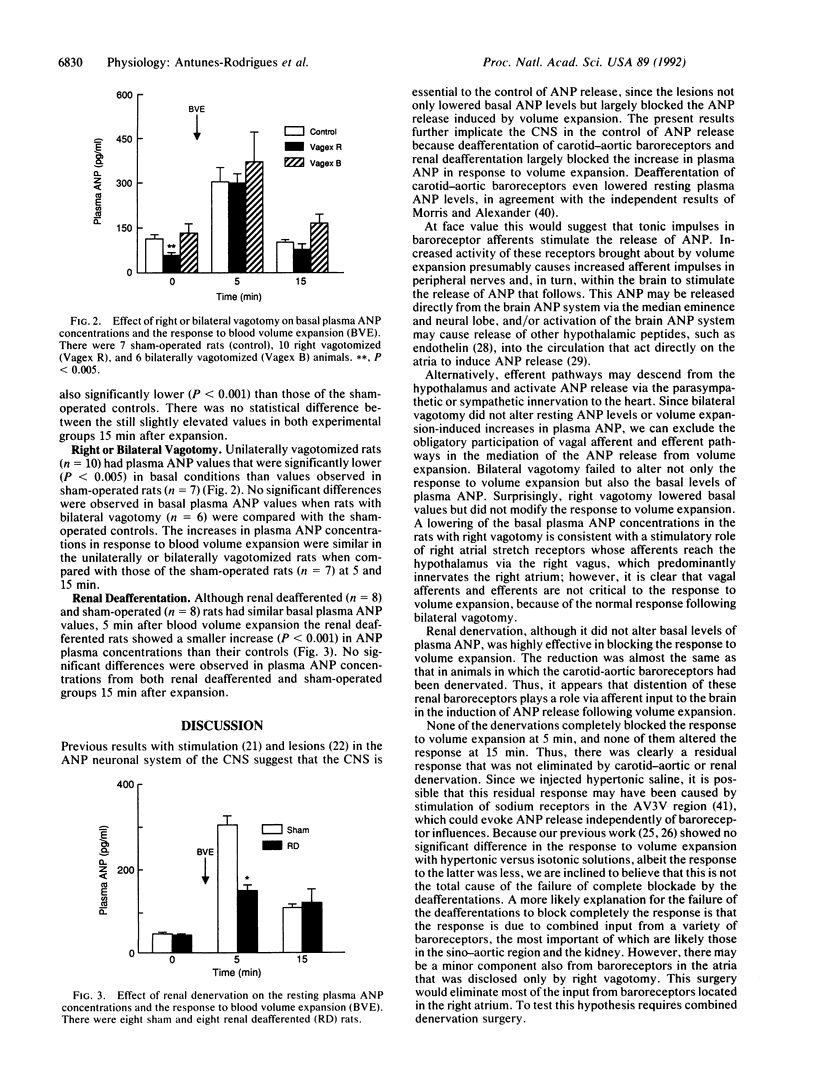
Our previous studies have shown that stimulation of the anteroventral third ventricle (AV3V) region of the brain increases atrial natriuretic peptide (ANP) release, whereas lesions of the AV3V region or median eminence of the tuber cinereum block the release of ANP caused by blood volume expansion. These results suggest that participation of the central nervous system is critical to this response. The role of baroreceptors in the response was evaluated in the current research by studying the response of plasma ANP to blood volume expansion induced by intravenous injection of hypertonic saline solution (0.3 M NaCl, 2 ml/100 g of body weight, over 1 min) in conscious, freely moving male rats. Plasma samples were assayed for ANP by radioimmunoassay. In sham-operated rats, blood volume expansion induced a rapid increase in plasma ANP: the concentration peaked at 5 min and remained elevated at 15 min after saline injection. One week after deafferentation of the carotid-aortic baroreceptors, basal plasma ANP concentrations were highly significantly decreased on comparison with values of sham-operated rats; plasma ANP levels 5 min after blood volume expansion in the deafferented rats were greatly reduced. Unilateral right vagotomy reduced resting levels of plasma ANP but not the response to blood volume expansion; resting concentrations of plasma ANP and responses to expansion were normal in bilaterally vagotomized rats. In rats that had undergone renal deafferentation, resting levels of ANP were normal but the response to blood volume expansion was significantly suppressed. The evidence indicates that afferent impulses via the right vagus nerve may be important under basal conditions, but they are not required for the ANP release induced by blood volume expansion. In contrast, baroreceptor impulses from the carotid-aortic sinus regions and the kidney are important pathways involved in the neuroendocrine control of ANP release. The evidence from these experiments and our previous stimulation and lesion studies indicates that the ANP release in response to volume expansion is mediated by afferent baroreceptor input to the AV3V region, which mediates the increased ANP release via activation of the hypothalamic ANP neuronal system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. P. Structure and biologic properties of the atrial natriuretic peptides. Endocrinol Metab Clin North Am. 1987 Mar;16(1):1–17. [PubMed] [Google Scholar]
- Alper R. H., Jacob H. J., Brody M. J. Central and peripheral mechanisms of arterial pressure lability following baroreceptor denervation. Can J Physiol Pharmacol. 1987 Aug;65(8):1615–1618. doi: 10.1139/y87-253. [DOI] [PubMed] [Google Scholar]
- Andersson B., Jobin M., Olsson K. Stimulation of urinary salt excretion following injections of hypertonic NaCl-solution into the 3rd brain ventricle. Acta Physiol Scand. 1966 May;67(1):127–128. doi: 10.1111/j.1748-1716.1966.tb03293.x. [DOI] [PubMed] [Google Scholar]
- Andersson B. Regulation of body fluids. Annu Rev Physiol. 1977;39:185–200. doi: 10.1146/annurev.ph.39.030177.001153. [DOI] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., McCann S. M., Rogers L. C., Samson W. K. Atrial natriuretic factor inhibits dehydration- and angiotensin II-induced water intake in the conscious, unrestrained rat. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8720–8723. doi: 10.1073/pnas.82.24.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., McCann S. M., Samson W. K. Central administration of atrial natriuretic factor inhibits saline preference in the rat. Endocrinology. 1986 Apr;118(4):1726–1728. doi: 10.1210/endo-118-4-1726. [DOI] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., Ramalho M. J., Reis L. C., Menani J. V., Turrin M. Q., Gutkowska J., McCann S. M. Lesions of the hypothalamus and pituitary inhibit volume-expansion-induced release of atrial natriuretic peptide. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2956–2960. doi: 10.1073/pnas.88.7.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., Turrin M. Q., Gutkowska J., McCann S. M. Blockade of volume expansion-induced release of atrial natriuretic peptide by median eminence lesions in the rat. Braz J Med Biol Res. 1990;23(3-4):355–359. [PubMed] [Google Scholar]
- Baldissera S., Menani J. W., dos Santos L. F., Favaretto A. L., Gutkowska J., Turrin M. Q., McCann S. M., Antunes-Rodrigues J. Role of the hypothalamus in the control of atrial natriuretic peptide release. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9621–9625. doi: 10.1073/pnas.86.23.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bealer S. L., Haywood J. R., Gruber K. A., Buckalew V. M., Jr, Fink G. D., Brody M. J., Johnson A. K. Preoptic-hypothalamic periventricular lesions reduce natriuresis to volume expansion. Am J Physiol. 1983 Jan;244(1):R51–R57. doi: 10.1152/ajpregu.1983.244.1.R51. [DOI] [PubMed] [Google Scholar]
- Bell-Reuss E., Trevino D. L., Gottschalk C. W. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Invest. 1976 Apr;57(4):1104–1107. doi: 10.1172/JCI108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine E. H., Davis J. O., Prewitt R. L. Evidence for a renal vascular receptor in control of renin secretion. Am J Physiol. 1971 Jun;220(6):1593–1597. doi: 10.1152/ajplegacy.1971.220.6.1593. [DOI] [PubMed] [Google Scholar]
- Cogan M. G. Atrial natriuretic factor can increase renal solute excretion primarily by raising glomerular filtration. Am J Physiol. 1986 Apr;250(4 Pt 2):F710–F714. doi: 10.1152/ajprenal.1986.250.4.F710. [DOI] [PubMed] [Google Scholar]
- Dorn J., Antunes-Rodrigues J., McCann S. M. Natriuresis in the rat following intraventricular carbachol. Am J Physiol. 1970 Nov;219(5):1292–1298. doi: 10.1152/ajplegacy.1970.219.5.1292. [DOI] [PubMed] [Google Scholar]
- Dorn J., Porter J. C. Diencephalic involvement in sodium excretion in the rat. Endocrinology. 1970 May;86(5):1112–1117. doi: 10.1210/endo-86-5-1112. [DOI] [PubMed] [Google Scholar]
- Epstein M., Loutzenhiser R., Friedland E., Aceto R. M., Camargo M. J., Atlas S. A. Relationship of increased plasma atrial natriuretic factor and renal sodium handling during immersion-induced central hypervolemia in normal humans. J Clin Invest. 1987 Mar;79(3):738–745. doi: 10.1172/JCI112879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest J., Cantin M. The atrial natriuretic factor: its physiology and biochemistry. Rev Physiol Biochem Pharmacol. 1988;110:1–145. doi: 10.1007/BFb0027530. [DOI] [PubMed] [Google Scholar]
- Gutkowska J., Horký K., Thibault G., Januszewicz P., Cantin M., Genest J. Atrial natriuretic factor is a circulating hormone. Biochem Biophys Res Commun. 1984 Nov 30;125(1):315–323. doi: 10.1016/s0006-291x(84)80370-8. [DOI] [PubMed] [Google Scholar]
- Gutkowska J., Racz K., Debinski W., Thibault G., Garcia R., Kuchel O., Cantin M., Genest J. An atrial natriuretic factor-like activity in rat posterior hypophysis. Peptides. 1987 May-Jun;8(3):461–465. doi: 10.1016/0196-9781(87)90010-6. [DOI] [PubMed] [Google Scholar]
- Harms P. G., Ojeda S. R. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol. 1974 Mar;36(3):391–392. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Lewicki J., Johnson L. K., Cogan M. G. Renal mechanism of action of rat atrial natriuretic factor. J Clin Invest. 1985 Feb;75(2):769–773. doi: 10.1172/JCI111759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. J., Alper R. H., Brody M. J. Lability of arterial pressure after baroreceptor denervation is not pressure dependent. Hypertension. 1989 Nov;14(5):501–510. doi: 10.1161/01.hyp.14.5.501. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D. M., Skofitsch G., Keiser H. R., Eskay R. L., Zamir N. Evidence for the existence of atrial natriuretic factor-containing neurons in the rat brain. Neuroendocrinology. 1985 Jan;40(1):92–94. doi: 10.1159/000124058. [DOI] [PubMed] [Google Scholar]
- KRIEGER E. M. NEUROGENIC HYPERTENSION IN THE RAT. Circ Res. 1964 Dec;15:511–521. doi: 10.1161/01.res.15.6.511. [DOI] [PubMed] [Google Scholar]
- Machado B. H. Arterial pressure responses to adrenoceptor antagonism in rats with sino-aortic deafferentation. Braz J Med Biol Res. 1990;23(3-4):343–353. [PubMed] [Google Scholar]
- Masotto C., Negro-Vilar A. Inhibition of spontaneous or angiotensin II-stimulated water intake by atrial natriuretic factor. Brain Res Bull. 1985 Nov;15(5):523–526. doi: 10.1016/0361-9230(85)90044-9. [DOI] [PubMed] [Google Scholar]
- Morris M., Alexander N. Baroreceptor influences on plasma atrial natriuretic peptide (ANP): sinoaortic denervation reduces basal levels and the response to an osmotic challenge. Endocrinology. 1988 Jan;122(1):373–375. doi: 10.1210/endo-122-1-373. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Katsuura G., Nakao K., Imura H. Antidipsogenic action of alpha-human atrial natriuretic polypeptide administered intracerebroventricularly in rats. Neurosci Lett. 1985 Jul 4;58(1):1–6. doi: 10.1016/0304-3940(85)90319-2. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Eskay R. L., Antoni F. A. Atrial natriuretic peptide in the median eminence is of paraventricular nucleus origin. Neuroendocrinology. 1987 Dec;46(6):542–544. doi: 10.1159/000124878. [DOI] [PubMed] [Google Scholar]
- Phillips M. I. Functions of angiotensin in the central nervous system. Annu Rev Physiol. 1987;49:413–435. doi: 10.1146/annurev.ph.49.030187.002213. [DOI] [PubMed] [Google Scholar]
- Rauch A. L., Callahan M. F., Buckalew V. M., Jr, Morris M. Regulation of plasma atrial natriuretic peptide by the central nervous system. Am J Physiol. 1990 Feb;258(2 Pt 2):R531–R535. doi: 10.1152/ajpregu.1990.258.2.R531. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Standaert D. G., Currie M. G., Schwartz D., Geller D. M., Needleman P. Atriopeptin-immunoreactive neurons in the brain: presence in cardiovascular regulatory areas. Science. 1985 Mar 1;227(4690):1047–1049. doi: 10.1126/science.2858127. [DOI] [PubMed] [Google Scholar]
- Silva-Netto C. R., Jackson R. H., Colindres R. E. Cholinergic stimulation of the hypothalamus and natriuresis in rats: role of the renal nerves. Am J Physiol. 1986 Feb;250(2 Pt 2):F322–F328. doi: 10.1152/ajprenal.1986.250.2.F322. [DOI] [PubMed] [Google Scholar]
- Silva-Netto C. R., Jackson R. H., Colindres R. E. Factors causing natriuresis after hypothalamic injection of a cholinergic drug in rats. Am J Physiol. 1983 Jan;244(1):F64–F69. doi: 10.1152/ajprenal.1983.244.1.F64. [DOI] [PubMed] [Google Scholar]
- Standaert D. G., Saper C. B. Origin of the atriopeptin-like immunoreactive innervation of the paraventricular nucleus of the hypothalamus. J Neurosci. 1988 Jun;8(6):1940–1950. doi: 10.1523/JNEUROSCI.08-06-01940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch J. P., Hirth-Dietrich C., Kazda S., Neuser D. Endothelin stimulates release of atrial natriuretic peptides in vitro and in vivo. Life Sci. 1989;45(10):869–875. doi: 10.1016/0024-3205(89)90200-2. [DOI] [PubMed] [Google Scholar]
- Synhorst D. P., Gutkowska J. Atrial distension of isolated rabbit hearts and release of atrial natriuretic factor. Am J Physiol. 1988 Aug;255(2 Pt 2):R232–R236. doi: 10.1152/ajpregu.1988.255.2.R232. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Shinmi O., Giaid A., Yanagisawa M., Gibson S. J., Kimura S., Uchiyama Y., Polak J. M., Masaki T., Kanazawa I. Endothelin: a novel peptide in the posterior pituitary system. Science. 1990 Jan 26;247(4941):462–464. doi: 10.1126/science.2405487. [DOI] [PubMed] [Google Scholar]



