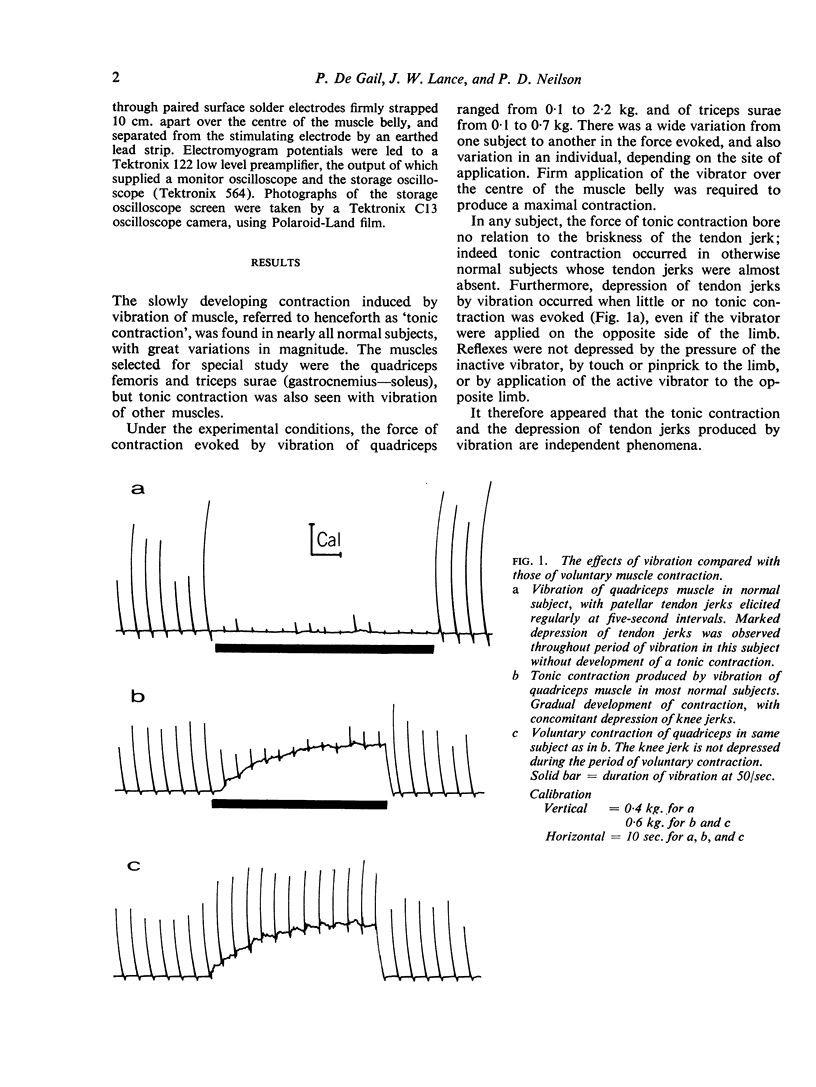
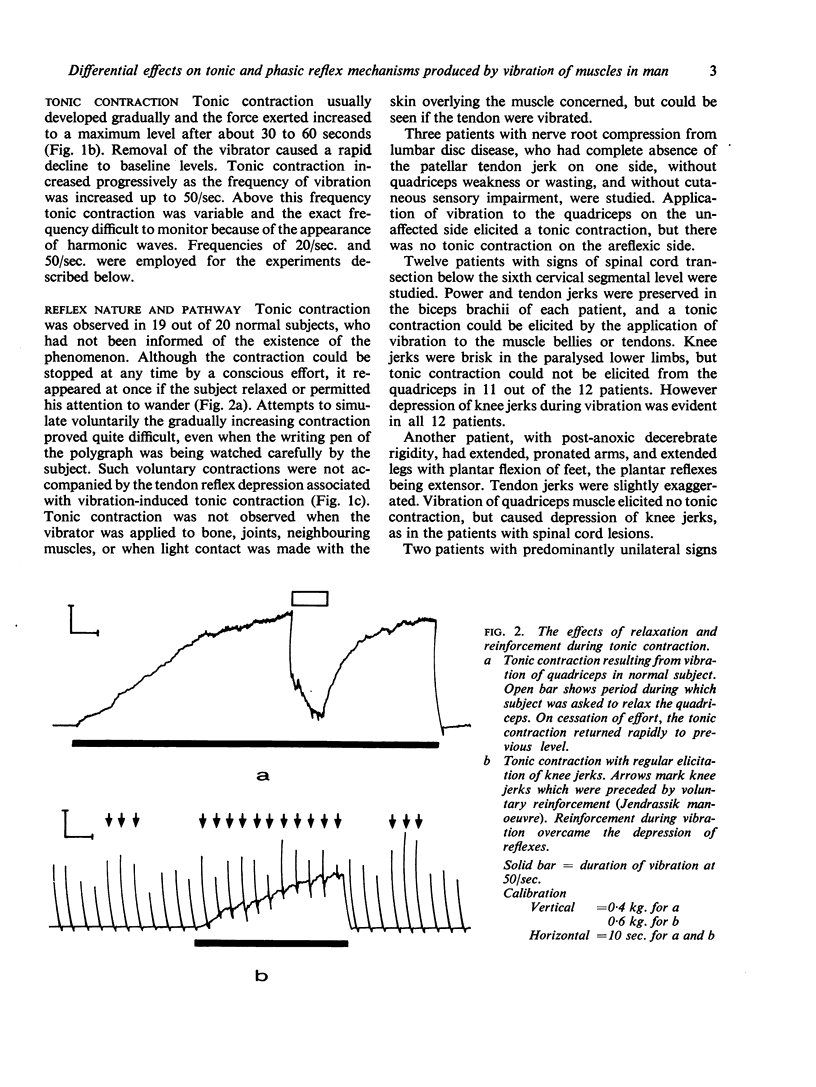
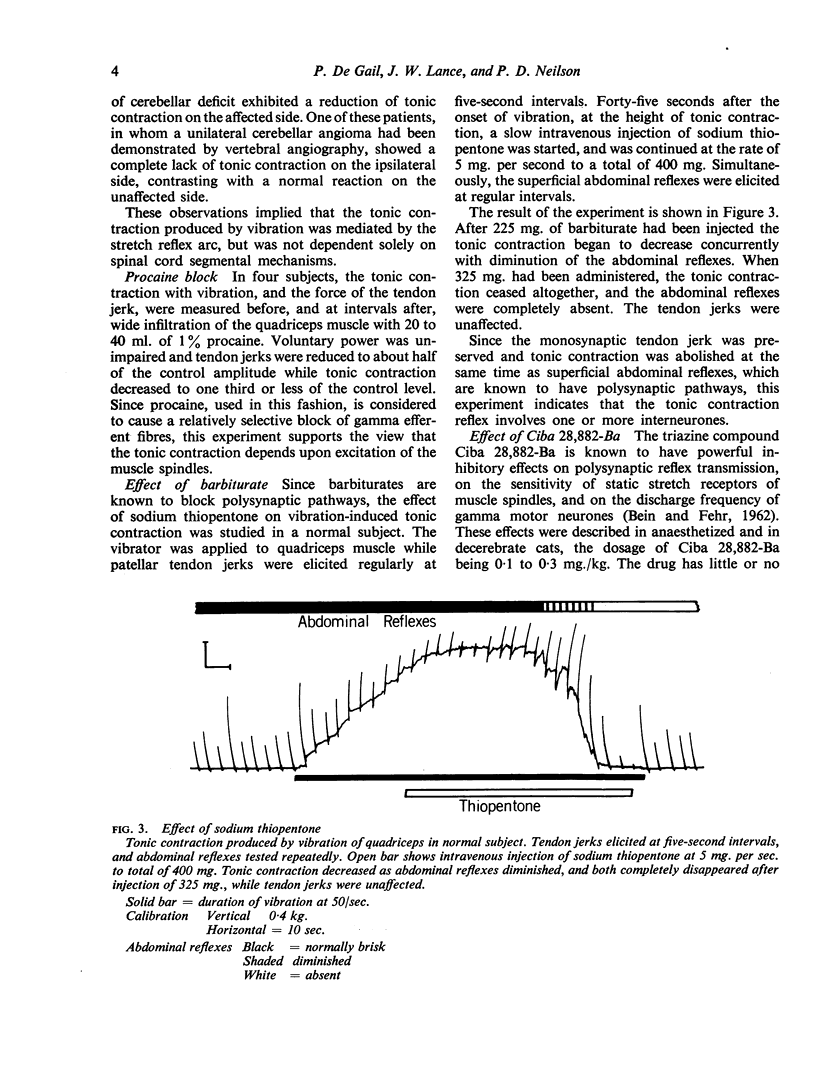
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEIN H. J., FEHR H. U. Depression of muscle spindle activity--a new type of pharmacological action? Br J Pharmacol Chemother. 1962 Dec;19:375–384. doi: 10.1111/j.1476-5381.1962.tb01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCONI R., van der MEULEN J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963 Jan;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- COOPER S. The responses of the primary and secondary endings of muscle spindles with intact motor innervation during applied stretch. Q J Exp Physiol Cogn Med Sci. 1961 Oct;46:389–398. doi: 10.1113/expphysiol.1961.sp001558. [DOI] [PubMed] [Google Scholar]
- CORRIE W. S., HARDIN W. B., Jr POST-TETANIC POTENTIATION OF H REFLEX IN NORMAL MAN; QUANTITATIVE STUDY. Arch Neurol. 1964 Sep;11:317–323. doi: 10.1001/archneur.1964.00460210095010. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D. E., Liddell E. G. Observations on the motor twitch and on reflex inhibition of the tendon-jerk of m. supraspinatus. J Physiol. 1927 Jun 7;63(1):70–80. doi: 10.1113/jphysiol.1927.sp002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., RALL W. Repetitive monosynaptic activation of motoneurones. Proc R Soc Lond B Biol Sci. 1951 Oct 30;138(893):475–498. doi: 10.1098/rspb.1951.0036. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C. THE EXCITATORY RESPONSES OF SPINAL NEURONES. Prog Brain Res. 1964;12:1–34. doi: 10.1016/s0079-6123(08)60614-7. [DOI] [PubMed] [Google Scholar]
- Echlin F., Fessard A. Synchronized impulse discharges from receptors in the deep tissues in response to a vibrating stimulus. J Physiol. 1938 Sep 16;93(4):312–334. doi: 10.1113/jphysiol.1938.sp003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASSEL M. M., DIAMANTOPOULOS E. THE EFFECT OF PROCAINE NERVE BLOCK ON NEUROMUSCULAR REFLEX REGULATION IN MAN. AN APPRAISAL OF THE ROLE OF THE FUSIMOTOR SYSTEM. Brain. 1964 Dec;87:729–742. doi: 10.1093/brain/87.4.729. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D. Gamma control of dynamic properties of muscle spindles. J Neurophysiol. 1956 Jul;19(4):356–366. doi: 10.1152/jn.1956.19.4.356. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D., STEG G. Tonic and phasic ventral horn cells differentiated by post-tetanic potentiation in cat extensors. Acta Physiol Scand. 1956 Sep 26;37(2-3):114–126. doi: 10.1111/j.1748-1716.1956.tb01347.x. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G., SKOGLUND S., STEG G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957 Sep;20(5):470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- HAGBARTH K. E. Post-tetanic potentiation of myotatic reflexes in man. J Neurol Neurosurg Psychiatry. 1962 Feb;25:1–10. doi: 10.1136/jnnp.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENATSCH H. D., MANNI E., DOW R. S. EFFECTS OF GAMMA LOOP INTERRUPTION ON CEREBELLAR CONTROL OF INDIVIDUAL ALPHA MOTONEURON STRETCH REFLEXES. J Neurophysiol. 1964 Mar;27:193–209. doi: 10.1152/jn.1964.27.2.193. [DOI] [PubMed] [Google Scholar]
- HENATSCH H. D., MANNI E., WILSON J. H., DOW R. S. LINKED AND INDEPENDENT RESPONSES OF TONIC ALPHA AND GAMMA HIND-LIMB MOTONEURONS TO DEEP CEREBELLAR STIMULATION. J Neurophysiol. 1964 Mar;27:172–192. doi: 10.1152/jn.1964.27.2.172. [DOI] [PubMed] [Google Scholar]
- LANGUTH H. W., TEASDALL R. D., MAGLADERY J. W. Electrophysiological studies of reflex activity in patients with lesions of the nervous system. III. Motoneurone excitability following afferent nerve volleys in patients with rostrally adjacent spinal cord damage. Bull Johns Hopkins Hosp. 1952 Oct;91(4):257–passim. [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B., RUSHWORTH G. The selective effect of proCaine on the stretch reflex and tendon jerk of soleus muscle when applied to its nerve. J Physiol. 1957 Feb 15;135(2):245–262. doi: 10.1113/jphysiol.1957.sp005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAYAMA S., SMITH C. M. RIGIDITY OF HIND LIMBS OF CATS PRODUCED BY OCCLUSION OF SPINAL CORD BLOOD SUPPLY. Neurology. 1965 Jun;15:565–577. doi: 10.1212/wnl.15.6.577. [DOI] [PubMed] [Google Scholar]
- RUSHWORTH G., LISHMAN W. A., HUGHES J. T., OPPENHEIMER D. R. Intense rigidity of the arms due to isolation of motoneurones by a spinal tumour. J Neurol Neurosurg Psychiatry. 1961 May;24:132–142. doi: 10.1136/jnnp.24.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]