Abstract
Background
The protoporphyrin IX-triplet state lifetime technique (PpIX-TSLT) is proposed as a potential clinical non-invasive tool to monitor mitochondrial function. This technique has been evaluated in several animal studies. Mitochondrial respirometry allows measurement in vivo of mitochondrial oxygen tension (mitoPO2) and mitochondrial oxygen consumption (mitoVO2) in skin. This study describes the first use of a clinical prototype in skin of humans.
Methods
The clinical prototype was tested in 30 healthy volunteers. A self-adhesive patch containing 2 mg 5-aminolevulinic acid (ALA) was applied on the skin of the anterior chest wall (sternal) for induction of mitochondrial protoporphyrin IX and was protected from light for 5 h. MitoPO2 was measured by means of oxygen-dependent delayed fluorescence of protoporphyrin IX. MitoVO2 was determined by dynamic mitoPO2 measurements on the primed skin, while locally blocking oxygen supply by applying local pressure with the measurement probe. MitoPO2 was recorded before and during a 60-s period of compression of the microcirculation, at an interval of 1 Hz. Oxygen consumption (i.e. the local oxygen disappearance rate) was calculated from the decay of the mitoPO2 slope.
Results
Oxygen-dependent delayed fluorescence measurements were successfully performed in the skin of 27 volunteers. The average value (± SD) of mitoPO2 was 44 ± 17 mmHg and mean mitoVO2 values were 5.8 ± 2.3 and 6.1 ± 1.6 mmHg s-1 at a skin temperature of 34°C and 40°C, respectively. No major discomfort during measurement and no long-term dermatological abnormalities were reported in a survey performed 1 month after measurements.
Conclusion
These results show that the clinical prototype allows measurement of mitochondrial oxygenation and oxygen consumption in humans. The development of this clinically applicable device offers opportunities for further evaluation of the technique in humans and the start of first clinical studies.
Introduction
An adequate supply of oxygen to tissue and its subsequent use in oxidative phosphorylation in the mitochondria is essential for preserving cellular integrity and, ultimately, life. Therefore, a non-invasive and in vivo monitoring system able to monitor mitochondrial parameters, like oxygenation and oxygen consumption, could be a valuable tool for clinicians [1].
The most common ex vivo techniques are oxygen consumption measurements using oxygen electrodes [2], such as high-resolution respirometry [3]. These ex vivo techniques measure in suspensions of isolated cells and mitochondria, or small tissue biopsies and may, therefore, not adequately reflect the in vivo situation. Another method, the XF24 Extracellular Flux Analyze (Seahorse Bioscience) [4, 5] allows ex vivo measuring of mitochondrial oxygen consumption in intact cells or tissue by oxygen-sensing fluorophores. Although these approaches provide highly specific information on the function of the mitochondrial respiratory chain, the ex vivo use is a well-recognized limitation [6]. Ex vivo techniques are incapable for bed-side montoring and determination of mitochondrial function under physiological or pathophysiological circumstances is essential to understand and evaluate changes in oxidative phosphorylation [7].
To overcome limitations of ex vivo approaches, numerous in vivo techniques have been used to study mitochondrial function within the context of the physiological environment. Most commonly used methods to measure mitochondrial function in vivo are Nuclear Magnetic Resonance (NMR) spectroscopy and near-infrared spectroscopy (NIRS). NMR spectroscopy measures changes in phosphormetabolites to determine rates of resting and maximal mitochondrial ATP production. Unfortunately, the need of a Magnetic Resonance Imaging (MRI) scan makes NMR spectroscopy expensive and unable for bed-side monitoring. In contrast, NIRS enables bedside monitoring of mitochondrial function by continuous monitoring of variations in hemoglobin oxygenation and in the redox state of cytochrome c oxidase. A new application of NIRS allows measurement of skeletal muscle oxidative capacity by following the change in the rate of oxygen consumption during recovery from ischemia or exercise [8–10]. Unfortunately, recovery of the cytochrome oxidase signal from NIRS data remains controversial [11]. NIRS is influenced by tissue-specific effects, such as the wavelength dependence of the optical path length and changes in light scattering [12, 13].
Indirect calorimetry is the current golden standard for examining respiration in vivo. This technique measures inspired and expired gas flows, volumes and concentrations of oxygen (O2) and carbon dioxide (CO2), and allows measurement of total body oxygen consumption (VO2). Also Fick’s principle has been applied in humans for many years to measure VO2 by combining data of regional blood flow and arterial-venous oxygen content difference. However, both measurements can generate data with a high variability and, therefore, its use for clinical decision-making has been questioned [14, 15].
The protoporphyrin IX-triplet state lifetime technique (PpIX-TSLT) is proposed as a potential novel approach to determine mitochondrial oxygen consumption [16]. The first detailed description of the PpIX-TSLT was published in 2006 [17]. PpIX-TSLT enables mitochondrial oxygen (mitoPO2) measurements by means of the oxygen-dependent optical properties of protoporphyrin IX (PpIX). PpIX-TSLT was the first technique to allow measurements of mitoPO2 in living cells and can be applied in vivo [18–20]. Our group has been working on the development of PpIX-TSLT from its use in cell cultures to a monitoring system of mitochondrial function in humans [21]. This technique has been tested and calibrated for use on isolated organs [19, 20] and in vivo [20]. Subsequently, oxygen-dependent quenching of delayed fluorescence of PpIX has been observed in skin [18] and validation of the quenching constants needed to calculate mitoPO2 from the signals has been successful [22]. In addition to direct non-invasive measurement of mitoPO2, it is also technically possible to gain information on mitochondrial function. Information on mitochondrial oxygen consumption (mitoVO2) can be obtained by dynamic mitoPO2 measurements while blocking local oxygen supply. The local oxygen disappearance rate is a measure of oxygen consumption and can be calculated from the decay of the mitoPO2 [23]. As proposed earlier [18, 22–26], it should be technically possible and safe to apply PpIX-TSLT in humans.
In the present study, we demonstrate for the first time the ability to measure mitoPO2 and mitoVO2 in human skin, using a clinical prototype of PpIX-TSLT. The safety and feasibility of our method is investigated and data are presented on the inter- and intra-individual distribution of mitochondrial oxygen measurements. Our ultimate goal is to develop the PpIX-TSLT as a non-invasive monitor that allows direct assessment of mitochondrial oxygenation and (dys)function in critically ill patients. Although mitochondrial dysfunctions are thought to be related to the pathogenesis of sepsis and multiorgan failure [26–28], inconsistent data have been reported. These conflicting results may be due to the wide variety of methods used to determine mitochondrial dysfunction in sepsis [1]. Therefore, a standard method to monitor mitochondrial dysfunction could further elucidate the role of the mitochondria during septic conditions. When the PpIX-TSLT has proven to do this successfully, opportunities will arise for novel strategies in the treatment of severe sepsis.
Methods
The study was performed in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the Institutional Review Board at the Erasmus MC CCMO-register NL37911.078.11). A total of 30 volunteers were drawn from a pool of young, healthy students and hospital staff aged 18–30 years. Written informed consent was obtained from all volunteers prior to study participation.
Principle of mitoPO2 measurements
The background of the PpIX-TSLT is described in detail elsewhere [17, 20]. In short, PpIX is the final precursor of heme in the heme biosynthetic pathway. PpIX is synthesized in the mitochondria, and administration of 5-aminolevulinic acid (ALA) substantially enhances the PpIX concentration. PpIX possesses a triplet state that reacts strongly with oxygen, making its lifetime oxygen-dependent. Population of the first excited triplet state occurs upon photo-excitation with a pulse of light, and causes the emission of red delayed fluorescence. The delayed fluorescence lifetime is related to mitoPO2 according to the Stern-Volmer equation:
| (1) |
in which τ is the measured delayed fluorescence lifetime, kq is the quenching constant and τ0 is the lifetime at zero oxygen. Eq 1 is valid for a homogenous oxygen distribution and after excitation with a pulse of light of which the lifetime is much shorter than τ [29]. In case of a non-homogenous oxygen distribution inside the measurement volume, a reliable estimation of the average PO2 can be made by the rectangular distribution method (RDM) [30, 31].
The signal/noise ratio (SNR) of resulting traces was calculated and defined as the ratio of maximum signal amplitude to the peak-to-peak noise. Lifetime analysis operates stably at moderate SNR (SNR >20) [20]. Therefore, only delayed fluorescence signals with a SNR >20 were analyzed and included in the present dataset.
Principle of mitoVO2 measurements
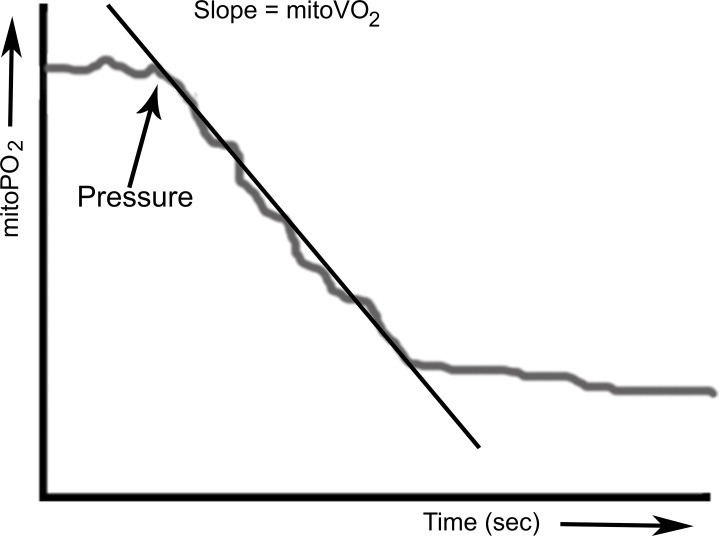
MitoVO2 is measured by means of the oxygen disappearance rate (ODR) after local occlusion of the oxygen supply. Local occlusion of the microcirculation in skin was obtained by application of pressure with the measurement probe. This simple procedure created reliable stop-flow conditions and induced measurable oxygen disappearance rates, due to cessation of microvascular oxygen supply and ongoing cellular oxygen consumption. MitoPO2 was measured before and during application of pressure at an interval of 1 Hz, using 4 laser pulses per measurement. We have described the basic principles behind the technology and have provided a working implementation of the technique for mitoVO2 measurements (15) as well as a method to calculate mitoVO2. Fig 1 presents an example of the analysis of mitoVO2.
Fig 1. Principle of respirometry by local cessation of the oxygen supply.
Mitochondrial oxygen consumption (mitoVO2) is calculated from the linear part of the oxygen disappearance curve following local compression of the microcirculation by the measurement probe.
Experimental setup
Prior to oxygen measurements the heart rate, blood pressure and peripheral oxygen saturation were determined in all volunteers, and a standard preoperative evaluation form was obtained from all these participants.
Oxygen measurements were performed by means of a clinical prototype PpIX-TSLT device [24]. A self-adhesive patch containing 8 mg ALA (Alacare®; Spirig AG, Egerkingen, Switzerland) was applied on the skin of the anterior chest wall (sternal), for induction of PpIX. To enhance ALA penetration adequate skin preparation proved essential. Hair was shaved (if present) and the skin was rubbed with a fine abrasive pad of a standard ECG sticker to remove the top parts of the stratum corneum. After ALA application, the skin was protected from light for 5 h. During these 5 h a suitable concentration of PpIX was synthesized to enable measurements of mitoPO2 and mitoVO2. To minimize the influence from ambient light, the experiments were performed in dimmed light. First, three successive mitoPO2 measurements were performed during a period of 90 s under baseline conditions. Subsequently, mitoVO2 was determined with a heated measurement probe at 34°C and 40°C. Warming of the measurement probe was done to prevent local vasoconstriction due to a cold probe, and to eliminate the effect of differences in skin temperature on the measurements. The temperature of 34°C was chosen because this was the maximal skin temperature determined in 10 healthy volunteers in a normal environment, measured by infrared thermography. To determine the effect of hyperthermia on mitoVO2 a probe temperature of 40°C was chosen. This temperature was well above the mean skin temperature [32] and low enough not to induce skin burns. One month after the experiments an evaluation form was completed by all volunteers. The form consisted of questions about any pain during the measurements and the appearance of dermatological symptoms, such as erythema, pruritus and hyperpigmentation of the skin.
Statistical analysis
Unless stated otherwise, reported values are mean ± SD. Normality of the data was tested using the Shapiro-Wilk test. Correlation between the two variables was tested by Pearson's correlation analysis.
Results
Basic characteristics of the 30 healthy volunteers are shown in Table 1. All participants had normal results for the physical parameters, such as heart rate, blood pressure and arterial oxygen saturation. Due to insufficient signal-to-noise ratio the measurements of three volunteers were excluded from further analysis.
Table 1. Characteristics healthy volunteers.
| Characteristics | Volunteers (n = 30) | |
|---|---|---|
| Age (years) | 26 ± 5 | |
| Sex, n (%) | ||
| Men | 19 (63) | |
| Women | 11 (37) | |
| Heart rate (bpm ± SD) | 69 ± 13 | |
| Systolic blood pressure (mmHg ± SD) | 128 ± 12 | |
| Diastolic blood pressure (mmHg ± SD) | 76 ± 10 | |
| Arterial saturation (% ± SD) | 99 ± 1 | |
MitoPO2 Measurements
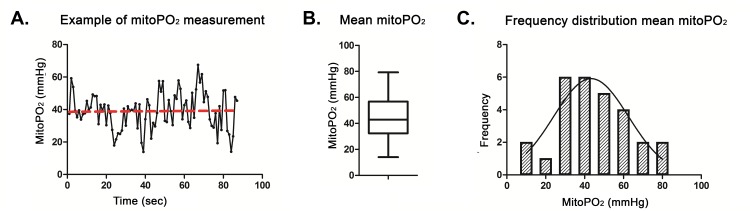
A typical example of a baseline mitoPO2 measurement is presented in Fig 2A. Due to the heterogeneous oxygenation in the skin a marked intra-individual variation was observed during the baseline measurement due to movement (breathing) in combination with a small measuring area (the diameter of the detection fiber was only 1 mm). Therefore, the mean mitoPO2 (red line) over a period of 90 s was used in the analysis of mitoPO2.
Fig 2.
A. Example of a baseline mitoPO2 measurement. The mean mitochondrial oxygen tension (mitoPO2) (red line) is calculated over a period of 90 second. B. The mitoPO2 is presented in a box-and-whisker graph, C. Frequency distribution of all ratio differences of the mitoPO2.
From the total of 27 experiments, mean mitoPO2 was 44 ± 17 mmHg (Fig 2B). This finding is consistent with previous values derived from animal studies [21, 33]. Despite the movement-induced variation in baseline mitoPO2, the mean data regress towards a normal distribution (p = 0.73; skewness = 0.07 ± 0.45, kurtosis = -0.46 ± 0.87) (Fig 2C).
MitoVO2 Measurements
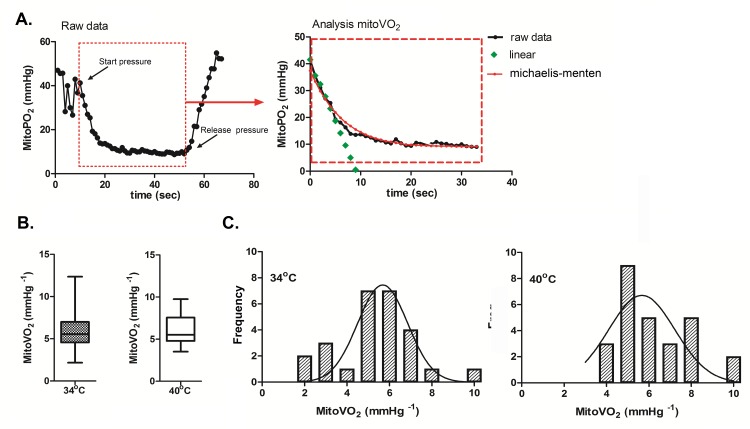
A typical example of an oxygen disappearance curve on human skin is presented in Fig 3A. The mean mitoVO2 values, measured in the skin of 27 healthy volunteers, were 5.8 ± 2.3 and 6.1 ± 1.6 mmHg s-1 for a probe temperature of 34°C and 40°C, respectively (Fig 3B). The distribution of the data is presented in Fig 3C. The Shapiro-Wilk test demonstrates that mitoVO2 measured at a temperature of 34°C follows a normal distribution (p-value = 0.069, skewness = 0.9, kurtosis = 1.9). However, the same analysis of mitoVO2 at a temperature of 40°C does not follow a normal distribution (p-value = 0.047, skewness = 0.72 ± 0.45, kurtosis = -0.29 ± 0.87).
Fig 3.
A. Typical example of the analysis of the mitochondrial oxygen consumption (mitoVO2). The first panel shows an example of the oxygen disappearance rate after local occlusion of the microcirculation. In the second panel we demonstrate the analysis of the mitochondrial oxygen tension (mitoPO2), the green line represents the linear fit (ΔPO2(t)/Δt) of the oxygen disappearance rate. B. The mitoVO2 are presented in a box-and-whiskers graph. The boxes extend from the 25th percentile to the 75th percentile, with a line at the median, the whiskers extend above and below the box to show the highest and lowest values. Presented data are measured with two different probe temperatures (34°C and 40°C, respectively). C. Frequency distribution of all ratio differences of the mitoVO2 at 34°C and 40°C.
Correlation between mitoPO2 and mitoVO2
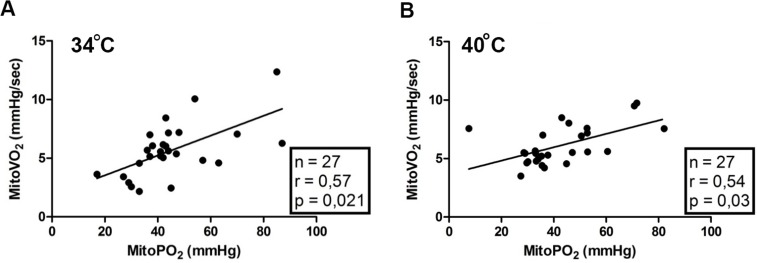
We found a weak positive correlation between mitoVO2 and baseline mitoPO2 for 27 data pairs (Fig 4). This indicates that mitoVO2 of the skin might not be completely independent of baseline mitoPO2 levels. However, the correlation is mainly caused by very high and/or very low mitoPO2 values.
Fig 4.
Correlation plot of the initial mitochondrial oxygen tension (mitoPO2) and the mitochondrial oxygen consumption (mitoVO2) at 34°C (A) and 40°C (B).
Safety of the measurements
All healthy volunteers experienced the skin preparation and measurements as non-problematic. Due to either the skin preparation or the ALA patch, 45% of the volunteers suffered from mild pruritus and/or erythema on the actual measurement day; these minor complaints were no longer present the day after the measurements. Only two volunteers had transient hyperpigmentation of the skin after the measurements, possibly due to premature exposure of the primed area to sunlight (against our advice). The hyperpigmentation was temporary and disappeared within one month. None of the volunteers sustained long-term skin damage, as established one month after the experiments.
Discussion
This study presents the first results of a clinical prototype of a novel non-invasive monitoring device based on PpIX-TSLT. This device enables measurement of mitochondrial oxygenation and oxygen consumption in human skin. The PpIX-TSLT appears to be a feasible and safe non-invasive measurement tool that allows performing a functional optical biopsy in intact skin.
Following topical application of ALA on the skin above the sternum, after 5 h we could detect delayed fluorescence signals, of which the lifetime kinetics could be analyzed in 27 of the 30 healthy volunteers. Three volunteers were excluded from the analysis because the SNR was insufficient; this was caused by technical problems with the light source at the time of measurement (laser output unstable and with reduced intensity). Mean mitoPO2 values were around 45 mmHg. This is slightly lower than previously measured in rats [24, 26] and is probably explained by inter-species differences, as well as differences in the fractional inspired oxygen concentration (FiO2). The FiO2 in rats was 0.40 versus an atmospheric FiO2 in healthy volunteers.
An intra-person variation in mitoPO2 was observed during the mitoPO2 measurements. Small movements of the measuring probe relative to the skin can lead to changes in mitoPO2 due to the small measurement volume in a heterogenic tissue surface. Therefore, our group is currently working on a measuring probe with revised optics to increase the detection area on the skin. Combined with the ability to be placed flat on the skin and be affixed above the measuring spot, this improved probe is expected to substantially reduce the intra-individual variation in mitoPO2 readings.
In this first set of human mitoVO2 measurements, under stop flow conditions, we chose to calculate the oxygen consumption by a simple fit of the ΔPO2/Δt curve. This resulted in a mitoVO2 of around 6 mmHg s-1. The measured values for mitoVO2 in human sternal skin are comparable to the results reported for the abdominal skin of rats [23, 26], i.e. 5.8 ± 2.3 mmHg s-1 and 5.0 ± 0.3 mmHg s-1 respectively.
The mitoVO2 measurements were performed with a heated measurement probe at 34°C and at 40°C. Although increased oxygen consumption was expected at a higher skin temperature, no significant difference was observed in these experiments.
Fig 4 demonstrated a correlation between mitoVO2 and initial mitoPO2. This finding is likely due to the used method for analysis of the oxygen disappearance rate, which assumes the measuring volume to be oxygen-tight. However, oxygen diffusion into the measuring volume during an ODR measurement increases at lower mitoPO2 and causes the deflection point and deviation from linearity at low mitoPO2. A disadvantage of the used linear fit procedure is that it is dependent on the deflection point. At low initial oxygen levels the deflection point has a greater influence on the fitting procedure and gives rise to the correlation between mitoVO2 and initial PO2. This limitation might be solved by using a different analysis method for the oxygen disappearance rate, which takes oxygen back diffusion into account [24, 34]. Although this more complex method of analyzing has been evaluated for use in rats [24], it has yet to be validated for use in humans; therefore, for the present study, we chose to use the more straightforward linear fit procedure.
The PpIX-TSLT is a novel noninvasive measurement tool that might be of considerable benefit in emergency, intensive care and peri-operative medicine. For example, mitoPO2 measurements could be useful to optimize oxygenation or hemodynamic status, or to guide treatment in critically ill patients [21]. PpIX-TSLT provides a new tool for mitochondrial research but does have some limitations. For example, unlike ex vivo respirometry, currently it cannot measure quantitatively the bioenergetic state of mitochondria and examine the different states of mitochondrial respiration. Also, measurements in the skin might not be representative of all other tissues in the body. Furthermore, although mitochondria in some tissues (like cardiac muscle) consume approximately 90% of oxygen [35] it is important to recognize that mitochondria are not the sole consumers of oxygen. In this respect it is important to note that in rat skin we found cyanide to be a very effective blocker of oxygen consumption within the measuring volume [23]. Assessment of mitochondrial respiration in vivo is also possible by dynamic near-infrared spectroscopy. In contrast to PpIX-TSLT, NIRS is measured in muscle and results have been demonstrated to correlate with respirometry in muscle biopsies (9). However, NIRS appears somewhat more cumbersome to use for clinical monitoring because of the need for calibration and the use of blood-pressure cuffs.
This study is the first to demonstrate that measurement of mitochondrial parameters by PpIX-TSLT is feasible in humans. Although the technique still needs further development and optimization, the prototype allows evaluation in humans and the clinical setting. Therefore, we conclude that the method itself is feasible to measure mitoPO2 and mitoVO2 in humans. A follow-up study comparing PpIX-TSLT to other mitochondrial function measurements remains to be performed, but use of PpIX-TSLT in pre-clinical studies showed promising results in the fields of sepsis and transfusion medicine [26, 36] We expect that clinical implementation of the PpIX-TSLT will make a valuable contribution to our knowledge on mitochondrial function and oxygen metabolism under healthy and pathophysiological circumstances. For example, this technique could be a useful tool to evaluate topical skin treatment, for guidance of systemic mitochondrial therapy, and to monitor mitochondrial function in critically ill patients.
Supporting Information
(XLSX)
Acknowledgments
This research was financially supported by a Life Sciences Pre-Seed Grant (grant no. 40-41300-98-9037) from the Netherlands Organization for Health Research and Development (ZonMW).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was financially supported by a Life Sciences Pre-Seed Grant (grant no. 40-41300-98-9037) from the Netherlands Organization for Health Research and Development (ZonMW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jeger V, Djafarzadeh S, Jakob SM, Takala J. Mitochondrial function in sepsis. Eur J Clin Invest. 2013;43(5):532–42. Epub 2013/03/19. 10.1111/eci.12069 . [DOI] [PubMed] [Google Scholar]
- 2.Piffaretti F, Santhakumar K, Forte E, van den Bergh HE, Wagnieres GA. Optical fiber-based setup for in vivo measurement of the delayed fluorescence lifetime of oxygen sensors. J Biomed Opt. 2011;16(3):037005 Epub 2011/04/05. 10.1117/1.3558846 . [DOI] [PubMed] [Google Scholar]
- 3.Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol. 1998;201(Pt 8):1129–39. Epub 1998/05/29. . [DOI] [PubMed] [Google Scholar]
- 4.Luz AL, Smith LL, Rooney JP, Meyer JN. Seahorse Xfe 24 Extracellular Flux Analyzer-Based Analysis of Cellular Respiration in Caenorhabditis elegans. Curr Protoc Toxicol. 2015;66:25 7 1–15. Epub 2015/11/03. 10.1002/0471140856.tx2507s66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen PB. Measuring Mitochondrial Defects: XF Bioenergetic Analysis Identifies Defects in Human Skin Fibroblasts. Genetic Engineering & Biotechnology News. 2014;34(10):19–. 10.1089/gen.34.10.10 [DOI] [Google Scholar]
- 6.Villani G, Attardi G. In vivo control of respiration by cytochrome c oxidase in human cells. Free Radic Biol Med. 2000;29(3–4):202–10. Epub 2000/10/18. S0891-5849(00)00303-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 7.Perry CG, Kane DA, Lanza IR, Neufer PD. Methods for assessing mitochondrial function in diabetes. Diabetes. 2013;62(4):1041–53. Epub 2013/03/23. 62/4/1041 [pii] 10.2337/db12-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motobe M, Murase N, Osada T, Homma T, Ueda C, Nagasawa T, et al. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn Med. 2004;3(1):2 Epub 2004/02/07. 10.1186/1476-5918-3-2 1476-5918-3-2 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan TE, Brophy P, Lin CT, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol. 2014;592(15):3231–41. Epub 2014/06/22. jphysiol.2014.274456 [pii] 10.1113/jphysiol.2014.274456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Beekvelt MC, Borghuis MS, van Engelen BG, Wevers RA, Colier WN. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci (Lond). 2001;101(1):21–8. Epub 2001/06/21. . [DOI] [PubMed] [Google Scholar]
- 11.Matcher SJ, Elwell CE, Cooper CE, Cope M, Delpy DT. Performance comparison of several published tissue near-infrared spectroscopy algorithms. Anal Biochem. 1995;227(1):54–68. Epub 1995/05/01. S0003-2697(85)71252-3 [pii] 10.1006/abio.1995.1252 . [DOI] [PubMed] [Google Scholar]
- 12.Boushel R, Piantadosi CA. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiol Scand. 2000;168(4):615–22. Epub 2000/04/12. aps713 [pii] 10.1046/j.1365-201x.2000.00713.x . [DOI] [PubMed] [Google Scholar]
- 13.Kakihana Y, Matsunaga A, Yasuda T, Imabayashi T, Kanmura Y, Tamura M. Brain oxymetry in the operating room: current status and future directions with particular regard to cytochrome oxidase. J Biomed Opt. 2008;13(3):033001 Epub 2008/07/08. 10.1117/1.2940583 . [DOI] [PubMed] [Google Scholar]
- 14.Marson F, Auxiliadora Martins M, Coletto FA, Campos AD, Basile-Filho A. Correlation between oxygen consumption calculated using Fick's method and measured with indirect calorimetry in critically ill patients. Arq Bras Cardiol. 2004;82(1):77–81, 72–6. Epub 2004/02/24. S0066-782X2004000100007 [pii]. . [DOI] [PubMed] [Google Scholar]
- 15.Smithies MN, Royston B, Makita K, Konieczko K, Nunn JF. Comparison of oxygen consumption measurements: indirect calorimetry versus the reversed Fick method. Crit Care Med. 1991;19(11):1401–6. Epub 1991/11/01. . [DOI] [PubMed] [Google Scholar]
- 16.Mik EG, Methods and devices for assessment of mitochondrial function. 2009: US 2011/0182825 A1.
- 17.Mik EG, Stap J, Sinaasappel M, Beek JF, Aten JA, van Leeuwen TG, et al. Mitochondrial PO2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nat Methods. 2006;3(11):939–45. Epub 2006/10/25. nmeth940 [pii] 10.1038/nmeth940 . [DOI] [PubMed] [Google Scholar]
- 18.Harms FA, de Boon WM, Balestra GM, Bodmer SI, Johannes T, Stolker RJ, et al. Oxygen-dependent delayed fluorescence measured in skin after topical application of 5-aminolevulinic acid. J Biophotonics. 2011;4(10):731–9. Epub 2011/07/20. 10.1002/jbio.201100040 . [DOI] [PubMed] [Google Scholar]
- 19.Mik EG, Ince C, Eerbeek O, Heinen A, Stap J, Hooibrink B, et al. Mitochondrial oxygen tension within the heart. J Mol Cell Cardiol. 2009;46(6):943–51. Epub 2009/02/24. S0022-2828(09)00061-3 [pii] 10.1016/j.yjmcc.2009.02.002 . [DOI] [PubMed] [Google Scholar]
- 20.Mik EG, Johannes T, Zuurbier CJ, Heinen A, Houben-Weerts JH, Balestra GM, et al. In vivo mitochondrial oxygen tension measured by a delayed fluorescence lifetime technique. Biophys J. 2008;95(8):3977–90. Epub 2008/07/22. S0006-3495(08)78536-9 [pii] 10.1529/biophysj.107.126094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mik EG. Special article: measuring mitochondrial oxygen tension: from basic principles to application in humans. Anesth Analg. 2013;117(4):834–46. Epub 2013/04/18. ANE.0b013e31828f29da [pii] 10.1213/ANE.0b013e31828f29da . [DOI] [PubMed] [Google Scholar]
- 22.Harms FA, Bodmer SI, Raat NJ, Stolker RJ, Mik EG. Validation of the protoporphyrin IX-triplet state lifetime technique for mitochondrial oxygen measurements in the skin. Opt Lett. 2012;37(13):2625–7. Epub 2012/06/30. 238990 [pii]. 10.1364/OL.37.002625 [DOI] [PubMed] [Google Scholar]
- 23.Harms FA, Voorbeijtel WJ, Bodmer SI, Raat NJ, Mik EG. Cutaneous respirometry by dynamic measurement of mitochondrial oxygen tension for monitoring mitochondrial function in vivo. Mitochondrion. 2013;13(5):507–14. Epub 2012/10/16. S1567-7249(12)00226-7 [pii] 10.1016/j.mito.2012.10.005 . [DOI] [PubMed] [Google Scholar]
- 24.Harms FA, Bodmer SI, Raat NJ, Mik EG. Cutaneous mitochondrial respirometry: non-invasive monitoring of mitochondrial function. J Clin Monit Comput. 2015;29(4):509–19. Epub 2014/11/13. 10.1007/s10877-014-9628-9 . [DOI] [PubMed] [Google Scholar]
- 25.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100(4):1093–106. Epub 2005/03/23. 100/4/1093 [pii] 10.1213/01.ANE.0000148691.33690.AC . [DOI] [PubMed] [Google Scholar]
- 26.Harms FA, Bodmer SI, Raat NJ, Mik EG. Non-invasive monitoring of mitochondrial oxygenation and respiration in critical illness using a novel technique. Crit Care. 2015;19(1):343 Epub 2015/09/24. 10.1186/s13054-015-1056-9 10.1186/s13054-015-1056-9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink MP. Cytopathic hypoxia. A concept to explain organ dysfunction in sepsis. Minerva Anestesiol. 2000;66(5):337–42. Epub 2000/08/31. . [PubMed] [Google Scholar]
- 28.Garrabou G, Moren C, Lopez S, Tobias E, Cardellach F, Miro O, et al. The effects of sepsis on mitochondria. J Infect Dis. 2012;205(3):392–400. Epub 2011/12/20. jir764 [pii] 10.1093/infdis/jir764 . [DOI] [PubMed] [Google Scholar]
- 29.Mik EG, Donkersloot C, Raat NJ, Ince C. Excitation pulse deconvolution in luminescence lifetime analysis for oxygen measurements in vivo. Photochem Photobiol. 2002;76(1):12–21. Epub 2002/07/20. . [DOI] [PubMed] [Google Scholar]
- 30.Bodmer SI, Balestra GM, Harms FA, Johannes T, Raat NJ, Stolker RJ, et al. Microvascular and mitochondrial PO(2) simultaneously measured by oxygen-dependent delayed luminescence. J Biophotonics. 2012;5(2):140–51. Epub 2011/11/25. 10.1002/jbio.201100082 . [DOI] [PubMed] [Google Scholar]
- 31.Golub AS, Popel AS, Zheng L, Pittman RN. Analysis of phosphorescence in heterogeneous systems using distributions of quencher concentration. Biophys J. 1997;73(1):452–65. Epub 1997/07/01. S0006-3495(97)78084-6 [pii] 10.1016/S0006-3495(97)78084-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JK, Miki K, Sagawa S, Shiraki K. Evaluation of mean skin temperature formulas by infrared thermography. Int J Biometeorol. 1997;41(2):68–75. Epub 1998/01/16. . [DOI] [PubMed] [Google Scholar]
- 33.Harms FA, Bodmer SI, Raat NJ, Mik EG. Cutaneous mitochondrial respirometry: non-invasive monitoring of mitochondrial function. J Clin Monit Comput. 2014. Epub 2014/11/13. 10.1007/s10877-014-9628-9 . [DOI] [PubMed] [Google Scholar]
- 34.Golub AS, Tevald MA, Pittman RN. Phosphorescence quenching microrespirometry of skeletal muscle in situ. Am J Physiol Heart Circ Physiol. 2011;300(1):H135–43. Epub 2010/10/26. ajpheart.00626.2010 [pii] 10.1152/ajpheart.00626.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77(3):731–58. Epub 1997/07/01. . [DOI] [PubMed] [Google Scholar]
- 36.Romers LH, Bakker C, Dollee N, Hoeks SE, Lima A, Raat NJ, et al. Cutaneous Mitochondrial PO2, but not Tissue Oxygen Saturation, Is an Early Indicator of the Physiologic Limit of Hemodilution in the Pig. Anesthesiology. 2016. Epub 2016/05/14. 10.1097/ALN.0000000000001156 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.