Abstract
Purpose
To report toxicity and early survival data for IDEAL-CRT, a trial of dose-escalated concurrent chemoradiotherapy (CRT) for non-small cell lung cancer.
Patients and Methods
Patients received tumor doses of 63 to 73 Gy in 30 once-daily fractions over 6 weeks with 2 concurrent cycles of cisplatin and vinorelbine. They were assigned to 1 of 2 groups according to esophageal dose. In group 1, tumor doses were determined by an experimental constraint on maximum esophageal dose, which was escalated following a 6 + 6 design from 65 Gy through 68 Gy to 71 Gy, allowing an esophageal maximum tolerated dose to be determined from early and late toxicities. Tumor doses for group 2 patients were determined by other tissue constraints, often lung. Overall survival, progression-free survival, tumor response, and toxicity were evaluated for both groups combined.
Results
Eight centers recruited 84 patients: 13, 12, and 10, respectively, in the 65-Gy, 68-Gy, and 71-Gy cohorts of group 1; and 49 in group 2. The mean prescribed tumor dose was 67.7 Gy. Five grade 3 esophagitis and 3 grade 3 pneumonitis events were observed across both groups. After 1 fatal esophageal perforation in the 71-Gy cohort, 68 Gy was declared the esophageal maximum tolerated dose. With a median follow-up of 35 months, median overall survival was 36.9 months, and overall survival and progression-free survival were 87.8% and 72.0%, respectively, at 1 year and 68.0% and 48.5% at 2 years.
Conclusions
IDEAL-CRT achieved significant treatment intensification with acceptable toxicity and promising survival. The isotoxic design allowed the esophageal maximum tolerated dose to be identified from relatively few patients.
Summary.
Eighty-four patients (6 IIB, 57 IIIA, 21 IIIB) were recruited to IDEAL-CRT, an early-phase trial of dose-per-fraction–escalated concurrent chemoradiation for NSCLC. Tumor doses of 63 to 73 Gy (mean, 67.7 Gy) were isotoxically prescribed to the ICRU reference point and delivered in 30 fractions over 40 days. Toxicity was acceptable. At 35 months' median follow-up, median OS was 36.9 months, 1-year OS and PFS were 87.8% and 72.0%, and 2-year OS and PFS were 68.0% and 48.5%.
Introduction
Intensification of local treatment has been associated with increased local control and overall survival (OS) for non-small cell lung cancer (NSCLC) patients. Improved 2-year OS was reported for CHART (Continuous Hyperfractionated Accelerated Radiotherapy) trial patients treated using 54 Gy delivered in just 12 days compared with the standard 60 Gy in 40 days (29% vs 20%) (1), and a recent meta-analysis has reported a hazard ratio (HR) of 0.86 for intensification of radiation-only or sequential chemoradiotherapy (CRT) treatments compared with control arms (2). A meta-analysis of trials of concurrent versus sequential CRT found an advantage for concurrent delivery of radiation and chemotherapy, with an HR of 0.77 for local progression-free survival (PFS) and 5.7% and 4.5% absolute benefit in OS at 3 and 5 years, respectively (3).
Radiation Therapy Oncology Group protocol 0617 recently examined the effect of increasing radiation therapy (RT) tumor doses from 60 to 74 Gy given in 5 2-Gy fractions per week (4). Unexpectedly, survival was significantly lower in the 74-Gy arm, perhaps partly because the 11-day increase in total treatment time required for additional fractions reduced the effectiveness of tumor dose escalation. Dose-per-fraction escalation circumvents this by fixing the number of fractions and treatment duration, hypofractionating and effectively accelerating RT 5, 6, 7. It may therefore provide a more effective means of local treatment intensification 8, 9, 10.
Radiation therapy toxicity is determined by doses delivered to normal tissues. Early-phase trials testing the toxicity of intensified CRT or RT combined with novel agents should control these normal tissue doses, while allowing prescribed tumor doses to vary within an acceptable range. Isotoxic dose escalation accomplishes this by prescribing the highest deliverable tumor doses without exceeding predetermined normal tissue limits.
We report toxicity and early survival data for IDEAL-CRT, a trial of tumor dose–escalated concurrent CRT for NSCLC. Dose-per-fraction escalation was used to achieve intensification without schedule protraction; tumor doses were prescribed isotoxically; and selected patients were prospectively assigned to cohorts receiving incrementally increasing esophageal RT doses.
Patients and Methods
This nonrandomized phase 1/2 trial enrolled stage II and III NSCLC patients, who received tumor RT doses between 63 Gy and 73 Gy in 30 once-daily fractions over 40 days, concurrent with 2 cycles of cisplatin and vinorelbine.
Patients
Inclusion criteria were histologically/cytologically confirmed stage IIA-IIIB NSCLC, World Health Organization performance status (PS) 0 to 1, suitability for CRT agreed by multidisciplinary team, no prior anticancer therapy, forced expiratory volume ≥40% predicted or ≥1 L, carbon monoxide diffusing capacity ≥40% predicted, hematology and biochemistry baselines suitable for chemotherapy, and glomerular filtration rate ≥60 mL/min. Patients with chronic liver disease and/or bilirubin >35 μmol/L, connective tissue disorders (eg, scleroderma, systemic lupus), or history of prior malignancy likely to interfere with protocol treatment were excluded.
Design
Patients received the highest prescribed tumor doses between 63 and 73 Gy deliverable while meeting the normal tissue dose constraints shown in Table 1. For lungs, spinal cord, brachial plexus, and heart these constraints were held at levels determined from an earlier review (11). However, insufficient data linking dose to toxicity existed for an esophageal constraint to be defined up front, and so an incrementally increasing esophageal limit was used during the trial. To facilitate this process, patients were split after RT treatment planning but before tumor dose prescription into 2 nonrandomized groups based on dosimetric findings. In group 1, prescribed tumor doses were limited by an escalating esophageal constraint, whereas in group 2 prescribed tumor doses were limited by lung and other normal tissue constraints. Allocation of patients to these groups was therefore determined purely on the basis of dosimetry and not clinician choice.
Table 1.
Summary of the radiation therapy planning and dose prescription process
| Process steps | ||||
|---|---|---|---|---|
| Tumor coverage aim | ||||
| PTV | 90% isodose to cover 98% of PTV | |||
| Tumor dose prescribed to the ICRU reference point initially selected to achieve | ||||
| Lung | EQD2mean 18.2 Gy | |||
| Prescribed tumor dose reduced by 10%, and further if needed to meet the following limits | ||||
| Heart | D100% <45 Gy, D67% <53 Gy, D33% <60 Gy | |||
| Spinal cord | D0.1cc ≤ 47 Gy | |||
| Brachial plexus | D30% ≤60 Gy, D0.1cc ≤65 Gy | |||
| Esophagus | Dose to 1 cm3=65 Gy | Dose to 1 cm3=68 Gy | Dose to 1 cm3=71 Gy | Dose to 1 cm3 ≤63 Gy∗ |
| Limit for | Group 1: cohort 1 | Group 1: cohort 2 | Group 1: cohort 3 | Group 2 |
Abbreviations: EQD2mean = equivalent dose in 2-Gy fractions averaged across lung, excluding gross tumor volume (GTV); ICRU = International Commission on Radiation Units and Measurements; PTV = planning target volume.
Prescribed tumor dose finally limited to 63-73 Gy, patients being ineligible for the trial if this causes lung V20Gy (the volume of lung excluding GTV receiving more than 20 Gy) or EQD2mean to exceed 35% or 19.3 Gy, respectively.
This dose level increased to 65 Gy, and then 68 Gy as safety data became available from group 1.
Group 1 was designed as a phase 1 study to establish an esophageal maximum tolerated dose (MTD), patients' prescribed tumor doses being limited by an escalating experimental constraint on dose delivered to the most highly irradiated 1 cm3 of esophagus. This esophageal dose constraint was progressively raised from 65 Gy to 68 Gy and then 71 Gy, following a 6 + 6 design (Fig. E1; available online at www.redjournal.org), treating 6 or 12 patients at each level. It was initially planned to include a 73-Gy esophageal dose cohort, but the 73-Gy upper limit on prescribed tumor dose meant that in practice it was not feasible to deliver 73 Gy to 1 cm3 of esophagus. Dose-limiting toxicities were grade ≥3 esophagitis (Common Terminology Criteria for Adverse Events, version 4.0) occurring during or within 4 weeks of completing RT and late esophageal toxicity, which was monitored closely.
Group 2 comprised all trial patients whose prescribed tumor doses were limited by other dose constraints, often lung or cord, and was designed to provide further toxicity data, particularly for radiation pneumonitis (RTPN). For some group 2 patients, however, prescribed tumor dose was limited by a lower esophageal constraint, already known to be safe, initially set at 63 Gy to 1 cm3 (Table 1). As the trial recruited, this group 2 esophageal limit was progressively raised to levels for which early toxicity had been found to be acceptable in cohorts of 12 patients in group 1.
Feasibility and survival data were analyzed jointly across both groups, comprising the phase 2 element of the study.
Interventions
Concurrent chemotherapy was 2 cycles of intravenous cisplatin (75 mg/m2) on days 1 and 29 of RT, and intravenous vinorelbine (15 mg/m2) on days 1, 8, 29, and 36. No induction or consolidation chemotherapy was allowed.
Radiation therapy planning was the same for groups 1 and 2. Three-dimensional (3D) or 4-dimensional (4D) CT images were collected during quiet breathing, and on 3D-CT images the gross tumor volume (GTV) was contoured and then expanded by 5 mm to create a clinical target volume and by a further 5-mm minimum radial and 10-mm minimum cranio-caudal to form a planning target volume. On 4D-CT, a composite volume was formed by merging GTVs outlined on the different scan phases, then expanded by 5 mm to form a clinical target volume and by 5 mm minimum further in all directions to form a planning target volume. Most patients were treated using 3D conformal plans comprising 3 to 5 photon fields of energy 5 to 8 MV. Some were treated using volumetric modulated arc therapy (VMAT), according to available center resources. Dose distributions were calculated using “type-b” superposition–convolution algorithms (12), and all tumor doses were prescribed to the International Commission on Radiation Units and Measurements (ICRU) reference point.
The tumor dose prescription process is summarized in Table 1. For each patient an initial prescribed tumor dose was selected to achieve a target value of 18.2 Gy for lung EQD2mean, the average equivalent dose in 2-Gy fractions delivered to all CT voxels of both lungs excluding the GTV 13, 14. This level is associated with a 20% rate of Southwest Oncology Group grade 2 to 5 RTPN and a presumed grade 3 to 5 rate of <10% 13, 15. The prescribed tumor dose was then reduced by 10% to allow for toxicity caused by concurrent chemotherapy, and further if necessary to meet the tabulated dose constraints for esophagus, brachial plexus, heart, and spinal cord (11). If this caused the prescribed tumor dose to fall below the trial minimum of 63 Gy, the lung EQD2mean limit was relaxed to 19.3 Gy, and the patient would still receive a prescribed tumor dose of 63 Gy provided this relaxed lung limit and all the other normal tissue constraints listed in Table 1 could be met. Prescribed tumor doses were capped at 73 Gy to limit damage to central blood vessels and airways.
Quality assurance was overseen by the Radiotherapy Trials Quality Assurance Group of the National Cancer Research Institute. Before starting the trial, clinicians and physicists from each center attended an outlining and dose prescription workshop. Clinicians outlined 2 benchmark cases (16), which were checked against contours drawn by the principal investigator, and planned 2 pre-outlined benchmark cases, which were reviewed to ensure that trial dosimetric aims were met. An additional arc-planned case was checked for centers introducing VMAT. Equipment details were collected via an online questionnaire. Contouring and dosimetry of each center's first recruited case was independently reviewed before treatment. Further reviews were requested, where deemed necessary, to ensure protocol compliance. Subsequently all treatment plan data were collected centrally and analyzed retrospectively to verify conformance to the trial protocol.
Staging CT of the thorax and abdomen and positron emission tomography scanning were performed for all patients, either one within 42 days of commencing RT. Clinical assessments of PS, hematology, weight, and dyspnea score were made weekly during RT. Posttreatment PS, weight, dyspnea score, pulmonary function, adverse events, and toxicity data were collected at clinical reviews held weekly during the first month, monthly to 6 months, every 3 months to 24 months, every 6 months to 36 months, and annually thereafter. Computed tomography thorax and abdomen and lung function tests were carried out 3, 6, 12, and 24 months after completion of RT, chest X ray at 1, 3, 6, 12, 18, and 24 months, and electrocardiogram at 6 and 12 months.
Outcomes and statistics
Trial endpoints were toxicity, particularly esophagitis and RTPN, OS, PFS, and tumor response (RECIST version 1.1). Attribution of toxicity to treatment was overseen by an independent data monitoring committee. Rates of OS and PFS were estimated using Kaplan-Meier methods. All patients who received at least 1 fraction of RT were included in this analysis. The database cutoff date was July 31, 2015.
The group 1 sample size depended on toxicity seen: up to 36 patients were possible, 12 each in the 3 feasible cohorts. In group 2, assuming a grade 2 to 5 RTPN rate of 20% was of further interest, 45 patients were required to exclude an unacceptable rate of 40% with a 1-sided 5% significance level and 90% power (17).
Role of the funding source
The funder, Cancer Research UK, was not involved in the conduct, analysis, or interpretation of the trial, or the writing of this report. The trial sponsor, responsible for trial conduct and analysis, was University College London. The corresponding author had full access to all the data in the study and final responsibility to submit for publication. The trial was run in accordance with the Declaration of Helsinki and with the approval of all relevant ethical bodies and regulatory authorities.
Results
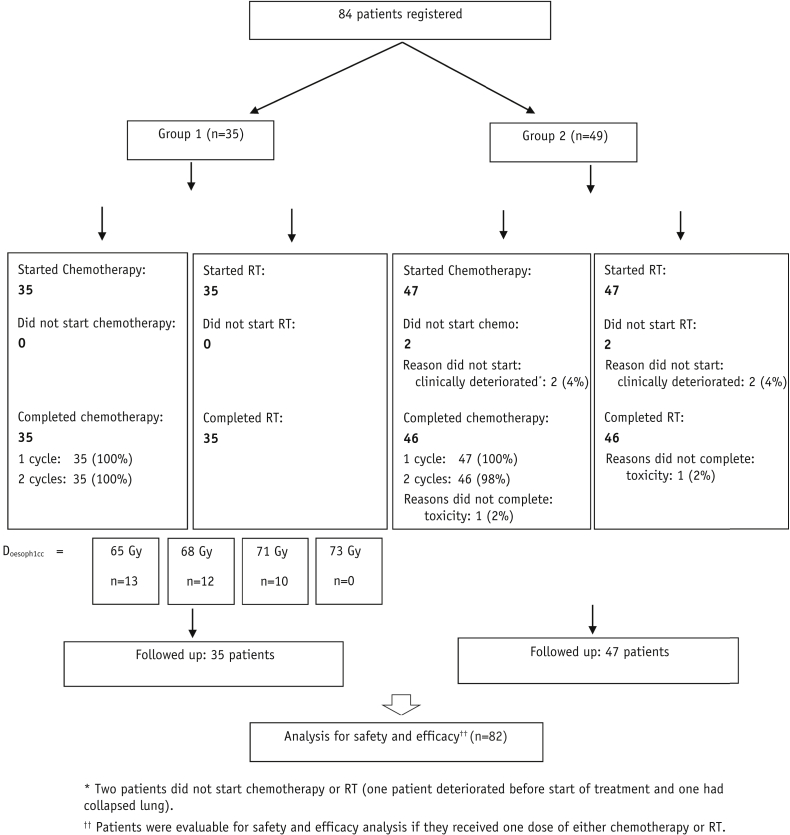
Between September 2010 and March 2013, 84 patients from 8 UK centers were enrolled, 35 in group 1 and 49 in group 2, with the baseline characteristics shown in Table 2. Of these, 34 patients (40.5%) were planned using 4D-CT and 50 (59.5%) with intravenous contrast. Three patients were treated using VMAT. An extra patient was recruited to the 65-Gy cohort of group 1 because replanning during treatment was required for 1 of the patients initially recruited, adding uncertainty to the delivered maximum esophageal dose. Twelve patients were recruited to the 68-Gy cohort of group 1, but only 10 to 71 Gy before trial funding ended (Fig. 1).
Table 2.
Demographics and baseline characteristics of all patients
| Characteristic | Group 1 (n=35) | Group 2 (n=49) | Total (n=84) |
|---|---|---|---|
| Age (y) | |||
| ≥70 | 10 (29) | 13 (27) | 23 (27) |
| <70 | 25 (71) | 36 (73) | 61 (73) |
| Mean (SD) | 65.6 (8.0) | 65.4 (8.0) | 65.5 (8.0) |
| Median (range) | 66 (46-84) | 66 (43-78) | 66 (43-84) |
| Sex | |||
| Female | 9 (26) | 13 (27) | 22 (26) |
| Male | 26 (74) | 36 (73) | 62 (74) |
| WHO PS | |||
| 0 | 12 (34) | 20 (41) | 32 (38) |
| 1 | 23 (66) | 29 (59) | 52 (62) |
| Histology | |||
| Adenocarcinoma | 12 (34) | 14 (29) | 26 (31) |
| Squamous | 17 (49) | 30 (61) | 47 (56) |
| Other NSCLC | 6 (17) | 5 (10) | 11 (13) |
| Stage | |||
| IIA | 0 | 0 | 0 |
| IIB | 0 | 6 (12) | 6 (7) |
| IIIA | 24 (69) | 33 (67) | 57 (68) |
| IIIB | 11 (31) | 10 (20) | 21 (25) |
| GTV size (cm3) | |||
| Mean (SD) | 127.7 (118.9) | 118.0 (83.3) | 122.1 (99.4) |
| Median (range) | 110 (14-602) | 92 (15-329) | 109 (14-602) |
Abbreviations: GTV = gross tumor volume; NSCLC = non-small cell lung cancer; WHO PS = World Health Organization performance status.
Values are number (percentage) unless otherwise noted.
Fig. 1.
CONSORT diagram. Abbreviation: RT = radiation therapy.
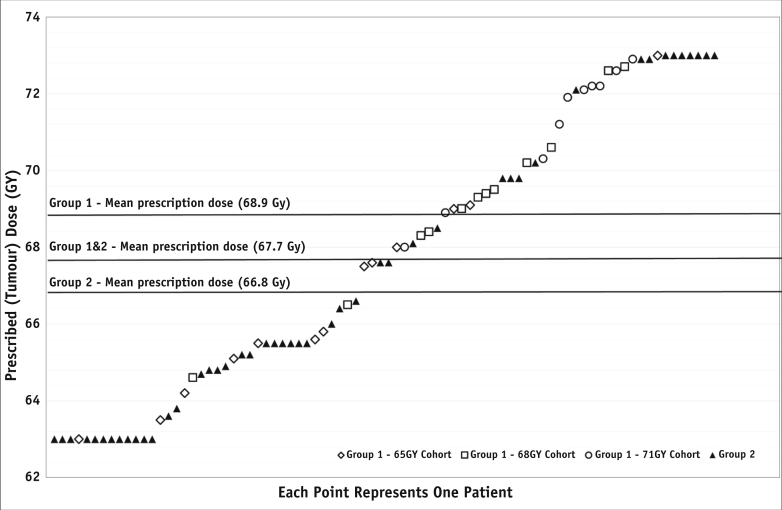
Two patients in group 2 did not begin treatment following clinical deterioration. Of the 82 patients starting CRT, 81 (98.8%) completed both cycles of chemotherapy, and 81 (98.8%) received all 30 RT fractions (Fig. 1); 1 patient withdrew due to toxicity (group 2). Median relative dose intensity was 99.6% for cisplatin and 99.0% for vinorelbine, and RT was generally delivered as scheduled, with a median duration of 5.6 weeks (range, 5.1-6.6 weeks; Table E1; available online at www.redjournal.org). Prescribed tumor doses are shown in Figure 2 and have an overall mean of 67.7 Gy, with means of 68.9 Gy and 66.8 Gy for groups 1 and 2, respectively.
Fig. 2.
Radiation therapy tumor doses delivered to the International Commission on Radiation Units and Measurements reference point.
Toxicity
Grade 2 to 5 RTPN was seen in 30.5% of patients who received trial treatment (1-sided upper 95% confidence limit 39.9%; 2-sided 95% confidence interval [CI] 20.8%-41.6%). Three of these RTPN events were grade 3 (3.7%; 95% CI 0.8%-10.3%).
The grade 2 to 5 esophagitis rate overall was 82.9% (2-sided 95% CI 73.0%-90.3%), with 5 grade 3 toxicities (6.1%; 2-sided 95% CI 2.0%-13.7%). A fatal esophageal perforation occurred in 1 patient in the 71-Gy cohort of group 1, 7 months after RT (Table 3), and was considered directly related to treatment. The esophageal MTD was therefore set at 68 Gy to 1 cm3 of esophagus.
Table 3.
Selected toxicities by grade among the 82 patients who began radiation therapy (safety population), according to trial group
| Toxicity | Grade | Group 1 (n=35) | Group 2 (n=47) | Total (N=82) |
|---|---|---|---|---|
| Esophagitis | 0 | 2 (6) | 7 (15) | 9 (11) |
| 1 | 1 (3) | 4 (9) | 5 (6) | |
| 2 | 30 (86) | 33 (70) | 63 (77) | |
| 3 | 2 (6) | 3 (6) | 5 (6) | |
| 4 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
| RTPN∗ | 0 | 11 (31) | 24 (51) | 35 (43) |
| 1 | 12 (34) | 10 (21) | 22 (27) | |
| 2 | 10 (29) | 12 (26) | 22 (27) | |
| 3 | 2 (6) | 1 (2) | 3 (4) | |
| 4 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
| All toxicities grades ≥3 | 25 (71) | 36 (77) | 61 (74) | |
| 3 | 20 (57) | 26 (55) | 46 (56) | |
| 4 | 3 (9) | 8 (17) | 11 (13) | |
| 5† | 2 (6) | 2 (4) | 4 (5) | |
| Grade ≥3%‡ hematologic | ||||
| White blood cell decreased | 2 (6) | 9 (19) | 11 (13) | |
| Lymphocyte decreased | 1 (3) | 8 (17) | 9 (11) | |
| Neutrophil decreased | 4 (11) | 8 (17) | 12 (15) | |
| Grade ≥3 other | ||||
| Lung infection | 9 (26) | 9 (19) | 18 (22) | |
| FEV decreased | 5 (14) | 7 (15) | 12 (15) | |
Abbreviation: FEV = forced expiratory volume.
Values are number (percentage).
RTPN = radiation therapy pneumonitis. Two patients received higher lung doses than allowed in the protocol. Both received prescribed tumor doses of 63 Gy, one with a lung V20 of 46.5% and one with 40.7%. Neither experienced RTPN.
Four patients had grade 5 events: in group 1, 1 patient experienced esophageal perforation and 1, hemoptysis. Two group 2 patients experienced hemoptysis.
There were no relevant hemoglobin-related events.
A further 3 patients had fatal events (Table 3), all hemoptysis. One occurred 14 months after RT with tumor recurrence and was considered possibly treatment related (group 1, prescribed tumor dose 72.6 Gy); another at 4.5 months after RT with residual tumor was considered unrelated (group 2, prescribed tumor dose 68.5 Gy); and the third at 4 weeks after RT was also considered unrelated (group 2, prescribed tumor dose 67.6 Gy).
Incidences of esophagitis and RTPN are listed by grade and trial group in Table 3, alongside a summary of other toxicities. Rates of other complications are listed in more detail in Table E2 (available online at www.redjournal.org), and the latency of grade 3 to 5 toxicities is summarized in Table E3 (available online at www.redjournal.org). Table E4 (available online at www.redjournal.org) summarizes the trial dosimetrically, listing prescribed tumor doses for all 3 cohorts of group 1 and for group 2, alongside details of delivered doses to the constrained normal tissues.
Efficacy
At the 3 months post-RT visit, 52 patients (63.4%) had a partial response, 21 (25.6%) had stable disease, 4 (4.9%) had progressive disease, 4 (4.9%) had non-evaluable disease, and 1 (1.2%) patient had died.
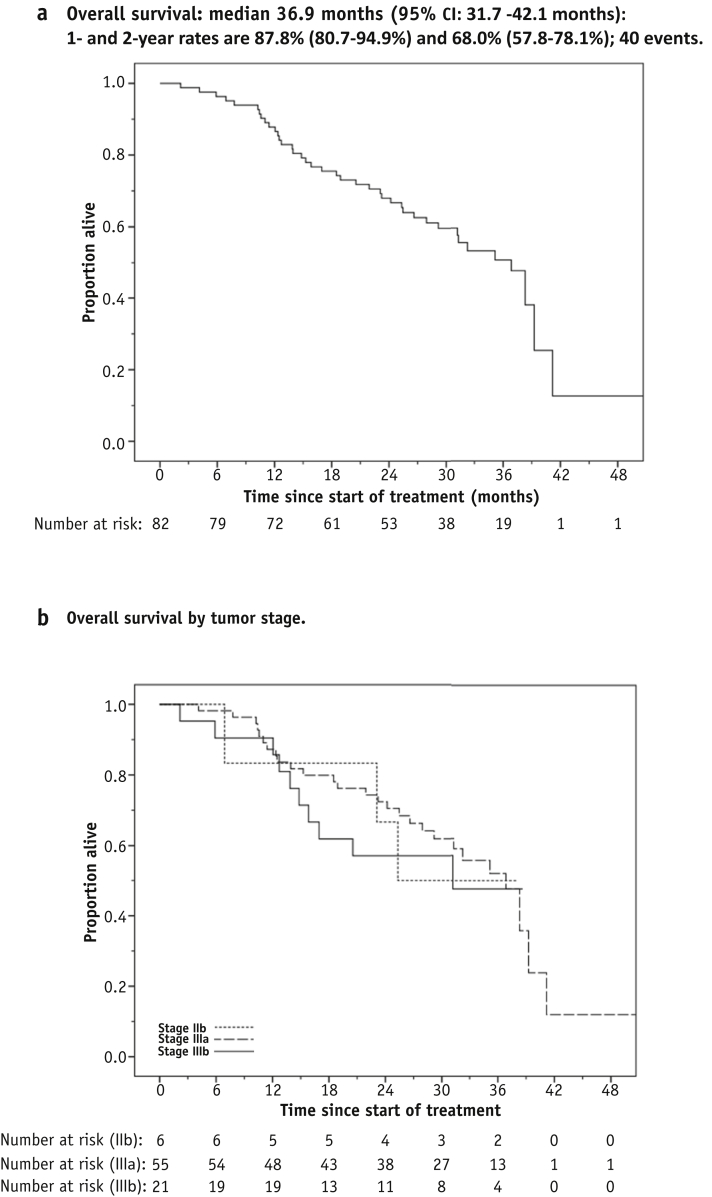
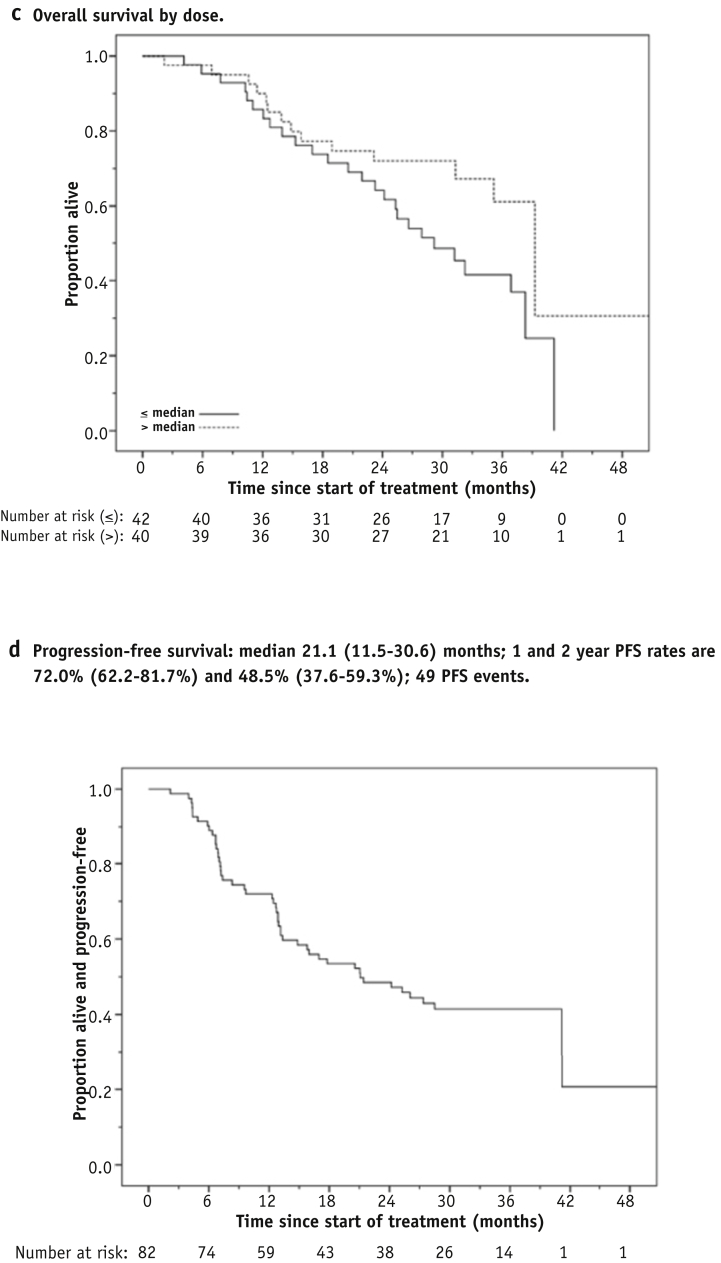
After a median follow-up of 34.9 months (range, 2.2-51.2 months) there were 40 deaths, the remaining 42 patients being censored at the last date known to be alive. One- and 2-year OS was 87.8% (95% CI 80.7%-94.9%) and 68.0% (95% CI: 57.8%-78.1%), respectively, and median OS was 36.9 months (95% CI 31.7-42.1 months) (Fig. 3a). Overall survival is plotted by tumor stage in Figure 3b. Figure 3c shows OS for the 82 patients split into 2 subgroups having prescribed tumor doses greater or less than the 68-Gy median; the risk of death was lower for the higher tumor dose subgroup (HR 0.53, 95% CI 0.28-1.02, P=.06). There were 49 PFS events overall. One- and two-year PFS was 72.0% (95% CI 62.2%-81.7%) and 48.5% (95% CI: 37.6%-59.3%), respectively, and median PFS was 21.1 months (95% CI 11.5-30.6 months) (Fig. 3d).
Fig. 3.
Overall and progression-free survival. (a) Overall survival. (b) Overall survival by tumor stage. (c) Overall survival by tumor dose. (d) Progression-free survival.
Discussion
IDEAL-CRT tested a novel, individualized, tumor-dose-per-fraction escalated concurrent CRT schedule for NSCLC. The trial demonstrated acceptable toxicity, feasibility, and promising clinical outcomes, as well as defining an esophageal MTD for the schedule.
The 6% rate of grade 3 to 5 esophagitis in IDEAL-CRT is lower than the 18% and 25% average rates found in 2 meta-analyses of concurrent CRT 3, 18 and the 7%, 19%, and 26% rates of the 2 dose arms of Radiation Therapy Oncology Group (RTOG) 0617 (4) and the MAASTRO study of isotoxically individualized concurrent CRT (10). Intensive clinical input resulting from mandated weekly patient assessments may have reduced the number of grade 3 to 5 cases in IDEAL-CRT, and the study's dosimetric focus may also have limited esophageal irradiation.
There was 1 late grade 3 to 5 esophageal toxicity, a fatal perforation in the 71-Gy cohort of trial group 1, and 68 Gy was defined as the esophageal MTD. Of 171 patients treated using concurrent CRT at Netherlands Cancer Institute, Amsterdam, 3 suffered esophageal fistula and 8, grade 3 stenosis (19). In the high-dose arm of RTOG 0617, 3 of 206 patients died of gastrointestinal toxicity (20), and an additional 9 of 442 patients overall experienced late grade 3 to 5 gastrointestinal toxicity (4). The most significant predictor of late esophageal toxicity in The Netherlands Cancer Institute series was esophageal volume receiving an EQD2 ≥76.7 Gy. Using the linear-quadratic model with an α/β ratio of 3 Gy to account for fractionation differences (14), this equates to the 71-Gy esophageal dose level of the IDEAL-CRT cohort in which the esophageal perforation occurred.
The ability of the isotoxic escalation scheme to limit the incidence of RTPN was confirmed by the grade 2 to 5 RTPN and grade 3 to 5 RTPN rates of 30.5% (95% CI 20.8%-41.6%) and 3.7% (95% CI 0.8%-10.3%), respectively, in the patients who received trial treatment, similar to the 7% and 4% grade 3 to 5 rates of the control and escalated arms of RTOG 0617, and the 7% rate in the Cochrane review of concurrent CRT (18). A detailed analysis of possible associations between dosimetry; observed pulmonary, cardiac, and esophageal toxicities and survival is underway.
Two deaths in IDEAL-CRT were treatment related. In the Cochrane review, treatment-related deaths were recorded in 3% of patients receiving concurrent CRT (18). In RTOG 0617, 8 treatment-related deaths occurred in the 74-Gy group, versus 7 in the 60-Gy group, and 10 patients receiving cetuximab had treatment-related deaths versus 5 not receiving cetuximab (4). Overall, despite substantial treatment intensification, toxicity in IDEAL-CRT does not seem higher than in other relevant concurrent CRT studies.
IDEAL-CRT patients were recruited and treated at multiple sites, supported by a rigorous quality assurance program (21). Their demographics and tumor characteristics were roughly comparable to those of patients in previous studies (Table 2) 3, 4. The average prescribed tumor dose of 67.7 Gy in 30 fractions corresponds to a 15% increase in EQD2 above the 60-Gy dose given in 30 fractions in the control arm of RTOG 0617, assuming a 10 Gy α/β ratio for NSCLC 14, 15. Although it remains to be proven in randomized trials whether this degree of intensification improves survival, the 36.9-month median OS seen in IDEAL-CRT is promising and compares well with median OS times of 28.7 and 20.3 months, respectively, in the control and escalated arms of RTOG 0617, and with 24.3 months in the concurrent CRT arm of the United Kingdom SOCCAR trial 4, 22.
The relatively high survival seen in IDEAL-CRT may originate from strict adherence to the CRT protocol, as well as from treatment intensification. It might also reflect the stage-mix of patients (7% stage IIB, more stage IIIA than IIIB), although we found no evidence of a difference in OS between IIIA and IIIB patients (HR 1.23; 95% CI 0.59-2.57; P=.58) (Fig. 3b). The borderline-significant survival advantage seen for patients treated with prescribed doses greater than the median could be interpreted as showing an increase in tumor control either with rising dose or with falling tumor size, because isotoxic schemes tend to prescribe higher doses to smaller tumors.
A key feature of IDEAL-CRT was its focus on doses to organs at risk, particularly in determining the safety of progressively increasing esophageal doses in a sequence of patient cohorts. Although it is not possible to plan exactly the same RT dose distribution in each patient, we have nevertheless demonstrated the feasibility of structured patient recruitment to cohorts defined by key dosimetric predictors of toxicity. This aspect of trial design proved to be effective and efficient in prospectively identifying an MTD for esophagus using relatively few patients, and is highly relevant to early-phase studies investigating intensified RT across many tumor types and sites and to studies exploring the addition to RT of systemic therapies, radiosensitizers, or radioprotectors.
Conclusions
Toxicity results and survival data from IDEAL-CRT are promising. Dose limits have been determined efficiently using the study's approach to dose escalation, namely by incrementally increasing key dose metrics in specific normal tissues. We have recently completed recruitment to a 5-week form of the IDEAL-CRT schedule, designed to further limit tumor repopulation during treatment, and are presently planning a randomized trial of the 6-week schedule described here versus standard dose CRT.
Footnotes
This trial was funded by Cancer Research UK, grant number C13530/A10424.
ISCRTN no. 12155469.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
Supplementary Data
Escalation of the esophageal dose constraint in group 1.
References
- 1.Saunders M., Dische S., Barrett A. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: A randomised multicentre trial. CHART Steering Committee. Lancet. 1997;350:161–165. doi: 10.1016/s0140-6736(97)06305-8. [DOI] [PubMed] [Google Scholar]
- 2.Yamoah K., Showalter T.N., Ohri N. Radiation therapy intensification for solid tumors: A systematic review of randomized trials. Int J Radiat Oncol Biol Phys. 2015;93:737–745. doi: 10.1016/j.ijrobp.2015.07.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auperin A., Le Pechoux C., Rolland E. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2290. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 4.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauguen A., Le Pechoux C., Saunders M.I. Hyperfractionated or accelerated radiotherapy in lung cancer: An individual patient data meta-analysis. J Clin Oncol. 2012;30:2788–2797. doi: 10.1200/JCO.2012.41.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salama J.K., Vokes E.E. New radiotherapy and chemoradiotherapy approaches for non-small-cell lung cancer. J Clin Oncol. 2013;31:1029–1038. doi: 10.1200/JCO.2012.44.5064. [DOI] [PubMed] [Google Scholar]
- 7.Machtay M., Bae K., Movsas B. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2012;82:425–434. doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Baardwijk A., Bosmans G., Bentzen S.M. Radiation dose prescription for non-small cell lung cancer according to normal tissue dose constraints: An in silico clinical trial. Int J Radiat Oncol Biol Phys. 2008;71:1103–1110. doi: 10.1016/j.ijrobp.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 9.De Ruysscher D., van Baardwijk A., Steevens J. Individualised isotoxic accelerated radiotherapy and chemotherapy are associated with improved long-term survival of patients with stage III NSCLC: A prospective population-based study. Radiother Oncol. 2012;102:228–233. doi: 10.1016/j.radonc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Van Baardwijk A., Reymen B., Wanders S. Mature results of a phase II trial on individualised accelerated radiotherapy based on normal tissue constraints in concurrent chemo-radiation for stage III non-small cell lung cancer. Eur J Cancer. 2012;48:2339–2346. doi: 10.1016/j.ejca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Fenwick J.D., Nahum A.E., Malik Z.I. Escalation and intensification of radiotherapy for stage III non-small cell lung cancer: Opportunities for treatment improvement. Clin Oncol. 2009;21:343–360. doi: 10.1016/j.clon.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Knöos T., Wieslander E., Cozzi I. On the dosimetric behaviour of photon dose calculation algorithms for treatment planning in external photon beam therapy for clinical situations. Phys Med Biol. 2006;51:5785–5807. doi: 10.1088/0031-9155/51/22/005. [DOI] [PubMed] [Google Scholar]
- 13.De Jaeger K., Hoogemans M.S., Engelsman M. Incorporating an improved dose-calculation algorithm in conformal radiotherapy of lung cancer: Re-evaluation of dose in normal lung tissue. Radiother Oncol. 2003;69:1–10. doi: 10.1016/s0167-8140(03)00195-6. [DOI] [PubMed] [Google Scholar]
- 14.Joiner M.C., Bentzen S.M. Dose-response relationships in radiotherapy. In: Steel G.G., editor. Basic Clinical Radiobiology. Arnold; London: 2002. p. 126. [Google Scholar]
- 15.Mehta M., Scrimger R., Mackie R. A new approach to dose escalation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2001;49:23–33. doi: 10.1016/s0360-3016(00)01374-2. [DOI] [PubMed] [Google Scholar]
- 16.Melidis C., Bosch W.R., Izewska J. Global harmonization of quality assurance naming conventions in radiation therapy clinical trials. Int J Radiat Oncol Biol Phys. 2014;90:1242–1249. doi: 10.1016/j.ijrobp.2014.08.348. [DOI] [PubMed] [Google Scholar]
- 17.A'Hern R.P. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 18.O'Rourke N., Roqué I., Figuls M. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010:CD002140. doi: 10.1002/14651858.CD002140.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Chen C., Uyterlinde W., Sonke J.J. Severe late esophagus toxicity in NSCLC patients treated with IMRT and concurrent chemotherapy. Radiother Oncol. 2013;108:337–341. doi: 10.1016/j.radonc.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 20.ASCO University. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. Available at: http://meetinglibrary.asco.org/content/82285?media=vm. Accessed June 1, 2015
- 21.Parsons E., Lester J., Hughes L. Can dose escalation be consistently carried out in a multi-centre trial? QA results for IDEAL-CRT and I-START trials. Radiother Oncol. 2015;115:S121. [Google Scholar]
- 22.Maguire J., Khan I., McMenemin R. SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III non-small cell lung cancer and good performance status. Eur J Cancer. 2014;50:2939–2949. doi: 10.1016/j.ejca.2014.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Escalation of the esophageal dose constraint in group 1.