Abstract
Increased resistance of Helicobacter pylori to clarithromycin and metronidazole has resulted in recommendation to substitute fluoroquinolones for eradication therapy. The aims of the study were to determine the prevalence and changes in primary levofloxacin resistance related to H. pylori gyrA sequences. The study utilized H. pylori strains isolated from patients undergoing gastroscopy in Bogotá, Colombia from 2009 to 2014. Levofloxacin susceptibility was assessed by agar dilution. Mutations in gyrA sequences affecting the quinolone resistance-determining region (QRDR) were evaluated by direct sequencing. Overall, the mean prevalence of primary levofloxacin resistance was 18.2% (80 of 439 samples). Resistance increased from 11.8% (12/102) in 2009 to 27.3% (21/77) in 2014 (p = 0.001). gyrA mutations in levofloxacin resistant strains were present in QRDR positions 87 and 91. The most common mutation was N87I (43.8%, 35/80) followed by D91N (28.8%, 23/80) and N87K (11.3%, 9/80). Levofloxacin resistance increased markedly in Colombia during the six-year study period. Primary levofloxacin resistance was most often mediated by point mutations in gyrA, with N87I being the most common QRDR mutation related to levofloxacin resistance.
Introduction
Helicobacter pylori (H. pylori) is an important human pathogen causing gastroduodenal inflammation that can result in duodenal ulcer, gastric ulcer, gastric adenocarcinoma and primary B-cell gastric lymphoma [1,2]. H. pylori has also been related to extragastric diseases, including iron deficiency anemia, idiopathic thrombocytopenic purpura and even colon adenocarcinoma [3]. The National Institutes of Health in the United States, the Maastricht Consensus conferences in Europe, Second Asia-Pacific Consensus Guidelines for H. pylori infection and the Canadian Consensus all recommended H. pylori eradication for the treatment/or prevention of these disorders in addition to reducing occurrence of new gastric cancers [4–9]. An increase H. pylori resistance to standard antibiotics such as clarithromycin, and metronidazole has been reported. Therefore, a reduction in triple therapy (proton pump inhibitors (PPI) plus clarithromycin and amoxicillin) treatment success has fallen below 80% for most countries. Escalating resistance to clarithromycin has urged the necessity to identify other treatment options [10–12]. Levofloxacin is a levorotary isomer of ofloxacin with known broad activity against Gram-negative and Gram-positive bacteria [13]. Levofloxacin mode of action is via bacterial type II topoisomerase inhibition, namely DNA gyrase and topoisomerase IV. The potential advantages of levofloxacin-containing triple therapy include the presence of an “in vivo” synergistic effect between quinolone antimicrobial agents and PPIs against H. pylori strains. However, quinolone resistance in H. pylori has been reported to be increasing and could undermine its efficacy [14–20].
The most common mechanism of fluoroquinolone resistance involves mutations in DNA gyrase and in DNA topoisomerase IV. These genes encode large enzymatic quaternary structures that consist of two pairs of subunits: GyrA and GyrB (DNA gyrase) and ParC and ParE (DNA topoisomerase IV). However, H. pylori lacks topoisomerase IV, thus fluoroquinolone resistance is likely due to mutations in the DNA gyrase gene (gyrA), encoding the DNA gyrase subunit A (GyrA) [14–19,21]. The absence of a secondary target for fluoroquinolones suggests a single modification in the gyrA gene could be sufficient to result in a fluoroquinolone-resistant phenotype. The amino acid substitutions observed in clinical strains have been primarily reported at positions 87 (Asn to Lys) and 91 (Asp to Gly, Asp to Asn, or Asp to Tyr) [22,23]. The presence of levofloxacin resistance in levofloxacin-containing therapy has a dramatic detrimental effect on treatment success [18]. Therefore, levofloxacin prescription therapy should be based on local resistance prevalence. The aims of the present study were to determine the rate of primary levofloxacin resistance in H. pylori (prevalence and changes) in patients from Bogotá, Colombia from 2009 to 2014. In addition, we determined gyrA sequence diversity of resistant strains.
Results
Patient Characteristics
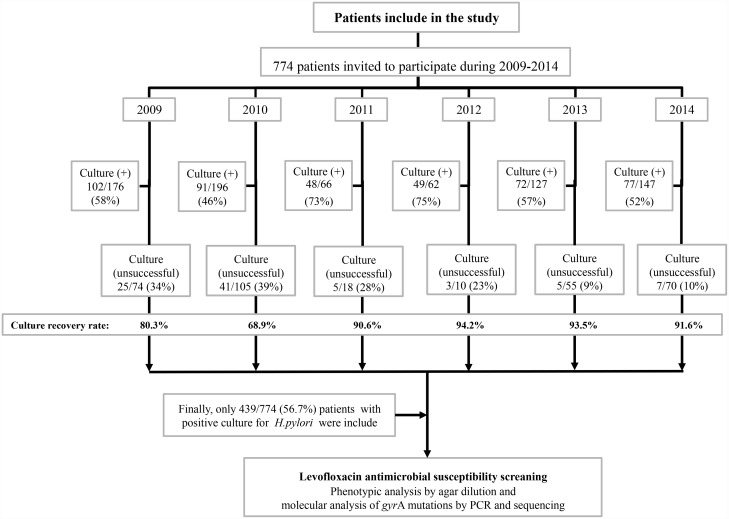
A total of 774 Colombian patients were enrolled from 2009 to 2014. After bacterial culture growth for H. pylori, only 439 (56.7%) patients were positive for H. pylori and were included in the study (Fig 1). Of the 439 H. pylori positive patients, 314/439 (71.6%) were women and 125/439 (38.4%) were men. Their ages ranged between 18 to 73 years old, with an average age of 46 ± 11 and 47 ± 13 for women and men, respectively. All of them were diagnosed with H. pylori chronic gastritis. All patients taking PPIs or H2-receptor antagonists were removed from this study, thus excluding patients with duodenal or gastric ulcers. The number of patients enrolled by year were: 2009: 102 subjects, 2010: 91 subjects, 2011: 48 subjects, 2012: 49 subjects, 2013: 72 subjects and 2014: 77 subjects.
Fig 1. Patients included in the study.
Diagram depicting details of number of patients/year and who was included in the study, based on H. pylori positive culture. Negative culture was related to H. pylori (negative), confirmed by histological analysis and rapid urease test or any other laboratory assay. Unsuccessful describes a sample where it was not possible to recover the isolate from a positive biopsy sample (positive for histological analysis and positive for rapid urease test and any other laboratory assay).
Prevalence of Levofloxacin Resistance
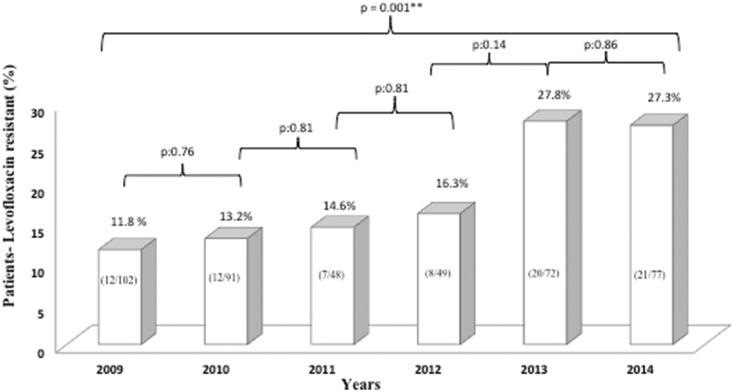
A total of 486 H. pylori isolates were obtained from 439 positive patients; meaning more than one isolate from the same patient, as has been previously reported [24–30]. The mean primary levofloxacin resistance rate was 18.2% (80/439). Minimum inhibitory concentrations (MICs) for levofloxacin resistant strains ranged from 1 to 64 μg/mL. The MICs distributions of susceptible strains ranged from 0.016 to 0.5 μg/mL. The levofloxacin resistance rate (per patient) studied for each year are in fig 2. Although resistance between each year was not significantly different, when comparing the resistant rate between 2009 and 2014 it was highly significant (p = 0.001) with a 15.5% difference (Fig 2).
Fig 2. Yearly prevalence.
Patients with H. pylori strains resistant to levofloxacin and statistical significance of differences in Bogotá-Colombia; between 2009 and 2014.
gyrA Mutations
Mutations in levofloxacin resistant strains were present in quinolone resistance-determining region (QRDR) at positions 87 and 91. The concordance score between phenotypic and genotypic test was 0.98. The most common resistant strain substitution was N87I, present in 43.8% (35/80), followed by D91N (28.8%; 23/80) and N87K present in 11.3% (9/80). Four of the patients with levofloxacin resistant strains (MICs from 1 to 8 μg/mL) did not present a gyrA mutation, and two patients with susceptible strains had N87I mutations. Levofloxacin MIC was 0.5 μg/mL for both N87I strains. Strains from two patients presented double mutations at 87 and 91 positions. Combination of double mutations at N87Y and D91G in one strain had a MIC of 1 μg/mL. The other double mutation (N87I and D91G) had a MIC of 32 μg/mL (Table 1).
Table 1. Association between minimal inhibitory concentrations levels (MICs) and gyrA nucleotides substitution and types of mutations in gyrA QRDR.
| Patients with gyrA mutations in H. pylori n (%) | MIC (μg/mL) | gyrA mutations data | ||
|---|---|---|---|---|
| Nucleotide change | Aminoacid change | gyrA mutation and position | ||
| 35/80 (43.8) | 1–32 | AAC → ATC | Asn → Ile | N87I |
| 9/80 (11.3) | 2–16 | AAC → AAA | Asn → Lys | N87K |
| 1/80 (1.3) | 4 | AAC → TAT | Asn → Tyr | N87Y |
| 23/80 (28.8) | 2–32 | GAT → GGT | Asp → Gly | D91G |
| 4/80 (5.0) | 1 | GAT → AAT | Asp → Asn | D91N |
| 1/80 (1.3) | 2 | GAT → TAT | Asp →Tyr | D91Y |
| 1/80 (1.3) | 1 | AAC → TAT GAT → AAT |
Asn → Tyr Asp → Asn |
N87Y D91N |
| 1/80 (1.3) | 32 | AAC → ATC GAT → AAT |
Asn → Ile Asp → Asn |
N87I D91G |
| 4/80 (5)* | 1–8 | AAC GAT |
Asn Asp |
No Mutation |
| 1/80 (1.3)* | 0.5 | AAC → ATC | Asn → Ile | N87I |
*conflicting results between genotypic and phenotypic resistant
Discussion
Cammarota et al. proposed fluoroquinolones, such as levofloxacin as a first line of treatment as an alternative to clarithromycin against H. pylori [13]. In this work, we evaluated changes in the prevalence of “primary” levofloxacin resistance of H. pylori in isolates obtained in Bogotá, Colombia from January 2009 to August 2014. Distribution of patients per year is shown in fig 1, depicting the irregular nature of patient entry, since several other institutions performed endoscopies and did not participate in this study. Although the inclusion of patients was consecutive the referrals to our unit for endoscopy varied over time. In addition, the culture recovery rate for H. pylori from biopsy was more stable (90 to 94%) between 2011 and 2014 (Fig 1). Hence, prevalence of resistance to levofloxacin could be possibly higher than reported in this paper.
During the observation period, levofloxacin resistance significantly increased (p = 0.001) from 11.8% (12/102) in 2009 to 27.3% (21/77) in 2014 (Fig 2). Remarkably, only 14 years after it was introduced as an alternative treatment for H. pylori eradication, levofloxacin resistance increased to the point it was no longer an acceptable choice as an empiric therapy. Indeed, given that patients did not receive previous H. pylori treatment, most likely resistance could be the result of treatment for other infections such as respiratory infections, urinary tract infections, or diarrheal diseases. In addition, it is not possible to document fluoroquinolone consumption in Colombia (dose per 1,000 inhabitants/day) because it is possible to purchase this antibiotic without medical prescription; which allows for self-medication, favoring an increase in resistance. Camargo et al., (2014) study support this hypothesis, where H. pylori resistance to quinolones was reported to be approximately 15% in Latin America [31].
In Colombia the first line of treatment is triple therapy containing metronidazole, clarithromycin or levofloxacin in addition to amoxicillin and proton pump inhibitors for seven days. Various studies performed in Colombia since 1998 have evaluated H. pylori resistance to metronidazole. Since then, resistance to this antimicrobial agent has been reported to be greater than 80% [32]. In contrast, clarithromycin resistance has been reported to be low. A first study in 2007 by Alvarez et al., in the coffee growing region of the country reported a low resistance of 2.2% [33]. These results contrast with those from Bogota, the country’s capital where Trespalacios et al., (2013) reported a 13.6% resistance [34]. In addition, for the south of Colombia Acosta et al., (2014) reported a clarithromycin resistance of 4% [35]. Given these resistance variability’s for clarithromycin and few available studies for the different regions of the country, many gastroenterologists prefer to prescribe clarithromycin in regions where resistance to this antimicrobial is low. In contrast, for Bogota levofloxacin is commonly prescribed for first and second line therapies.
The scheme for second line of treatment mostly used is therapy with levofloxacin or quadruple therapies. However, a Colombian survey revealed physicians don’t follow these recommendations or international guidelines, but in an intuitive manner prescribe different regimens that for the most part have not been previously investigated [36]. However, this is the first study carried-out in Colombia to evaluate H. pylori resistance to levofloxacin in Bogota.
Other countries have also reported an increase in quinolone resistance. A systematic review published in 2010 indicated a global worldwide levofloxacin resistance of 16.2% (95% CI 14.4–18.0) with the highest level of resistance in Europe (24.1%); followed by a mid- resistance level in Asia (11.6%), with differences among Asian countries: Japan (14.9%), Taiwan 11.9% and Hong Kong 2.6% [15]. In addition, recent reports have suggested that prevalence of levofloxacin-resistant H. pylori strains is increasing. A recently steady annual increase has been observed, ranging from 18% up to >30% for Europe and Asia, Italy (18%), Turkey (23.8 to 29.5%), Vietnam (41.3%) and China (Beijing, 54.8%), [37–41]. Based on these findings it is advisable to restrict the empiric use of levofloxacin [42].
Quinolone resistance-determining region mutations in levofloxacin resistant H. pylori isolates had previously been reported. For strains isolated in our study we did not find any correlation between the mutation present and the level of resistance. However, higher levels of resistance were most likely in isolates with N87I and D91G mutations, i.e. with MIC values of 32 μg/mL. Our data agrees with reports from Asia and Europe [14–16]. Two of our susceptible strains had N87I mutations with MICs close to breakpoint (0.5 μg/mL). Conflicting results between genotypic and phenotypic resistance (Table 1) were found in those with gyrA wild-type sequences. 1/4 (25%), of resistant isolates without mutations in gyrA, had the mutation E463K in gyrB, hence this mutation is a candidate explaining isolate resistance. No mutations were identified in gyrB for the other 3/4 (75%) resistant isolates, without mutations in gyrA, (supplementary material). These data suggest the presence of additional resistant mechanisms involved in levofloxacin resistance [16,43]. According Rimbara et al., (2012) results, H. pylori isolates resistant to fluoroquinolones and without gyrA mutations are likely to have mutations in gyrB at positions 463 (supplementary material).
Discordant results were observed in four patients, where no phenotypic resistance was observed in the presence of gyrA sequence mutation (Table 1). These results may be possibly due to the levofloxacin resistance breakpoint used in this study (1 μg/mL). However, other studies describe isolates with gyrA mutations with a MIC of 0.5 μg/mL [44]. Additional studies are needed to correlate MIC and gyrA mutations, in order to obtain an accurate breakpoint for levofloxacin.
Overall, QRDR mutations were most often found at positions 87 and 91, and included N87I, N87K, N87Y, D91G, D91N and D91Y (Table 1). In 75/80 (94%) patients with resistant strains (MIC > 1 μg/mL), mutations occurred in the gyrA gene. This finding is consistent with mutations in gyrA occurrence, as a principal mechanism for levofloxacin resistance. As previously stated, four of our patients with resistant strains (MICs from 1 to 8 μg/mL) did not have a detectable mutation in QRDR of the gyrA gene. Thus, further studies are necessary to identify the resistance mechanism in those strains. As a case in point, Rimbara et al., (2011, 2012) reported gyrB mutations as a potential novel mechanism of resistance to fluoroquinolones in H. pylori [2,43]. However, other still unknown mechanisms could be also involved.
N87I mutation was detected in 43.8% (35/80) of resistant strains followed by D91G with a frequency of 28.8% (23/80) and N87K present in 11.3% (9/80), (Table 1). This distribution is different from that reported in Europe, Asia or Canada (North America), where the major mutation sites were N87K and D91G [14,45]. N87I mutation results from amino acid wild type H. pylori J99 substitution of aminoacid Thr87 to Ileu, with a C to T substitution at codon 87 ACC→ATC [21], (Table 1).
In conclusion, the prevalence of primary resistance to levofloxacin has been increasing in Colombia. Although, levofloxacin resistance was due to point mutation in gyrA, the mutations pattern differed from than reported in Europe. Results from this study evidenced the most common mutation in isolates resistant to levofloxacin was gyrA mutation N87I. This result contrasts with studies performed in France, where the most frequent mutation was N87K, followed by D91N, D91G and D91Y [46]. Mutation N87I has not been reported by the aforementioned studies. In a similar manner studies carried-out in Malaysia and Beijing report the most frequent gyrA mutations were: N87K, D91N, D91G, D91Y [16,47]. These results are different from those reported from Senegal, Africa; where the most common mutation was N87I followed by D91N. Low frequencies were reported for D91G and D91Y [45,48]. These last results agree with the ones reported in our study, where the most frequent mutation was N87I, followed by D91G and N87K. Therefore, it is important to continue performing studies that allow identifying gyrA mutations responsible for fluoroquinolone resistance and the geographical explanation for these differences. Surveillance of quinolone resistance in H. pylori should include assessment of new responsible mutations. The geographical area should be considered, to update the increasingly popular molecular tests.
Materials and Methods
Patients
Adult outpatients referred for gastroscopy at the Gastroenterology Unit of “Clínica Fundadores”, Bogotá D.C., Colombia, between January 2009 and August 2014, both positive for H. pylori rapid urease test (RUT) and histopathology (Giemsa stain) were enrolled. Written informed consent was obtained from each participating patient before enrolling the study. Research protocol was approved by the ethical committee of “Pontificia Universidad Javeriana” and “Clínica Fundadores” in Bogotá D.C., Colombia. Patients were excluded if they were taking PPI or H2-receptor antagonists four weeks prior to enrollment. Since the objective of the study was to assess primary resistance, those with previous anti-H. pylori treatment were also excluded, as were patients with concomitant illness. Biopsies from antrum and body of the stomach underwent bacterial culture and susceptibility testing and DNA extraction.
Bacterial strains, culture conditions, and determination of susceptibility to levofloxacin
Biopsies samples from antrum and body of the stomach were crushed in 0.5 mL PBS and cultured in Wilkins Chalgren Agar (Becton Dickinson, Heidelberg, Germany) containing 7% (v/v) horse blood, vancomycin (10 mg/L) and trimethoprim (5 mg/L). Petri dishes were incubated at 37°C under microaerophilic conditions for up to 14 days. H. pylori growth was confirmed by typical colony morphology, Gram stain, positive oxidase, catalase and urease tests.
Levofloxacin MICs for the recovered H. pylori strains were measured using the agar dilution method according to the guidelines of the National Committee for Clinical Laboratory Standards (CLSI), [49]. The resistance breakpoint was defined as ≥1 μg/mL [50,51]. The agar dilution method was performed by serial two fold dilution, mixing the antibiotic with molten Mueller-Hinton agar II, supplemented with 5% (v/v) sheep blood and 0.4% (v/v) Isovitalex (Becton Dickinson, MD) to reach the desired antibiotic concentration. 1 to 3 μL of a McFarland 2.0 adjusted inoculum were dropped on the surface of the Petri dishes; containing the solid media, followed by 35 ± 2°C for 72 h under microaerophilic conditions. For quality control H. pylori NCTC 11637, was used.
DNA Extraction, PCR Amplification and Nucleotide Sequence Analysis
Genomic DNA of H. pylori strains was extracted using DNAzol® kits (Invitrogen—USA). DNA was stored at -20°C until use. To detect gene mutations in the QRDRs subunit A of the DNA gyrase (gyrA), degenerated oligonucleotide primers reported by Tankovic et al., (2003); 5’-TTT RGC TTA TTC MAT GAG CGT-3’ and 5’-GCA GAC GGC TTG GTA RAA TA-3’ were used. Primers were synthesized by Invitrogen-USA. The size of the amplified fragments of gyrA was 428 bp. The PCR was performed with PCR Master Mix—Promega (WI, USA) using 1.0 μL DNA template, 1.0 pmol/L of each primer, in a final mix reaction volume of 50 μL. The cycling conditions were first 3 minutes at 94°C followed by 35 cycles of 1 min denaturation at 94°C, 1 min annealing at 57°C and 1 min extension at 72°C [52]. PCR products were analyzed by visualizing in 1.5% (w/v) agarose gel electrophoresis, followed by SYBR Green (Invitrogen-USA) staining for 30 minutes. PCR product purification and direct sequencing was performed by Macrogen, Korea. The oligonucleotides used for gyrA PCR were also used for DNA sequencing. The sequences were compared to H. pylori gyrA gene published sequence (GeneBank accession No. L29481). PCR was performed for all strains (susceptible and resistant) and direct sequencing was obtained for all PCR products.
Data Analysis
Frequencies and percentages were used to describe H. pylori antimicrobial isolate resistance. Statistical significances for resistance rates among the different collection years were analyzed by the chi-squared test (χ2) with p < 0.05 considered as statistically significant. Phenotypic susceptibility was evaluated by MIC in μg/mL. Genotypic resistance was determined by the presence or lack of a mutation involved with resistance. Degree of agreement (concordance) between phenotypic and genotypic resistance was performed using Cohen’s kappa coefficient. All statistical analyses were performed using the statistical software package SPSS 18.0 for Windows (SPSS, Chicago, IL, USA).
Acknowledgments
We would like to express our special thanks to the staff of Gastroenterology Unit of “Clínica Fundadores”, Bogotá D.C., Colombia for technical support in this study. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. The authors thank Fiona Raikes and María Lucía Gutiérrez for English editing.
Data Availability
Data are available from Figshare: https://figshare.com/s/c1928ecda291ed2c61fa doi: 10.6084/m9.figshare.3386065.
Funding Statement
This work was supported by COLCIENCIAS (Colombia), Grant 120340820464, Pontificia Universidad Javeriana, Bogotá, D.C. (Colombia) Grant 00004554 and by Michael E. DeBakey Veterans Affairs Medical Center Internal Project and the Baylor College of Medicine, United States. Dr. Graham was supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service Grant DK56338, which funds the Texas Medical Center Digestive Diseases Center and DK067366.
References
- 1.Liu Y, Ponsioen CIJ, Xiao S, Tytgat GNJ, Ten Kate FJW (2005) Geographic pathology of Helicobacter pylori gastritis. Helicobacter 10: 107–113. [DOI] [PubMed] [Google Scholar]
- 2.Rimbara E, Fischbach LA, Graham DY (2011) Optimal therapy for Helicobacter pylori infections. Nature Review 8: 79–88. 10.1038/nrgastro.2010.210 [DOI] [PubMed] [Google Scholar]
- 3.Testerman TL, Morris J (2014) Beyond the stomach: An updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World Journal of Gastroenterology 20: 12781–12808. 10.3748/wjg.v20.i36.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howden CW, Hunt RH (1998) Guidelines for the management of Helicobacter pylori. Ad Hoc Committee on practice parameters of the American College of Gastroenterology. American Journal of Gastroenterology 93: 2330–2338. [DOI] [PubMed] [Google Scholar]
- 5.Bourke B, Ceponis P, Chiba N, Czinn S, Ferraro R, et al. (2005) Canadian Helicobacter study group consensus conference: update on the approach to Helicobacter pylori infection in children and adolescents—an evidence-based evaluation. Canadian Journal of Gastroenterology 19: 399–408. [PubMed] [Google Scholar]
- 6.Caselli M, Zullo A, Maconi G, Parente F, Alvisi V, et al. (2007) Cervia II Working Group Report 2006": guidelines on diagnosis and treatment of Helicobacter pylori infection in Italy. Digestive and Liver Disease 39: 782–789. [DOI] [PubMed] [Google Scholar]
- 7.Chey WD, Wong BC (2007) American college ofgastroenterology guideline on the management of Helicobacter pylori infection. American Journal of Gastroenterology 102: 1808–1825. [DOI] [PubMed] [Google Scholar]
- 8.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, et al. (2009) Second Asia-Pacific consensus guidelines for Helicobacter pylori infection. Journal of Gastroenterology and Hepatology 24: 1587–1600. 10.1111/j.1440-1746.2009.05982.x [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, et al. (2012) Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut 61: 646–664. 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- 10.Graham DY, Fischbach L (2010) Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59: 1143–1153. 10.1136/gut.2009.192757 [DOI] [PubMed] [Google Scholar]
- 11.Graham DY, Lu H, Yamaoka Y (2007) A report card to grade Helicobacter pylori therapy. Helicobacter 12: 275–278. [DOI] [PubMed] [Google Scholar]
- 12.Graham DY, Lu H, Yamaoka Y (2008) Therapy for Helicobacter pylori Infection can be improved: sequential therapy and beyond. Drugs 68: 725–736. [DOI] [PubMed] [Google Scholar]
- 13.Cammarota G, Cianci R, Cannizzaro O, Cuoco L, Pirozzi G, et al. (2000) Eficacy of two one-week rabeprazole/levofoxacin-based triple therapies for Helicobacter pylori infection. Alimentary Pharmacology Therapeutics 14: 1339–1343. [DOI] [PubMed] [Google Scholar]
- 14.Miyachi H, Miki I, Aoyama N, Shirasaka D, Matsumoto Y, et al. (2006) Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter 11: 243–249. [DOI] [PubMed] [Google Scholar]
- 15.De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, et al. (2010) Worldwide H. pylori antibiotic resistance: A systematic review Journal of Gastrointestinal Liver Disese 19: 409–414. [PubMed] [Google Scholar]
- 16.Wang LH, Cheng H, Hu FL, Li J (2010) Distribution of gyrA mutations in fluoroquinolone-resistant Helicobacter pylori strains. World Journal of Gastroenterology 16: 2272–2277. 10.3748/wjg.v16.i18.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, et al. (2013) Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 62: 34–42. 10.1136/gutjnl-2012-302254 [DOI] [PubMed] [Google Scholar]
- 18.Chuah S-K, Tai W-C, Lee C-H, Liang C-M, Hu T-H (2014) Quinolone-containing therapies in the eradication of Helicobacter pylori. BioMed Research International 2014: Article ID 151543 10.1155/2014/151543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou J-M, Chang C-Y, Chen M-J, Chen C-C, Fang Y-J, et al. (2015) The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and Its relation to virulence factors—A nationwide study. Plos One 10: e0124199 10.1371/journal.pone.0124199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogaerts P, Berhin C, Nizet H, Glupczynski Y (2006) Prevalence and mechanisms of resistance to fluoroquinolones in Helicobacter pylori strains from patients living in Belgium. Helicobacter 11: 441–445. 10.1111/j.1523-5378.2006.00436.x [DOI] [PubMed] [Google Scholar]
- 21.Cattoir V, Nectoux J, Lascols C, Deforges L, Delchier JC, et al. (2007) Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. International Journal Antimicrobial Agents 29: 389–396. [DOI] [PubMed] [Google Scholar]
- 22.Hung KH, Sheu BS, Chang WL, Wu HM, Liu CC, et al. (2009) Prevalence of primary fluoroquinolone resistance among clinical isolates of Helicobacter pylori at a University Hospital in Southern Taiwan. Helicobacter 14: 61–65. 10.1111/j.1523-5378.2009.00655.x [DOI] [PubMed] [Google Scholar]
- 23.Murakami K, Okimoto T, Kodama M, Tanahashi J, Fujioka T, et al. (2009) Sitafloxacin activity against Helicobacter pylori isolates, including those with gyrA mutations. Antimicrobial Agents and Chemotherapy 53: 3097–3099. 10.1128/AAC.01552-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arévalo-Galvis A, Trespalacios-Rangel AA, Otero W, Mercado-Reyes M, Poutou-Piñales RA (2012) Prevalence of cagA, vacA, babA2 and iceA genes in H. pylori strains isolated from Colombian patients with functional dyspepsia. Polish Journal of Microbiology 61: 33–40. [PubMed] [Google Scholar]
- 25.Podzorski RP, Podzorski DS, Wuerth A, Tolia V (2003) Analysis of the vacA, cagA, cagE, iceA, and babA2 genes in Helicobacter pylori from sixty-one pediatric patients from the Midwestern United States. Diagnostic Microbiology and Infectious Disease 46: 83–88. [DOI] [PubMed] [Google Scholar]
- 26.Cittelly DM, Huertas MG, Martínez JD, Oliveros R, Posso H, et al. (2002) Los genotipos de Helicobacter pylori en gastritis no atrófica difieren de los encontrados en úlcera péptica, lesiones premalignas y cáncer gástrico en Colombia. Revista Médica de Chile 130: 143–151. 10.4067/S0034-98872002000200003 [DOI] [PubMed] [Google Scholar]
- 27.Morales-Espinosa R, Castillo-Rojas G, Gonzalez-Valencia G, Ponce De León S, Cravioto A, et al. (1999) Colonization of Mexican Patients by Multiple Helicobacter pylori Strains with Different vacA and cagA Genotypes. Journal of Clinical Microbiology 37: 3001–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García CA, Barra TR, Delgado SchC, Kawaguchi PF, Trabal FN, et al. (2006) Genotipificación de aislados clínicos de Helicobacter pylori en base a genes asociados a virulencia cagA, vacA y babA2. Primer aislamiento de una cepa babA2 positiva en pacientes chilenos. Revista Médica de Chile 134: 981–988. 10.4067/S0034-98872006000800006 [DOI] [PubMed] [Google Scholar]
- 29.Figueiredo C, van Doorn LJ, Nogueira C, Soares JM, Pinho C, et al. (2001) Helicobacter pylori genotypes are associated with clinical outcome in Portuguese patients and show a high prevalence of infections with multiple strains. Scandinavian Journal of Gastroenterology 36: 128–135. [DOI] [PubMed] [Google Scholar]
- 30.Blaser MJ (1997) The versatility of Helicobacter pylori in the adaptation to the human stomach. Journal Physiology and Pharmacology 48: 307–314. [PubMed] [Google Scholar]
- 31.Camargo MC, García A, Riquelme A, Otero W, Camargo CA, et al. (2014) The Problem of Helicobacter pylori Resistance to antibiotics: a systematic review in Latin America. American Journal of Gastroenterology 109: 485–495. 10.1038/ajg.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutiérrez O, Otero W (1998) Resistencia del Helicobacter pylori al metronidazol en Colombia. Revista Colombiana de Gastroenterología 12: 31–36. [Google Scholar]
- 33.Álvarez A, Moncayo JI, Santacruz JJ, Corredor LF, Reinosa E, et al. (2009) Resistencia a metronidazol y claritromicina en aislamientos de Helicobacter pylori de pacientes dispépticos en Colombia. Revista Médica de Chile 137: 1309–1314. [PubMed] [Google Scholar]
- 34.Trespalacios AA, Otero W, Caminos JE, Mercado MM, Ávila J, et al. (2013) Phenotypic and genotypic analysis of clarithromycin-resistant Helicobacter pylori from Bogotá D.C., Colombia. The Journal of Microbiology 51: 448–452. 10.1007/s12275-013-2465-6 [DOI] [PubMed] [Google Scholar]
- 35.Acosta CP, Hurtado FA, Trespalacios A (2014) Determination of single nucleotide mutations in the 23S rRNA gene of Helicobacter pylori related to clarithromycin resistance in a population from Cauca, Colombia. Biomédica 34: 156–162. 10.1590/S0120-41572014000500018 [DOI] [PubMed] [Google Scholar]
- 36.Gómez M, Otero W, Gutiérrez Ó (2007) Helicobacter pylori infection treatment. Survey performed by a group of general practitioners and specialist in Colombia. Revista Colombiana de Gastroenterología 22: 7–16. [Google Scholar]
- 37.Paoluzi OA, Del Vecchio Blanco G, Visconti E, Coppola M, Fontana C, et al. (2015) Low efficacy of levofloxacin-doxycycline-based third-line triple therapy for Helicobacter pylori eradication in Italy. World Journal of Gastroenterology 21: 6698–6705. 10.3748/wjg.v21.i21.6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caliskan R, Tokman HB, Erzin Y, Saribas S, Yuksel P, et al. (2015) Antimicrobial resistance of Helicobacter pylori strains to fi ve antibiotics, including levofloxacin, in northwestern Turkey. Revista da Sociedade Brasileira de Medicina Tropical 48: 278–284. 10.1590/0037-8682-0027-2015 [DOI] [PubMed] [Google Scholar]
- 39.Phan TN, Santona A, Tran VH, Tran TNH, Le VA, et al. (2015) High rate of levofloxacin resistance in a background of clarithromycin-and metronidazole-resistant Helicobacter pylori in VietnamTrung. International Journal of Antimicrobial Agents 45: 244–248. 10.1016/j.ijantimicag.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y-X, Zhou L-Y, Song Z-Q, Zhang J-Z, He L-H, et al. (2015) Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: A prospective serial study. World Journal of Gastroenterology 21: 2786–2279. 10.3748/wjg.v21.i9.2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kocazeybek B, Tokman HB (2015) Prevalence of primary antimicrobial resistance of H. pylori in Turkey: a systematic review. Helicobacter IN PRESS. 10.1111/hel.12272 [DOI] [PubMed] [Google Scholar]
- 42.Megraud F (2012) The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therapeutic Advances in Gastroenterology 5: 103–109. 10.1177/1756283X11432492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rimbara E, Noguchi N, Kawai T, Sasatsu M (2012) Fluoroquinolone resistance in Helicobacter pylori: role of mutations at position 87 and 91 of GyrA on the level of resistance and identification of a resistance conferring mutation in GyrB. Helicobacter 17: 36–42. 10.1111/j.1523-5378.2011.00912.x [DOI] [PubMed] [Google Scholar]
- 44.Liou JM, Chang CY, Sheng WH, Wang YC, Chen MJ, et al. (2011) Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin and clarithromycin-based therapies. Antimicrobial Agents and Chemotherapy 55: 1123–1129. 10.1128/AAC.01131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cambau E, Allerheiligen V, Coulon C, Corbel C, Lascols C, et al. (2009) Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori. Journal of Clinical Microbiology 47: 3600–3607. 10.1128/JCM.00744-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia M, Raymond J, Garnier M, Cremniter J, Burucoa C (2012) Distribution of Spontaneous gyrA mutations in 97 fluoroquinolone-resistant Helicobacter pylori isolates collected in France. Antimicrobial Agents and Chemotherapy 56: 550–551. 10.1128/AAC.05243-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teh X, Khosravi Y, Lee WC, Leow AH, Loke MF, et al. (2014) Functional and molecular surveillance of Helicobacter pylori antibiotic resistance in Kuala Lumpur. Plos One 9: e101481 10.1371/journal.pone.0101481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seck A, Burucoa C, Dia D, Mbengue M, Onambele M, et al. (2013) Primary antibiotic resistance and associated mechanisms in Helicobacter pylori isolates from Senegalese patients. Annals of Clinical Microbiology and Antimicrobials 12: 3 10.1186/1476-0711-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clinical and Laboratory Standards Institute (2010) Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement M100-S20. Vol. 30 No. 31: 160 p. [Google Scholar]
- 50.Kim JM, Kim JS, Kim N, Jung HC, Song IS (2005) Distribution of fluoroquinolone MICs in Helicobacter pylori strains from Korean patients. Journal of Antimicrobial Chemotherapy 56: 965–967. 10.1093/jac/dki334 [DOI] [PubMed] [Google Scholar]
- 51.Wueppenhorst N, Stueger H-P, Kist M, Glocker E (2009) Identification and molecular characterization of triple- and quadruple-resistant Helicobacter pylori clinical isolates in Germany. Journal of Antimicrobial Chemotherapy 63: 648–653. 10.1093/jac/dkp003 [DOI] [PubMed] [Google Scholar]
- 52.Tankovic J, Lascols C, Sculo Q, Petit JC, Soussy CJ (2003) Single and double mutations in gyrA but Not in gyrB are associated with low- and high-level fluoroquinolone resistance in Helicobacter pylori. Antimicrobial Agents and Chemotheraphy 47: 3942–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from Figshare: https://figshare.com/s/c1928ecda291ed2c61fa doi: 10.6084/m9.figshare.3386065.