Abstract
The need for the development of new cancer therapies and push for the design of new targeting techniques is on the rise, and would be useful for cancers that are resistant to current drug treatments. The understanding of the genome has significantly advanced cancer therapy, as well as prevention and earlier detection. This research highlight discusses a potential new type of cancer-targeting molecule, Sweet-P, which is the first of its kind. Sweet-P specifically targets the microRNA-144 binding site in the 3′ untranslated region (3′ UTR) of the human glucocorticoid receptor β (GRβ), which has been demonstrated to increase expression. GRβ has been shown to be highly expressed in cells from solid tumors of uroepithelial carcinomas, gliomas, osteosarcomas, and hepatocellular carcinomas, as well as in liquid tumor cells from leukemia patients. In non-cancerous diseases, GRβ has been shown to be highly expressed in glucocorticoid-resistant asthma. These maladies brought the need for the development of the Sweet-P anti-GRβ molecule. Sweet-P was shown to repress the migration of bladder cancer cells, and may serve as a new therapeutic for GRβ-related diseases.
Keywords: Glucocorticoid receptor, GR, GR alpha, GR beta, glucocorticoids, cancer, bladder cancer, asthma, growth, migration, microRNA, miRNA, Sweet-P
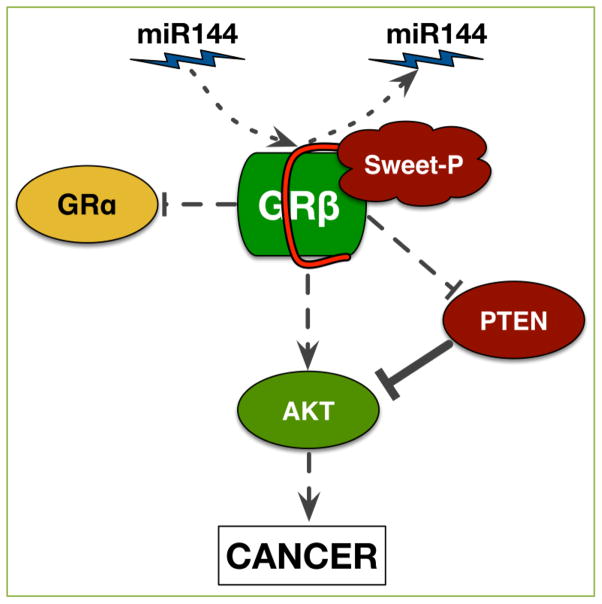
In our recent work, published in Oncotarget, we have discovered that the glucocorticoid receptor β (GRβ) causes migration (movement) of human bladder cancer cells [1]. Bladder cancer is the 4th most common cancer in men, and the 5th most overall [2]. Almost three-quarters of bladder cancer patients may have a recurrence, and one-third experience progression, causing the need for constant lifelong surveillance and treatment [3]. The long-term therapy results in bladder cancer being the most costly cancer for lifetime regimen [4], which brings the need for new and better treatments. Because we showed that GRβ plays a role in bladder cancer migration, we set out to construct the first anti-GRβ molecule, which we termed Sweet-P, with the goal of providing a potential new therapy. Sweet-P was designed as a peptide nucleic acid (PNA), conjugated to the Trans-Activator of Transcription (TAT) protein from HIV (for cellular delivery) to specifically target the 3′ untranslated region (3′ UTR) of human GRβ. Sweet-P functions by specifically blocking the microRNA-144 (miR-144) binding site in the 3′UTR of human GRβ (Figure 1), which we showed increases expression. Furthermore, Sweet-P and shRNA suppression of GRβ in human bladder cancer cells attenuated migration [1].
Figure 1. Sweet-P inhibition of GRβ reduces signaling that leads to cancer.
Sweet-P inhibits miR144 binding to the 3′UTR of human GRβ, resulting in reduced expression. Sweet-P inhibition of GRβ increases GRα and PTEN activity and decreases AKT, which leads to reduced cancer growth and migration.
The gene that codes for GR in humans is found on the q arm of chromosome 5 [5, 6], and is a single GR gene that is alternative spliced to give rise to at least five isoforms α, β, γ, A, and P [5, 7–9]. GRα and GRβ have been the most investigated isoforms. GRα is identical to GRβ from exons 2–8 and is distinguished by alternative splicing of exon 9 in humans resulting in the differing of the C-terminus [10]. GRα contains an additional fifty amino acids derived from the proximal portion of exon 9 that constructs helix 12 for ligand binding. GRβ does not have the capacity to bind glucocorticoids because of an additional fifteen amino acids derived from the distal portion of exon 9 that causes a degenerate helix 12 [5, 9, 11, 12]. The alternative splicing mechanism in humans is different than in mouse [11], rat [13], and zebrafish [14], but in these species that GRβ has been identified, GRα and GRβ are identical through exon 8 with an addition of an alternatively spliced intron 8. In humans, the 3′ UTR of GRβ and GRα are different [10] and are targeted differently by miRNAs. For instance, miR-144 increased GRβ but had no effect on GRα expression in human bladder cancer cells [1]. However, GC resistance in sepsis is influenced by miR-124, which downregulated GRα [15]. The effect of miR-124 on GRβ is unknown and miRNAs that target GRβ or GRα are very limited.
GRβ has been shown to antagonize GRα, which has been demonstrated to be due to the competition with GRα for glucocorticoid response elements (GREs)/coregulators, coactivator squelching through the transactivation domain, and through inactive α/β dimers that bind in the nucleus [6, 11, 16, 17]. Therefore, increasing GRβ levels can lead to a GC-resistant state that allows for an elevation of proinflammatory cytokines and transcription factors [10, 11, 18–20]. The ratio of GRα:GRβ is a critical factor in GC disease states [10, 17, 18, 20]. A high GRα:GRβ ratio can be indicative of a GC-sensitive state while a low ratio would be considered GC-resistant [18]. Importantly, Sweet-P inhibition of GRβ increased the responsiveness to GCs [1], which indicates that it may reverse GRβ induced GC-resistant diseases. Also, GRβ has recently been shown to have positive and negative GRα independent transcriptional activity [6, 12]. We recently demonstrated that mouse GRβ specifically binds to the promoter of phosphatase and tensin homolog (PTEN), which increased Akt1 guided proliferation [21]. We also showed that Sweet-P inhibition of human GRβ increased PTEN expression in bladder cancer cells [1] (Figure 1). There may be other GRβ-specific gene targets that are increased in cancer, and microarray or RNA-seq studies would help strengthen our understanding of the involvement of GRβ in cancer. However, this work is yet to be done.
Sweet-P may have several clinical applications as GRβ has been shown to be involved in other cancer types. For example, treatment with GCs as a first line therapy in acute lymphoblastic leukemia (ALL) is effective due to its ability to arrest cell growth and trigger apoptosis. Unfortunately, resistance to therapeutic GCs is common, which has been attributed to increased levels of GRβ or decreased GRα [22]. The GRβ interaction with β-catenin and transcription factor-4 (TCF-4) was shown to positively regulate astrocyte activity, leading to increased proliferation [23, 24]. This observation further supports our previous finding of GRβ stimulation of growth [21], albeit via Akt1 activation and PTEN inhibition. Also, GRβ was shown to increase migration of glioblastoma cells [25]. However, the interaction of miR-144 with the GRβ 3′UTR in glioblastoma or ALL is unknown. In LNCaP-ARA70β prostate cancer cells, which express increased levels of GRβ, Ligr et al. reported increased cellular growth and proliferation [26]. Furthermore, treatment with methotrexate in peripheral mononuclear and lymphocyte cells resulted in decreased GRβ expression, thus increasing GC sensitivity [27]. Additionally, Piotrowska et al. demonstrated in Hut-78 and Raji B-lymphoma cells, MCF-7 breast cancer cells, and HT-29 colon carcinoma cells that known growth inhibitors trichostatin, sodium butyrate, and 5-Aza-20-deoxycytidine treatment suppressed GRβ and enhanced GRα with an increase in GC sensitivity [28, 29]. However, miR-144 levels, proliferation, or migration were not assessed in these studies. Nevertheless, these observations indicate the necessity of developing an anti-GRβ therapy to specifically target GRβ-related cancers.
In non-cancerous diseases, the resistance to GCs due to high levels of GRβ have been reported, and Sweet-P can potentially be used as a novel therapy. Most clinically relevant is the anti-inflammatory and immunosuppressant effects of GCs, which have been shown to decrease levels of cytokines, chemokines, and vasoactive agents. GCs reduce the movement of leukocytes to inflamed areas, and the function of immunocompetent cells [8]. In mice, increased GC levels induce apoptosis in thymocytes [30]. Because of the anti-inflammatory effects of GCs, they are commonly prescribed to asthma patients. Many studies have demonstrated an elevated expression of GRβ and GC-insensitivity in the airways of asthma patients [31–34]. Christodoulopoulos et al. showed that approximately 8% of cells in large and 2% of cells in small airways of patients were GRβ positive. However, mild asthma patients had an increase of 14% (7 fold) in GRβ positive cells in the small airways, but no change in expression in large airways. Alarmingly, in fatal asthmatic patients, the airways showed a dramatic increase in GRβ positive cells to 21% (2.5 fold) in large and 35% (17 fold) in small airways [31]. Hamid et al. reported an increased number of GRβ immunoreactive inflammatory cells in the airway T-cells of GC-resistance patients when compared to GC-sensitive or healthy patients [33]. In tuberculin-driven cutaneous inflammatory lesions of patients with GC-resistance asthma, increased number of cells expressing GRβ was also reported [34]. Furthermore, Goleva et al. demonstrated in bronchoalveolar lavage macrophages that GC-insensitive asthmatics have elevated GRβ mRNA and protein levels in comparison to GC-sensitive patients [32]. Of most interest, the authors reported enhancement of dexamethasone-induced GRα transactivation in GC-insensitive asthmatics after RNAi silencing of GRβ. As such, Sweet-P suppression of GRβ may serve useful for GC-insensitive asthmatic patients.
Our work highlights miR-144’s role in inducing migration of bladder cancer cells via GRβ; however, miR-144 has been demonstrated to play roles, both positive and negative, in many other forms of cancers and diseases. For example, miR-144 has been shown to contribute to the pathogenesis of Alzheimer’s disease through the downregulation of ADAM10 [35] but is essential for proper erythropoiesis by downregulating RAB14 [36]. Also, miR-144 has been shown to promote nasopharyngeal carcinoma through the downregulation of PTEN, a regulator of the PI3K/AKT pathway [37], and induce breast cancer and hepatocarcinoma cell proliferation through the downregulation of Runx1, a tumor-suppressor gene [38]. Interestingly, estrogen treatment (E2) in SkBr3 breast cancer and HepG2 hepatocarcinoma cells increased the expression of miR-144 through the PI3K/ERK/Elk1 transduction pathway [39], which may serve as a positive activator of GRβ. Solakidi et al. showed in HepG2 and SaOS-2 cells that GRβ and ERα were localized mainly in the nucleus, particularly concentrated in nuclear structures which suggest a direct involvement of GRβ and ERα in nucleolar-related processes [40]. However, the interaction of ERα and miR-144 signaling to increase GRβ activity has not been studied. In contrast to oncogenic properties of miR-144, the loss of miR-144 expression has been shown to be related to the progression of colorectal cancer through the derepression of mTOR, a cell growth and metabolism regulator [41]. However, because the decrease in miR-144 expression leads to colorectal cancer progression, GRβ may not have an involvement. Also, miR-144 has been shown to inhibit the migration, invasion, and proliferation of carcinomas such as rectal cancer [42] and osteosarcoma [43], which was attributed to the downregulation of ROCK1 [42]. High levels of GRβ was shown in SaOS-2 osteosarcoma cells, which suggest that miR-144 and GRβ signaling may be differentially regulated in bone cancer. Similarly, miR-144 inhibited migration and proliferation of hepatocarcinoma cells by the downregulation of AKT3 [44] and non-squamous cell lung carcinoma through the downregulation of ZFX [45]. Due to the diverse targeting of many different genes, inhibiting the global function of miR-144 during cancer therapy could be detrimental through off-target effects, and result in the de-repression of oncogenes. The specificity of Sweet-P blocking only the interaction of miR-144 with the 3′UTR of GRβ (Figure 1) to suppress cancer cell migration may be particularly useful due to the presentation of fewer side effects.
In conclusion, our discovery of Sweet-P targeting GRβ in bladder cancer sheds light on a novel drug therapy that specifically targets a gene known to cause growth, proliferation, migration, and GC hormonal therapy resistance. At this point, we have shown that the Sweet-P molecule suppresses GRβ in bladder cancer. In addition, we have shown that Sweet-P only targets GRβ and not other miR-144 regulated genes. More importantly, Sweet-P inhibits the ability of cancer cells to migrate. We will also be testing the effect of the Sweet-P molecule on other types of cancer. Essentially, Sweet-P may be used as a treatment option for several different carcinomas where GRβ is highly expressed including bladder, prostate, lung, or glioblastoma, as well as for liquid tumors such as in leukemia. Sweet-P can be beneficial for non-cancerous diseases also, such as asthma and GC-insensitive disease states caused by increased GRβ. Thus, Sweet-P serves as the first anti-GRβ molecule that may provide a new therapy.
Acknowledgments
This work was supported by the University of Toledo deArce-Memorial Endowment Fund (T.D.H). Research reported in this publication was also supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K01HL125445 (T.D.H.) and L32MD009154 (T.D.H.).
Footnotes
Conflicting interests
The authors have declared that no conflict of interests exist.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.McBeth L, Nwaneri AC, Grabnar M, Demeter J, Nestor-Kalinoski A, Hinds TD., Jr Glucocorticoid receptor beta increases migration of human bladder cancer cells. Oncotarget. 2016 doi: 10.18632/oncotarget.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Society AC. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015. [Google Scholar]
- 3.Chamie K, Litwin MS, Bassett JC, Daskivich TJ, Lai J, Hanley JM, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013:3219–3227. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sievert KD, Amend B, Nagele U, Schilling D, Bedke J, Horstmann M, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009:295–300. doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol. 2006:11–21. doi: 10.1016/j.jsbmb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform beta: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci. 2009:3435–3448. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funder JW. Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Annu Rev Med. 1997:231–240. doi: 10.1146/annurev.med.48.1.231. [DOI] [PubMed] [Google Scholar]
- 8.Reichardt H. Encyclopedic Reference of Molecular Pharmacology. Van Godewijckstraat: Springer Science and Business Media B.V; 2008. Gluco-/Mineralocorticoid Receptors; pp. 543–547. [Google Scholar]
- 9.Dunderski J, Matic G. GLUCOCORTICOID RECEPTOR IN HEALTH AND DISEASE. Journal of Molecular Biology. 2009:248–261. [Google Scholar]
- 10.John K, Marino JS, Sanchez ER, Hinds TD., Jr The glucocorticoid receptor: cause of or cure for obesity? Am J Physiol Endocrinol Metab. 2016:E249–257. doi: 10.1152/ajpendo.00478.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinds TD, Jr, Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Najjar SM, et al. Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Mol Endocrinol. 2010:1715–1727. doi: 10.1210/me.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanchi N, Filho M. Glucocorticoids: Extensive Physiological Actions Modulated Through Multiple Mechanisms of Gene Regulation. Cellular Physiology. 2010:311–315. doi: 10.1002/jcp.22141. [DOI] [PubMed] [Google Scholar]
- 13.DuBois DC, Sukumaran S, Jusko WJ, Almon RR. Evidence for a glucocorticoid receptor beta splice variant in the rat and its physiological regulation in liver. Steroids. 2013:312–320. doi: 10.1016/j.steroids.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaaf MJ, Champagne D, van Laanen IH, van Wijk DC, Meijer AH, Meijer OC, et al. Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinology. 2008:1591–1599. doi: 10.1210/en.2007-1364. [DOI] [PubMed] [Google Scholar]
- 15.Ledderose C, Mohnle P, Limbeck E, Schutz S, Weis F, Rink J, et al. Corticosteroid resistance in sepsis is influenced by microRNA-124--induced downregulation of glucocorticoid receptor-alpha. Crit Care Med. 2012:2745–2753. doi: 10.1097/CCM.0b013e31825b8ebc. [DOI] [PubMed] [Google Scholar]
- 16.Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yudt MR, Jewell CM, Bienstock RJ, Cidlowski JA. Molecular origins for the dominant negative function of human glucocorticoid receptor beta. Mol Cell Biol. 2003:4319–4330. doi: 10.1128/MCB.23.12.4319-4330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis-Tuffin LJ, Cidlowski JA. The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Ann N Y Acad Sci. 2006:1–9. doi: 10.1196/annals.1351.001. [DOI] [PubMed] [Google Scholar]
- 19.Hinds TD, John K, McBeth L, Trabbic CJ, Sanchez ER. Timcodar (VX-853) Is a Non-FKBP12 Binding Macrolide Derivative That Inhibits PPARγ and Suppresses Adipogenesis. PPAR Research. 2016:1–10. doi: 10.1155/2016/6218637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinds TD, Peck B, Shek E, Stroup S, Hinson J, Arthur S, et al. Overexpression of Glucocorticoid Receptor beta Enhances Myogenesis and Reduces Catabolic Gene Expression. Int J Mol Sci. 2016 doi: 10.3390/ijms17020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stechschulte LA, Wuescher L, Marino JS, Hill JW, Eng C, Hinds TD., Jr Glucocorticoid receptor beta stimulates Akt1 growth pathway by attenuation of PTEN. J Biol Chem. 2014:17885–17894. doi: 10.1074/jbc.M113.544072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longui CA, Vottero A, Adamson PC, Cole DE, Kino T, Monte O, et al. Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Horm Metab Res. 2000:401–406. doi: 10.1055/s-2007-978661. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Lu PH, Shi ZF, Xu YJ, Xiang J, Wang YX, et al. Glucocorticoid Receptor beta Acts as a Co-activator of T-Cell Factor 4 and Enhances Glioma Cell Proliferation. Mol Neurobiol. 2015:1106–1118. doi: 10.1007/s12035-014-8900-9. [DOI] [PubMed] [Google Scholar]
- 24.Avgeris M, Mavridis K, Tokas T, Stravodimos K, Fragoulis EG, Scorilas A. Uncovering the clinical utility of miR-143, miR-145 and miR-224 for predicting the survival of bladder cancer patients following treatment. Carcinogenesis. 2015:528–537. doi: 10.1093/carcin/bgv024. [DOI] [PubMed] [Google Scholar]
- 25.Yin Y, Zhang X, Li Z, Deng L, Jiao G, Zhang B, et al. Glucocorticoid receptor beta regulates injury-mediated astrocyte activation and contributes to glioma pathogenesis via modulation of beta-catenin/TCF transcriptional activity. Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Ligr M, Li Y, Logan SK, Taneja S, Melamed J, Lepor H, et al. Mifepristone inhibits GRbeta coupled prostate cancer cell proliferation. J Urol. 2012:981–988. doi: 10.1016/j.juro.2012.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goecke IA, Alvarez C, Henriquez J, Salas K, Molina ML, Ferreira A, et al. Methotrexate regulates the expression of glucocorticoid receptor alpha and beta isoforms in normal human peripheral mononuclear cells and human lymphocyte cell lines in vitro. Mol Immunol. 2007:2115–2123. doi: 10.1016/j.molimm.2006.07.303. [DOI] [PubMed] [Google Scholar]
- 28.Piotrowska H, Jagodzinski PP. Trichostatin A, sodium butyrate, and 5-aza-2′-deoxycytidine alter the expression of glucocorticoid receptor alpha and beta isoforms in Hut-78 T- and Raji B-lymphoma cell lines. Biomed Pharmacother. 2007:451–454. doi: 10.1016/j.biopha.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Piotrowska H, Jagodzinski PP. Glucocorticoid receptor alpha and beta variant expression is associated with ASF/SF2 splicing factor upregulation in HT-29 colon cancer and MCF-7 breast carcinoma cells. Arch Med Res. 2009:156–162. doi: 10.1016/j.arcmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Reichardt HM, Umland T, Bauer A, Kretz O, Schutz G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Molecular and Cellular Biology. 2000:9009–9017. doi: 10.1128/mcb.20.23.9009-9017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christodoulopoulos P, Leung DY, Elliott MW, Hogg JC, Muro S, Toda M, et al. Increased number of glucocorticoid receptor-beta-expressing cells in the airways in fatal asthma. J Allergy Clin Immunol. 2000:479–484. doi: 10.1067/mai.2000.109054. [DOI] [PubMed] [Google Scholar]
- 32.Goleva E, Li LB, Eves PT, Strand MJ, Martin RJ, Leung DY. Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 2006:607–616. doi: 10.1164/rccm.200507-1046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, et al. Increased glucocorticoid receptor beta in airway cells of glucocorticoid–insensitive asthma. Am J Respir Crit Care Med. 1999:1600–1604. doi: 10.1164/ajrccm.159.5.9804131. [DOI] [PubMed] [Google Scholar]
- 34.Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol. 2000:943–950. doi: 10.1067/mai.2000.106486. [DOI] [PubMed] [Google Scholar]
- 35.Cheng C, Li W, Zhang Z, Yoshimura S, Hao Q, Zhang C, et al. MicroRNA-144 is regulated by activator protein-1 (AP-1) and decreases expression of Alzheimer disease-related a disintegrin and metalloprotease 10 (ADAM10) J Biol Chem. 2013:13748–13761. doi: 10.1074/jbc.M112.381392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim M, Tan YS, Cheng WC, Kingsbury TJ, Heimfeld S, Civin CI. MIR144 and MIR451 regulate human erythropoiesis via RAB14. Br J Haematol. 2015:583–597. doi: 10.1111/bjh.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang LY, Ho-Fun Lee V, Wong AM, Kwong DL, Zhu YH, Dong SS, et al. MicroRNA-144 promotes cell proliferation, migration and invasion in nasopharyngeal carcinoma through repression of PTEN. Carcinogenesis. 2013:454–463. doi: 10.1093/carcin/bgs346. [DOI] [PubMed] [Google Scholar]
- 38.Psarra AM, Solakidi S, Trougakos IP, Margaritis LH, Spyrou G, Sekeris CE. Glucocorticoid receptor isoforms in human hepatocarcinoma HepG2 and SaOS-2 osteosarcoma cells: presence of glucocorticoid receptor alpha in mitochondria and of glucocorticoid receptor beta in nucleoli. Int J Biochem Cell Biol. 2005:2544–2558. doi: 10.1016/j.biocel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Vivacqua A, De Marco P, Santolla MF, Cirillo F, Pellegrino M, Panno ML, et al. Estrogenic gper signaling regulates mir144 expression in cancer cells and cancer-associated fibroblasts (cafs) Oncotarget. 2015:16573–16587. doi: 10.18632/oncotarget.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solakidi S, Psarra AM, Sekeris CE. Differential distribution of glucocorticoid and estrogen receptor isoforms: localization of GRbeta and ERalpha in nucleoli and GRalpha and ERbeta in the mitochondria of human osteosarcoma SaOS-2 and hepatocarcinoma HepG2 cell lines. J Musculoskelet Neuronal Interact. 2007:240–245. [PubMed] [Google Scholar]
- 41.Iwaya T, Yokobori T, Nishida N, Kogo R, Sudo T, Tanaka F, et al. Downregulation of miR-144 is associated with colorectal cancer progression via activation of mTOR signaling pathway. Carcinogenesis. 2012:2391–2397. doi: 10.1093/carcin/bgs288. [DOI] [PubMed] [Google Scholar]
- 42.Cai SD, Chen JS, Xi ZW, Zhang LJ, Niu ML, Gao ZY. MicroRNA144 inhibits migration and proliferation in rectal cancer by downregulating ROCK1. Mol Med Rep. 2015:7396–7402. doi: 10.3892/mmr.2015.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J, Shi Y, Li H, Yang M, Liu G. MicroRNA-144 acts as a tumor suppressor by targeting Rho-associated coiled-coil containing protein kinase 1 in osteosarcoma cells. Mol Med Rep. 2015:4554–4559. doi: 10.3892/mmr.2015.3937. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y, She XG, Ming YZ, Wan QQ, Ye QF. MicroRNA144 suppresses tumorigenesis of hepatocellular carcinoma by targeting AKT3. Mol Med Rep. 2015:1378–1383. doi: 10.3892/mmr.2014.2844. [DOI] [PubMed] [Google Scholar]
- 45.Zha W, Cao L, Shen Y, Huang M. Roles of Mir-144-ZFX pathway in growth regulation of non-small-cell lung cancer. PLoS One. 2013:e74175. doi: 10.1371/journal.pone.0074175. [DOI] [PMC free article] [PubMed] [Google Scholar]