Abstract
Background
The benefit of combined chemoradiation in elderly patients with human papillomavirus (HPV)-positive locally advanced oropharyngeal squamous cell carcinoma (SCC) must be balanced with the potential for higher toxicity rates. We performed a retrospective review of our institutional experience.
Methods
Patients 70 years or older with p16-positive oropharyngeal SCC treated with definitive chemoradiation from 2005 to 2013 were evaluated. Overall survival (OS), disease-free survival (DFS), and locoregional failure–free survival were calculated.
Results
Twenty-one eligible patients had a follow-up of 22.4 months. Estimated 5-year OS, DFS, and locoregional failure–free survival were 76.0%, 40%, and 95%, respectively. There was 1 death from acute toxicity, and 50% had unplanned hospitalizations. Sixty percent had late toxicity, and 6-month feeding tube dependence was 25%.
Conclusion
Elderly patients with HPV-positive locally advanced SCC of the oropharynx treated with definitive chemoradiation had good OS but high rates of acute and long-term toxicity.
Keywords: oropharyngeal cancer, human papillomavirus-positive (HPV+), chemoradiation, elderly
INTRODUCTION
The optimal treatment of elderly patients with locally advanced head and neck cancer is controversial. Curative chemoradiation (CRT) is associated with relatively high rates of acute and late toxicities of up to 90% and 43%, respectively, in large randomized series.1,2 Despite higher rates of toxicity, there is a clear survival benefit with the addition of chemotherapy to radiotherapy alone, as was convincingly shown by the meta-analysis of chemotherapy in head and neck cancer.3 However, the benefit of CRT in the elderly population is not clear. The meta-analysis of chemotherapy in head and neck cancer results demonstrated a statistically significant survival benefit of 6.5% with the addition of chemotherapy to radiation therapy in locally advanced head and neck squamous cell carcinoma (HNSCC),3 but a subset analysis showed a diminishing benefit of chemotherapy in ages 70 years or older. Elderly patients are often excluded from clinical trials or are underrepresented.4 They tend to have higher rates of comorbidities and reduced renal, cardiac, and respiratory reserves, which can lead to higher toxicities and worse quality of life after aggressive combined modality therapy.5 Additionally, the presence of comorbidities may independently have a negative effect on disease-specific and overall survival (OS).6 Age has been suggested to be an independent prognostic factor in HNSCC, with lower OS and disease-specific survival in patients older than 65 years relative to those younger than 65.7 Comparative effectiveness analyses have also suggested that there is no benefit of combined modality treatment in the elderly cohort.8 On the other hand, some retrospective series of CRT in the elderly have demonstrated outcomes similar to younger patients, albeit at the cost of greater acute toxicity,5,9,10 leading to the recommendation that age alone should not be used as a criterion for withholding aggressive treatment in the elderly. Retrospective data and national registry-based analyses are often limited by significant heterogeneity within the study cohorts. Disease-specific and patient-specific prognostic and predictive factors, which can have a major impact on treatment outcomes, are not always accounted for. In particular, the prevalence of human papillomavirus (HPV)-positivity in oropharyngeal carcinoma among these datasets remained unknown, although it is estimated that, in the general population, up to 70% of oropharyngeal squamous cell carcinomas (SCCs) are caused by HPV.11
Cancers of the oropharynx are unique in that HPV-associated SCCs represent a distinct clinical and prognostic entity compared to tobacco-associated and alcohol-associated SCCs. Epidemiologically, these cancers often occur in younger patients12 and have significantly better OS and locoregional control rates than non-HPV associated or smoking-related oropharyngeal SCC.11,13,14 On the other hand, HPV-associated cancers display a tendency for distant metastases, often at prolonged intervals from initial therapy.15 Thus, efforts to deescalate treatment in this favorable subgroup of oropharyngeal cancers must be balanced with reducing the risk of distant failure in the long term. Radiation Therapy Oncology Group (RTOG) 1016, a phase III randomized trial comparing cetuximab to cisplatin as concurrent systemic treatment with definitive radiation therapy in patients with HPV-related oropharyngeal cancers, is currently closed to accrual and is pending results, and attempts to determine if treatment can be deintensified for HPV-positive oropharyngeal SCC.16 A reduced dose of radiotherapy with cetuximab after complete response to induction chemotherapy has been shown to have excellent short-term outcomes in HPV-positive patients with head and neck cancers in a phase III Eastern Cooperative Oncology Group study.17 The feasibility of eliminating or reducing the dose of radiation therapy after limited surgery, including transoral robotic surgery, is also being investigated.19
Elderly patients with HPV-associated oropharyngeal SCC account for only a small proportion of all HPV-positive tumors.13 As mentioned above, optimal management of this specific population is unclear. Notably, current guidelines do not recommend modification of therapy based on either age or HPV status and concurrent CRT remains the standard of care for locally advanced disease. Given the lack of prospective data in this subgroup of patients, we sought to determine clinical outcomes within our institution for definitive treatment for HPV-positive oropharyngeal SCC in patients 70 years or above.
MATERIALS AND METHODS
This was a retrospective chart review for which institutional review board approval was obtained. The Emory University institutional database was queried for patients diagnosed with primary SCC of the oropharynx (base of tongue, tonsil, pharyngeal wall, soft palate, oropharynx, or not otherwise specified) who were treated with definitive concurrent CRT between January 1, 2005, and December 31, 2013. Patients included in the analysis were at least 70 years old at diagnosis or at any time before completing radiation therapy. Patients receiving radiation therapy alone without concurrent systemic therapy, patients with metastatic disease, or those who were not confirmed to be HPV-positive were excluded from this study. Patients who had received prior head and neck radiation therapy, had recurrent disease, or were treated primarily by surgical resection were also excluded from this study. HPV-positivity by either positive p16 immunostaining or in situ hybridization (ISH) for HPV DNA was required and was determined from pathology reports. For patients without p16 or HPV status documentation, we attempted to retrieve their pathology slides/blocks and p16 staining was then performed. Please see below for details regarding p16 staining. Patients were excluded from this study if we were unable to locate the tissue or had insufficient material to perform immunohistochemistry analysis.
p16 ink4a immunohistochemical staining
In cases in which the HPV status of the oropharyngeal tumor was unknown, p16 ink4a (p16) immunostaining was performed and funded through institutional departmental sources. In a manner described per the manufacturer’s protocol (CINtec 9517, MTM Laboratories, Westborough, MA), the p16 immunostain process was performed on sections cut from formalin-fixed paraffin-embedded tissue blocks on an open system automated immunostainer (DAKO Autostainer Link 48). Utilizing a diaminobenzidine reagent to visualize the antibody–antigen complex, the slides were then counterstained with hematoxylin, and washed. Positive and negative control slides were performed with each case. The proportion of tumor cells demonstrating nuclear and cytoplasmic p16 immunostaining were dichotomously classified as p16-positive (>75% tumor cells exhibiting strong and diffuse nuclear and cytoplasmic staining) or p16-negative (<75% tumor cells exhibiting strong and diffuse nuclear and cytoplasmic staining.20 In archival study cases, the proportion of tumor cells demonstrating nuclear and cytoplasmic p16 immunostaining were dichotomously classified as p16-positive (>50% tumor cells exhibiting strong and diffuse nuclear and cytoplasmic staining) or p16-negative (<50% tumor cells exhibiting strong and diffuse nuclear and cytoplasmic staining.20 Beginning in 2014, positive p16 immunostaining of an oropharyngeal SCC is accepted as a surrogate of HPV-positivity, a joint decision supported by the institutional Head and Neck Tumor Board.
Human papillomavirus 16/18 in situ hybridization
In cases studied with ISH, 5 micron tissue sections were processed with a biotin-labeled HPV 16/18 DNA probe (ENZO Life Sciences, Farmingdale, NY) and Gen-Point Tyramide Signal Amplification System (DAKO) with development of the final signal using 3, 3’-diaminobenzidine. These results were subsequently counterstained with hematoxylin. All steps, including positive and negative control slides, were performed on the Leica Bond III automated system in a manner as instructed by the supplier. Any punctate, dot-like nuclear staining was interpreted as evidence of DNA integration, a positive result.
All patients had to be deemed medically fit to undergo definitive radiation therapy with concurrent systemic therapy. The choice of systemic agent was per provider preference, and included cisplatin, carboplatin/paclitaxel, cetuximab, or docetaxel. Patients receiving induction chemotherapy (as part of the PARADIGM trial21) were included, provided they also received concurrent chemotherapy during the radiation treatment. Patients prospectively enrolled on institutional or multicenter trials were included in the current study. Radiation therapy was delivered using modern linear accelerators with 6 MV photon energy using intensity-modulated radiation therapy or volumetric modulated arc therapy. Treatments were generally given once a day, 5 days a week, except for patients enrolled on the RTOG 1016 protocol who received 6 fractions per week, and patients on the PARADIGM trial who received twice daily radiation after the initial 36 Gy.
Patient demographics and tumor characteristics were extracted from electronic medical records and recorded. Primary endpoints were OS, disease-free survival (DFS), and locoregional failure-free survival. All intervals were calculated from the date of diagnosis. Secondary endpoints included acute toxicity, hospitalization during treatment, late toxicity, and percutaneous endoscopic gastrostomy (PEG) tube dependence at 6 months. Acute toxicity was routinely assessed by the treating physician as part of weekly treatment checks and recorded in the radiation therapy chart. Grading of acute toxicity was according to RTOG grading criteria. Late toxicity was defined as any toxicity noted after 30 days from the last treatment. Data for late toxicity was retrieved from clinical notes at each follow-up visit. Deaths were recorded from patient charts or from the social security death index via commercial genealogical search websites. Crude rates of grade 3 or higher acute and late toxicities were calculated. Survival analyses were performed using the Kaplan–Meier method.
RESULTS
Patient and tumor characteristics
Twenty-one patients who met inclusion criteria were identified. Clinical characteristics are summarized in Table 1. The majority of patients were men (85.7%). Median age at diagnosis was 72.6 years (range, 69.8–75.8 years). Notably, only 6 patients were never smokers and 11 patients (52%) had 30 pack-year or greater smoking history, 12 patients had a 10 or greater pack-year smoking history (57%); 2 additional patients were documented smokers without quantitative data on pack-years. The most frequent primary site was the base of tongue in 66.7%, followed by tonsil (23.8%), and pharyngeal wall (4.8%). One patient had synchronous tonsil and base of tongue cancers (contralateral sides). The majority of the patients were American Joint Committee on Cancer (AJCC) stage IVA (17 patients), 3 were stage IVB, and 1 was stage III (see Table 2 for details of the stages). Two patients received induction chemotherapy (on PARADIGM trial); all patients received concurrent chemotherapy with cisplatin (9 patients), carboplatin/paclitaxel (10 patients), cetuximab (1 patient), or docetaxel (1 patient; Table 3). Median RT dose was 70 Gy (range, 69.9–74.5 Gy). Median follow-up was 22.4 months.
TABLE 1.
Patient characteristics.
| Characteristic | No. of patients | % |
|---|---|---|
| Age at diagnosis, median (range), y | 72.6 y (69.8–75.8 y) | |
| Sex | ||
| Male | 18 | 85.7 |
| Female | 3 | 14.3 |
| Smoking | ||
| Never | 6 | 28.6 |
| Unknown pack-years | 2 | 9.5 |
| <10 pack-years | 1 | 4.8 |
| >10 pack-years | 12 | 57.1 |
| Mean pack-year (range) | 35.05 (0–180) | |
| Primary site | ||
| Base of tongue | 14 | 66.7 |
| Tonsil | 5 | 23.8 |
| Pharyngeal wall | 1 | 4.8 |
| Base of tongue and tonsil | 1 | 4.8 |
TABLE 2.
Tumor characteristics by TNM classification.
| T1 | T2 | T3 | T4a | T4b | Total | |
|---|---|---|---|---|---|---|
| N0 | 1 | 1 | 2 | |||
| N1 | 2 | 2 | ||||
| N2a | 1 | 1 | ||||
| N2b | 2 | 4 | 3 | 9 | ||
| N2c | 2 | 1 | 2 | 1 | 6 | |
| N3 | 1 | 1 | ||||
| Total | 2 | 7 | 3 | 7 | 2 | 21 |
TABLE 3.
Treatment characteristics.
| No. of patients | % of patients | |
|---|---|---|
| RT dose, median (range) | Median dose 70 Gy (range 69.9–74.5) |
|
| Chemotherapy | ||
| Induction | 2 | 9.5 |
| Concurrent | 21 | 100 |
| Cisplatin | 9 | 42.9 |
| Carboplatin/paclitaxel | 10 | 47.6 |
| Cetuximab | 1 | 4.8 |
| Docetaxel | 1 | 4.8 |
| Chemotherapy tolerance | ||
| Completed as planned | 10 | 47.6 |
| Dose reduction | 2 | 9.5 |
| Delayed cycles | 6 | 28.6 |
| Changed regimen | 4 | 19 |
Abbreviation: RT, radiotherapy.
Treatment completion
All patients completed the planned course of radiation therapy, 3 patients had reported treatment breaks secondary to side effects requiring hospitalization. The length of treatment break ranged from 4 to 8 days. In terms of chemotherapy, 10 patients (47.6%) received all cycles of chemotherapy as planned (Table 3). The remaining patients had intolerance or significant toxicity to the initial regimen, leading to dose reduction (2 patients; 9.6%), delay in subsequent cycles (6 patients; 28.6%), or change in the chemotherapy regimen (4 patients; 19%). One patient had both dose reduction and delay in the next cycle.
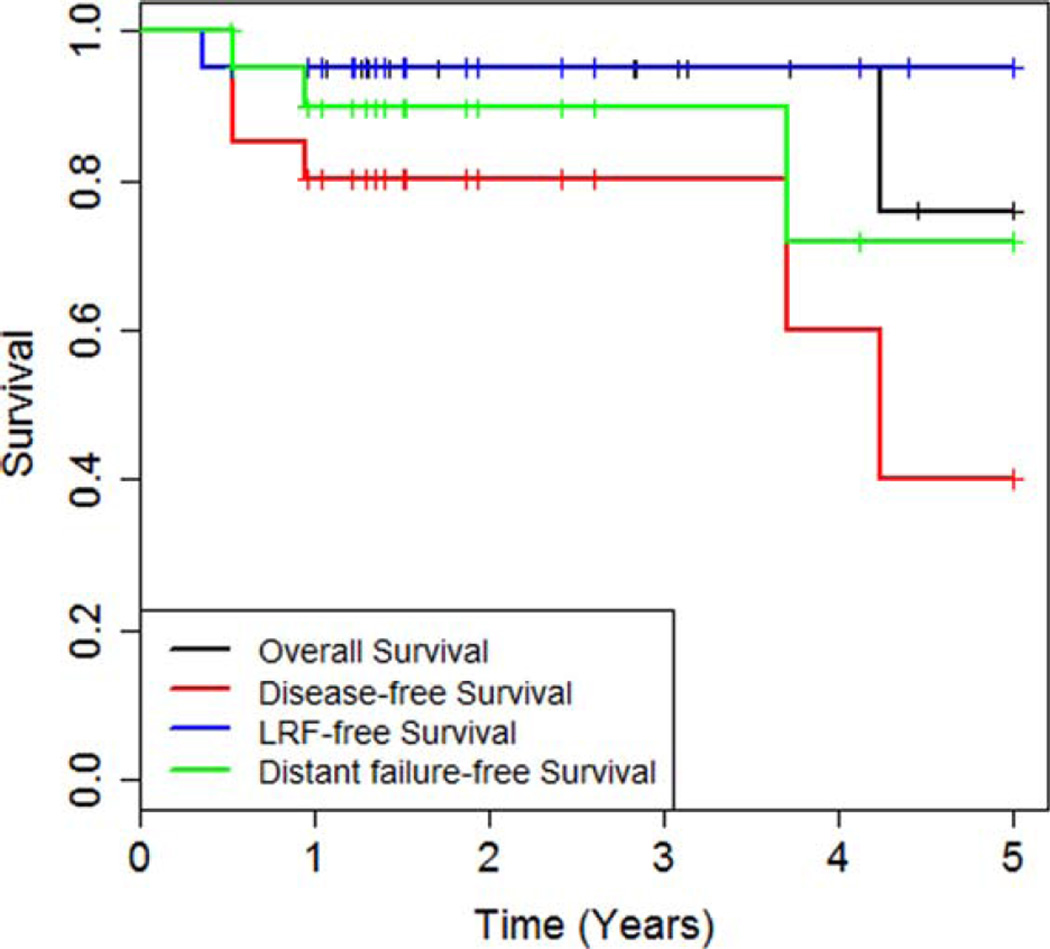
Locoregional failure–free survival and overall survival
Median OS was not reached in our study (Figure 1). Estimated 5-year OS was 76.0%. There was 1 death from acute toxicity (see below) and 1 death from an unknown cause, who had no evidence of disease at the last follow-up. Median DFS was 4.2 years; estimated 5-year DFS was 40%. No patient developed local failure, and only 1 patient had regional failure (4.8%). This patient had persistent palpable cervical adenopathy and underwent subsequent neck dissection. He was found to have 1 positive lymph node on final pathology; however, he was alive and disease-free after 5.7 years of follow-up after neck dissection. One patient who died of acute toxicity could not be assessed for disease control and was censored at the time of death. Of the remaining 19 patients, 3 developed distant failures in the lung. Of these, 2 underwent wedge resections and 1 patient subsequently received systemic therapy. Both patients remained alive and disease-free until the last follow-up. The third patient with distant metastasis developed pleural effusion for which he underwent thoracentesis and talc pleurodesis, multiple cycles of palliative chemotherapy, and was ultimately discharged to hospice. However, a documentation of death cannot be found for this patient.
FIGURE 1.
Kaplan Meier estimates of overall survival, disease-free survival, locoregional failure-free survival (LRF-free survival), and distant failure-free survival.
The remaining 15 patients all remained alive and disease-free. The 5-year locoregional failure-free rate was 95%. Thus, distant metastasis was the predominant failure pattern with lung metastases occurring in 3 patients for a 5-year distant metastasis-free rate of 71.8%. There was only 1 regional failure that was successfully salvaged with neck dissection.
Toxicity
One patient (enrolled on the PARADIGM trial of induction chemotherapy) died of acute toxicity shortly after completion of treatment. This patient developed respiratory failure secondary to bilateral pneumonia presumed to be resulting from aspiration. There were no other treatment-related deaths.
During treatment, most patients developed low-grade fatigue, anorexia, dysphagia, mucositis, and xerostomia. Grade 3 or higher acute toxicity was seen in 37.5% (see Table 4 for details). Specifically, 2 patients developed grade 4 anorexia, 3 patients had grade 4 dysphagia, and 1 patient developed grade 4 mucositis. Thirteen patients (61.9%) had unplanned hospitalizations during the treatment course for acute toxicities. All patients had gastrostomy tubes placed prophylactically before treatment. Five patients (25%) remained PEG-dependent at 6 months. Late toxicity was noted in 12 of 20 patients (60%). These included 3 patients with osteoradionecrosis and 1 patient with soft tissue necrosis, and 6 patients with dysphagia or esophageal strictures requiring dilation (Table 5). Mild to moderate xerostomia occurred in 4 patients (20%). A variety of management approaches was utilized ranging from physical therapy/ speech and swallowing therapy, dilation, hyperbaric oxygen, medications, and surgery.
TABLE 4.
Acute toxicity.
| No. of patients with acute toxicity |
% of patients with acute toxicity |
|
|---|---|---|
| Unplanned hospitalization | 13 | 61.9 |
| Treatment related death | 1 | 4.8 |
| Grade 3 or higher toxicity | ||
| Mucositis | 5 | 31.3 |
| Dysphagia | 4 | 25 |
| Skin reactions | 0 | 0 |
| Fatigue | 2 | 12.5 |
| Anorexia | 6 | 37.5 |
| Mood | 1 | 6.3 |
Data on acute toxicity was available for only 16 patients.
TABLE 5.
Late toxicity – all grades.
| No. of patients with late toxicity |
% of patients with late toxicity |
|
|---|---|---|
| PEG dependence at 6 mo | 5 | 25 |
| Necrosis (soft tissue or ORN) | 4 | 20 |
| Xerostomia/loss of taste | 4 | 20 |
| Dysphagia/eso stricture | 6 | 30 |
Abbreviations: PEG, percutaneous endoscopic gastrostomy; ORN, osteoradionecrosis.
Data for late toxicity was available for only 20 patients because 1 patient died of acute toxicity.
DISCUSSION
HNSCC is primarily a disease of the elderly, with a median age at diagnosis of 62 to 65 years.22 In many head and neck subsites, locally advanced therapy is treated with a combination of chemotherapy and radiation. This is based on prospective data, and a large meta-analysis showed that there was a survival advantage with the addition of chemotherapy, particularly concurrent chemotherapy.3 However, in this meta-analysis, there was noted to be a decreasing effect of the survival benefit with increasing age on a subset analysis. Of note, only about 7.4% of the patients included in the study were older than 70 years of age. The lack of a robust survival benefit coupled with the perception among providers that older patients have poor tolerance to aggressive multimodality therapy has led to the argument that these patients could be candidates for deintensified treatment. The notion that fewer elderly patients are offered aggressive or combined modality therapy is supported by studies conducted within population-based registries such as the Surveillance, Epidemiology, and End Results database.
The rising incidence of HPV-associated oropharyngeal SCC and the favorable prognosis carried by this pathologic entity has led to studies investigating treatment deintensification. This disease is more common in a younger population, and their anticipated long-term survival has also raised concerns about late toxicity with combined modality therapy. However, it is being increasingly recognized that HPV-positive cancers have a propensity for late distant metastasis, often to nonpulmonary sites. In particular, patients with HPV-positive oropharyngeal SCC who were stage N2c were found to have increased incidence of distant metastasis when treated with RT alone compared with CRT.15 Clearly, some subsets of patients stand to benefit from aggressive therapy even in the context of HPV-positive disease. Given that HPV-positive disease is less common in older patients, the paradigm for their management is even less established.
In this present study, clinical outcomes for elderly patients with confirmed HPV-positive oropharyngeal SCC are reported. All patients were treated with standard definitive chemoradiation, including some who were enrolled on multi-institutional national protocols, notably the PARAGIDM and RTOG 1016 randomized control trials. HPV-positive status either by positive p16 immunostaining or ISH for HPV was a requisite to ensure patient homogeneity. Our series demonstrated excellent locoregional failure-free survival of 95% at 5 years, with only 1 patient with persistent neck disease after completion of definitive treatment, which was subsequently salvaged with neck dissection. This rate is similar if not higher than those reported in the literature, in which HPV-positive tumors had a hazard ratio of 0.49 for progression-free survival compared with HPV-negative tumors, and a 3-year progression-free survival rate of 13%.14 We also noted an impressive OS in our cohort, with a 5-year OS rate of 75.8%, even though the majority of our patients were heavy smokers, thus exceeding survival rates reported in literature.
In terms of toxicity of treatment, single institutional series have previously demonstrated reasonable tolerance of chemoradiation within the elderly population. A series of 89 patients older than 70 years treated with 5 fluorouracil and hydroxyurea-based CRT for locally advanced head and neck cancer revealed that over 86% of the patients were able to complete the planned treatment.5 Forty-four percent of these patients had oropharyngeal primaries. Sixty-two percent required gastrostomy tube placement, 47% being unplanned; and aspiration (40%) and hematologic toxicities were some of the other toxicities reported. Another retrospective study showed no differences in the rates of acute toxicity or survival between older and younger patients undergoing CRT for locally advanced head and neck cancer, defined as >70 or <70 years.10 Rates of unplanned hospitalization, myelosuppression, gastrostomy tube placement, and completion of all cycles of chemotherapy was noted to be significantly different in another retrospective series; however, this study did not find any differences in disease control or survival between patients aged 70 and older versus younger patients.9 In the current study, there was 1 treatment-related death occurring within 30 days after conclusion of CRT. Rates of other acute grade 3 and 4 toxicities were in the range of 37%, and long-term toxicity was also not trivial, at 60%. However, this includes mild as well as severe effects, as long-term toxicity was not always explicitly graded by providers at follow-up visits.
At our institution, it is routine practice to offer prophylactic gastrostomy tube placement to all patients about to start a course of definitive CRT for locally advanced head and neck cancer. All patients undergo comprehensive evaluation by speech pathology with assessment of swallowing function, both during treatment and after completion of treatment. Removal of the gastrostomy tube is recommended once the patient is noted to be tolerating all nutrition orally for at least 2 weeks, and is usually performed during a follow-up visit. In our series, we noted a long-term (defined as 6 months after completion of CRT) PEG-dependence rate of 25%. This is not different from rates reported in literature, and may be at least partly related to noncompliance with speech and swallowing exercises, as recently reported in the literature.23 It must be noted, however, that compliance with therapy was not assessed in our study.
The majority of the published literature analyzing outcomes in elderly patients with head and neck cancer suffer from lack of HPV data. HPV-positivity is possibly one of the strongest predictors of outcome in oropharyngeal SCC. With HPV projected to be causative in up to 70% of oropharyngeal SCC in the near future, information about HPV status is very critical in predicting outcomes and attempting to tailor therapy. As seen in our cohort, the actuarial rate of distant metastasis was 28.2% with combined modality therapy at 5 years. Two of these patients had N2c disease, which has been reported to be a predictor of distant relapse15 and the third patient had N2b disease. It is likely that the distant failure rate will be higher at longer follow-up.
Our study expectedly had limitations associated with retrospective reviews in general. Our study population was small, largely because of the rarity of this disease in the elderly population, as well as nonstandardized HPV testing in the early years of the study period. Additionally, comorbidity data were not available and median follow-up was 22.4 months. Treatment was administered in a relatively homogenous fashion under the care of 3 different medical oncologists and 3 radiation oncologists. Our rate of unplanned hospitalization was high at 62% and is likely reflective of our institutional practice patterns. OS rates in this study were comparable to those reported in randomized trials within the HPV-positive oropharyngeal population, however, it should be noted that retrospective data are not subject to the same level of scrutiny as data collected in a randomized trial, and prospective results of elderly patients undergoing CRT would be the ideal way to define the optimal treatment for this population.
Another caveat to this study includes receipt of CRT at a single institution with a high volume of patients with head and neck cancer. It is unknown if similar results would be attainable when treatment is given in the community setting, as it is likely that much of the success of CRT treatment in this group of patients is secondary to aggressive management of toxicities during treatment.
In conclusion, our study demonstrates that OS after CRT for patients >70 years with HPV-positive oropharyngeal SCC is similar to OS in the nonelderly population. We observed excellent rates of locoregional failure-free survival, with the dominant pattern of failure being distant failure, with a 28.2% rate of distant metastasis. Our rates of acute and late toxicity were not insignificant. Our findings suggest that standard CRT may be tolerated by the elderly population, and, clinically, outcomes are comparable to that of the general population.
Acknowledgments
Contract grant sponsor: Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University and National Institutes of Health/National Cancer Institute under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignon JP, le Maître A, Maillard E, Bourhis J MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Maggiore RJ, Curran EK, Witt ME, Haraf DJ, Vokes EE, Cohen EE. Survival and selected outcomes of older adults with locally advanced head/neck cancer treated with chemoradiation therapy. J Geriatr Oncol. 2013;4:327–333. doi: 10.1016/j.jgo.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VanderWalde NA, Fleming M, Weiss J, Chera BS. Treatment of older patients with head and neck cancer: a review. Oncologist. 2013;18:568–578. doi: 10.1634/theoncologist.2012-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan Camilon P, Stokes WA, Nguyen SA, Lentsch EJ. The prognostic significance of age in oropharyngeal squamous cell carcinoma. Oral Oncol. 2014;50:431–436. doi: 10.1016/j.oraloncology.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 8.VanderWalde NA, Meyer AM, Deal AM, et al. Effectiveness of chemoradiation for head and neck cancer in an older patient population. Int J Radiat Oncol Biol Phys. 2014;89:30–37. doi: 10.1016/j.ijrobp.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Michal SA, Adelstein DJ, Rybicki LA, et al. Multi-agent concurrent chemoradiotherapy for locally advanced head and neck squamous cell cancer in the elderly. Head Neck. 2012;34:1147–1152. doi: 10.1002/hed.21891. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen NP, Vock J, Chi A, et al. Impact of intensity-modulated and image-guided radiotherapy on elderly patients undergoing chemoradiation for locally advanced head and neck cancer. Strahlenther Onkol. 2012;188:677–683. doi: 10.1007/s00066-012-0125-0. [DOI] [PubMed] [Google Scholar]
- 11.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380–386. doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 16. ClinicalTrials.gov. A service of the U.S. National Institutes of Health. [Accessed March 1, 2015];Radiation therapy with cisplatin or cetuximab in treating patients with oropharyngeal cancer. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01302834.
- 17.Cmelak A, Li S, Marur S, et al. E1308: Reduced dose IMRT in human papilloma virus (HPV)-associated resectable oropharyngeal squamous carcinomas (OPSCC) after complete clinical response (cCR) to induction chemotherapy (IC) J Clin Oncol. 2014 Jun 20;32(Supplement) abstract LBA6006. [Google Scholar]
- 18. ClinicalTrials.gov. A service of the U.S. National Institutes of Health. Transoral surgery followed by low-dose or standard-dose radiation therapy with or without chemotherapy in treating patients with HPV positive stage III-IVA oropharyngeal cancer. [Accessed March 1, 2015]; Available at: http://www.clinicaltrials.gov/ct2/show/NCT01898494.
- 19.Lewis JS., Jr p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl 1):S75–S82. doi: 10.1007/s12105-012-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14:257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 21.Camilon PR, Stokes WA, Nguyen SA, Lentsch EJ. Are the elderly with oropharyngeal carcinoma undertreated? Laryngoscope. 2014;124:2057–2063. doi: 10.1002/lary.24660. [DOI] [PubMed] [Google Scholar]
- 22.Shinn EH, Basen–Engquist K, Baum G, et al. Adherence to preventive exercises and self-reported swallowing outcomes in post-radiation head and neck cancer patients. Head Neck. 2013;35:1707–1712. doi: 10.1002/hed.23255. [DOI] [PMC free article] [PubMed] [Google Scholar]