Abstract
Rationale:
Catheter-based renal denervation (RDN) is currently under development for the treatment of resistant hypertension and is thought to reduce blood pressure via interruption of sympathetic pathways that modulate cardiovascular function. The sympathetic nervous system also plays a critical role in the pathogenesis of acute myocardial infarction and heart failure.
Objective:
We examined whether treatment with radiofrequency (RF)-RDN would protect the heart against subsequent myocardial ischemia/reperfusion injury via direct effects on the myocardium.
Methods and Results:
Spontaneously hypertensive rats received either bilateral RF-RDN or sham-RDN. At 4 weeks after RF-RDN (n=14) or sham-RDN (n=14) treatment, spontaneously hypertensive rats were subjected to 30 minutes of transient coronary artery occlusion and 24 hours –7 days reperfusion. Four weeks after RF-RDN, myocardial oxidative stress was markedly attenuated, and transcription and translation of antioxidants, superoxide dismutase 1 and glutathione peroxidase-1, were significantly upregulated compared with sham-RDN spontaneously hypertensive rats. RF-RDN also inhibited myocardial G protein–coupled receptor kinase 2 pathological signaling and enhanced myocardial endothelial nitric oxide synthase function and nitric oxide signaling. RF-RDN therapy resulted in a significant reduction in myocardial infarct size per area at risk compared with sham-RDN (26.8 versus 43.9%; P<0.01) at 24 hours postreperfusion and significantly improved left ventricular function at 7 days after myocardial ischemia/reperfusion.
Conclusions:
RF-RDN reduced oxidative stress, inhibited G protein–coupled receptor kinase 2 signaling, increased nitric oxide bioavailability, and ameliorated myocardial reperfusion injury in the setting of severe hypertension. These findings provide new insights into the remote cardioprotective effects of RF-RDN acting directly on cardiac myocytes to attenuate cell death and protect against ischemic injury.
Keywords: G protein-coupled receptor kinases, nervous system, nitric oxide, radiofrequency, oxidative stress, renal denervation, sympathetic
Because hypertension and cardiovascular disease rates rise in the developed world,1 effective interventions and pharmacotherapies are required to optimally manage blood pressure and impede the development of comorbidities associated with hypertension. Recent enthusiasm for the treatment of resistant hypertension arose from preliminary clinical trials that reported effective, sustained reductions in blood pressure after catheter-based radiofrequency renal denervation (RF-RDN), which inhibits activity of renal sympathetic efferent and afferent nerves that lie within and immediately adjacent to the wall of the renal artery.2–4 However, data from the SYMPLICITY HTN-3 trial, which was the first randomized, sham-controlled trial, failed to show significant reductions of systolic blood pressure in patients with resistant hypertension 6 months after RDN as compared with control.5
Although the clinical use of RDN for the treatment of hypertension remains controversial, it is possible that the sympathoinhibitory effects of RF-RDN may have beneficial effects on end-organ function in the setting of ischemia–reperfusion injury. It is well established that the sympathetic nervous system plays a role in the pathogenesis of acute myocardial infarction and heart failure.6,7 Sustained sympathetic signaling results in elevated oxidative stress and overactive β-adrenergic receptor (βAR) stimulation, which triggers deranged βAR prodeath signaling pathways that exacerbate myocardial injury.8–10 When βAR signaling goes awry, it is in large part because of increased levels and activity of cardiac G protein–coupled receptor kinase 2 (GRK2).11,12 GRK2 is in a family of G protein–coupled receptor serine/threonine kinases that phosphorylates and desensitizes G protein–coupled receptors.13,14 Upregulated GRK2 not only attenuates procontractile signaling, but also elicits noncanonical signaling pathways that promote cell death.15–17 In one such pathway, heightened GRK2 activity inhibits endothelial nitric oxide (NO) synthase (eNOS), which results in diminished levels of the cytoprotective molecule, NO.18,19
In this study, we hypothesized that RF-RDN protects the heart from subsequent myocardial ischemia/reperfusion (MI/R) injury in the spontaneously hypertensive rat (SHR), a rodent model of established hypertension, by attenuating myocardial GRK2 signaling and promoting NO signaling.
Methods
Experimental Animals
Male SHRs and male Wistar-Kyoto (WKY) rats 19 to 20 weeks of age (Charles River Laboratories) were used in the present study. All animals were housed in a temperature-controlled animal facility with a 12-hour light/dark cycle, with water and rodent chow provided ad libitum. All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication No 85-23, Revised 1996). The Louisiana State University Health Sciences Center New Orleans Institutional Animal Care and Use Committee approved all animal procedures.
Blood Pressure Telemetry Measurements
At 19 weeks of age, SHRs were implanted with a radiotelemetry transmitter (Data Sciences International, DSI, St Paul, MN) for measurement of blood pressure and heart rate. Under isoflurane (2% in O2 flow rate 0.5 L/min), an incision was made to expose the right femoral artery. The tip of the transmitter catheter was advanced into the abdominal aorta and secured into position via the femoral artery access. The wound was sutured, and the rats were given 1 to 2 weeks to recover. Baseline blood pressure recording was then performed daily for 4 consecutive days. After the sham-RDN or RF-RDN procedure, blood pressure recording was performed daily for the first 2 weeks and then 3 times each week for the remaining study (weeks 3–4). Telemetry data were analyzed with Dataquest ART Acquisition Software (version 4.33). The average arterial blood pressure and heart rate for each day were calculated from values recorded during a 3-hour period between 9:00 am and 12:00 pm.
Radiofrequency Renal Denervation Procedure
After baseline measurement of systemic cardiovascular function, SHRs were randomly divided into either an RF-RDN or a sham-RDN group. For these procedures, SHRs were anesthetized with isoflurane (2%), and a flank incision was made to expose the left renal artery. For RF-RDN, a segment (≈3 mm in length) of the proximal renal artery adjacent to the renal artery ostium (ie, bifurcation from the aorta) was carefully dissected, leaving any visible extravascular nerves intact. A small piece of plastic cut in the shape of a triangle was then placed under the renal artery as a platform and to protect the underlying tissue. The tip of the radiofrequency probe (6F, Celsius electrophysiology catheter) was then applied to 4-quadrants (circumferential) of the renal artery for 20 s each at 10 watts for the RF-RDN group or at 0 watts for the sham-RDN group (Stockert 70 radiofrequency generator and probes graciously provided by Biosense Webster). During RF-RDN, the temperature of the probe was not permitted to be higher than 65°C. After completion of the RF-RDN or sham-RDN procedure, the plastic was removed, and the muscle and skin were sutured closed in layers. The same RF-RDN or sham-RDN procedure was then performed on the contralateral renal artery.
Tyrosine Hydroxylase Staining of Renal Arteries in SHRs
At 5 weeks after sham-RDN or RF-RDN, renal arteries were excised, fixed (paraformaldehyde, 4.0%), paraffin-embedded, and sectioned (3 μm). Sections were deparaffinized, and antigen retrieval was performed. Sections were incubated with rabbit polyclonal anti-tyrosine hydroxylase (Millipore AB152) followed by biotinylated anti-rabbit IgG. In a blinded fashion, stain intensity (degree of tyrosine hydroxylase staining) was scored as 0, negative; 1, weak (blush); 2, mild; 3, moderate; or 4, strong. Proportion of nerves showing decreased tyrosine hydroxylase staining was scored as 1, ≈1% to 25%; 2, ≈25% to 50%; 3, ≈50% to 75%; and 4, ≈75% to 100%.
Plasma Norepinephrine and Epinephrine Measurement
At 4 weeks after sham-RDN or RF-RDN, plasma was collected, and catecholamine levels were measured using ELISA technique according to the manufacturer’s recommendations (Abnova Co).
Myocardial Ischemia/Reperfusion
Rats were fully anesthetized as mentioned earlier. The animals were then attached to a surgical board, orally intubated, and connected to a model 683 rodent ventilator (Harvard Apparatus; Natick, MA). The tidal volume was set at 3.5 mL, and the respiratory rate was set at 80 breaths/min. A median sternotomy was performed, and the proximal left anterior descending coronary artery was visualized and completely ligated with 6-0 silk suture mounted on a tapered needle (Ethicon). Rats were subjected to 30 minutes of ischemia and either 24 hours or 7 days of reperfusion.
Myocardial Infarct Size Determination
At 24 hours of reperfusion, rats were anesthetized, intubated, and connected to a rodent ventilator. A catheter was placed in the common carotid artery to allow for Evans blue dye injection. A median sternotomy was performed, and the left main coronary artery was religated in the same location as the original ligation. Evans blue dye (2.0 mL of a 2% solution) was injected into the carotid artery catheter into the heart to delineate the ischemic zone from the nonischemic zone. The heart was rapidly excised and serially sectioned along the short axis in 2-mm-thick sections, which were then incubated in 1.0% 2,3,5-triphenyltetrazolium chloride for 5 minutes at 37°C to demarcate the viable and nonviable myocardium within the risk zone. Each of the five 1-mm-thick myocardial slices was weighed, and the areas of infarction, risk, and nonischemic left ventricle (LV) were assessed in a blinded manner using computer-assisted planimetry (ImageJ).
Plasma Troponin Measurements
At 4 hours after reperfusion, plasma was collected, and cardiac troponin-I levels were measured using ELISA technique according to the manufacturer’s recommendations (Life Diagnostics).
Echocardiography
Before myocardial infarction, baseline transthoracic echocardiogram was performed using MS250 13–24-MHz probe on a Vevo 2100 (Visualsonics) under anesthesia with isoflurane (1%) supplemented with 100% O2. Seven days later, echocardiography was also performed in the same manner. To determine cardiac structure and function, LV end-diastolic dimension and LV end-systolic dimension were measured from EKV (ECG-Gated Kilohertz Visualization; Visualsonics)-generated m-mode long-axis images. LV ejection fraction (%) was calculated using EKV-generated B-mode long-axis images coupled with LV trace software (Visualsonics), whereby the endocardial posterior and anterior walls were traced at end-systole and end-diastole.
Western Blot Analysis
Protein quantification was evaluated as previously described.20 Protein samples obtained from LV were analyzed by immunoblotting using specific antibodies to eNOS (BD Biosciences), P-eNOS (Cell Signaling), GRK2 (Santa Cruz), P-GRK2 (Millipore), glutathione peroxidase-1 (GPX-1; Abcam), and superoxide dismutase-1 (SOD1; Abcam).
Myocardial and Plasma Measurements of NO2
Plasma nitrite concentrations were quantified by an automated ion chromatography system (ENO30 Analyzer, Eicom). Nitrite was separated by a column (NO-PAK with polystyrene polymer, Eicom). The mobile phase, delivered at a pump rate of 0.33 mL/min, was 10% methanol containing 0.15 mol/L NaCl-NH4Cl and 0.5 g/L of tetrasodium EDTA. The Griess reagent, which was 1.25% HCl containing 5 g/L sulfanilamide with 0.25 g/L N-naphthylethylenediamine, was delivered at a rate of 0.1 mL/min.
Myocardial Measurements of S-Nitrosothiols
Myocardial tissue nitroso compounds were quantified by using group-specific reductive denitrosation by iodine–iodide with subsequent detection of the liberated NO by using gas-phase chemiluminescence (Eco Physics CLD 88 Y). S-nitrosothiol levels were detected by preincubation with 2% mercuric chloride followed by acidified sulfanilamide.
Measurement of Malondialdehyde Levels
Malondialdehyde levels is LV tissue were measured as described previously.21
Measurement of Protein Carbonyl Content
Protein carbonyl content in LV tissue was measured as described previously.21
Measurement of 8-Isoprostane
LV and plasma total 8-isoprostane way assayed using an ELISA kit (Cell Biolabs) according to the manufacturer’s recommendations.
RNA Isolation and Reverse Transcriptase RT-PCR
mRNA levels were assessed by using quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted from LV tissue. Purified RNA was quantified, and cDNA was synthesized using an I-script cDNA synthesis kit (Bio-Rad). TaqMan primers from Life Technology were used to amplify quantitative real-time RT-PCR. 18s was used as a housekeeping gene and 2ΔΔCT was used for data analysis.
Statistical Analysis
All data in this study are expressed as the mean±SEM. Differences in data between the groups were compared using Prism 6 (GraphPad Software) with Student unpaired, 2-tailed t test when only 2 groups were compared. Two-way analysis of variance with Bonferroni post-test was used for blood pressure and heart rate analysis. Mann–Whitney tests were used for ranked histological analysis. A chi-squared test was used for survival analysis. P value of <0.05 was considered statistically significant.
Results
Renal Artery Nerve Staining and Norepinephrine Spillover
Renal artery nerve tyrosine hydroxylase staining at 35 days after RF-RDN or sham-RDN in SHRs revealed significantly reduced, but somewhat variable reductions in renal nerve viability after RF-RDN as compared with sham-RDN procedures (Figure 1). As a marker of sympathetic nerve function, spillover norepinephrine and epinephrine levels were measured 28 days after RF-RDN or sham-RDN. There was a significant reduction in circulating norepinephrine after RF-RDN compared with the sham-RDN. There were no significant changes in plasma epinephrine levels.
Figure 1.
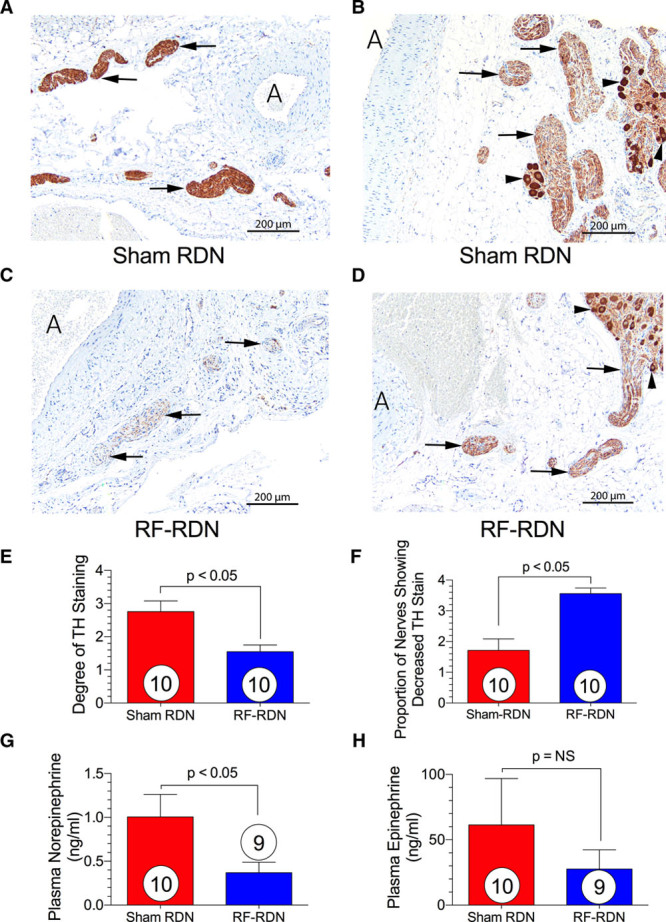
Viable renal artery nerve staining and catecholamine spillover after radiofrequency renal denervation (RF-RDN) in spontaneously hypertensive rats (SHRs). Tyrosine hydroxylase staining 35 days after sham-RDN or RF-RDN. A, Tyrosine hydroxylase (TH) stain of renal artery section from sham-RDN-treated SHRs. Arrows indicate normal nerves showing score 4 TH staining; and A, indicates renal artery. B, TH stain of renal artery section from sham-RDN-treated SHRs. Arrows indicate nerves showing score 3 TH staining; A, renal artery; and arrowheads, ganglion cells showing full-intensity cytoplasmic TH staining. C, TH staining of renal artery section from RF-RDN-treated SHRs. Arrows indicate nerves showing score 2 TH staining; A, renal artery; and arrowheads, ganglion cells showing full-intensity cytoplasmic TH staining. D, TH stain of renal artery section from RF-RDN-treated SHRs. Arrows indicate atrophic nerves showing score 1 TH staining; and A, renal artery. (E) Degree of TH staining and (F) proportion of nerves showing decreased TH staining. (G) Plasma norepinephrine and (H) epinephrine 28 days after sham or RF-RDN. Values are expressed as mean±SEM.
Effects of RF-RDN on Arterial Blood Pressure in SHRs
Twenty-one-week-old male SHR were subjected to either bilateral RF-RDN or sham-RDN of the nerves within the renal arteries. RF-RDN produced a small, but significant decrease in systolic blood pressure as compared with sham-RDN at days 15 to 28 after the procedure, but systolic blood pressures remained significantly elevated (ie, >170 mm Hg) compared with normotensive animals (Figures 2 and 7). Furthermore, systolic blood pressures in SHR treated with RF-RDN were not significantly reduced when compared with baseline values in the SHR group. RF-RDN did not result in a significant reduction in diastolic pressure in SHRs compared with the sham-RDN procedure. Mean arterial blood pressure was significantly lower at days 24 to 28 after RF-RDN (P<0.05 versus sham-RDN). We next examined the rate–pressure product (pressure–rate index) as a parameter of oxygen consumption. Interestingly, despite a reduction in arterial pressure, an accompanied modest increase in HR after RF-RDN resulted in no difference in the rate–pressure product between sham-RDN and RF-RDN SHR (Figure 2E).
Figure 2.
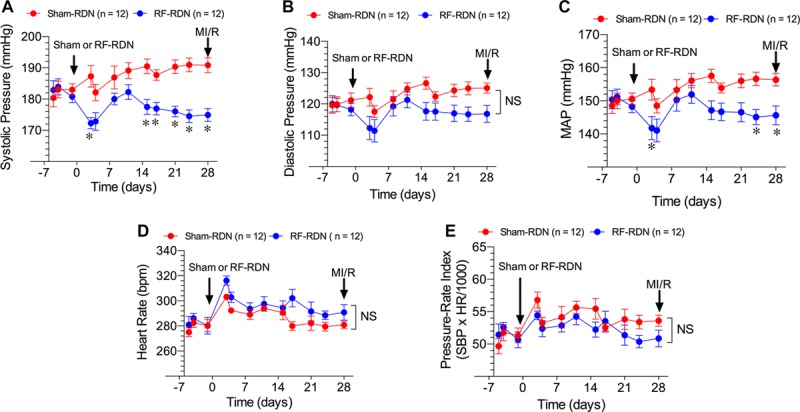
Arterial blood pressure and heart rate in spontaneously hypertensive rats (SHRs) after radiofrequency renal denervation (RF-RDN) or sham-RDN. Systolic pressure (mm Hg; A), diastolic pressure (mm Hg; B), mean arterial pressure (MAP; mm Hg; C), heart rate (bpm; D), and pressure–rate index (systolic pressure×heart rate/1000; E) in 21-week-old male SHRs before and for 4 weeks after treatment. RF-RDN and sham-RDN procedures were performed on day 0 when rats were 21 weeks of age. Values are expressed as mean±SEM. *P<0.05 between groups. MI/R indicates myocardial ischemia/reperfusion.
Figure 7.
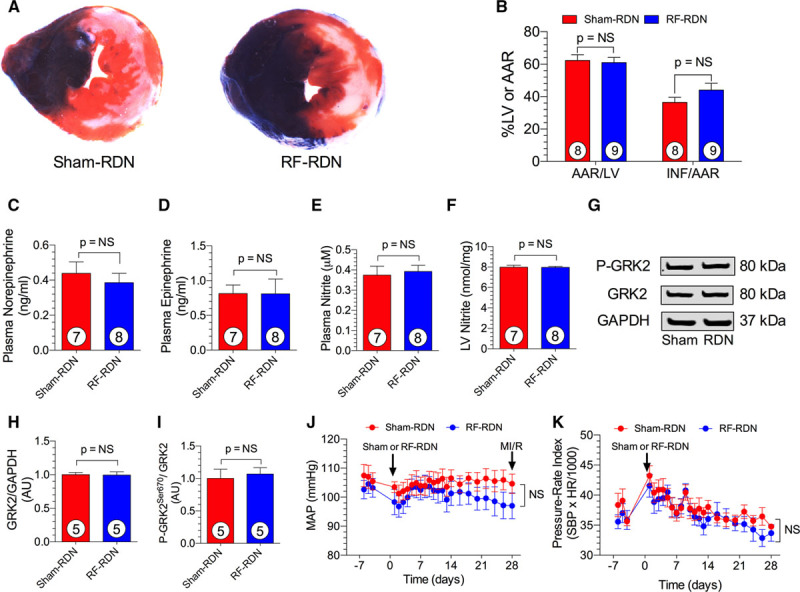
Radiofrequency renal denervation (RF-RDN) does not protect against myocardial ischemia/reperfusion injury in normotensive Wistar-Kyoto (WKY) rats. A, Representative midventricular photomicrographs of rat hearts after 30 min of myocardial ischemia and 24 h reperfusion. B, Bar graphs of myocardial area at risk (AAR)/left ventricular (LV) and infarct (INF)/AAR. Plasma norepinephrine (C) and epinephrine levels (D) in WKY rats 4 weeks after sham-RDN or RF-RDN. Plasma (E) and LV nitrite levels (F) in WKY rats 4 weeks after sham-RDN or RF-RDN. G, Representative immunoblot of LV G protein–coupled receptor kinase 2 (GRK2) from WKY rats after either RF-RDN or sham-RDN. Relative intensity of total GRK2 (H) and P-GRK2Ser670 (I). Mean arterial pressure (MAP; J) and pressure–rate index (K) calculated from radiotelemetry recordings in 21-week-old male WKY rats before and for 4 weeks after sham-RDN or RF-RDN treatment. Circles inside bars denote number of animals per group. N=6/group for blood pressure and pressure–rate index data (telemetry recordings). HR indicates heart rate; and SBP, systolic blood pressure.
RF-RDN Attenuates Oxidative Stress in SHRs
Previous studies clearly demonstrate that oxidative stress is elevated during hypertension and contributes to disease progression.22,23 As depicted in Figure 3, myocardial oxidative stress was significantly reduced, and multiple antioxidant proteins were upregulated at 28 days after RF-RDN (Figure 3). Plasma and LV 8-isoprostane and LV malondialdehyde levels were reduced in SHRs 28 days after RF-RDN compared with sham-RDN, suggesting attenuated oxidative stress after RDN. Similarly, LV carbonyl content was attenuated in the RF-RDN-treated group. We next examined antioxidant enzyme levels, including GPX-1 and SOD mRNA and proteins levels in myocardial tissue. mRNA levels of GPX-1, cytosolic SOD (SOD1), and mitochondrial SOD (SOD2) were all increased after RF-RDN. Myocardial GPX-1 and SOD1 protein levels accompanied mRNA changes and were both elevated in the RF-RDN-treated animals.
Figure 3.
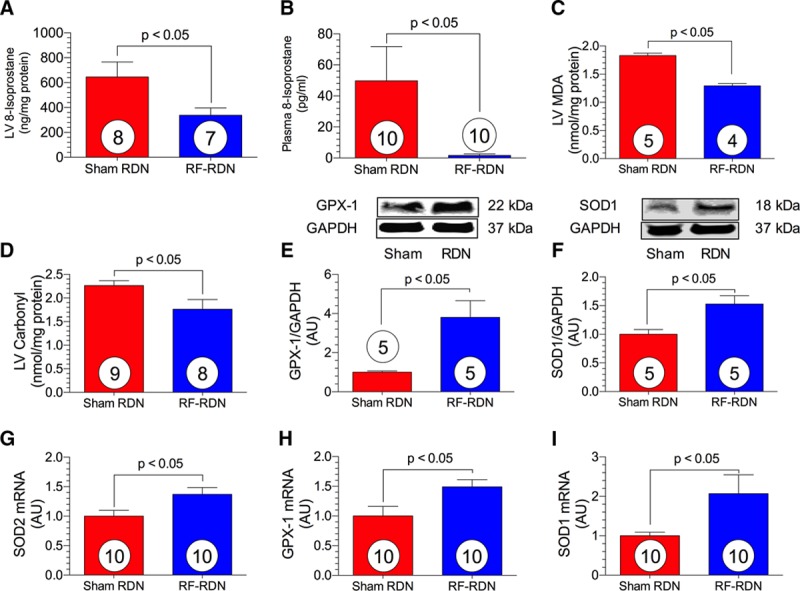
Radiofrequency renal denervation (RF-RDN) attenuates myocardial oxidative stress in spontaneously hypertensive rats (SHRs). Left ventricular (LV) 8-isoprostane (ng/mg protein; A), plasma 8-isoprostane (pg/mL; B), LV malondialdehyde (MDA; nmol/mg protein; C), and LV carbonyl protein content (nmol/mg protein; D). Representative immunoblots and relative intensity of LV glutathione peroxidase-1 (GPX-1; E) and superoxide dismutase-1 (SOD1; F). Relative LV mRNA levels of superoxide dismutase 2 (SOD2; G), GPX-1 (H), and SOD1 (I). Circles inside bars denote number of animals per group.
Reduced GRK2 Signaling After RF-RDN in SHRs
Myocardial GRK2 signaling is enhanced in the settings of redox imbalance and myocardial ischemia.24 As shown in Figure 4, RF-RDN produced a significant decrease in LV GRK2 mRNA to 60% of sham-RDN levels. Although we did not observe a difference in whole cell GRK2 protein levels between groups, phosphorylation at residue Ser670 was significantly downregulated after RF-RDN. Phosphorylation at Ser670 results in GRK2 mitochondrial translocation and cell death induced by mitochondrial permeability transition pore opening.16
Figure 4.
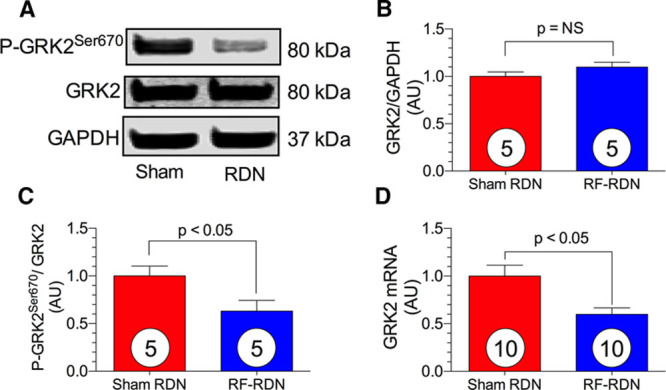
Reduced G protein–coupled receptor kinase 2 (GRK2) signaling after radiofrequency renal denervation (RF-RDN) in spontaneously hypertensive rats (SHRs). A, Representative immunoblot of left ventricular (LV) GRK2 from SHR safter either RF-RDN or sham-RDN. Relative intensity of total GRK2 (B) and P-GRK2Ser670 (C). D, Relative mRNA levels of GRK2. Circles inside bars denote number of animals per group.
Myocardial eNOS Activity and NO Levels After RF-RDN in SHRs
eNOS activity is tightly regulated by post-translational phosphorylation and coupling of the homodimer structure. Hypophosphorylation at Ser1177 is associated with eNOS uncoupling and enzyme inactivation.25 Reduced phosphorylation at this residue results in deficient NO production and the exacerbation of cardiovascular disease states.26 Four weeks after RF-RDN in SHRs, we observed enhanced myocardial eNOS Ser1177 phosphorylation compared with the sham-RDN (Figure 5). There were unchanged LV whole cell eNOS levels between groups. eNOS generation of NO is also regulated by available enzymatic substrates and cofactors.25 Asymmetrical dimethylarginine inhibits eNOS by competing with the eNOS substrate, L-arginine.27 The RF-RDN-treated group exhibited markedly reduced myocardial tissue asymmetrical dimethylarginine levels compared with sham-RDN. Myocardial mRNA levels of the 3 NOS isoforms, neuronal NOS, inducible NOS, and eNOS, were unchanged between the RF-RDN- and sham-RDN-treated groups. Improved eNOS activity in the RF-RDN group resulted in increased NO levels in the heart and circulation. Myocardial protein S-nitrosylation and nitrite levels were both significantly increased after RF-RDN. Plasma nitrite levels were also increased in the hypertensive SHRs after RF-RDN, providing further evidence of a sustained increase in NO bioavailability subsequent to renal nerve ablation.
Figure 5.
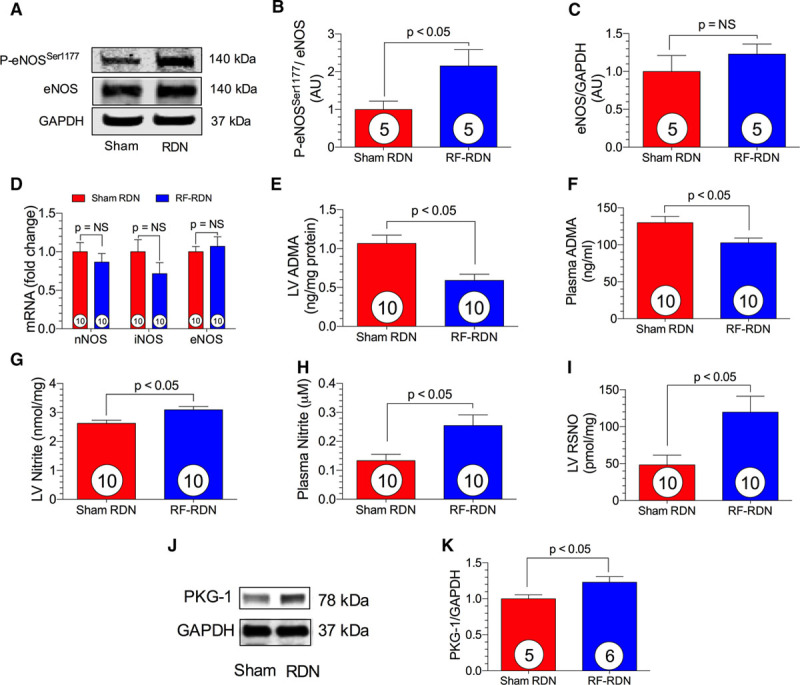
Myocardial endothelial nitric oxide synthase (eNOS) activity and nitric oxide (NO) levels after radiofrequency renal denervation (RF-RDN) in spontaneously hypertensive rats (SHRs). A, Representative immunoblots of eNOS from SHRs after either RF-RDN or sham-RDN. Relative intensity of P-eNOSSer1177 (B) and total eNOS protein expression (C). D, Relative mRNA levels of neuronal NOS (nNOS), inducible NOS (iNOS), and eNOS in left ventricular (LV) tissue. LV asymmetrical dimethyl arginine (ADMA, ng/mg protein; E) and plasma ADMA (ng/mL; F). LV nitrite (nmol/mg; G), plasma nitrite (μM; H), and LV S-nitrosothiols (RSNO, nmol/mg; I). (J) Representative immnoblots of protein kinase G-1 (PKG-1) from SHRs after either RF-RDN or sham-RDN and (K) relative intensity of PKG-1. Circles inside bars denote number of animals per group.
RF-RDN Protects Against Myocardial Ischemia/Reperfusion Injury and Improves LV Function in SHRs
We next evaluated whether reductions in GRK2 signaling coupled with restoration of NO-mediated signaling would protect against MI/R injury. At 4 weeks after RF-RDN, SHRs were subjected to 30 minutes of left anterior descending ligation followed by 24 hours reperfusion. RF-RDN rats displayed a significant reduction in myocardial infarct size per area at risk and reduced plasma troponin-I levels compared with the sham-RDN group (Figure 6). Survival during myocardial ischemia was significantly improved in the RF-RDN-treated group as compared with sham-RDN-treated group. In an additional set of animals, LV function and dimension were measured in SHRs with and without RF-RDN. Seven days after MI/R, LV function was improved in the RF-RDN group as evidenced by improved LV ejection fraction and LV fractional shortening (Figure 6). There were no changes in LV end-diastolic diameter between groups 7 days post MI/R; however, there were significantly improved LV end-systolic diameter dimension in the RF-RDN-treated animals.
Figure 6.
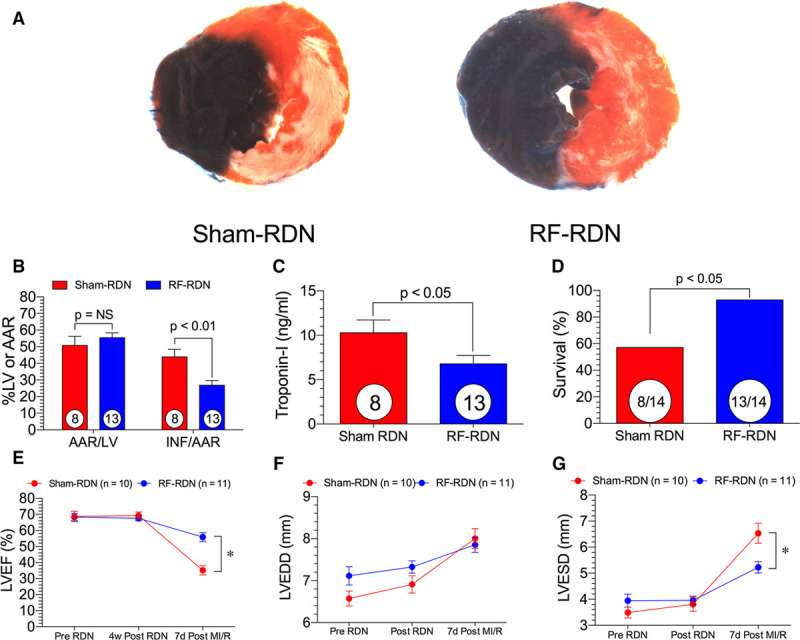
Radiofrequency renal denervation (RF-RDN) protects against myocardial ischemia/reperfusion injury and improves left ventricular (LV) function in spontaneously hypertensive rats (SHRs). A, Representative midventricular photomicrographs of rat hearts after 30 min of myocardial ischemia and 24 h reperfusion. B, Bar graphs of myocardial area at risk (AAR)/LV and infarct (INF)/AAR. C, Cardiac troponin-1 levels after 4 h reperfusion (ng/mL) and (D) survival rate during ischemia. E, LV ejection fraction (LVEF) before RDN, 4 weeks after RDN, and 7 days after ischemia–reperfusion injury. F, LV fractional shortening before RDN, 4 weeks after RDN, and 7 days after ischemia–reperfusion injury. G, LV end-diastolic diameter (LVEDD) before RDN, 4 weeks after RDN, and 7 days after ischemia–reperfusion injury. Circles inside bars denote number of animals per group. *P<0.05 between groups.
RF-RDN Does Not Protect Against Myocardial Ischemia/Reperfusion Injury in Normotensive Rats
We next examined whether RF-RDN could protect against MI/R injury in normotensive WKY rats (Figure 7). At 4 weeks after RF-RDN or sham-RDN, WKY rats were subjected to 30 minutes of left anterior descending ligation followed by 24 hours of reperfusion. There were no significant differences in myocardial infarct per area at risk between groups. Blood pressure and heart rate measurements reveal that RF-RDN does not significantly alter blood pressure or heart rate in normotensive animals. We next examined catecholamine levels, NO signaling, and GRK2 expression in WKY rats after RF-RDN. RF-RDN did not reduce spillover norepinephrine levels as was observed in SHRs. Moreover, plasma and LV nitrite levels were unaffected by RF-RDN therapy, indicating that RF-RDN does not augment NO signaling in healthy, normotensive animals. Additionally, GRK2 signaling was unaltered in WKY rats after RF-RDN procedures. These results suggest that RF-RDN-mediated MI/R protection is optimal in the setting of a hyperactive sympathetic nervous system with elevated oxidative stress and NOS dysfunction.
Discussion
For years, physicians have controlled excessive sympathetic nervous system activity associated with cardiovascular disease using pharmacological approaches. However, many of the pharmacological agents have unintended and undesirable off-target side effects, and their ultimate effectiveness is limited by the complex pathology of hypertension and by patient compliance. RDN is currently under clinical investigation as a strategy to reduce blood pressure in resistant hypertensive patients by disruption of the sympathetic nerves that lie within and around the renal artery. We used a reverse translational approach to investigate the effects of complete RDN on myocardial injury in the setting of hypertension and acute myocardial infarction in the SHRs. Given that the sympathetic nervous system plays a critical role in the pathogenesis of myocardial infarction, we examined whether RF-RDN could protect the heart against MI/R injury in the setting of established hypertension.
Recently, several groups have proposed that RDN may have beneficial effects on ventricular remodeling in postmyocardial infarction heart failure.28–30 These studies have examined RDN as a potential postinfarction therapy similar to β-blockers. In the current study, we propose that elevated activity of the sympathetic nervous system in the setting of hypertension results in myocardial oxidative stress, diminished eNOS-NO signaling, and activation of pro death signaling pathways. Our data clearly demonstrate that RF-RDN treatment before the onset of myocardial ischemia and reperfusion attenuates oxidative stress, restores NO signaling, and downregulates cytotoxic pathways that exacerbate MI/R injury in the setting of hypertension.
Oxidative stress associated with hypertension results in endothelial dysfunction and has detrimental effects on vascular tone, thrombosis, and vascular inflammation. Hypertensive patients have elevated circulating levels of superoxide and hydrogen peroxide,31 as well as reduced antioxidant defenses.22 Elevated reactive oxygen species (ROS) in hypertension is largely because of the abundance of toxic oxygen-derived free radicals ensuing from catecholamine metabolism, NADPH oxidase, and mitochondrial protein adduct formation.32,33 Elevated ROS combined with depressed host antioxidant activity results in significant end-organ cellular death.34 In SHRs, excess oxygen radicals are produced from several sources, including activated circulating leukocytes.23 In the current study, we used the SHR as a clinically relevant model of neurogenic hypertension with elevated oxidative stress.
Endothelial damage resulting from ROS in hypertensive subjects is, in part, because of eNOS dysfunction.32 Oxidative stress uncouples the functional homodimer eNOS enzyme, which results in diminished NO, reduced vasodilation, and exacerbated vascular damage.21,26 In the myocardium, heightened oxidative stress resulting from hypertension and MI/R correlates with cardiac dysfunction and injury.35 We, therefore, hypothesized that RF-RDN would reduce myocardial oxidative stress, attenuate pathological GRK2 signaling, promote NO signaling, and improve myocardial injury and function. The RF-RDN–treated group had significantly reduced circulating and myocardial markers lipid peroxidation, as well as decreased protein carbonylation. In addition, RF-RDN upregulated mRNA and protein expression of key antioxidants, SOD1 and GPX-1, in the heart, providing strong evidence that RF-RDN attenuates oxidative stress associated with severe hypertension.
GRKs are classically recognized as G protein–coupled receptor desensitization agents.36 However, more recent studies suggest that in the setting of elevated ROS or ischemia–reperfusion injury, persistent binding of catecholamines to the βAR results in GRK-mediated cytotoxic cellular signaling pathways.17 GRK2 is the most abundant GRK in the myocardium.16 Interestingly, GRK2 and eNOS cross talk and interact in the heart.37 Previous studies indicate that increased GRK2 activity results in eNOS dysfunction,19 whereas others propose that eNOS inhibits GRK2 through post-translational modifications.37 Ultimately, when eNOS dysfunction accompanies GRK2 activity, prodeath signaling pathways ensue. We proposed that improved eNOS function and reduced norepinephrine activation of the βAR would result in the inhibition of intracellular GRK2 signaling. Under normal conditions, GRK2 is primarily located in the cytosol and is primarily associated with membrane proteins. However, during pathological conditions, GRK2 levels have been shown to be elevated in the mitochondria, resulting in mitochondrial permeability transition pore opening and detrimental calcium imbalance.16 Mitochondrial translocation is directed by a stress-induced phosphorylation event at Ser670 via MAP (mitogen-activated protein) kinase.17 This activation of GRK2 allows binding to HSP90 (heat shock protein 90), which directs it to the mitochondria within the myocyte.16 In the current study, we observed reduced phosphorylation at Ser670 after RF-RDN. Interestingly, decreased GRK2 mRNA levels and hypo-phosphorylation of GRK2 were accompanied by activated eNOS in the cardiac cells. This suggests a healthy homeostasis of GRK2 and eNOS activity that has been reported to protect the heart from ischemia–reperfusion injury.
eNOS-NO cytoprotective signaling also acts independently of inhibiting the GRK2 pathways. NO plays a protective role in the homeostasis of blood pressure, cardiovascular function, and cell survival during pathological disease states. NO is a potent antioxidant.38 NO decreases apoptosis,39 increases mitochondrial biogenesis,40 and promotes angiogenesis.41,42 Altered redox signaling coupled with NO deficiency is critically associated with the development of hypertension and hypertension-induced organ damage, such as myocardial infarction. In the current study, we found that RF-RDN recoupled myocardial eNOS as evidenced by increased phosphorylation at Ser1177. The reduction of oxidative stress that accompanies RF-RDN likely plays a role in the recoupling of eNOS, whereas the NO that is produced from the recoupled eNOS upregulates the antioxidant defenses and further attenuates oxidative stress in the heart and circulation. It is possible that elevated NO signaling and endothelial improvements that result from healthy eNOS function and reduced ROS play a role in the reduction in blood pressure after RF-RDN. However, further studies are required to more fully elucidate this possibility.
Because increased sympathetic tone, eNOS uncoupling, GRK2 signaling, and oxidative stress play pivotal roles in the pathogenesis of MI/R injury, we examined the possibility that RF-RDN protects the myocardium against ischemia/reperfusion injury. We observed significant reductions in myocardial infarct size after RF-RDN. Importantly, this preservation of viable myocardium was accompanied by improved LV function after MI/R injury.
Interestingly, we failed to observe a reduction in myocardial infarct size after RF-RDN in normotensive WKY rats. It is well established that normotensive WKY rats do not have heightened sympathetic drive, exacerbated baseline oxidative stress, or eNOS dysfunction seen in SHRs.43–45 We have also confirmed that the absence of protection afforded by RF-RDN in WKY rats is explained by low baseline GRK2 signaling and limited eNOS dysfunction. In the current study, we report that RF-RDN protects against MI/R injury in SHRs by reducing elevated oxidative stress, restoring eNOS activity, and downregulating GRK-2. Although we have reported a relationship between the infarct-sparing effect of RF-RDN and NO/GRK signaling, our study does not provide direct evidence that the cardioprotective effects of RF-RDN are a direct result of eNOS activation, increased NO bioavailability, and GRK2 inhibiton. Further studies are required to demonstrate direct causality.
In the present study, we observed similar blood pressure–lowering effects in SHRs that have been reported in recent catheter-based RDN clinical trials in patients with resistant hypertension.5,46 We do not think that this modest reduction in pressure (6–8 mm Hg) is entirely responsible for the significant infarct-sparing actions of RF-RDN. We think that this effect is, in large part, because of upregulation of cardioprotective signaling and reduced oxidative stress in the myocardium.
In conclusion, our study is the first to report that RF-RDN protects against myocardial ischemia/reperfusion injury in the setting of established hypertension and that it does so, in part, by inhibiting prodeath signaling pathways that are associated with an overactive sympathetic outflow. These RF-RDN pathways include reduction in systemic and myocardial oxidative stress, inhibition of GRK2 signaling, and enhanced eNOS-NO signaling. These findings provide new insights into the remote cardioprotective effects of RF-RDN. Our data suggest that RF-RDN may exert therapeutic benefits that extend beyond blood pressure reduction in the setting of hypertension and acute myocardial infarction.
Acknowledgments
We are grateful to Jessica Bradley, PhD, and Kazi Islam, PhD, for their assistance during the course of these studies.
Sources of Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (National Institutes of Health; 1R01 HL092141 [D.J. Lefer], 1R01 HL093579 (D.J. Lefer), 1U24 HL 094373 (D.J. Lefer), 1P20 HL113452 (D.J. Lefer), and P30GM106392 (D.R. Kapusta). This work was also supported by Biosense Webster IIS-175 to D.R. Kapusta and F. Smart and the American Heart Association 14POST20450173 to J. Gao. We are also grateful for the generous funding from the LSU Medical School Foundation and the LSU Medical School Alumni Association.
Disclosures
D.J. Lefer, D.J. Polhemus, and D.R. Kapusta have pending patents for the use of renal denervation for the treatment of myocardial infarction and heart failure.
Nonstandard Abbreviations and Acronyms
- βAR
- β adrenergic receptor
- eNOS
- endothelial nitric oxide synthase
- GPCR
- G protein–coupled receptor
- GPX-1
- glutathione peroxidase-1
- GRK2
- G protein–coupled receptor kinase 2
- LV
- left ventricle
- MI/R
- myocardial ischemia/reperfusion
- NO
- nitric oxide
- RDN
- renal denervation
- RF-RDN
- radiofrequency renal denervation
- ROS
- reactive oxygen species
- SHR
- spontaneously hypertensive rat
- SOD
- superoxide dismutase
- WKY
- Wistar-Kyoto
In May 2016, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.93 days.
Novelty and Significance
What Is Known?
Renal nerve denervation (RDN) is a minimally invasive, endovascular procedure currently under investigation for the treatment for resistant hypertension, and mixed results of clinical trials have raised questions about its ability to effectively reduce blood pressure.
High blood pressure is a significant risk factor for coronary heart disease, and the sympathetic nervous system plays a critical role in the pathogenesis of acute myocardial infarction.
What New Information Does This Article Contribute?
We show that RDN remotely protects the heart against ischemic injury by inhibiting prodeath signaling pathways and reducing oxidative stress in hypertension.
In this study, we examined whether RDN could have remote infarct-sparing effects on the heart in the setting of essential hypertension. We found that RDN limits myocardial ischemic injury by inhibiting prodeath signaling pathways, improving redox status, and enhancing nitric oxide signaling in cardiac myocytes. Our data suggest that in the setting of hypertension, RDN may have therapeutic potential beyond blood pressure reduction.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 3.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 4.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–2140. doi: 10.1093/eurheartj/eht197. doi: 10.1093/eurheartj/eht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 6.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 7.Schömig A. Catecholamines in myocardial ischemia. Systemic and cardiac release. Circulation. 1990;82:II13–II22. [PubMed] [Google Scholar]
- 8.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 9.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tachibana H, Naga Prasad SV, Lefkowitz RJ, Koch WJ, Rockman HA. Level of beta-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation. 2005;111:591–597. doi: 10.1161/01.CIR.0000142291.70954.DF. doi: 10.1161/01.CIR.0000142291.70954.DF. [DOI] [PubMed] [Google Scholar]
- 11.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–323. doi: 10.1038/nm1553. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 12.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 13.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 14.Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Receptor-specific in vivo desensitization by the g protein-coupled receptor kinase-5 in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW., II Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, Pan S, Sheu SS, Gao E, Koch WJ. Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circ Res. 2013;112:1121–1134. doi: 10.1161/CIRCRESAHA.112.300754. doi: 10.1161/CIRCRESAHA.112.300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodall MC, Ciccarelli M, Woodall BP, Koch WJ. G protein-coupled receptor kinase 2: a link between myocardial contractile function and cardiac metabolism. Circ Res. 2014;114:1661–1670. doi: 10.1161/CIRCRESAHA.114.300513. doi: 10.1161/CIRCRESAHA.114.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taguchi K, Kobayashi T, Takenouchi Y, Matsumoto T, Kamata K. Angiotensin II causes endothelial dysfunction via the GRK2/Akt/eNOS pathway in aortas from a murine type 2 diabetic model. Pharmacol Res. 2011;64:535–546. doi: 10.1016/j.phrs.2011.05.001. doi: 10.1016/j.phrs.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 20.Polhemus DJ, Bradley JM, Islam KN, Brewster LP, Calvert JW, Tao YX, Chang CC, Pipinos II, Goodchild TT, Lefer DJ. Therapeutic potential of sustained-release sodium nitrite for critical limb ischemia in the setting of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2015;309:H82–H92. doi: 10.1152/ajpheart.00115.2015. doi: 10.1152/ajpheart.00115.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun. 1993;19:59–66. doi: 10.3109/10715769309056499. [DOI] [PubMed] [Google Scholar]
- 23.Schmid-Schönbein GW, Seiffge D, DeLano FA, Shen K, Zweifach BW. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension. 1991;17:323–330. doi: 10.1161/01.hyp.17.3.323. [DOI] [PubMed] [Google Scholar]
- 24.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res. 2010;107:1140–1149. doi: 10.1161/CIRCRESAHA.110.221010. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 26.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 27.Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 28.Hu J, Li Y, Cheng W, Yang Z, Wang F, Lv P, Niu C, Hou Y, Yan Y, Ge J. A comparison of the efficacy of surgical renal denervation and pharmacologic therapies in post-myocardial infarction heart failure. PLoS One. 2014;9:e96996. doi: 10.1371/journal.pone.0096996. doi: 10.1371/journal.pone.0096996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension. 2014;64:745–755. doi: 10.1161/HYPERTENSIONAHA.114.03699. doi: 10.1161/HYPERTENSIONAHA.114.03699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe H, Iwanaga Y, Miyaji Y, Yamamoto H, Miyazaki S. Renal denervation mitigates cardiac remodeling and renal damage in Dahl rats: a comparison with β-receptor blockade. Hypertens Res. 2016;39:217–226. doi: 10.1038/hr.2015.133. doi: 10.1038/hr.2015.133. [DOI] [PubMed] [Google Scholar]
- 31.Lacy F, O’Connor DT, Schmid-Schönbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens. 1998;16:291–303. doi: 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- 32.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(suppl 2):S170–S180. doi: 10.2337/dc08-s247. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 33.Costa VM, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F. Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Curr Med Chem. 2011;18:2272–2314. doi: 10.2174/092986711795656081. [DOI] [PubMed] [Google Scholar]
- 34.Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res. 2001;89:279–286. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari R, Alfieri O, Curello S, Ceconi C, Cargnoni A, Marzollo P, Pardini A, Caradonna E, Visioli O. Occurrence of oxidative stress during reperfusion of the human heart. Circulation. 1990;81:201–211. doi: 10.1161/01.cir.81.1.201. [DOI] [PubMed] [Google Scholar]
- 36.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. doi: 10.1146/annurev.ph.69.013107.100021. [DOI] [PubMed] [Google Scholar]
- 37.Huang ZM, Gao E, Fonseca FV, Hayashi H, Shang X, Hoffman NE, Chuprun JK, Tian X, Tilley DG, Madesh M, Lefer DJ, Stamler JS, Koch WJ. Convergence of G protein-coupled receptor and S-nitrosylation signaling determines the outcome to cardiac ischemic injury. Sci Signal. 2013;6:ra95. doi: 10.1126/scisignal.2004225. doi: 10.1126/scisignal.2004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 39.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki H, Swei A, Zweifach BW, Schmid-Schönbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- 44.Zerrouk A, Champeroux P, Safar M, Brisac AM. Role of endothelium in the endothelin-1-mediated potentiation of the norepinephrine response in the aorta of hypertensive rats. J Hypertens. 1997;15:1101–1111. doi: 10.1097/00004872-199715100-00008. [DOI] [PubMed] [Google Scholar]
- 45.Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res. 1994;74:416–421. doi: 10.1161/01.res.74.3.416. [DOI] [PubMed] [Google Scholar]
- 46.Mahfoud F, Lüscher TF, Andersson B, et al. European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013;34:2149–2157. doi: 10.1093/eurheartj/eht154. doi: 10.1093/eurheartj/eht154. [DOI] [PubMed] [Google Scholar]


