Abstract
Tissue-resident memory T cells (TRM) are a recently defined, noncirculating subset with the potential for rapid in situ protective responses, although their generation and role in vaccine-mediated immune responses is unclear. Here, we assessed TRM generation and lung-localized protection following administration of currently licensed influenza vaccines, including injectable inactivated influenza virus (IIV, Fluzone) and i.n. administered live-attenuated influenza virus (LAIV, FluMist) vaccines. We found that, while IIV preferentially induced strain-specific neutralizing antibodies, LAIV generated lung-localized, virus-specific T cell responses. Moreover, LAIV but not IIV generated lung CD4+ TRM and virus-specific CD8+ TRM, similar in phenotype to those generated by influenza virus infection. Importantly, these vaccine-generated TRM mediated cross-strain protection, independent of circulating T cells and neutralizing antibodies, which persisted long-term after vaccination. Interestingly, intranasal administration of IIV or injection of LAIV failed to elicit T cell responses or provide protection against viral infection, demonstrating dual requirements for respiratory targeting and a live-attenuated strain to establish TRM. The ability of LAIV to generate lung TRM capable of providing long-term protection against nonvaccine viral strains, as demonstrated here, has important implications for protecting the population against emergent influenza pandemics by direct fortification of lung-specific immunity.
The generation of lung tissue–resident memory T cells to live-attenuated influenza vaccines promotes cross-strain protection.
Introduction
Influenza virus is a severe, acute respiratory pathogen with the potential to generate novel strains capable of global pandemics. Current vaccines protect against disease by eliciting neutralizing antibodies to the strain-specific glycoproteins hemagglutinin (HA) and neuraminidase (NA). Such neutralizing antibodies are the correlate of protection by which current vaccines are assessed (1, 2). However, antigenic shift and drift drive alterations in these molecules, limiting protective efficacy of antibody responses and necessitating the annual production of new vaccines (2). Developing vaccines that provide universal protection to current and emerging influenza strains remains a major public health challenge.
Influenza infection generates both lasting antibody and T cell responses (3). While antibody responses are strain dependent, virus-specific T cells recognize epitopes derived from conserved internal viral proteins in both mice and humans (4–6) and have been shown to provide heterosubtypic, cross-strain protection in animal models (3, 7). Promoting T cell–mediated protection through vaccination, however, has remained elusive, and the precise subsets involved in protection are still being defined. We and others have identified subsets of noncirculating, lung tissue–resident memory (TRM) CD4+ and CD8+ T cells generated following influenza infection; these cells mediate enhanced viral clearance, survival to lethal challenge, and protection to heterosubtypic challenge (8–10). Establishment of TRM, which mediate protection against site-specific infection, has also been described in other tissues, including the skin, female reproductive tract, and brain (11–14). The protective capacities of TRM suggest that vaccination strategies targeting their generation and persistence may provide enhanced immunity compared with vaccines relying on circulating responses. However, roles for circulating versus tissue-localized immunity in vaccine-mediated protection remain undefined.
Two classes of influenza vaccines are currently available: injectable inactivated influenza (IIV) vaccines and live-attenuated influenza (LAIV) vaccines administered i.n. Both generate HA-specific neutralizing antibodies and exhibit similar protection against influenza-like illness (1, 15–18), with LAIV more efficacious in children (19). Whether protective influenza-specific T cells are generated in humans following vaccination has been difficult to establish (20, 21). Moreover, it is not known whether influenza vaccines promote TRM development in humans or animal models.
Here, we evaluated the capacity of currently used quadrivalent Fluzone IIV or FluMist LAIV vaccines to promote lung T cell responses and protective TRM. We found that vaccination with LAIV, but not IIV, elicited robust lung T cell responses, while IIV promoted primarily neutralizing antibody responses as observed by hemagglutination-inhibition assay (HAI). Importantly, LAIV — but not IIV — elicited the establishment of long-term, virus-specific lung TRM and provided heterosubtypic protection to nonvaccine viral strains similar to previous influenza infection. Vaccine-mediated lung T cell responses and protection required both the live-attenuated strain and respiratory targeting, revealing a requirement for site-specific productive infection for establishing lung TRM. Our findings demonstrate that LAIV may be an effective strategy to generate influenza-specific lung TRM capable of cross-strain protection in a pandemic situation.
Results
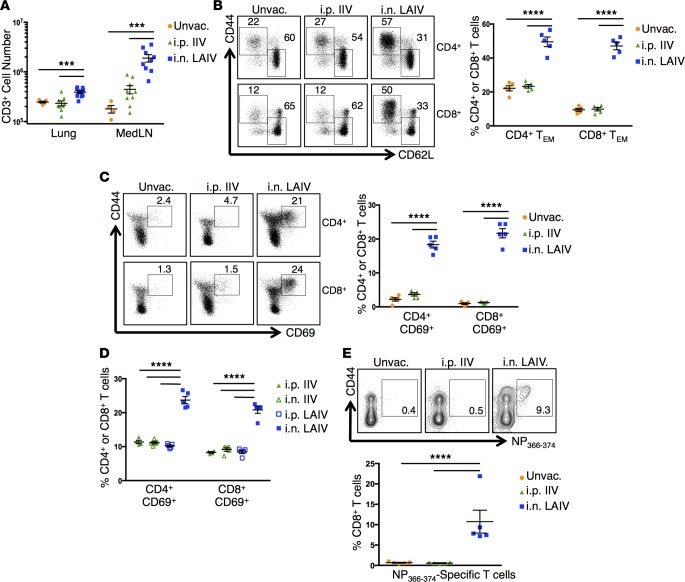
Distinct localization of primary responses generated from vaccination with IIV and LAIV.
We compared primary immune responses, including T cell and antibody responses, in mice vaccinated i.p. or s.c. with Fluzone IIV versus i.n. with FluMist LAIV. The injection routes (i.p. and s.c.) for IIV were chosen based on patterns of antigen drainage with i.p. immunization draining to the lung-draining, mediastinal lymph nodes (MedLN) (22, 23) and s.c. immunization at the base of the neck, draining to the axillary lymph nodes, similar to intradeltoid injection in humans (24, 25). We observed increased numbers of CD3+ T cells in the lungs and MedLN and increased percentages of CD44+CD62Llo effector/memory CD4+ and CD8+ T cells in the lungs of i.n. LAIV mice compared with i.p. or s.c. IIV mice or unvaccinated groups (Figure 1, A and B, and Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/jci.insight.85832DS1). The majority of T cells in the lungs of LAIV mice expressed the early-activation marker CD69, while T cells in the lungs of IIV or unvaccinated mice were predominantly CD69-negative (Figure 1, C and D), indicating generation of tissue-localized, effector CD4+ and CD8+ T cells following administration of LAIV but not IIV. These results demonstrate that vaccination with LAIV promotes the generation and/or migration of early effector T cells in the lung.
Figure 1. Distinct localization of primary T cell responses following vaccination with IIV or LAIV.
(A) CD3+ cells in the lung and MedLN of mice 10 days after vaccination with 2014–2015 IIV or LAIV. Graph displays mean absolute CD3+ cell numbers ± SEM (n = 5–10 mice per group compiled from 2 independent experiments; significance determined by multiple Student’s t tests with Welch’s correction, ***P < 0.001). (B) Lung effector/memory T cells 10 days after vaccination with 2014–2015 IIV or LAIV. Left: Representative flow cytometry plots displaying percentages of cells with naive (CD44loCD62Lhi) and effector/memory (CD44hiCD62Llo) phenotypes. Right: Individual percentages of lung CD4+ and CD8+ effector/memory phenotype T cells (TEM) ± SEM (n = 5 mice per group, representative of 3 experiments; significance determined by 2-way ANOVA with Holm-Sidak’s multiple comparisons test, ****P < 0.0001). (C) CD69 expression by lung T cells 10 days after vaccination with 2014–2015 IIV or LAIV. Left: Representative flow cytometry plots showing percentages of cells with CD44hiCD69hi phenotype. Right: Individual percentages of lung CD4+ and CD8+ T cells expressing CD69 ± SEM (n = 5 mice per group, representative of 3 experiments; significance determined by 2-way ANOVA with Holm-Sidak’s multiple comparisons test, ****P < 0.0001). (D) CD69 expression by lung T cells 10 days after vaccination with 2014–2015 IIV (i.n. or i.p.) or LAIV (i.n. or i.p). Graph displays individual percentages of lung CD4+ and CD8+ T cells expressing CD69 ± SEM (n = 4–5 mice/group; significance determined by 2-way ANOVA with Holm-Sidak’s multiple comparisons test, ****P < 0.0001). (E) Influenza-specific CD8+ T cells in the lungs 10 days after vaccination with 2015–2016 IIV or LAIV. Top: Representative flow cytometry plots with percentages of lung NP366-374–specific CD8+ T cells. Bottom: Individual percentages of lung NP366-374–specific CD8+ T cells ± SEM (n = 5 mice per group, representative of 2 experiments; significance determined by 1-way ANOVA with Holm-Sidak’s multiple comparisons test, ***P < 0.001).
We further investigated whether the increased lung T cell response from LAIV, compared with IIV, was due to direct targeting of vaccine into the respiratory tract. We found that respiratory targeting of IIV by i.n. administration did not generate increased numbers of lung or MedLN CD3+ T cells (Supplemental Figure 2A) or increased percentages of CD69+ or CD44hi effector/memory CD4+ and CD8+ T cells as observed with i.n. LAIV (Figure 1D and Supplemental Figure 2B), indicating that mucosal administration alone is not sufficient to promote lung T cell responses. Similarly, systemic i.p. administration of LAIV failed to promote lung T cell responses (Figure 1D and Supplemental Figure 2B). These results demonstrate that both the live-attenuated vaccine formulation and respiratory targeting are required for the generation of lung-localized primary T cell responses.
Our initial studies used commercially available IIV and LAIV vaccines from the 2014–2015 season directed against the following 4 strains: A/California/7/2009 (H1N1)pdm09, A/Texas/50/2012 (H3N2), B/Massachusetts/2/2012, and B/Brisbane/60/2008. However, since vaccine formulations are changed annually, it was important to establish whether the distinct responses observed with 2014–2015 IIV and LAIV were to due differences in vaccine type and not specific to the vaccine strains. We therefore assessed the primary responses to vaccination using the current 2015–2016 quadrivalent IIV and LAIV formulations directed against new A/Switzerland/9715293/2013 (H3N2) and B/Phuket/3073/2013 strains, in addition to A/California/7/2009 (H1N1)pdm09 and B/Brisbane/60/2008, similar to the 2014–15 version. In agreement with our results using 2014–2015 vaccines, we observed increased percentages of CD44+CD62Llo effector/memory and CD69-expressing CD4+ T cells in the lungs of mice vaccinated with 2015–2016 LAIV compared with 2015–2016 IIV–treated or unvaccinated mice (Supplemental Figure 3, A and B). Vaccination with LAIV also resulted in the accumulation of influenza nucleoprotein–specific (influenza NP-specific) CD8+ T cells in the lung, which was not observed in IIV-vaccinated or unvaccinated mice (Figure 1E). Together, these results demonstrate that i.n.-administered LAIV generates influenza-specific primary lung T cell responses, while IIV immunization does not result in significant lung-localized primary responses, even when delivered directly into the lung.
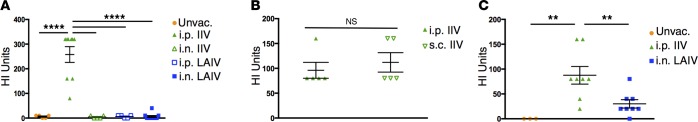
Serum neutralizing antibody responses, as measured by HAI, are currently the gold standard for assessing vaccine efficacy with titers greater than or equal to 40 generally protective against homologous infection in humans (26). Given the disparate primary T cell responses to vaccination with IIV and LAIV, we further assessed serum HAI titers during the primary response at day 10 after vaccination with 2014–2015 IIV or LAIV by different routes. Significant HAI titers were only observed in mice administered IIV by i.p. or s.c. vaccination, with minimal titers in i.n. IIV recipients or in i.n. or i.p. LAIV–vaccinated mice (Figure 2, A and B), demonstrating the importance of both vaccine formulation and route of vaccination in this response. For the 2015–2016 formulations, HAI titers were higher in mice vaccinated with i.p. IIV compared with i.n. LAIV (Figure 2C). Titers were generally higher in mice vaccinated with 2015–16 LAIV compared with 2014–15 LAIV on an absolute scale, although the lack of concurrent availability of both seasonal vaccines precluded direct comparisons. Taken together, IIV and LAIV generate dichotomous primary immune responses with IIV inducing predominantly humoral immunity and LAIV generating robust T cell–mediated responses targeted to the lung.
Figure 2. Distinct primary serum neutralizing antibody responses following vaccination with IIV or LAIV.
(A) Serum HA–neutralizing antibody titers to vaccine antigen 10 days after vaccination in mice receiving 2014–2015 IIV (i.n. or i.p.) or LAIV (i.n. or i.p.). Graph shows individual HAI titers ± SEM (n = 5–10 mice per group compiled from 2 independent experiments; significance determined by 1-way ANOVA with Holm-Sidak’s multiple comparisons test, ****P < 0.0001). (B) Serum HA-neutralizing antibody titers to vaccine antigen 10 days after vaccination in mice administered 2014–2015 IIV i.p. or s.c. Graph shows individual HAI titers ± SEM (n = 5 mice per group; significance determined by 2-tailed Student’s t test with Welch’s correction, ns P > 0.05). (C) Serum HA–neutralizing antibody titers to vaccine antigen 10 days after vaccination in mice receiving 2015–2016 i.p. IIV or i.n. LAIV. Graph shows individual HAI titers ± SEM (n = 3–8 mice per group compiled from 2 independent experiments; significance determined by 1-way ANOVA with Holm-Sidak’s multiple comparisons test, **P < 0.01).
LAIV generates persisting lung TRM.
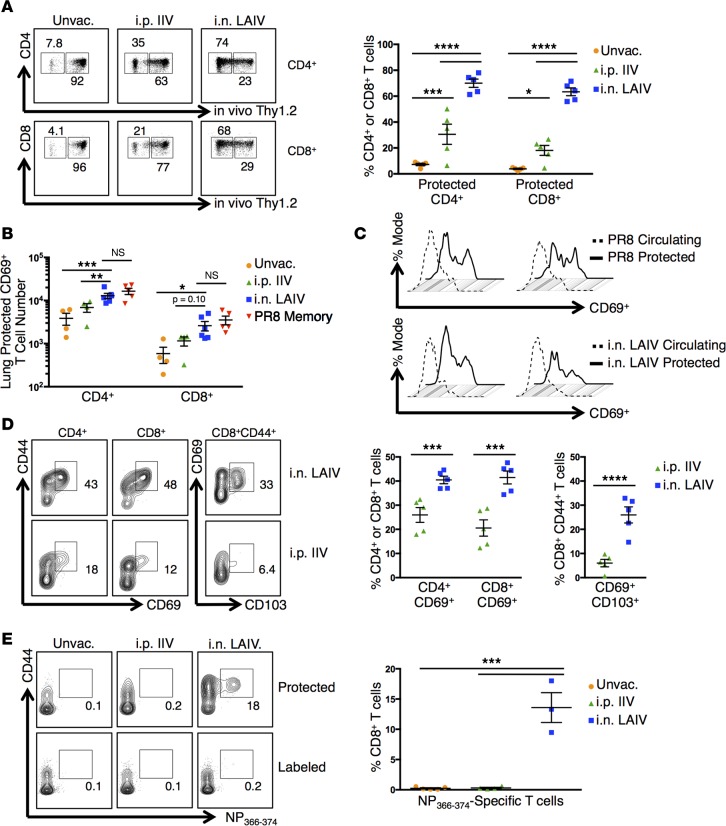
We next assessed whether immunization with IIV or LAIV generated persisting lung TRM assessed at longer times (>6 weeks) after vaccination. To distinguish circulating T cells from tissue-localized TRM, we used an i.v. in vivo antibody labeling technique (i.v. Ab) whereby i.v. infusion of fluorescently conjugated anti–T cell antibodies resulted in fluorescent labeling of circulating T cells, while T cells retained within tissues are protected from antibody binding (9, 27). “Protected” and “labeled” T cells distinguished by i.v. Ab binding exhibit phenotypic markers of TRM and circulating T cells, respectively, and segregate into distinct niches of the lung (9, 27). Using this technique, we found that the majority of lung CD4+ and CD8+ T cells (>75%) in mice vaccinated with LAIV were protected from i.v. Ab labeling compared with only 20% protected in IIV-vaccinated mice and 5% protected in unvaccinated mice, on average (Figure 3A). The majority of lung T cells in IIV and unvaccinated groups were labeled, indicating that they comprised circulating subsets (Figure 3A). Similar patterns of lung T cell segregation to protected and labeled niches were observed following vaccination with both 2014–2015 and 2015–2016 IIV and LAIV formulations (data not shown). Protected CD4+ and CD8+ T cells in the lungs of mice vaccinated with LAIV were present in similar numbers and upregulated the TRM marker CD69 to similar extents as those resulting from live PR8 influenza infection (Figure 3, B–D). These results demonstrate that TRM generated following vaccination with LAIV are similar in phenotype and localization to those generated from influenza infection.
Figure 3. LAIV-vaccination generates lung TRM.
(A) Lung T cells labeled by i.v. anti-Thy1 antibody in mice 6 weeks after vaccination with 2014–2015 IIV or LAIV. Left: Representative flow cytometry plots displaying CD4+ and CD8+ cells in tissues (“Protected”) or circulating (“Labeled”). Right: Individual percentages of protected T cells ± SEM (n = 5 mice per group, representative of 2 experiments; significance determined by 2-way ANOVA with Holm-Sidak’s multiple comparisons test, ****P < 0.0001, ***P < 0.001, *P < 0.05). (B) Protected lung CD69+ T cells in mice 16 weeks after vaccination with 2014–2015 IIV or LAIV or after infection with PR8 influenza. Graph displays protected CD69+ cell numbers ± SEM (n = 4–5 mice per group, representative of 2 experiments; significance determined by multiple Student’s t tests with Welch’s correction, *P < 0.05). (C) CD69 expression by protected versus circulating lung T cells 16 weeks after 2014–2015 LAIV vaccination or PR8 infection. Top: Representative histogram of CD69 expression by protected/labeled lung T cells after PR8 infection. Bottom: CD69 expression by protected/labeled lung T cells after LAIV vaccination. (D) TRM marker expression in protected lung T cells 6 weeks after vaccination with 2015–2016 IIV or LAIV. Left: Representative flow cytometry plots displaying protected CD4+ and CD8+ T cells with CD44hiCD69+ phenotypes or protected CD8+CD44+ T cells with CD69+CD103hi phenotype. Right: Individual percentages of protected CD4+ or CD8+ T cells expressing CD69 or protected CD8+CD44+ T cells expressing CD103 ± SEM (n = 5 mice per group, representative of 3 experiments; significance determined by multiple Student’s t tests with Holm-Sidak’s correction, ****P < 0.0001, ***P < 0.001). (E) Protected lung influenza–specific CD8+ T cells in mice 5 weeks after vaccination with 2015–2016 IIV or LAIV. Left: Representative flow cytometry plots displaying protected or labeled lung NP366-374–specific CD8+ T cells. Right: Individual percentages of protected lung NP366-374–specific CD8+ T cells ± SEM (n = 3–5 mice per group; significance determined by 1-way ANOVA with Holm-Sidak’s multiple comparisons test, ***P < 0.001).
We further investigated whether LAIV-generated CD8+ TRM cells also expressed the canonical CD8+ TRM marker CD103 and/or were enriched for influenza-specific T cells as observed following influenza infection (8, 9, 28). Protected CD8+ TRM generated following vaccination with LAIV expressed CD103, while lung T cells in mice vaccinated with IIV were CD103-negative (Figure 3D). In addition, NP-specific lung CD8+ memory T cells were readily detected in mice vaccinated with LAIV, but not IIV, and nearly all of these NP-specific cells were protected from i.v. Ab labeling (Figure 3E), indicating that they belonged to the TRM subset. Thus, vaccination with LAIV, but not IIV, generates lung-localized memory CD4+ and influenza-specific CD8+ TRM populations.
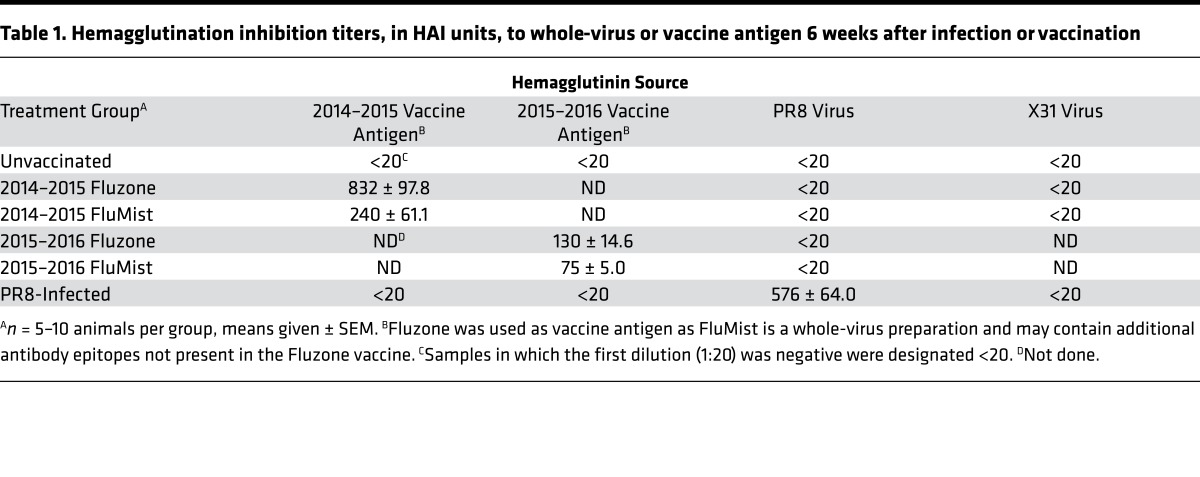
Persisting serum HAI antibody titers specific for vaccine viral strains were detected in the sera of mice 6 weeks after vaccination with IIV and LAIV (Table 1, columns 1 and 2). Vaccination with IIV generated higher titers than LAIV for both 2014–2015 and 2015–2016 formulations (Table 1, columns 1 and 2). We did not detect neutralizing HAI titers specific for the nonvaccine influenza strains PR8 or X31 in any of the vaccinated mice at any time point (Table 1, columns 3 and 4 and data not shown), further confirming the strain-specific nature of vaccine-elicited antibody responses. These results indicate key differences in the persisting memory immune response following immunization with IIV and LAIV, with high levels of durable, strain-specific antibodies induced by IIV, while LAIV promotes TRM in addition to neutralizing antibodies.
Table 1. Hemagglutination inhibition titers, in HAI units, to whole-virus or vaccine antigen 6 weeks after infection or vaccination.
LAIV-generated TRM mediate heterosubtypic protection.
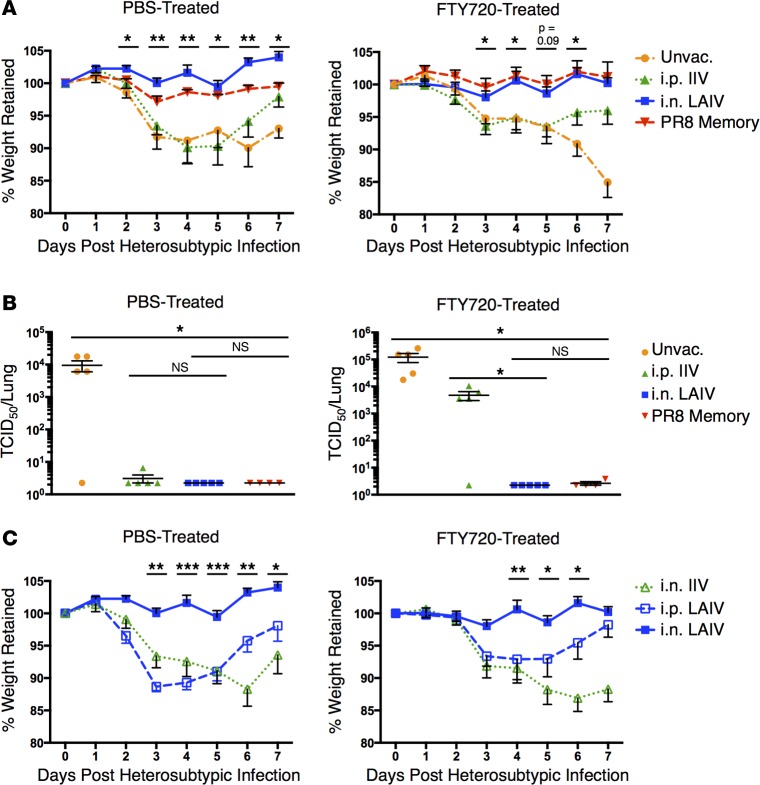
In order to assess whether vaccine-generated, lung-localized memory T cell responses were protective, we challenged mice vaccinated with IIV and LAIV with X31 or PR8 heterosubtypic strains of influenza virus. These stains are known to be serologically distinct from the vaccine strains, and vaccinated mice lack neutralizing antibodies to X31 and PR8 HA (Table 1). To specifically assess TRM-mediated protection independent from that mediated by circulating T cells, we treated mice throughout infection with the sphingosine 1-phosphate receptor-1 agonist FTY720, which sequesters circulating T cells within the secondary lymphoid tissues, while TRM within the lung remain intact (9). We also challenged PBS-treated, vaccinated mice to assess total T cell–mediated protection from both resident and circulating subsets.
Following X31 challenge, mice vaccinated with LAIV (and mice previously infected with PR8 influenza as a positive control) were fully protected from morbidity of infection as measured by weight loss in both PBS- and FTY720-treated groups. In contrast, mice vaccinated with IIV and unvaccinated mice exhibited similar kinetics of weight loss and recovery over 7 days after challenge in PBS-treated groups; however, in FTY720-treated groups, recovery was only observed by IIV-immunized mice, while unvaccinated mice exhibited worsening morbidity throughout the infection course (Figure 4A). These results demonstrate that vaccination with LAIV protects from morbidity of infection independent of circulating lymphocytes, while IIV does not, although it may shorten the disease course compared with naive mice.
Figure 4. LAIV generates TRM-mediated heterosubtypic protection.
(A) Morbidity following heterosubtypic X31 (H3N2) infection in unvaccinated mice or mice vaccinated 6 weeks prior with 2014–2015 IIV or LAIV or infected with PR8 (H1N1) influenza. Left: Mean percentage weight retention in infected animals receiving daily PBS treatment ± SEM. Right: Mean percentage weight retention in infected animals receiving daily FTY720 treatment ± SEM (n = 5 mice per group, representative of 2 experiments; significance determined by multiple Student’s t tests comparing i.n. LAIV-vaccinated to i.p. IIV-vaccinated, **P < 0.01, *P < 0.05). (B) Viral clearance 5 days after X31 infection in mice administered 2014–2015 IIV or LAIV or infected with PR8 influenza 6 weeks prior. Left: Individual D5 lung viral titers in infected animals receiving daily PBS treatment ± SEM. Right: Individual D5 lung viral titers in infected animals receiving daily FTY720 treatment ± SEM (n = 5 mice per group, representative of 3 experiments; significance determined by 1-way ANOVA with Holm-Sidak’s multiple comparisons test, *P < 0.05). (C) Morbidity following X31 infection in animals vaccinated with i.n. 2014–2015 IIV or i.n. or i.p. LAIV 6 weeks prior. Left: Mean percentage weight retention in infected animals with daily PBS treatment ± SEM. Right: Mean percentage weight retention in infected animals with daily FTY720 treatment ± SEM (n = 5 mice per group; significance determined by multiple Student’s t tests comparing i.n. LAIV-vaccinated with other recipient groups with the least significant P value reported, ***P < 0.001, **P < 0.01, *P < 0.05). TCID50, 50% tissue culture infective dose.
In terms of lung viral clearance, mice vaccinated with LAIV exhibited enhanced lung viral clearance in both PBS- and FTY720-treated groups (Figure 4B), indicating that protection is independent of circulating responses. By contrast, mice vaccinated with IIV exhibited viral clearance in PBS-treated but not FTY720-treated groups (Figure 4B), suggesting that, while the circulating T cell response induced by IIV (29, 30) could provide a degree of protection, lung-resident protection was not generated. Additionally, targeting IIV to the lung through i.n. administration did not result in protection in either FTY720- or PBS-treated mice, demonstrating that respiratory targeting of antigen alone — even in the form of inactivated, split virions — does not promote long-term protective immunity (Figure 4C).
We further investigated the breadth and persistence of lung-specific protection generated by LAIV. Mice vaccinated with LAIV (and treated with FTY720) were also protected against challenge with PR8 influenza (Supplemental Figure 4, A and B), and this protection persisted 45 weeks (10 months) after vaccination (Supplemental Figure 4C). Furthermore, vaccination with 2015–2016 LAIV or IIV resulted in a similar pattern of lung-specific protection in the presence of FTY720, with LAIV providing enhanced protection from weight loss compared with IIV (Supplemental Figure 4D). Together, these results demonstrate that LAIV generates long-term lung-localized protection to multiple influenza virus strains.
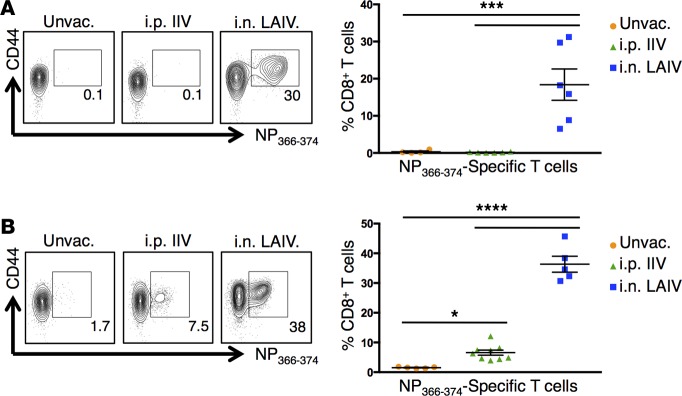
We investigated whether the putative TRM-mediated protection to heterosubtypic challenge in FTY720-treated, vaccinated mice was associated with a virus-specific recall response in the lung. We detected substantial percentages (>30%) of influenza-specific CD8+ T cells in the lung at early time points after heterosubtypic challenge with PR8 or X31 in mice vaccinated with LAIV. In contrast, low to negligible virus-specific CD8+ T cells were observed in the lungs of similarly challenged IIV-vaccinated or unvaccinated mice (Figure 5, A and B). The preferential mobilization of a lung-localized influenza-specific CD8+ T cell recall response in LAIV, compared with IIV-vaccinated mice, was observed with both 2014–2015 and 2015–2016 vaccine formulations (Figure 5, A and B), as further evidence that i.n.-administered live-attenuated vaccines promote lung TRM. Taken together, these results demonstrate that LAIV promotes generation and long-term persistence of lung TRM capable of controlling both viral load and preventing infection morbidity.
Figure 5. Heterosubtypic infection induces a significant lung influenza–specific CD8+ T cell recall response in LAIV-vaccinated mice.
(A) Influenza-specific CD8+ T cells in the lungs 5 days after PR8 (H1N1) influenza infection in unvaccinated mice or mice vaccinated with 2014–2015 IIV or LAIV 6 weeks prior. Animals were treated daily throughout infection with FTY720. Left: Representative flow cytometry plots showing percentages of influenza NP366-374–specific CD8+ T cells in the lungs. Right: Individual percentages of lung NP366-374–specific CD8+ T cells ± SEM (n = 5–6 mice per group; significance determined by 1-way ANOVA with Holm-Sidak’s multiple comparisons test, ***P < 0.001). (B) Influenza-specific CD8+ T cells in the lungs 6 days after X31 (H3N2) influenza infection in unvaccinated mice or mice vaccinated with 2015–2016 IIV or LAIV 6 weeks prior. Animals were treated daily throughout infection with FTY720. Left: Representative flow plots showing percentages of NP366-374–specific CD8+ T cells in the lungs. Right: Individual percentages of lung NP366-374–specific CD8+ T cells ± SEM (n = 5–9 mice per group; significance determined by 1-way ANOVA with Holm-Sidak’s multiple comparisons test, ****P < 0.0001, *P < 0.05).
Discussion
The identification of TRM and their robust protective capacities to site-specific infections has provided a new paradigm by which to assess T cell–mediated responses (31) and an important new target for vaccine design (14, 32). Here, we compared the ability of commercially available influenza vaccines Fluzone Quadrivalent IIV, and FluMist Quadrivalent LAIV to generate lung T cell responses and establish protective lung TRM. We demonstrate that vaccination with LAIV elicits lung CD4+ and virus-specific CD8+ T cell responses and ultimately establishes lung TRM capable of providing long-term, heterosubtypic protection to multiple, nonvaccine influenza strains. In contrast, vaccination with IIV generates durable, strain-specific humoral immunity but does not promote the establishment of significant lung TRM or heterosubtypic protection. Our results have important implications in the testing, monitoring, and administration of different influenza vaccine formulations for promoting protection from seasonal and pandemic strains.
Influenza-specific T cells in mice and humans recognize conserved internal viral proteins that are similar in diverse serotypes (4, 33) and could therefore promote universal protection to new and emerging influenza strains — the ultimate goal of a successful influenza vaccine. Importantly, mouse studies of T cell–mediated protection now indicate that the tissue location of persisting memory T cells greatly impacts their protective capacities. Lung TRM have been shown to provide enhanced protective immunity to influenza infection compared with circulating T cells (8) and are necessary for effective heterosubtypic protection (10). These findings raise the question of whether vaccines can promote TRM-mediated long-term immunity — an issue that is not currently possible to address in humans. By administering clinically available influenza vaccines (IIV and LAIV) to mice, we were able to recapitulate the distinct systemic humoral responses observed in humans, with IIV promoting enhanced HAI titers compared with LAIV (15–18), indicating that this system may provide an effective model by which to examine potential TRM formation to vaccines.
Factors necessary for the establishment of TRM are not currently well understood. Both the route of antigen encounter and the nature of the antigen itself may play critical roles in this process. Recent vaccine studies have demonstrated that mucosal administration of antigen is important for the establishment of localized T cell responses, which may go on to establish TRM (14, 32, 34–37). We found that a single i.n. vaccination with LAIV from either 2 seasonal vaccine formulations was sufficient to generate lung CD4+ and CD8+ TRM enriched in influenza-specific T cells, which provided long-lasting protection to heterosubtypic challenge. By contrast, vaccination with IIV by systemic or i.n. routes was not sufficient to promote lung TRM generation or lung-specific protection from infection. Moreover, systemic administration of LAIV did not generate lung TRM, indicating an essential role for tissue targeting. These results demonstrate that a site-specific infection is necessary for TRM generation, while antigen introduced at the tissue site, even in the context of the pathogen-associated molecular patterns (PAMPs) present within inactivated virus preparations for activation of innate immunity, was not sufficient for TRM establishment.
Our influenza challenge studies with FTY720 treatment indicate that LAIV-mediated protection is targeted to the lung and can occur independent of circulating T cell responses or infiltration from the lymphoid tissue. The enrichment of significant influenza-specific lung memory CD8+ T cells following heterosubtypic influenza challenge in FTY720-treated, LAIV-vaccinated mice (Figure 5) suggests in situ expansion of influenza-specific lung TRM. Focused, local responses by TRM involving rapid cytokine production have been reported in skin and the female reproductive tract following secondary challenge (14, 38–40), although expansion was not assessed in those studies. We propose that local and limited expansion of lung TRM may be responsible for the enhanced viral clearance and protection from morbidity of infection that we observed in mice vaccinated with LAIV.
While studies of TRM generation and protection have been limited to mouse models, TRM-phenotype cells are present in human lymphoid and mucosal tissues and represent the majority of memory T cells in tissues (41, 42). Moreover, analysis of human lung samples has revealed that influenza-specific memory T cells are present in most healthy humans (43, 44), with a proportion of these lung virus–specific T cells expressing CD69 (9), representing putative TRM. Whether TRM in human lungs are generated in response to infection or vaccination, however, is unknown. In general, studies of human influenza–specific T cell responses to infection and vaccines have largely been limited to the analysis of peripheral blood subsets. Circulating virus–specific T cells in the peripheral blood of children were detected following LAIV but not IIV vaccination (19, 20), although whether such responses provide protection is unclear. In human influenza infection, the presence of virus-specific CD4+ T cells in circulation has been correlated to reduced overall disease severity (45). Whether circulating T cell responses predict lung TRM generation will be important to establish in order to improve vaccine response monitoring.
We propose that assessing the relative contribution of TRM versus systemic T cells and humoral responses for clinical vaccine strains in a mouse model can be informative for predicting protective efficacy required for different conditions of seasonal or pandemic infections. Our findings here highlight the differing mechanisms of protection between LAIV and IIV vaccines and establish TRM as an important correlate of vaccine-mediated protection to influenza virus, with potential implications for the design of a universal influenza vaccine.
Methods
Mice.
Female 6- to 8-week-old C57BL/6 mice (Charles River Laboratories) were purchased. Vaccination, infection with influenza virus, and maintenance under specific pathogen–free conditions occurred in a BSL-2 biocontainment facility within CUMC animal facilities.
Reagents.
The following influenza vaccine formulations were purchased from Moore Medical: FluMist Quadrivalent, 2014–2015, live-attenuated vaccine (MedImmune) and Fluzone Quadrivalent, 2014–2015, inactivated virus vaccine (Sanofi Pasteur) directed against A/California/7/2009 (H1N1)pdm09, A/Texas/50/2012 (H3N2), B/Massachusetts/2/2012, and B/Brisbane/60/2008; and 2015-2016 FluMist Quadrivalent and Fluzone Quadrivalent formulations directed against A/California/7/2009 (H1N1)pdm09, A/Switzerland/9715293/2013 (H3N2), B/Phuket/3073/2013, and B/Brisbane/60/2008 strains. Fluorescently conjugated antibodies for Thy1.2 (clone 53-2.1, BioLegend), CD4 (clones RM4-5, BD Biosciences, and GK1.5, eBioscience), CD8 (clone 53-6.7, BioLegend), CD44 (clone IM7, BioLegend), CD62L (clone MEL-14, BioLegend), CD69 (clone H1.2F3, eBioscience), and CD103 (clone 2E7, eBioscience) were purchased. The influenza NP–specific (NP366-374) H-2Db tetramer was purchased from MBL International. FTY720 was purchased from Cayman Chemical Company.
Vaccination of mice.
For vaccinations, mice were administered 20 μl of 2014–2015 or 2015–2016 FluMist (1/10 the adult human dose) i.n. or i.p. for a final dose of 105.5–106.5 FFU/viral strain/mouse, or 50 μl of 2014–2015 or 2015–2016 Fluzone (1/10 the adult human dose) i.p. or s.c. in the neck scruff or i.n. for a final dose of 1.5 μg of HA/viral strain/mouse.
Influenza virus infection.
Mice were infected i.n. with 250 TCID50 PR8 influenza virus (A/PR/8/34[H1N1]) or 5000 TCID50 X31 (A/X31[H3N2]) for primary infection. For heterosubtypic challenge, 5,000 TCID50 PR8 or 10,000 TCID50 X31 was administered i.n. Morbidity was monitored by daily examination and weight assessment.
FTY720 treatment.
Mice were treated with 1 mg/kg FTY720 (25 μg/mouse) or PBS i.p. 2–3 days prior to infection and daily throughout infection.
In vivo antibody labeling and flow cytometry.
For in vivo antibody labeling, naive or vaccinated mice were given 3 μg PE-conjugated anti-Thy1.2 antibody (clone 53-2.1) i.v. and allowed to rest for 10 minutes. Lungs were then perfused with PBS and collected, and cells were isolated by mechanical disruption with collagenase digestion as described (9). In heterosubtypic infection experiments, right lung lobes were collected and homogenized for viral titer analysis, left lobes were collected and collagenase digested, and cells were isolated for flow cytometric analysis as described (9). Cells were stained with fluorochrome-conjugated antibodies or NP366-374 tetramer reagent and analyzed using an LSRII flow cytometer (BD Biosciences), and data were assessed using FlowJo software (Tree Star Inc.).
Viral titers.
Influenza titers in lung homogenates were measured by a 50% tissue culture infective dose (TCID50) assay as described (46), with titers expressed as the reciprocal of the dilution of lung extract that corresponded to 50% virus growth in Madine-Darby canine kidney (MDCK) cells, as calculated by the Reed-Muench method.
Hemagglutination inhibition assay.
Serum neutralizing antibodies titers were measured by HAI as previously described (47). In brief, serum was collected by cardiac puncture and then treated overnight with Receptor Destroying Enzyme (Denka Seiken). Treated serum was serially diluted, incubated with vaccine antigen (2014–2015 or 2015–2016 Fluzone to match the formulation used for vaccination) or PR8 or X31 whole virus particles as HA sources; it was then incubated with 0.5% chicken red blood cells (Lampire). Titers were expressed as the reciprocal of the last dilution of serum that completely inhibited hemagglutination.
Statistics.
Analyses were performed with Prism (GraphPad Software). Results are expressed as the mean value from individual groups ± SEM, unless otherwise designated, indicated by error bars. Significance between experimental groups was determined by 1- or 2-way ANOVA or Student’s t test and corrected for multiple comparisions as indicated, assuming a normal distribution for all groups.
Study approval.
All animal studies and procedures were conducted according to the NIH guidelines for the care and use of laboratory animals and were approved by the CUMC IACUC.
Author contributions
KDZ and DLF designed the research studies. KDZ and JKC conducted experiments and analyzed data. KDZ and DLF wrote the manuscript.
Supplementary Material
Acknowledgments
We thank Esi Lamouse-Smith and Renee McKell for experimental assistance and Michelle Miron and Dustin Carpenter for critical reading of this manuscript. Work was supported by NIH grants AI100119 and HL116136 (to D.L. Farber). K.D. Zens was supported by NIH grant T32 AI106711. Research reported here was performed in the CCTI Flow Cytometry Core, supported in part by award S10RR027050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:JCI Insight. 2016;1(10):e85832. doi:10.1172/jci.insight.85832.
References
- 1.Fiore AE, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 2.McIntyre AF, Gonzalez-Feliciano AG, Bryan LN, Santibanez TA, Williams WW, Singleton JA. Seasonal influenza vaccination coverage - United States, 2009-10 and 2010-11. MMWR Surveill Summ. 2013;62 Suppl 3(3):65–68. [PubMed] [Google Scholar]
- 3.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12(1):48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assarsson E, et al. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J Virol. 2008;82(24):12241–12251. doi: 10.1128/JVI.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belz GT, Xie W, Doherty PC. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J Immunol. 2001;166(7):4627–4633. doi: 10.4049/jimmunol.166.7.4627. [DOI] [PubMed] [Google Scholar]
- 6.Richards KA, Topham D, Chaves FA, Sant AJ. Cutting edge: CD4 T cells generated from encounter with seasonal influenza viruses and vaccines have broad protein specificity and can directly recognize naturally generated epitopes derived from the live pandemic H1N1 virus. J Immunol. 2010;185(9):4998–5002. doi: 10.4049/jimmunol.1001395. [DOI] [PubMed] [Google Scholar]
- 7.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152(4):1653–1661. [PubMed] [Google Scholar]
- 8.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner DL, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7(3):501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu T, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014;95(2):215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 12.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107(42):17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491(7424):463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki S, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81(1):215–228. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71(12):1591–1622. doi: 10.2165/11206860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis. 2011;204(12):1879–1885. doi: 10.1093/infdis/jir661. [DOI] [PubMed] [Google Scholar]
- 18.Cao RG, et al. Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children. J Infect Dis. 2014;210(2):224–233. doi: 10.1093/infdis/jiu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belshe RB, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 20.He XS, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80(23):11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis. 2013;207(2):297–305. doi: 10.1093/infdis/jis684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parungo CP, et al. Lymphatic drainage of the peritoneal space: a pattern dependent on bowel lymphatics. Ann Surg Oncol. 2007;14(2):286–298. doi: 10.1245/s10434-006-9044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson MR, McDermott DS, Varga SM. The initial draining lymph node primes the bulk of the CD8 T cell response and influences memory T cell trafficking after a systemic viral infection. PLoS Pathog. 2012;8(12):e85832. doi: 10.1371/journal.ppat.1003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirone N, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 25.Jackson LA, et al. Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 adjuvant system in healthy adults and older persons. J Infect Dis. 2012;206(6):811–820. doi: 10.1093/infdis/jis427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 27.Anderson KG, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189(6):2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laidlaw BJ, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41(4):633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards KA, Chaves FA, Alam S, Sant AJ. Trivalent inactivated influenza vaccines induce broad immunological reactivity to both internal virion components and influenza surface proteins. Vaccine. 2012;31(1):219–225. doi: 10.1016/j.vaccine.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MA, Ganesan AP, Luckashenak N, Mendonca M, Eisenlohr LC. Endogenous antigen processing drives the primary CD4+ T cell response to influenza. Nat Med. 2015;21(10):1216–1222. doi: 10.1038/nm.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21(7):688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stary G, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348(6241):e85832. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitiello A, et al. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two new dominant and subdominant Kb-restricted epitopes. J Immunol. 1996;157(12):5555–5562. [PubMed] [Google Scholar]
- 34.Li J, Arévalo MT, Chen Y, Chen S, Zeng M. T-cell-mediated cross-strain protective immunity elicited by prime-boost vaccination with a live attenuated influenza vaccine. Int J Infect Dis. 2014;27:37–43. doi: 10.1016/j.ijid.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol. 2004;173(10):6357–6365. doi: 10.4049/jimmunol.173.10.6357. [DOI] [PubMed] [Google Scholar]
- 36.Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol. 2015;8(5):1060–1071. doi: 10.1038/mi.2014.133. [DOI] [PubMed] [Google Scholar]
- 37.Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol. 2005;174(12):7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 38.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol. 2013;14(5):509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med. 2015;212(9):1405–1414. doi: 10.1084/jem.20142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38(1):187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thome JJ, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159(4):814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE. 2011;6(1):e85832. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med. 2005;202(10):1433–1442. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkinson TM, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18(2):274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 46.Cottey R, Rowe CA, Bender BS. Influenza virus. Curr Protoc Immunol. 2001;Chapter 19:e85832. doi: 10.1002/0471142735.im1911s42. [DOI] [PubMed] [Google Scholar]
- 47.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84(18):9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.