Synopsis
Pulmonary arterial hypertension (PAH) is a debilitating disease characterized by pathological remodeling of the resistance pulmonary arteries, ultimately leading to right ventricular (RV) failure and death [1]. In this review, we discuss the definition of PAH, the initial epidemiology based on the NIH Registry and the updated epidemiology gleaned from contemporary registries, pathogenesis of pulmonary vascular dysfunction and proliferation, and RV failure in PAH.
DEFINITION and CLASSFICATION
Pulmonary hypertension (PH) is defined as mean pulmonary arterial pressure (PAP) measured by right heart catheterization ≥ 25 mm Hg at rest. The most recent World Health Organization (WHO) classification has categorized PH in to five different groups based on the underlying mechanism (Box 1).
Box 1. WHO Classification of Pulmonary Hypertension Subtypes.
-
Pulmonary arterial hypertension (PAH)
Idiopathic PAH
Heritable PAH
1.2.1 BMPR2
1.2.2 ALK-1, ENG, SMAD9, CAV1, KCNK3
1.2.3 Unknown
1.3 Drug and toxin induced
1.4 Associated with:
1.4.1 Connective tissue disease
1.4.2 HIV infection
1.4.3 Portal hypertension
1.4.4 Congenital heart diseases
1.4.5 Schistosomiasis
1’ Pulmonary veno-occlusive disease and/or pulmonary capillary Hemangiomatosis
1” Persistent pulmonary hypertension of the newborn (PPHN)
- Pulmonary hypertension due to left heart disease
- Left ventricular systolic dysfunction
- Left ventricular diastolic dysfunction
- Valvular disease
- Congenital/acquired left heart inflow/outflow tract obstruction and congenital cardiomyopathies
- Pulmonary hypertension due to lung diseases and/or hypoxia
- Chronic obstructive pulmonary disease
- Interstitial lung disease
- Other pulmonary diseases with mixed restrictive and obstructive pattern
- Sleep-disordered breathing
- Alveolar hypoventilation disorders
- Chronic exposure to high altitude
- Developmental lung diseases
Chronic thromboembolic pulmonary hypertension (CTEPH)
- Pulmonary hypertension with unclear multifactorial mechanisms
- Hematologic disorders: chronic hemolytic anemia, myeloproliferative disorders, splenectomy
- Systemic disorders: sarcoidosis, pulmonary histiocytosis, Lymphangioleiomyomatosis
- Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders
- Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure, segmental PH
WHO Group I PH or PAH
Hemodynamic-based definition of PAH:
Mean PAP ≥ 25 mm Hg
Pulmonary capillary wedge pressure (PCWP) < 15 mm Hg
Pulmonary vascular resistance (PVR) ≥ 3 Wood units.
PAH includes a group of disorders with similar pulmonary vascular pathophysiological mechanisms and clinical characteristics. PAH can be idiopathic (IPAH), hereditable (HPH), or associated with other conditions such as connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV) infection, anorexigen exposure, or schistosomiasis.
EPIDEMIOLOGY
The NIH Registry
The landmark National Institutes of Health (NIH) registry was a multicenter registry that collected data prospectively from 1981 to 1985 from 32 centers in the United States [2]. 187 patients with idiopathic, hereditary, or anorexigen-associated PAH were included. PAH was defined as mean PAP ≥ 25 mm Hg at rest or ≥ 30 mm Hg with exercise and PCWP < 12 mm Hg. The mean age at presentation was 36 ± 15 years and the majority of the patients were females. The distribution of patients based on race was 85.4% Caucasian, 12.3% African American, and 2.3% Hispanic. The mean time interval between onset of symptoms and diagnosis was 2 years. Dyspnea was the most common presenting symptom followed by fatigue and syncope. Patients in the registry had severe PAH based on hemodynamics with a mean PA pressure of 60 ± 18 mm Hg, a pulmonary vascular resistance index of 26 ± 14 Wood Units, and a cardiac index (CI) of 2.3 ± 0.9 L/min/m2. Treatment consisted of diuretics, digoxin, and supplemental nasal oxygen and, in a minority of cases, anticoagulation with warfarin. PAH-specific vasodilator therapy was not available, but a small number of patients were treated with calcium-channel blockers and/or hydralazine. The estimated median survival was 2.8 years with a 1-year survival of 68%, 3-year survival of 48%, and a 5-year survival of 34% [3]. The registry developed a regression equation to predict survival based on the baseline hemodynamics (mean right atrial pressure, CI, and mean PAP) at the time of diagnosis, and it was subsequently used in many therapeutic trials to demonstrate improved survival in idiopathic or hereditary PAH patients by comparing the observed versus the survival rates predicted by the equation. However, the NIH equation underestimates survival in the current era and is therefore is no longer valid [4].
Contemporary PAH Registries
Since the NIH registry, there has been a remarkable progress in both the understanding of the pathophysiology and treatment options of PAH. Unlike the NIH registry, the current WHO classification of PAH includes IPAH, HPH, anorexigen-associated PH, PH associated with connective tissue disease, congenital heart disease, portal hypertension, and HIV infection. Furthermore, the Food and Drug Administration (FDA) has approved 12 drugs for the treatment of PAH over the past two decades [5]. Due to these changes, several contemporary registries were developed to update both the epidemiological and prognostic data of PAH. There are several differences between these registries including: the time period of enrollment, etiology of PAH patient population enrolled (idiopathic/heritable PAH vs. associated PAH), study design (retrospective vs. prospective), and study cohort (incident cases vs. prevalent cases). Table 1 describes the 11 major PAH registries [2, 6-15].
Table 1.
Characterization of 11 Major PAH Registries
| Registry | Study Cohort | Number | Time Period | Study Design | Study cohort | IPAH/HPAH vs. APAH |
|---|---|---|---|---|---|---|
| NIH | IPAH | 187 | 1981-1985 | Prospective | Incident cases | NA |
| Chinese | IPAH/HPAH | 72 | 1999-2004 | Prospective | Incident cases | NA |
| COMPERA§ | IPAH/HPAH | 587 | 2007-2011 | Prospective | Incident cases | NA |
| French | Group I PAH | 674 | 2002-2003 | Prospective | Incident and prevalent cases | 39% vs. 61% |
| Mayo | Group I PAH | 484 | 1995-2004 | Prospective | Incident and prevalent cases | |
| New Chinese | Group I PAH | 956 | 2008-2011 | Prospective | Incident cases | 35% vs. 65% |
| PHC | Group I PAH | 578 | 1982-2006 | Retrospective & Prospective |
Incident and prevalent cases | 48% vs. 52% |
| REVEAL | Group I PAH | 3515 | 2006-2009 | Prospective | Incident and prevalent cases | 46% vs. 54% |
| Scottish-SMR | Group I PAH | 374 | 1986-2001 | Retrospective | Incident cases | 47% vs. 54% |
| Spanish | Group I PAH CTEPH |
866/162 | 1998-2008 | Retrospective & Prospective |
Incident and prevalent cases | 36% vs. 64% |
| UK and Ireland | IPAH, HPAH, and Anorexigen-APAH |
482 | 2001-2009 | Incident cases | 98.3% vs. 1.7% |
- COMPERA registry enrolled patients with any WHO group PH. The data presented here is for the incident IPAH cohort only.
NIH – national institutes of health, PHC – pulmonary hypertension connections, PAH – pulmonary arterial hypertension, IPAH – idiopathic PAH, and CTEPH – chronic thromboembolic pulmonary hypertension, HPAH – heritable PAH, and APAH – associated PAH.
Epidemiology of PAH in the Modern Era
Although the incidence and prevalence of PAH in North America are unknown, studies from Scotland and France revealed an incidence of 2.5-7.1 cases/million and a prevalence ranging from 5-52/million adults [6, 13]. In the major PAH registries, approximately half of the patients had IPAH or HPAH and the remaining had associated PAH. Of the associated PAH patients, the most common etiology was connective tissue disease followed by congenital heart disease [6-8]. Scleroderma was the most common connective tissue disease associated with PAH. In the French Registry, there was a higher prevalence of PAH associated with anorexigen use (9.5 % vs. 3-5.3%) and HIV infection (6.2% vs. 1-2.3%) compared to US based registries [16]. In China, PAH associated with congenital heart disease was the predominant cause of WHO Group I PAH, a finding that differed from all other countries [12].
Analysis of contemporary PAH registries suggest that PAH epidemiology has changed significantly over the last three decades [16]. The mean age of the patients with IPAH or HPAH in the contemporary registries from the Western world ranged from 45-65 years, which is much higher than the NIH registry. The reason for the dramatic increase in mean age at the time of presentation is unclear, but there are several probable explanations. First of all, most of the modern registries enrolled predominantly prevalent cases (~85%) PAH, which was not used in the NIH registry, which may have introduced survivor bias. However, in the incident cases in both the French registry and the pulmonary hypertension registry of the United Kingdom and Ireland that studied only treatment-naïve incident IPAH, HPAH, and anorexigen-associated PAH, the median age at the time of enrollment was 50 years (vs. 36 years in the NIH registry) with 13.5% of the total UK and Ireland cohort older than 70 years of age at the time of diagnosis [6, 9]. Secondly, there is increased awareness of PAH in the western world due to the availability of Doppler echocardiography as a PH screening tool and the availability of effective PAH-specific therapies. Interestingly, patients in the Chinese IPAH cohort had a profile as the NIH cohort, arguing that the increased age in the Western cohort was caused by detection of PAH in older patients and not an actual change in the biological phenotype of PAH [14]. Moreover, it is also possible that the increasing age at the time of presentation was due to misclassification of PH, especially PH associated with HFpEF being misclassified as PAH due to overreliance on a single measurement of a resting pulmonary capillary wedge pressure (PCWP) [17]. These two clinical entities have common clinical characteristics [18], and the differentiation between these two rests exclusively on PCWP value, which can be erroneous in many situations [19, 20]. This concept is further supported by the enrollment pattern observed in the most recently completed PAH clinical trial. AMBITION was a randomized, double blind, placebo-controlled, clinical trial designed to compare the benefits of initial double combination therapy with ambrisentan and tadalafil vs. monotherapy with either ambrisentan or tadalafil. The steering committee had to stop enrollment briefly and modify inclusion criteria, as the phenotype of the first one hundred patients entered in to this clinical trial was very similar to PH associated with HFpEF as opposed to PAH [21].
PAH shows a female predominance with a female to male ratio of 1.7: 1 in the NIH registry [2]. Contemporary registries of PAH also showed a female dominant distribution, although, the female to male ratio was significantly higher in the modern US based registries: PHC registry − 3:1, REVEAL registry − 4.8: 1, and the Mayo registry − 3.2: 1 [16, 22]. The reason for the increasing female predominance, particularly in the modern US based PAH registries, compared with the European registries is unclear. One possible explanation is the increased use of hormone replacement therapy in the United States in the 1980’s and the 1990’s; however, this is a speculation and has not been tested.
Racial distribution of PAH was explored in the REVEAL registry, the patient distribution was 72.8% Caucasians, 12.2 % African Americans, 8.9% Hispanics, 3.3 % Asians or Pacific Islanders, and 2.8% other or unknown. The proportion of Caucasians in the REVEAL registry was very similar to the age and sex-adjusted expected distribution of Caucasians in the US population; however, there was overrepresentation of African Americans (12.2% vs. 10.9%) and underrepresentation of Hispanics (8.9% vs. 11.5%), and Asians/pacific islander (3.3% vs. 4.3%) [16]. Other contemporary US and non-US based registries lacked data on race.
Despite increasing awareness of PAH, a significant lag between the onset of symptoms and the diagnosis of PAH still exists. The mean interval between onset of symptoms and diagnosis of PAH in the contemporary registries ranged from 18-32 months (vs. 2 years in the NIH registry) [9, 23]. In the REVEAL registry, 20% of patients had symptoms for >2 years before diagnosis. Similar to the NIH registry, majority of the PAH in contemporary registries had advanced WHO functional III and IV symptoms at the time of diagnosis (56%-91%). The mean six minute walk distance (6MWD) at the time of presentation in the contemporary registries ranged from 292 ± 129 meters to 382 ± 117 meters. Assessment of invasive hemodynamics in PAH patients in the contemporary registries revealed significantly elevated mean PAP and PVR with moderately reduced CI at the time of presentation, very similar to the NIH registry. Only a minority of patients (4.5–10%) had a positive acute vasodilator response, and IPAH was the predominant etiology of those with a positive response. In contrast to the NIH registry, contemporary registry patients have multiple co-morbities such as systemic hypertension, diabetes, coronary artery disease, and metabolic syndrome, which could result in an inaccurate underlying diagnosis
PATHOLOGY
The Pulmonary Vasculature
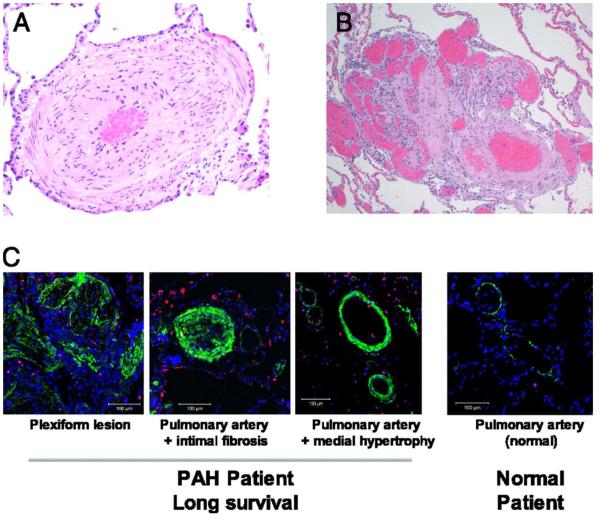
PAH predominantly affects the small resistance pulmonary arteries, characterized by intimal hyperplasia, medial hypertrophy, adventitial proliferation, in situ thrombosis, and inflammation (Fig. 1) [24]. Plexiform arteriopathy, which refers to capillary-like, angioproliferative vascular channels within the lumina of small muscular arteries, is the pathognomonic lesion of PAH [24]. Plexiform lesions often appear at branch points, frequently have fibrin thrombi within the lumen, and have varying channel diameter, giving them a disordered appearance.
Figure 1.
Examples of pathological changes observed in lung samples from patients with PAH Adapted from Rich S, Pogoriler J, Husain AN, et al. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest 2010;138(5):1234-9; with permission.
The Right Ventricle (RV)
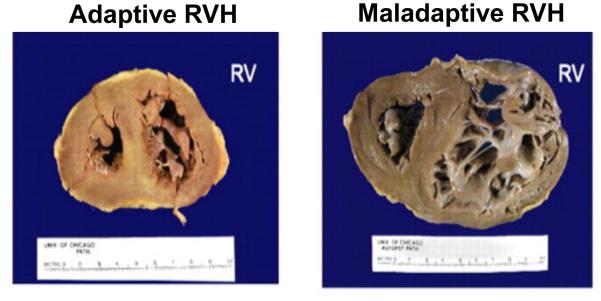
The chronic elevation in RV afterload due to increased PVR induces right ventricular hypertrophy (RVH), which can be either adaptive or maladaptive (Fig. 2). Adaptive RVH, characterized by concentric hypertrophy with minimal eccentric dilatation and fibrosis, maintains normal ejection fraction, cardiac output, and filling pressures [24]. However, maladaptive RVH shows eccentric dilatation, increased fibrosis, and capillary rarefaction with reduction in ejection fraction and cardiac output and elevation in filling pressures [24, 25]. At a metabolic level, maladaptive RVH is characterized by increased aerobic glycolysis, fatty acid oxidation, and glutaminolysis [26]. In addition maladaptive RVH is associated with increased sympathetic activation and down regulation of α, β, and dopaminergic receptors in myocytes of the RV [27]. Furthermore, maladaptive RVH is associated with increased beta-myosin heavy chains (hypo contractile) and decreased alpha-myosin heavy chains (hyper contractile) similar to the fetal heart [28]. Some patients, especially those with congenital heart disease associated PAH, remain stable with adaptive RVH for a prolonged period [29, 30]. However, certain PAH patients, specifically those with scleroderma associated PAH, develop maladaptive RVH relatively early, leading to RV failure and death [31]. The mechanisms that mediate the switch from adaptive, compensatory hypertrophy of the RV to maladaptive RV dilatation and ultimately RV failure are unclear and are under investigation [27, 32]. More research is needed to answer this questions as long-term outcomes in PAH are largely determined by the response of the RV to the increased afterload [33].
Figure 2.
Examples of adaptive and maladaptive RV remodeling in PAH Adapted from Rich S, Pogoriler J, Husain AN, et al. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest 2010;138(5):1234-9; with permission.
PATHOGENESIS
Pulmonary Vascular Dysfunction
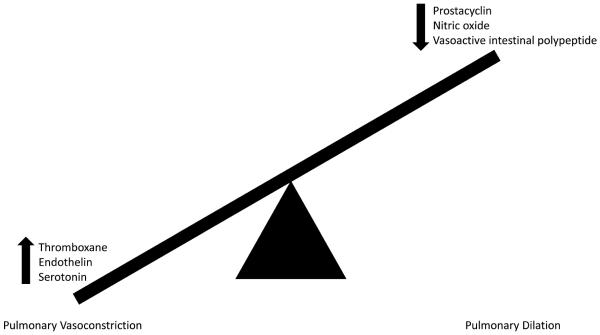
The pathogenesis of PAH likely involves multiple pathways rather than a single mechanism. There is an imbalance of vasoconstricting and vasodilating substances in the small pulmonary arteries. Increased production of the vasoconstrictors thromboxane, endothelin, and serotonin and reduced synthesis of vasodilators prostacyclin, nitric oxide, and vasoactive intestinal polypeptide were observed in PAH patients [1]. This imbalance in favor of vasoconstrictors causes small pulmonary artery vasoconstriction. The change in abundance of these vasoactive chemicals has important implications beyond simply vasoactivity as thromboxane induces platelet proliferation, prostacyclin inhibits smooth muscle proliferation and has anti-platelet properties, nitric oxide inhibits smooth muscle proliferation and inhibits platelet activation, and endothelin induces smooth muscle cell proliferation [1]. Figure 3 summarizes the known mechanisms that contribute to pulmonary vascular dysfunction in PAH.
Figure 3.
Schematic representation of imbalance of vasodilatory and vasocontricting substances present in the pulmonary circulation in PAH leading to pulmonary vascular dysfunction in PAH.
Pulmonary Vascular Proliferation
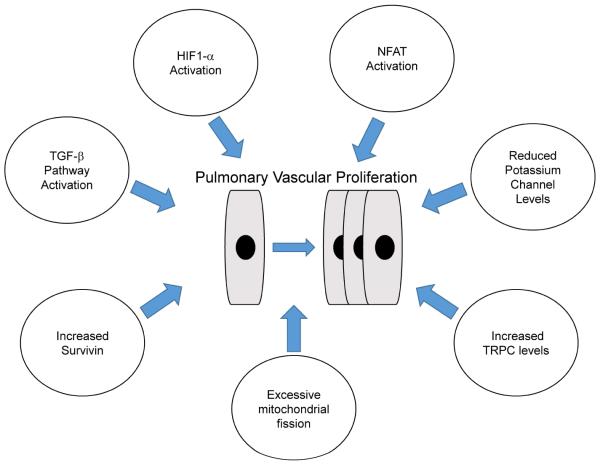
There are changes in multiple pathways in the pulmonary artery smooth muscle cells that promote cellular replication and decreased apoptosis that contribute to the pulmonary vascular proliferation. Inappropriate activation of transcription factors hypoxia inducible factor (HIF)-1 alpha and nuclear factor of activated T-cells (NFAT), decreased expression of voltage gated potassium channels (e.g., Kv1.5 and Kv2.1), increased expression of the anti-apoptotic protein survivin, and increased expression of transient receptor potential channels (TRPC 6) which leads to calcium overload are all pathways that promote smooth muscle propagation [34]. Finally, there is strong evidence that imbalance of mitochondrial fission and fusion leading to excessive mitochondrial fission, also stimulates pulmonary arterial smooth muscle proliferation [35]. Thus, multiple pathways converge to contribute to excessive pulmonary arterial smooth muscle proliferation in PAH (Fig. 4).
Figure 4.
Multiple pathways converge to induce pulmonary vascular proliferation in PAH.
Extracellular Matrix Remodeling
There is emerging evidence that extracellular matrix remodeling may be one of the inciting events in PAH pathophysiology. Firstly, in the monocrotaline-induced rat PH model, fragmentation of the internal elastic lamina in the hilar pulmonary arteries occurs two days after monocrotaline injection, and 14 days before pulmonary artery smooth muscle hypertrophy [36]. Disruption of the internal elastic lamina is associated with increased elastolytic activity of serine elastases and matrix metalloproteinases in this animal model. Similar findings were reported in animal models of chronic hypoxic PH [37]. Inhibition of serine elastases prevented development of PH in both these animal models confirming the pathogenic role of extracellular matrix remodeling in distal pulmonary arterial smooth muscle proliferation [37-40].
Inflammation
Pathological examination of the pulmonary vasculature, both in animal models of PH and PAH patients, showed inflammatory cell infiltration, suggesting inflammation might contribute to pulmonary vascular remodeling [41]. Further investigation of the role of inflammation in PH led to the observations that serum levels of inflammatory cytokines (IL-1β, IL2, IL-6, IL-8, IL-10, IL-12, and TNFα) were elevated in PAH patients [42, 43], and serum levels of IL-6, IL-8, IL-10, and IL-12 predicted survival in PAH [43]. IL-6 has been the most intensively studied inflammatory cytokine in PAH. Animal data supported a role of IL-6 in promoting PAH as IL-6 knockout mice were resistant to hypoxia-induced PH [44], while overexpression of IL-6 induced PH [45]. Moreover, use of the IL-6 receptor antibody Tocilizumab reduced the severity of PH in patients with Castelman’s disease [46, 47], providing further evidence that elevated IL-6 levels promote a more severe PAH phenotype.
Right Ventricular Pathological Changes
RV Metabolic Derangements
Work aimed at understanding the switch between adaptive and maladaptive RV remodeling have identified metabolic derangements as a likely mediator. In particular, mitochondrial dysfunction marked by reduced oxidative metabolism [25] and ectopic glutaminolysis [48] has been shown to play a role in RV dysfunction in PAH.
A shift from oxidative to anaerobic metabolism of glucose occurs in the RV in PAH. This can lead to an energy starved state as anaerobic metabolism of glucose produces less ATP than aerobic metabolism of glucose (2 ATP per glucose in anaerobic metabolism versus 36 ATP with coupled oxidative metabolism). To compensate for reduced metabolic efficacy of anaerobic glycolysis, glucose uptake by the RV is increased in order to generate more ATP [49].
Similar to cancer cell metabolism, there is normoxic activation of HIF-1 alpha (Warburg effect) in the RV in PAH, which activates pyruvate dehydrogenase kinase (PDK). Activation of PDK inhibits pyruvate dehydrogenase, the rate-limiting enzyme for aerobic glucose oxidation [25].
Activation of oxidative metabolism through inhibition of pyruvate dehydrodenase kinase using dichloroacetate improved RV function in animal models of PAH [50, 51].
There is increased fatty acid oxidation in the RV in PAH. Inhibition of fatty acid oxidation, using either ranolazine or trimetazidine, led to a reciprocal increase in glucose oxidation and improved RV function (Randall’s cycle) in animal models of PH [52].
Glutaminolysis, metabolism of glutamine, a metabolic pathway that is usually only detected in cancer cells, was identified in the RV of animals with RV hypertrophy. Inhibition of glutaminolysis enhanced glucose oxidation and improved RV function and exercise capacity [48].
RV Ischemia
Another factor contributes to maladaptive RV remodeling and metabolic changes is RV ischemia due to reduced right coronary artery epicardial flow and capillary rarefaction associated with PAH.
In the normal RV, perfusion from the right coronary artery occurs during systole and diastole. However, in PAH, elevated RV systolic pressure minimizes the pressure differences between the right coronary artery and the aorta and thus reduces filling time (only during diastole as opposed to both systole and diastole) and RV perfusion [53].
Impaired angiogenesis demonstrated by reduced number of capillaries and intramuscular arterioles can also contribute to RV ischemia [54], a phenotype often observed in Scleroderma-associated PAH. Scleroderma patients typically display maladaptive RV remodeling [31], providing more evidence that relative RV ischemia contributes to RV failure in PAH.
RV Calcium Mishandling
While the cellular mechanisms of LV dysfunction has been extensively studied, the understanding of the causes of RV dysfunction in PAH has lagged behind. However, recent data has emerged that suggests calcium mishandling contributes to RV dysfunction in PAH.
In a canine model of PH caused by PA banding, decreased amounts of SERCA-2a and phosphorylated phospholamban which was relative to the degree of increased afterload were observed [55].
In the monocrotaline rat model, decreased levels of SERCA-2a protein and mRNA levels of phospholamban and ryanodine receptor were documented in the RV [56].
In the monocrotaline rat model, calcium transients were noted to be smaller with slower kinetics to peak and decay. This was associated with a reduction in L-type calcium channel protein levels [57].
Sarcomere Remodeling
As observed in cardiomyocyte dysfunction in the left ventricle, there are derangements of the sarcomeric proteins in the RV in PAH.
Myosin isoform switches: Increased β-myosin heavy chain mRNA and decreased α-myosin heavy chain mRNA was documented in samples of patients with PAH and RV failure [28]. Animal models with similar myosin isoform switching had reduced contractility [58] likely due to different ATPase activities of the α- and β-myosin heavy chains.
Change is actin isoforms: A ten-fold increase in the amount of skeletal muscle α-actin mRNA in the right ventricle was found in the high altitude calf model of PH [59]. The functional consequences of this change remain unknown.
Cytoskeletal Remodeling
Increased stabilization and subsequent proliferation of the microtubule cytoskeleton was documented in pre-clinical models of pulmonary hypertension. However, there are mixed results on the pathological consequences of microtubule proliferation in the RV.
Microtubule stabilization and proliferation was documented in the PA-banded feline model. Microtubule depolymerization normalized cardiac contractility in isolated cardiomyocytes [60, 61].
The rat monocrotaline PAH model also showed increased microtubules, but colchicine-induced microtubule depolymerization did not enhance contractility in isolated cardiomyocytes [62].
Genetic Causes of PAH
PAH can also be inherited. Approximately, 10% of patients have hereditable PAH which are outlined below.
- Transforming growth factor beta (TGF-ß) pathway mutations
- ○ Bone morphogenetic protein receptor (BMPR2): Regulates vascular smooth muscle cell growth by activating the intracellular pathways of SMAD and LIM kinase. Loss of function mutation of the BMPR2 gene leads to decreased activation of SMAD, ultimately leading to increased proliferation of pulmonary artery smooth muscle cells [63].
- ○ Endoglin: Membrane bound TGF-β receptor, which when activated induces cellular replication. [65]
Caveloin-1: A membrane protein that forms caveolae, which are flask-shaped invaginations of the plasma membrane. Caveolae regulates membrane trafficking, cell signaling, cholesterol homeostasis, and mechanotransduction [66].
KCNK3: Two-pore potassium channel expressed in pulmonary artery smooth muscle cells. This potassium channel modulates resting membrane potential, pulmonary vascular tone, and hypoxic pulmonary vasoconstriction [67].
Conclusions
Although our knowledge about the epidemiology and pathophysiology of PAH has increased greatly in the past 20 years, PAH continues to be a debilitating disease with poor survival and high economic burden. There are still many unanswered questions that will need to be investigated to reduce symptomatic burden and promote patient survival in the future.
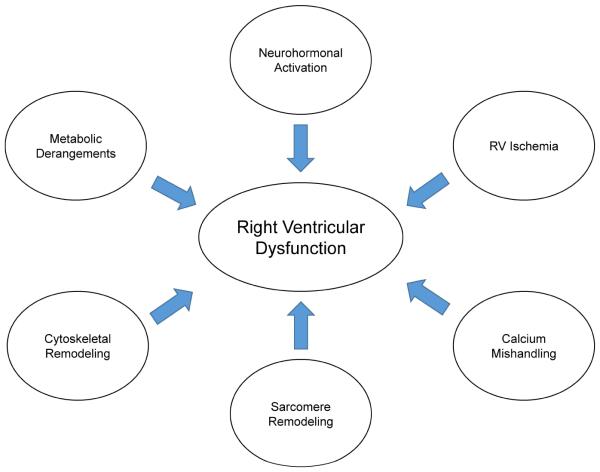
Figure 5.
Mechanisms that contribute to RV failure in PAH.
Key Points.
Pulmonary arterial hypertension (PAH) is characterized by pathological vascular remodeling resulting in elevated pulmonary artery pressures and eventual right ventricular (RV) dysfunction.
PAH remains a rare disease with a female predominance and an evolving epidemiology as our awareness of the disease improves.
The pathological changes in the pulmonary vasculature include pulmonary vascular dysfunction and excessive proliferation, with multiple molecular mechanisms contributing to both phenotypes.
RV is the major predictor of outcomes in PAH, but our knowledge about RV failure in PAH is limited.
Available evidence on RV dysfunction suggests several pathways converge to promote RV failure in PAH.
Acknowledgments
Funding: TT was funded by AHA Scientist Development Grant 15SDG25560048 and KWP was funded by NIH F32 grant HL129554
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655–65. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 2.Rich S, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107(2):216–23. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 3.D'Alonzo GE, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 4.Thenappan T, et al. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35(5):1079–87. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galiè N, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60–72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 7.Thenappan T, et al. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Respir J. 2007;30(6):1103–10. doi: 10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 8.Badesch DB, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–87. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 9.Ling Y, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186(8):790–6. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 10.Escribano-Subias P, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. 2012;40(3):596–603. doi: 10.1183/09031936.00101211. [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168(2):871–80. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, et al. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest. 2011;140(2):301–9. doi: 10.1378/chest.10-2327. [DOI] [PubMed] [Google Scholar]
- 13.Peacock AJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30(1):104–9. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 14.Jing ZC, et al. Registry and survival study in chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest. 2007;132(2):373–9. doi: 10.1378/chest.06-2913. [DOI] [PubMed] [Google Scholar]
- 15.Kane GC, et al. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest. 2011;139(6):1285–93. doi: 10.1378/chest.10-1293. [DOI] [PubMed] [Google Scholar]
- 16.Frost AE, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest. 2011;139(1):128–37. doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 17.Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136(1):37–43. doi: 10.1378/chest.08-2784. [DOI] [PubMed] [Google Scholar]
- 18.Thenappan T, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4(3):257–65. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 19.Ryan JJ, et al. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012;163(4):589–94. doi: 10.1016/j.ahj.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs G, et al. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med. 2014;190(3):252–7. doi: 10.1164/rccm.201402-0269PP. [DOI] [PubMed] [Google Scholar]
- 21.Galiè N, et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med. 2015;373(9):834–44. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 22.McGoon MD, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25 Suppl):D51–9. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Brown LM, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest. 2011;140(1):19–26. doi: 10.1378/chest.10-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rich S, et al. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest. 2010;138(5):1234–9. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res. 2014;115(1):176–88. doi: 10.1161/CIRCRESAHA.113.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archer SL, et al. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ. 2013;3(1):144–52. doi: 10.4103/2045-8932.109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piao L, et al. GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: therapeutic implications in pulmonary hypertension. Circulation. 2012;126(24):2859–69. doi: 10.1161/CIRCULATIONAHA.112.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowes BD, et al. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest. 1997;100(9):2315–24. doi: 10.1172/JCI119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy S, Bernstein D. Molecular Mechanisms of Right Ventricular Failure. Circulation. 2015;132(18):1734–42. doi: 10.1161/CIRCULATIONAHA.114.012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonk-Noordegraaf A, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25 Suppl):D22–33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Tedford RJ, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6(5):953–63. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake JI, et al. Chronic carvedilol treatment partially reverses the right ventricular failure transcriptional profile in experimental pulmonary hypertension. Physiol Genomics. 2013;45(12):449–61. doi: 10.1152/physiolgenomics.00166.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Veerdonk MC, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58(24):2511–9. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 34.Tuder RM, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D4–12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archer SL. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369(23):2236–51. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 36.Todorovich-Hunter L, et al. Increased pulmonary artery elastolytic activity in adult rats with monocrotaline-induced progressive hypertensive pulmonary vascular disease compared with infant rats with nonprogressive disease. Am Rev Respir Dis. 1992;146(1):213–23. doi: 10.1164/ajrccm/146.1.213. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama K, et al. Chronic hypoxic pulmonary hypertension in rats and increased elastolytic activity. Am J Physiol. 1991;261(6 Pt 2):H1716–26. doi: 10.1152/ajpheart.1991.261.6.H1716. [DOI] [PubMed] [Google Scholar]
- 38.Zaidi SH, et al. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation. 2002;105(4):516–21. doi: 10.1161/hc0402.102866. [DOI] [PubMed] [Google Scholar]
- 39.Ilkiw R, et al. SC-39026, a serine elastase inhibitor, prevents muscularization of peripheral arteries, suggesting a mechanism of monocrotaline-induced pulmonary hypertension in rats. Circ Res. 1989;64(4):814–25. doi: 10.1161/01.res.64.4.814. [DOI] [PubMed] [Google Scholar]
- 40.Ye CL, Rabinovitch M. Inhibition of elastolysis by SC-37698 reduces development and progression of monocrotaline pulmonary hypertension. Am J Physiol. 1991;261(4):H1255–67. doi: 10.1152/ajpheart.1991.261.4.H1255. Pt 2. [DOI] [PubMed] [Google Scholar]
- 41.Hassoun PM, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 Suppl):S10–9. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Humbert M, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151(5):1628–31. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 43.Soon E, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122(9):920–7. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 44.Savale L, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steiner MK, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104(2):236–44. doi: 10.1161/CIRCRESAHA.108.182014. 28p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi K, et al. Tocilizumab is effective for pulmonary hypertension associated with multicentric Castleman's disease. Int J Hematol. 2009;90(1):99–102. doi: 10.1007/s12185-009-0346-x. [DOI] [PubMed] [Google Scholar]
- 47.Arita Y, et al. The efficacy of tocilizumab in a patient with pulmonary arterial hypertension associated with Castleman's disease. Heart Vessels. 2010;25(5):444–7. doi: 10.1007/s00380-009-1215-5. [DOI] [PubMed] [Google Scholar]
- 48.Piao L, et al. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 2013;91(10):1185–97. doi: 10.1007/s00109-013-1064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan JJ, et al. Right ventricular adaptation and failure in pulmonary arterial hypertension. Can J Cardiol. 2015;31(4):391–406. doi: 10.1016/j.cjca.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMurtry MS, et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95(8):830–40. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 51.Michelakis ED, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105(2):244–50. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 52.Fang YH, et al. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle's cycle. J Mol Med (Berl) 2012;90(1):31–43. doi: 10.1007/s00109-011-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Wolferen SA, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29(1):120–7. doi: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]
- 54.Bogaard HJ, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120(20):1951–60. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 55.Moon MR, et al. Differential calcium handling in two canine models of right ventricular pressure overload. J Surg Res. 2012;178(2):554–62. doi: 10.1016/j.jss.2012.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kögler H, et al. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ Res. 2003;93(3):230–7. doi: 10.1161/01.RES.0000085042.89656.C7. [DOI] [PubMed] [Google Scholar]
- 57.Xie YP, et al. Sildenafil prevents and reverses transverse-tubule remodeling and Ca(2+) handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension. 2012;59(2):355–62. doi: 10.1161/HYPERTENSIONAHA.111.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90(11):1150–2. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- 59.Bakerman PR, Stenmark KR, Fisher JH. Alpha-skeletal actin messenger RNA increases in acute right ventricular hypertrophy. Am J Physiol. 1990;258(4):L173–8. doi: 10.1152/ajplung.1990.258.4.L173. Pt 1. [DOI] [PubMed] [Google Scholar]
- 60.Tsutsui H, Ishihara K, Cooper G. Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science. 1993;260(5108):682–7. doi: 10.1126/science.8097594. [DOI] [PubMed] [Google Scholar]
- 61.Tsutsui H, et al. Role of microtubules in contractile dysfunction of hypertrophied cardiocytes. Circulation. 1994;90(1):533–55. doi: 10.1161/01.cir.90.1.533. [DOI] [PubMed] [Google Scholar]
- 62.Stones R, et al. Microtubule proliferation in right ventricular myocytes of rats with monocrotaline-induced pulmonary hypertension. J Mol Cell Cardiol. 2013;56:91–6. doi: 10.1016/j.yjmcc.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Best DH, et al. Genetics of pulmonary hypertension. Curr Opin Cardiol. 2014;29(6):520–7. doi: 10.1097/HCO.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 64.Abdalla SA, et al. Primary pulmonary hypertension in families with hereditary haemorrhagic telangiectasia. Eur Respir J. 2004;23(3):373–7. doi: 10.1183/09031936.04.00085504. [DOI] [PubMed] [Google Scholar]
- 65.Harrison RE, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40(12):865–71. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Austin ED, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5(3):336–43. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma L, Chung WK. The genetic basis of pulmonary arterial hypertension. Hum Genet. 2014;133(5):471–9. doi: 10.1007/s00439-014-1419-3. [DOI] [PubMed] [Google Scholar]