Abstract
Aim:
Lower respiratory tract infections including mainly pneumonia represent an important public health problem leading to high mortality and mobidity rates in children aged below five years in developing countries including our country. Vitamin D deficiency has been associated with increased risk of rickets/osteomalacia, various cancers, autoimmune diseases, hyperproliferative skin diseases, cardiovascular system diseases and infectious diseases. Vitamin D has an important role in cellular and humoral immunity and pulmonary functions. Vitamin D deficiency and lower respiratory tract infection are common health problems in children in our country and no clinical study investigating the relationship between these problems has been conducted so far. In this case-control study, we aimed to assess the association between vitamin D level and lower respiratory tract infection in children.
Material and Methods:
Sixty-three children aged between six months and five years with lower respiratory infections and 59 age-matched children who had no history of respiratory symptoms in the last month and no accompanying chronic disease were compared in terms of vitamin D levels. The children in the patient group were also evaluated by the clinical picture.
Results:
No significant correlation was found between vitamin D levels and lower respiratory tract infection in terms of disease and its severity. However, it was found that vitamin D deficiency/ insufficiency was observed with a high rate in all children included in the study.
Conclusions:
Although no correlation was found between vitamin D level and lower respiratory tract infection, it is recommended that vitamin D level should be measured in children with lower respiratory tract infection and vitamin D supplementation should be given to all children especially in winter months based on the fact that the level of vitamin D was lower than normal in approximately half of the children included in the study and considering the effects of vitamin D on infections, pulmonary functions and immunity.
Keywords: Child, lower respiratory tract infection, vitamin D
Introduction
Lower respiratory tract infections (LRTI) are among the most important causes of morbidity and mortality in the childhood. Approximately two million children aged below five years die each year because of LRTI (1). The factors which predispose to lower respiratory tract infections include age (<1 year), low birth weight, premature delivery, malnutrition, underlying morbidity, breast-feeding failure, low socioeconomical level, crowded life conditions (large family, nursery care etc.), poor access to healthcare services, maternal age and education, indoor and outdoor air pollution (including mainly smoking), inadequate immunization and vitamin D deficiency (2). Both vitamin D deficiency and LRTI are significant public health problems in developing countries like our country. Currently, it is known that vitamin D deficiency constitutes a risk in terms of various conditions including mainly rickets/osteomalacia, various cancers, autoimmune diseases, hyperproliferative skin diseases, cardiovascular system diseases and infectious diseases (3). Vitamin D has an important role in cellular and humoral immunity and pulmonary functions (4, 5). In our country, no study examining the relationship between LRTI and vitamin D deficiency in children has been conducted. In this case-control study, we aimed to examine the relationship between LRTI and vitamin D deficiency in children.
Material and Methods
A total of 122 children aged between six months and five years who presented to the pediatric outpatient clinic between December 2010 and February 2011 including 63 children who were diagnosed with LRTI for the first time and 59 children who had no chronic disease and who did not show any LRTI sing in the last one month were included in the study.
While planning the study, Epi Info version 6.0 (CDC) package program was used and it was planned to include at least 55 children in the study and control groups each, with calculation of sample size for unmatched case-control studies considering a confidence interval of 95%, a power of 80%, a disease prevalence of 13.4% and an odds ratio of 4.0 (2).
The age, antropometric measurements and percentiles at the time of enrollment, complaints at presentation, durations of complaints, seasons of birth, nutritional status, immunization status, heating conditions at home, number of people living in house, indoor smoking status and vitamin D support in the first one year were interrogated in all children included in the control and study groups. The children who were not in the appropriate height and weight percentile for age, who had a history of premature delivery, who had malnutrition or chronic disease, who received breastmilk for less than 6 months, whose immunizations were incomplete, who were warmed by stove or open fire at home, who were exposed to indoor smoking, who had crowded life conditions, who had a history of recurrent LRTI and who had not received vitamin D supplementation in the first year of life were not included in the study (In our country, a supplement of 400 IU vitamin D has been given to all babies aged between 0 and 12 months since 2005 for the aim of preventing vitamin D deficiency).
Fever, cough and tachypnea were considered signs of lower respiratory tract infection. Tachypnea was defined as a respiratory rate above 60/minute in babies aged younger than two months, above 50/minute in children aged between two months and one year and above 40/minute in children aged between one and five years according to the criteria of the World Health Organization (2). Subcostal, intercostals and suprasternal retractions and nasal flaring, if present, and oxygen saturations were recorded. Lung auscultation findings were recorded as rales, ronchi, wheezing and prolonged expiration. A body temperature above 38°C was considered high fever. The radiological findings, white cell counts, presence of shifting to left on peripheral smear, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) values, treatment given and hospitalization were recorded. A C-reactive protein value of 0–6 mg/L and an ESR value of <20 mm/h were considered normal. The patients were classified as bronchiolitis, bronchopneumonia and pneumonia according to the clinical and laboratory findings.
The patients who were diagnosed with lower respiratory tract infections were treated in the outpatient or inpatient setting according to the severity of the disease. Hospitalization durations and the therapies administered in all patients were recorded. Whether or not Vitamin D status changed according to season of birth, white blood cell counts, CRP levels, peripheral smear findings, oxygen saturations, lung graphy findings, hospitalization requirements, intensive care requirements and antibiotic treatment requirements was evaluated in the patient group. The relationship between vitamin D status and the diagnoses and hospitalization durations was also investigated.
25 (OH) vitamin D level was studied with Shimadzu device using the HPLC technique and ImmunChrom (IC3401rp) kit. Separation was performed using reversed phase column in the HPLC method and vitamin D level was determined on UV detector. The results were given as ng/mL. A 25 (OH) vitamin D level of <20 ng/mL was considered vitamin D deficiency, a level of 20–30 ng/mL was considered vitamin D insufficiency, a level of >30 ng/mL was considered normal and a level of >150–200 ng/mL was considered intoxication (6). The blood samples for studying vitamin D were obtained at the time of presentation and kept at −20°C. At the end of the study, vitamin D level was studied all together in all blood samples.
The study was conducted with the approval obtained from Gazi University School of Medicine Ethics Committee dated 03/23/2011 with number B.30.2.GÜN.0.20-3657 - 16. Written informed consent was obtained from the parents of all children included in the study.
Statistical analysis
The data were recorded in the SSPS (Statistical Package for the Social Science, Inc.; Chicago, IL, USA) 15.0 package program and statistical analyses were performed. The variables specified with measurements were expressed as mean ± standard deviation and median (minimum, maximum). The variables specified by counting were expressed as percentages. Chi-square test was used in comparison of counting values of the patient and control groups. Compatibility with the normal distribution of the measurement variables was tested with Kolmogorov-Smirnov test. Nonparametric tests were used when the variables were not compatible with the normal distribution. Non-parametric correlation test (Spearman) was used to find the factors related with vitamin D levels. A p value of <0.05 was considered statistically significant.
Results
The mean age was found to be 21.05±13.7 months (6–59 months) in the patient group and 21.10±13.6 months (6–48 months) in the control group. No statistically significant difference was found between the patient and control groups in terms of mean age (p>0.05).
The complaint of cough was present in all children in the patient group at presentation and 53 patients (84%) had tachypnea. On physical examination, tachypnea was found in 52 patients (82%) and retractions (subcostal, intercostals and suprasternal) were found in 52 patients (82%). Infiltration on lung graphy was present in 52 (82%) of the patients. C-reaktive protein was found to be positive in 46 (73%) of the patients. The complaints, physical examination findings and laboratory findings of the patient group are shown in Table 1.
Table 1.
Complaints, physical examination findings and laboratory findings in the patient group
| (n=63) | Present n (%) | Absent n (%) |
|---|---|---|
| Complaint | ||
| Tachypnea | 53 (84) | 10 (16) |
| Cough | 63 (100) | - |
| Physical examination | ||
| Fever | 29 (46) | 34 (54) |
| Tachypnea | 52 (82) | 11 (18) |
| SpO2 >92% | 46 (73) | 17 (27) |
| Rales | 44 (70) | 19 (30) |
| Wheezing | 51 (81) | 12 (19) |
| Prolonged expiration | 19 (30) | 44 (70) |
| Retractions | 52 (82) | 11 (18) |
| Laboratory findings | ||
| Infiltration on lung graphy | 52 (82) | 11 (18) |
| Increased white blood cells | 30 (47) | 33 (53) |
| Shifting to left on peripheric blood smear | 32 (49) | 31 (51) |
| CRP>6 mg/L | 46 (73) | 17 (27) |
| ESR>20 mm/h* | 33 (78) | 9 (22) |
ESR was measured in 42 patients.
CRP: c-reactive protein; ESR: erythrocyte sedimentation rate
The mean vitamin D level was found to be 34.9±22.1 ng/mL in the patient group and 37.2±21.4 ng/mL in the control group. No statistically significant difference was found between the two groups in terms of these values (p=0.389). Vitamin D deficiency was found with a rate of 25% and vitamin D insufficiency was found with a rate of 22% in all children included in the study. Vitamin D deficiency and insufficiency were found with a rate of 31% and 19%, respectively, in the patient group and with a rate of 19% and 25%, respectively, in the control group (Figure 1). No difference was found between the patient and control groups in terms of vitamin D deficiency and insufficiency (p>0.05).
Figure 1.
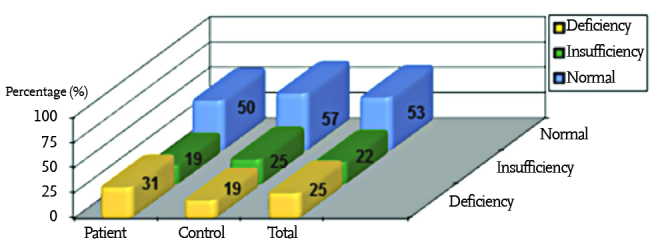
Vitamin D status in the patient group, in the control group and in all subjects included in the study (p>0.05)
Twenty six (41%) of the children in the patient group and 30 (51%) of the children in the control group were born in winter. Thirty seven (59%) of the children in the patient group and 29 (49%) of the children in the control group were born in summer. No difference was found between the patient and control groups in terms of birth season (p>0.05). The mean number of people living in house in the patient and control groups was four.
Thirty two (51%) of the patients were treated in an outpatient setting and 31 patients (49%) were treated in an inpatient setting. Three patients (5%) needed intensive care. Sixty five percent of the patients received antibiotic treatment.
No significant correlation was found between vitamin D status of the patients and the parameters presented in Table 2 (p>0.05).
Table 2.
Vitamin D status by characteristics of the patient group
| Vitamin D deficiency n (%) | Vitamin D insuffiency n (%) | Normal n (%) | P | ||
|---|---|---|---|---|---|
| Season of birth | Winter | 11 (42.3) | 2 (7.7) | 13 (50) | 0.102 |
| Summer | 9 (24.3) | 10 (27) | 18(48.6) | ||
| Increased white blood cells | Present | 9 (30) | 8 (26.7) | 13 (43.3) | 0.333 |
| Absent | 11 (33.3) | 4 (12.1) | 18 (54.5) | ||
| CRP | Normal | 7 (41.2) | 3 (17.6) | 7 (41.2) | 0.612 |
| Increased | 13 (28.3) | 9 (19.6) | 24 (52.2) | ||
| Shifting to the left on peripheric blood smear | Present | 11 (34.4) | 7 (21.9) | 14 (43.8) | 0.688 |
| Absent | 9 (29) | 5 (16.8) | 17 (54.8) | ||
| SpO2 | <92% | 8 (44.4) | 3 (16.7) | 7 (38.9) | 0.387 |
| >92% | 12 (26.7) | 9 (20) | 24 (53.3) | ||
| Infiltration on lung graphy | Present | 18 (34.6) | 11 (21.2) | 23 (44.2) | 0.061a |
| Absent | 2 (18.2) | 1 (9.1) | 8 (72.7) | ||
| Hospitalization | Present | 11 (34.4) | 8 (25) | 13 (40.6) | 0.313 |
| Absent | 9 (29) | 4 (12.9) | 18 (58.1) | ||
| ICU | Required | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0.999a |
| Not required | 19 (31.7) | 11 (18.3) | 30 (50) | ||
| Antibiotic | Received | 12 (29.3) | 9 (22) | 20 (48.8) | 0.678 |
| Did not receive | 8 (36.4) | 3 (13.6) | 11 (50) |
CRP: c-reactive protein; ICU: Intensive care unit
The groups of vitamin D deficiency and insufficiency were combined in statistical analyses performed with the lines of infiltration on lung graphy and ICU (Fisher’s exact test was performed).
When the vitamin D status of the patients were compared according to the diagnoses they received, no significant correlation was found between the diagnosis and vitamin D level (p>0,05) (Table 3).
Table 3.
Vitamin D status by diagnoses of the patient group
| Vitamin D deficiency <20 ng/mL n (%) | Vitamin D insufficiency 20–30 ng/mL >30 ng/mL n (%) | Vitamin D Normal n (%) | p | |
|---|---|---|---|---|
| Bronchiolitis (n=22) | 8 (36) | 3 (13) | 11 (50) | |
| Pneumonia (n=9) | 1 (11) | 2 (22) | 6 (66) | 0.609 |
| Bronchopneumonia (n=32) | 11 (34) | 7 (21) | 14 (43) |
The mean hospitalization duration was 5.9±2.5 (3–12) days in the patient group. When the relationship between hospitalization durations and vitamin D level was examined, a positive, weak (r=0.08) and statistically insignificant (p=0.665) correlation was found between the hospitalization duration and vitamin D levels.
Discussion
It is thought that vitamin D deficiency is endemic and affects one billion people worldwide (7). In children, vitamin D deficiency and insufficiency are important health problems and variable prevalences have been reported in studies conducted in many countries (8–11). In a study conducted in Greece, vitamin D deficiency was found with a rate of 14% in children aged between three and 18 years (8). In USA, vitamin D deficiency was found with a rate of 14% and vitamin D insufficiency was found with a rate of 63% in 1799 children aged between one and five years (9). In another study, vitamin D deficiency was found with a rate of 12% and vitamin D insufficiency was found with a rate of 40% in 133 infants aged between eight and 24 months (10). In Toronto, vitamin D deficiency was found with a rate of 32% and vitamin D insufficiency was found with a rate of 82% in 91 children aged between 24 and 30 months (11).
In our country, the number of studies related with vitamin D level in children aged below five years is quite limited. The prevalence of rickets related with nutrition was found to be 6% by Özkan et al. (12) in the 0-3-year age group in Erzurum region. In another study conducted in the same region by Özkan et al. (13) together with the Ministry of Health with approximately 50 000 subjects in 2007, the prevalence of rickets related with nutrition (vitamin D deficiency) was found to be below 1% in the 0-3-year age group. In a study conducted in 2002, vitamin D deficiency was found with a rate of 25% and vitamin D insufficiency was found with a rate of 15% in breastfed babies (14).
In this study, the relationship between vitamin D level and LRTI was investigated in children. Vitamin D deficiency was found with a rate of 25% and vitamin D insufficiency was found with a rate of 22% in all children included in this study. The frequency of vitamin D deficiency is increasing, because sun light can reach the skin to a lesser extent in winter months and natural food usually cannot meet daily vitamin D requirement (15). It has been thought that staying inside, air pollution, covered clothing and lack of regular vitamin D supplementation in addition to decreased vitamin D synthesis in the skin during winter months have a role in low vitamin D levels. However, it was notable that we found higher rates of vitamin D deficiency and insufficiency in our study compared to the previous studies conducted in our country (12–14), although the children included in our study showed an age-appropriate development, received vitamin D supplementation during the first year of life and breastfed for at least six months and had no accompanying chronic disease.
In a study conducted in Bangladesh which examined the relationship between vitamin D level and lower respiratory tract infections, the 25 (OH) D levels were compared between 25 babies aged between one and 18 months who were hospitalized because of LRTI and 25 age and gender matched control babies who lived in the same village. 25 (OH) D was found to be lower in babies with LRTI and it was concluded that vitamin D deficiency was related with LRTI in the early childhood (16). In our study, no significant difference was found between the patient and control groups in terms of vitamin D level. However, the rates of vitamin D deficiency and insufficiency in both groups were found to be higher compared to the studies conducted in our country and in the world (8, 10, 13, 14).
Studies have reported that vitamin D deficiency has a negative impact on the prevalence of LRTI and disease severity and increases intensive care and oxygen requirements of LRTI patients (17–20). In this study, no significant correlation was found between vitamin D status and LRTI or disease severity, but the fact that vitamin D levels was below normal in 47% of all children included in the study suggested that this result was important for our country in which both LRTIs and vitamin D deficiency are prevalent.
Although there is no other study conducted in our country examining the relationship between vitamin D and LRTI, studies examining the relationship between vitamin D and asthma showed that the disease severity and frequency of exacerbations and emergency department admissions were increased in children with low vitamin D levels (21–23).
Although no correlation was found between LRTI and vitamin D in our study, it is recommended that vitamin D level should be measured in children with LRTI and all children should be given vitamin D supplementations especially in winter months considering the effects of vitamin D on infections, pulmonary functions and immunity. Studies with larger series with long follow-up periods should be conducted for definite results.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from Gazi University School of Medicine Ethics Committee (23/03/2011 - B.30.2. Day.0.20-3657/16).
Informed Consent: Informed consent was obtained from the parents.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.Ş., A.T.A.; Design - T.Ş., A.T.A.; Supervision - T.Ş., A.T.A., Ö.G., S.Ö.; Funding - T.Ş., A.T.A., Ö.G., S.Ö.; Materials - T.Ş., Ö.G.; Data Collection and/ or Processing - T.Ş., A.T.A., Ö.G., S.Ö.; Analysis and/or Interpretation - T.Ş., A.T.A., Ö.G., S.Ö.; Literature Review - T.Ş., A.T.A., Ö.G., S.Ö.; Writing - T.Ş., A.T.A.; Critical Review - T.Ş., A.T.A., Ö.G., S.Ö.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study was supported by Gazi University Unit of Scientific Research Projects with the 01/2011-73 project number.
References
- 1.WHO . The World Health Report 2005: Redesigning child care: Survival, growth and development. Geneva: World Health Organization; 2005. pp. 127–43. [Google Scholar]
- 2. Türk Toraks Derneği Çocuklarda toplumda gelişen pnömoni tanı ve tedavi uzlaşı raporu 2009. ( http://www.turkishthoracicjournal.com/upload/documents/pdf_Toraksder_633.pdf). Erişim Tarihi: 21.03.2016.
- 3.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. http://dx.doi.org/10.1007/978-1-59259-740-6_25. [DOI] [PubMed] [Google Scholar]
- 4.Bikle D. Nonclassic of Actions vitamin D. JCEM. 2009;94:26–34. doi: 10.1210/jc.2008-1454. http://dx.doi.org/10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boullion R, Carmeliet G, Verlinden L, et al. Vitamin D and human healt: Lessons from vitamin D receptor null mice. Endocrin Rev. 2008;11:1–103. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. http://dx.doi.org/10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. http://dx.doi.org/10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Lapatsanis D, Moulas A, Cholevas V, Soukakos P, Papadopoulou ZL, Challa A. Vitamin D: a necessity for children and adolescents in Greece. Calcif Tissue Int. 2005;77:348–55. doi: 10.1007/s00223-004-0096-y. http://dx.doi.org/10.1007/s00223-004-0096-y. [DOI] [PubMed] [Google Scholar]
- 9.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics. 2009;124:1404–10. doi: 10.1542/peds.2008-2041. http://dx.doi.org/10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon CM, Feldman HA, Sinclair L, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162:505–12. doi: 10.1001/archpedi.162.6.505. http://dx.doi.org/10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maguire JL, Birken CS, O’Connor DL, et al. Prevalence and predictors of low vitamin D concentrations in urban Canadian toddlers. Paediatr Child Health. 2011;16:e11–e15. doi: 10.1093/pch/16.2.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozkan B, Büyükavcı M, Aksoy H, Tan H, Akdag R. Erzurumda 0–3 yas grubu çocuklarda nutrisyonel rikets sıklığı. Çocuk Sağlığı ve Hastalıkları Dergisi. 1999;42:389–96. [Google Scholar]
- 13.Ozkan B, Doneray H, Karacan M, et al. Prevalence of vitamin D deficiency rickets in the eastern part of Turkey. Eur J Pediatr. 2009;168:95–100. doi: 10.1007/s00431-008-0821-z. http://dx.doi.org/10.1007/s00431-008-0821-z. [DOI] [PubMed] [Google Scholar]
- 14.Andiran N, Yordam N, Ozon A. Risk factors for vitamin D deficiency in breast-fed newborns and their mothers. Nutrition. 2002;39:47–50. doi: 10.1016/s0899-9007(01)00724-9. http://dx.doi.org/10.1016/S0899-9007(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. http://dx.doi.org/10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 16.Roth DE, Shah R, Black RE, Baqui AE. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Pædiatr. 2010;99:389–93. doi: 10.1111/j.1651-2227.2009.01594.x. http://dx.doi.org/10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 17.Wayse W, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory tract infections in Indian children under 5 year. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. http://dx.doi.org/10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 18.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D Deficiency in Young Children With Severe Acute Lower Respiratory Infection. Pediatr Pulmonol. 2009;44:981–8. doi: 10.1002/ppul.21089. http://dx.doi.org/10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- 19.Banajeh SM. Nutritional Rickets and Vitamin D Deficiency Association With the Outcomes of Childhood Very Severe Pneumonia: A Prospective Cohort Study. Pediatr Pulmonol. 2009;44:1207–15. doi: 10.1002/ppul.21121. http://dx.doi.org/10.1002/ppul.21121. [DOI] [PubMed] [Google Scholar]
- 20.Inamo Y, Hasegawa M, Saito K, et al. Serum vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pediatr Int. 2011;53:199–201. doi: 10.1111/j.1442-200x.2010.03224.x. http://dx.doi.org/10.1111/j.1442-200X.2010.03224.x. [DOI] [PubMed] [Google Scholar]
- 21.Dogru M, Kirmizibekmez H, Yesiltepe Mutlu RG, Aktas A, Ozturkmen S. Clinical effects of vitamin D in children with asthma. Int Arch Allergy Immunol. 2014;164:319–25. doi: 10.1159/000366279. http://dx.doi.org/10.1159/000366279. [DOI] [PubMed] [Google Scholar]
- 22.Arikoglu T, Kuyucu S, Karaismailoglu E, Batmaz SB, Balci S. The association of vitamin D, cathelicidin, and vitamin D binding protein with acute asthma attacks in children. Allergy Asthma Proc. 2015;36:51–8. doi: 10.2500/aap.2015.36.3848. http://dx.doi.org/10.2500/aap.2015.36.3848. [DOI] [PubMed] [Google Scholar]
- 23.Uysalol M, Mutlu LC, Saracoglu GV, et al. Childhood asthma and vitamin D deficiency in Turkey: is there cause and effect relationship between them? Ital J Pediatr. 2013;39:78. doi: 10.1186/1824-7288-39-78. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]


