Abstract
Background
Few therapeutic options exist for the millions of persons living with severe arm impairment after stroke to increase their dose of arm rehabilitation. This study compared self-guided, high-repetition home therapy with a mechanical device (the Resonating Arm Exerciser - RAE) to conventional therapy in patients with chronic stroke, and explored RAE use for patients with subacute stroke.
Methods
Sixteen participants with severe upper extremity impairment (mean Fugl-Meyer (FM) score = 21.4 ± 8.8 out of 66) > 6 months post stroke were randomized to three-weeks of exercise with RAE or conventional exercises. Primary outcome measure was FM score one month post-therapy. Secondary outcome measures included MAL, Visual Analog Pain scale, and Ashworth spasticity scale. After a one-month break, individuals in the conventional group also received a three-week course of RAE therapy.
Results
The change in FM score was significant in both the RAE and conventional groups after training (2.6 ± 1.4 and 3.4 ± 2.4, p = 0.008 and 0.016, respectively). These improvements were not significant at one-month. Exercise with RAE led to significantly greater improvements in distal FM score than conventional therapy at the one-month follow-up (p = 0.02). In a separate cohort of patients with subacute stroke, RAE was found feasible for exercise.
Discussion
In subjects with severe arm impairment after chronic stroke, home-based training with RAE was feasible and significantly reduced impairment without increasing pain or spasticity. Gains with RAE were comparable to those found with conventional training, and also included distal arm improvement.
Introduction
There are more than five million stroke survivors living in the United States, with over 650,000 people experiencing a new stroke each year1. An estimated 40% of this population live with moderate to severe impairment of their upper extremity1–4, but evidence has shown that intensive rehabilitation exercise can reduce this impairment if an appropriate type and dose of therapy is performed5–10. Unfortunately, while there is little work showing what the adequate dose of rehabilitation is, current methods likely provide an order of magnitude too few repetitions to be most effective, if animal models are used as a reference11,12. Intensive one-on-one exercise with a therapist can significantly increase the amount of exercise performed13, but this is costly and may be impractical for many patients. Another approach is to develop home-based rehabilitation programs that allow patients to augment the amount of therapist-guided therapy they receive with additional self-guided exercise. However, self-guided rehabilitation programs have not been rigorously tested14, and individuals with severe arm impairment may be further limited by an inability to complete exercises at home without assistance15,16.
A machine-based approach could improve home therapy options by providing the motivation, real-time and long-term feedback, assistance, and automation needed to promote a large number of additional exercise repetitions. For example, the BATRAC system uses rhythmic auditory cueing to prompt hundreds of assisted reaching movements in a short amount of time17–19. A similar approach has been used for exercise of the lower extremity20. However, although BATRAC has been successful in clinical trials, there has been no report to our knowledge of a controlled home-based test of BATRAC. Machine-based systems may also include virtual reality components, which have been shown to be motivating and thus might increase patient compliance with home-based therapy programs21–23. Robotic rehabilitation devices can provide assistance and automate therapy, and they have been shown to improve outcomes, limit patient frustration, enhance sensory input, and promote self efficacy15,24–26. Unfortunately, due to the cost and complexity of robotic devices, they have not been rigorously tested outside of clinical settings and are largely unavailable for home use. Thus, while previous machine-based approaches are promising, there is still a need for more home therapy options27.
We recently developed a device called the Resonating Arm Exerciser (RAE) that might be an appropriate tool for improving self-guided home-based rehabilitation28. RAE consists of a lever that attaches to the push rim of a wheelchair, a forearm support, and an elastic band attached between the lever and the wheelchair frame (Figure 1). A user operates RAE by pushing and pulling on the lever, which rolls the wheelchair back in forth in place by 20–30 cm. Similar to the BATRAC system, the lever acts as a mechanical constraint that guides the arm through a movement with coordinated shoulder and elbow flexion and extension similar to reach-and-retrieval tasks. This configuration is also mechanically resonant, so if individuals push and pull on the lever at the system’s resonant frequency, they are rewarded with a larger movement. We found that this assistance amplifies the active range of motion of a user’s arm to approximately twice his unassisted range of motion, and every volunteer who tried RAE in the clinic was able to exercise with it, regardless of their impairment level, weight, age, or other factors28. A similar type of exercise (using the impaired arm to rock in a rocking chair) has previously been shown to reduce long-term impairment after stroke29,30. Furthermore, in a pilot study (n = 8), we found chronic stroke patients who exercised with RAE in a clinic performed thousands of repetitions, significantly increased their FM score and arm active range of motion, and had no increase in arm pain28.
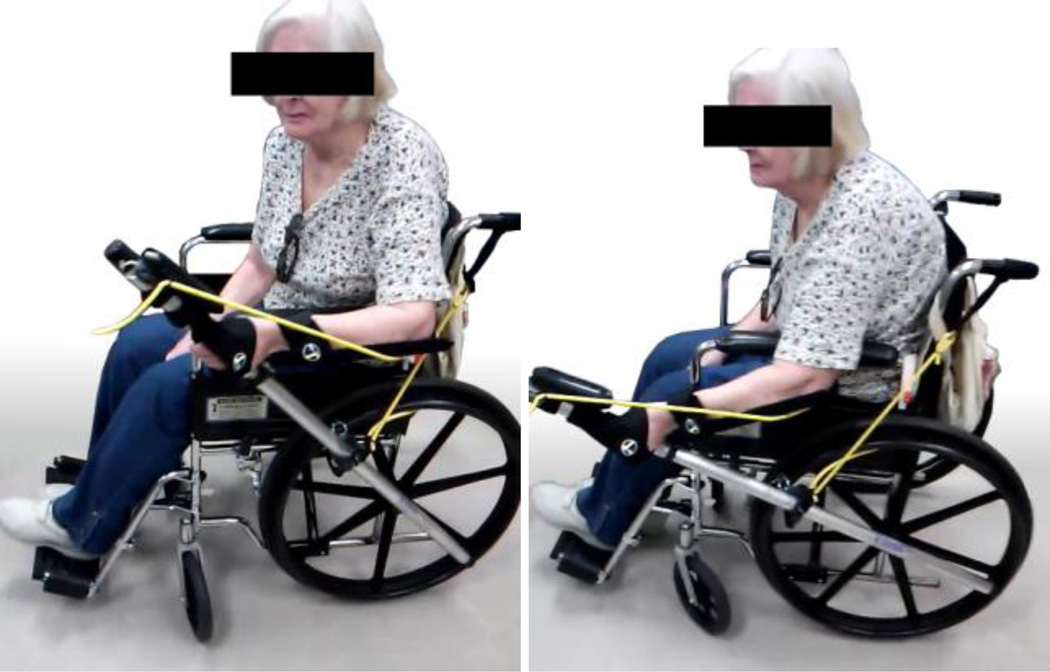
Figure 1.
The Resonating Arm Exerciser (RAE). RAE quickly attaches onto the wheelchair push rim. The user exercises rhythmically with the device at about 1 Hz by extending the shoulder and flexing the elbow (left) and flexing the shoulder and extending the elbow (right) in order to activate the resonance of the device. The device rolls back and forth in place during the exercise.
The goal of this study was to test whether RAE can provide a safe, motivating, and effective method of self-guided arm exercise to users at home, and to determine whether home-based, assisted exercise with RAE leads to greater reductions in arm impairment than conventional, self-guided home-based exercises. In order to answer these questions, we performed a randomized, controlled trial that compared self-guided home-based RAE therapy to conventional home-based therapy. We hypothesized that participants who performed RAE therapy would both exercise more and have significantly greater reductions in arm impairment than participants who performed conventional arm exercises, as assessed at a one-month follow-up. We chose the one-month follow-up as the primary outcome because persistent benefits are important to individuals with stroke.
Of additional interest was whether RAE could be an appropriate tool for providing early, high-repetition arm therapy for individuals with subacute stroke (i.e. < 6 months post-stroke). Therefore, we also performed a small pilot study of RAE in a clinic with subacute stroke patients in order to gain the experience needed to design a larger efficacy study with this dynamic and complex population. Here, we hypothesized that the participants would be able to perform a large number of repetitions with RAE without experiencing arm pain.
Methods
All experiments were approved by UC Irvine’s Institutional Review Board. Participants provided informed consent.
Chronic stroke trial in the home
Study Design and Participants
The first study was a randomized, controlled trial that compared self-guided, home-based exercise with RAE to conventional self-guided, home-based therapy for individuals in the chronic phase of stroke. Inclusion criteria were: experienced one or multiple strokes more than six months previously, Upper Extremity Fugl-Meyer (FM) Score < 30 out of 66, absence of shoulder pain, ability to understand the instructions to operate the device, and age < 80 (as older age could be a confounding variable). We provided subjects with a loaner manual wheelchair if they did not own one.
Outcome Measures and Data Collection
All assessments were performed at UC Irvine by a single blinded evaluator. The primary outcome measure was the Upper Extremity FM Score31. The secondary measures included the Modified Ashworth Scale of spasticity32, the Visual Analog Pain scale, the Motor Activity Log33, Box and Blocks score34, and quantitative measures of active range of motion at the shoulder and elbow acquired with a goniometer. For the goniometer measurements, only one measurement was taken to avoid fatigue. The single trained evaluator used the same goniometer and followed the same set of placement instructions for each participant.
Interventions
Participants returned one week after the initial evaluation to repeat the baseline clinical exam and verify whether they had a stable baseline. At this time the supervising therapist placed the participants into either the RAE (n = 8) or conventional therapy (n = 8) groups via adaptive randomization based on their initial FM score35. That is, subjects with a FM score greater than 30 were alternately placed in the two groups, as were subjects with a FM score less than 30. Based on our pilot data28, 8 subjects would provide a 90% chance of detecting a significant difference of 8 points on the FM scale at the 5% significance level (the FM scale has a minimal clinically significant difference of 4.25 points36). The participants in the RAE group were given a RAE device and instructed on how to use it safely. The participants in the conventional therapy group were given a booklet of exercises developed by experienced occupational therapists at the Rehabilitation Institute of Chicago for home therapy (see Appendix). These exercises included passive range of motion, weight bearing, and active movement exercises for the shoulder and elbow, some of which used a tabletop for support. Both groups were instructed to exercise for three hours per week over at least three sessions per week for three weeks. This intensity has been shown to produce a therapeutic effect9,37,38. An upper limit was not placed on the amount of exercise they could perform, however the participants were instructed to stop exercising and consult the supervising therapist if they began to experience any pain or discomfort.
To monitor amount of use of RAE, we mounted a smartphone to the main shaft of the device and developed an application that counts and logs the number of repetitions a user performs. The RAE group was instructed to run the application during each exercise session. The conventional group was asked to self-record the amount of time they spent exercising on a written log sheet; such self-report methods have been shown to be reliable39. The supervising therapist contacted all participants weekly by phone to ensure that they were not experiencing any difficulties with their exercise, and to query about any adverse events or pain.
After the three-week exercise period, the participants returned for post-therapy clinical assessments. At this assessment, participants also took the Intrinsic Motivation Inventory (IMI) to evaluate their perceptions of their therapy. The IMI is a series of questions answered on a scale from 1 to 7 that are related to perceived enjoyment, usability, stressfulness, motivation, and value of the exercises they performed40. We also retrieved the data from the smartphone on RAE at this session. Participants returned one month later for follow-up assessments.
After the one-month follow-up assessments, the participants in the conventional therapy group were also given a RAE device and instructed on how to use it safely. These participants then repeated the therapy regimen (3 hours/week for 3 weeks) using RAE to exercise. At the end of this crossover exercise period, these participants again returned for post-therapy assessments. These participants also returned one-month later for a final crossover follow-up assessment.
Data Analysis
We calculated individual differences in each outcome measure at the post-therapy and one-month follow-up assessments from the average of the two baseline assessments, as no significant differences were found between the two baseline assessments for any measure (see Results). We tested for significantly greater improvements in the RAE group compared to the conventional therapy group using a one-tailed, non-parametric Wilcoxon rank sum test, since normality is difficult to confirm with such a small sample size. We tested for significant within-group changes using a two-tailed Wilcoxon signed rank test. Since exercise with RAE primarily features proximal movements, we analyzed the FM results further by separating out the distal and proximal components. We also examined the absolute scores from the Visual Analog Pain scale at the post-therapy and one-month follow-up assessments, since individuals with stroke may have difficulty reporting accurate changes in pain over time41, but the absolute scores are an accurate indicator of the intensity of an individual’s current pain42.
In addition to this analysis, we decided post-hoc to analyze the effect of RAE therapy on the combined RAE group and conventional therapy group during the crossover period (n = 16). We calculated individual differences for the crossover group compared to the assessment performed one-month after the initial conventional therapy. These changes can likely be safely attributed to exercise with RAE since there was no significant change in the assessment values at one-month after conventional therapy compared to the assessments performed immediately after therapy. We again tested for significant differences between groups using a one-tailed Wilcoxon rank sum test and for significant changes within the combined RAE group using a two-tailed Wilcoxon signed rank test.
Sub-acute stroke study in the clinic
We also performed a small pilot study of clinical RAE therapy with individuals in the sub-acute phase of stroke (i.e. < 6 months post-stroke). Inclusion criteria were the same as for the home-based study, except for the time since stroke, which was greater than 2 weeks and less than 6 months. The primary outcome measures were the Visual Analog Pain scale to assess arm pain and the total number of exercise repetitions recorded by the smartphone on RAE. Secondary outcome measures were Upper-Extremity FM Score and the Modified Ashworth Scale of spasticity.
The study followed a similar protocol as the home-based study (i.e. 2 baseline assessments 1 week apart, exercise for 3 hours/week over 3 weeks, a post-therapy assessment, and a one-month follow-up assessment). The only differences were that there was no conventional therapy group and the exercises were performed under partial supervision in a clinic. That is, caregivers set RAE up on the participants’ wheelchairs and reminded them to exercise, but then let them exercise on their own.
The data from this study were not analyzed for significance, but individual results are presented in full. Changes were measured from the second baseline assessment only, since baseline values were not stable for this group.
Results
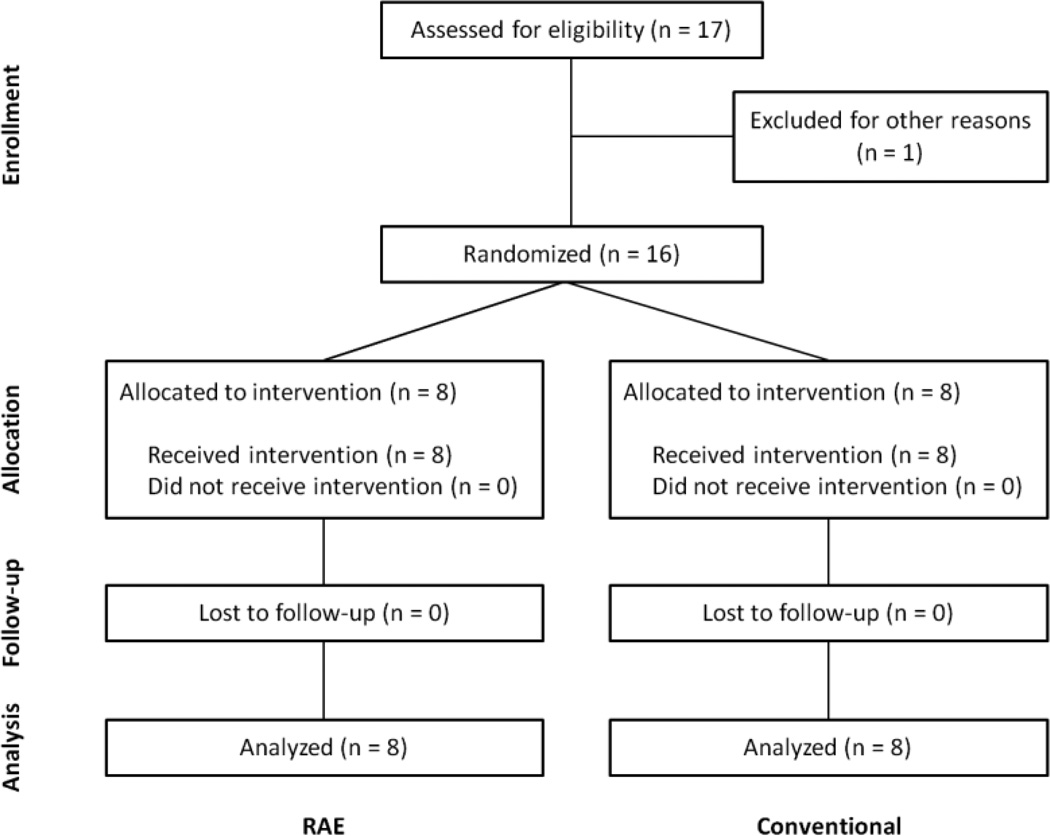
We recruited 17 participants with chronic stroke to participate in the home-based study (Figure 2). One subject dropped out due to issues unrelated to the study. No adverse events occurred during the course of the study. There were no significant differences between the two baseline measurements of each outcome measure taken one week apart for either group. There were also no significant differences between the groups’ baseline measures, except the RAE group had a lower initial MAL score (Table 1).
Figure 2.
A flow of individuals participating in the study.
Table 1. Subject Demographics.
| RAE | Control | |
|---|---|---|
| Total number of Subjects | 8 | 8 |
| Age | 61 ± 17 | 54 ± 14 |
| Months Post-stroke | 39 ± 46 | 19 ± 9 |
| Gender (M/F) | 6 / 2 | 7 / 1 |
| Impaired Side (R/L) | 3 / 5 | 1 / 7 |
| Baseline FM | ||
| Distal (24 max) | 4 ± 4 | 6 ± 4 |
| Proximal (36 max) | 11 ± 6 | 14 ± 6 |
| Total (66 max) | 19 ± 9 | 24 ± 8 |
| Baseline MAL* | 0.04 ± 0.08 | 0.70 ± 0.76 |
| Baseline Visual Analog Pain score | 0.3 ± 0.7 | 0.3 ± 0.6 |
| Baseline Ashworth score | 2.3 ± 0.8 | 3.3 ± 1.0 |
= significant difference between groups, p < 0.05
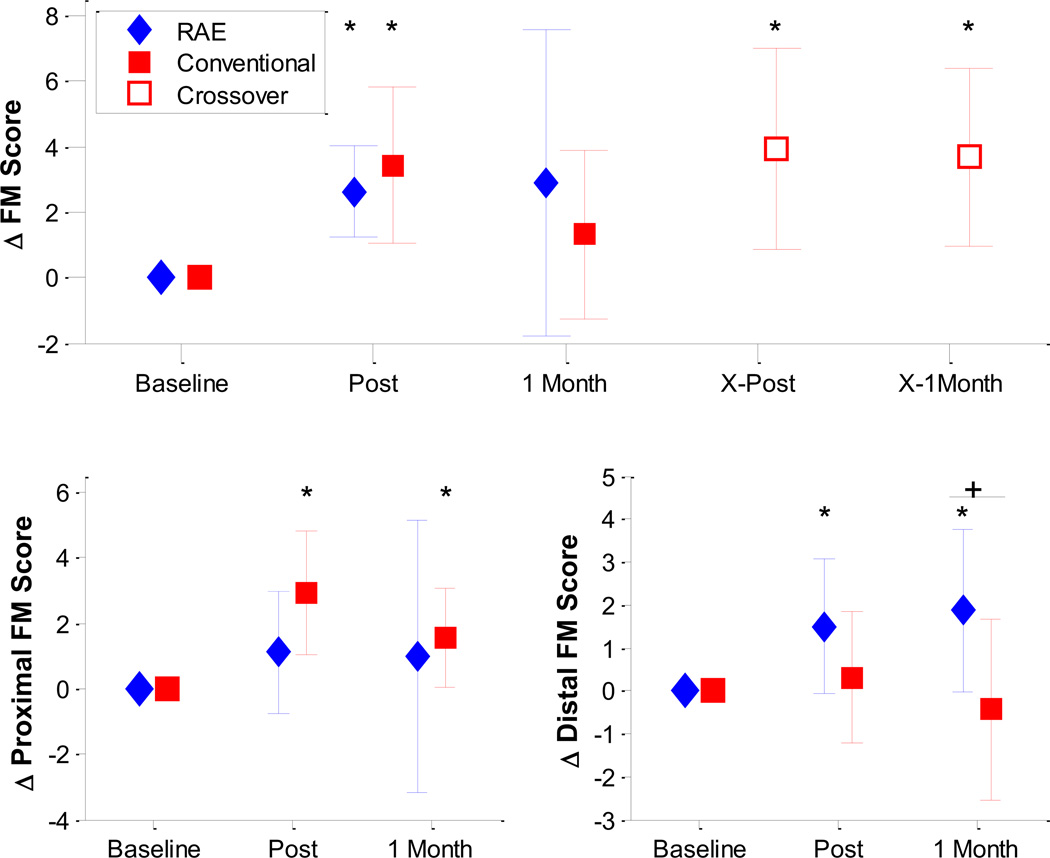
A significant increase in FM score was found for both the RAE group and the conventional therapy group at the post-therapy assessment (changes of 2.6 ± 1.4 and 3.4 ± 2.4, p = 0.008 and 0.016, respectively, Figure 3). However, no significant change in FM score compared to baseline was found for either group at the one-month follow-up assessment. At this assessment, the mean change in FM score for the RAE group was greater than that for the conventional group, with a difference of 1.56 points (95% CI, −3.50 to 6.62). However, the difference was not significant (Table 2). After crossing-over to train with RAE, the conventional therapy group had significant improvements in FM score at both the post-therapy and one-month follow-up crossover assessments (changes of 3.9 ± 3.1 and 3.7 ± 2.7, p = 0.008, and 0.008, respectively).
Figure 3.
Top: Change in Fugl-Meyer score for both groups throughout the experiment (n=8). The diamonds (RAE group) and squares (conventional) show the mean change in FM Score at the Post-Exercise and 1 Month follow-up evaluations. The open squares denote the crossover period for the conventional group, and mark the mean change in FM Score for that group immediately after exercise with RAE, and 1 month after exercise with RAE. Bottom: Change in Fugl-Meyer score between the two groups separated into Proximal (bottom left) and Distal (bottom right) components. There was a significantly greater increase in distal FM score for subjects who exercised with RAE compared to the conventional group (p = 0.02, shown with a ‘+’). * denotes significant changes compared to baseline at p < 0.05. Error bars denote ± 1 standard deviation.
Table 2. Home-Based Study Results.
The RAE vs Conventional Therapy data shows a comparison of changes in outcome measures between the RAE (n = 8) and conventional therapy (n = 8) groups at the one month follow-up after the initial 3 week exercise period. TheCombined results show the changes in outcome measures for both the RAE group and the crossover group at both the post-therapy and one-month follow-up assessments (n = 16). For the crossover subjects, the changes were calculated from the one month follow-up after the initial conventional therapy. Unless otherwise indicated, all values are given as the mean ± 1 standard deviation.
| RAE vs Conventional Therapy (n = 8) | Combined RAE and Crossover Results (n = 16) | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Measure | RAE Therapy | Conventional Therapy |
Mean Difference (95% CI) |
P Val* | Post Therapy | P Val** | One Month Post Therapy |
P Val** |
|
Change in score on Fugl- Meyer Assessment (max 66) |
2.88 ± 4.68 | 1.31 ± 2.59 | 1.56 (−3.50, 6.62) | 0.43 | 2.56 ± 2.45 | 0.001 | 2.56 ± 3.85 | 0.010 |
|
Change in score on Fugl- Meyer Assessment, Proximal Portion (max 36) |
1.0 ± 4.17 | 1.56 ± 1.52 | −0.56 (−4.77, 3.65) | 0.21 | 1.31 ± 1.99 | 0.026 | 1.25 ± 3.07 | 0.185 |
|
Change in score on Fugl- Meyer Assessment, Distal Portion (max 24) |
1.88 ± 1.89 | −0.44 ± 2.09 | 2.31 (−0.45, 5.08) | 0.02 | 1.56 ± 1.03 | < 0.001 | 1.69 ± 1.35 | < 0.001 |
|
Change in score on Visual Analog Pain Scale |
0.0 ± 1.07 | −0.31 ± 0.59 | 0.31 (−0.71, 1.33) | 0.32 | 1.03 ± 2.36 | 0.106 | 0 ± 3.05 | 0.992 |
|
Change in score on Modified Ashworth Scale |
−0.31 ± 0.75 | −0.50 ± 0.38 | 0.25 (−0.36, 0.73) | 0.43 | −0.25 ± 0.68 | 0.313 | −0.79 ± 0.75 | 0.508 |
|
Change in shoulder active range of motion |
10.6 ± 24.3 | 15.9 ± 54.3 | −5.3 (−58.2, 47.7) | 0.43 | 26.4 ± 32.2 | 0.004 | 6.8 ± 28.4 | 0.412 |
|
Change in elbow active range of motion |
10.8 ± 19.2 | 12.6 ± 22.1 | −1.9 (−29.9, 26.1) | 0.48 | 3.2 ± 30.1 | 0.203 | 3.6 ± 21.2 | 0.417 |
|
Change in Box and Blocks Score |
1.88 ± 4.22 | 0.63 ± 1.19 | 1.25 (−2.0, 4.5) | 0.43 | 0.2 ± 1.7 | 1.000 | 0.8 ± 3.2 | 0.531 |
| Change in MAL Score | 0.46 ± 0.85 | 0.12 ± 1.03 | 0.34 (−0.69, 1.37) | 0.33 | 0.07 ± 0.12 | 0.025 | 0.32 ± 0.64 | 0.001 |
P value calculated using one-tailed wilcoxon rank sum test for equal medians.
P value calculated using two-tailed wilcoxon signed rank test for zero median
No significant difference was found between the two groups’ changes in secondary outcome measures at one month. The RAE group showed non-significant decreases in score on the Visual Analog Pain scale (change of −0.69 ± 3.53 points) and Modified Ashworth Scale (change of −0.25 ± 0.89).
We further analyzed the data by separating out the distal and proximal components of the FM exam. Notably, exercise with RAE lead to significant improvements in distal FM score at both the post-therapy and one-month follow-up assessments (changes of 1.5 ± 1.56 and 1.88 ± 1.89, p = 0.047 and 0.047) while the conventional exercises had no apparent effect on distal FM score, a significant difference (p = 0.02 at one-month follow up, Figure 3).
When we analyzed the combined data for all subjects before and after RAE therapy, we found significant increases in FM score and MAL score at both the post-therapy assessment and the one-month follow-up (FM changes of 2.56 ± 2.45 and 2.56 ± 3.85, p = 0.001 and 0.01; MAL changes of 0.07 ± 0.12 and 0.32 ± 0.64, p = 0.025 and 0.001), and a significant increase in active range of motion at the shoulder at the post-therapy assessment (change of 26.4 ± 32.2 degrees, p = 0.004, Table 2). Again, no significant increase in pain or spasticity was found after therapy. One subject reported a moderate level of arm pain after exercise with RAE, but this did not persist at the one-month follow-up.
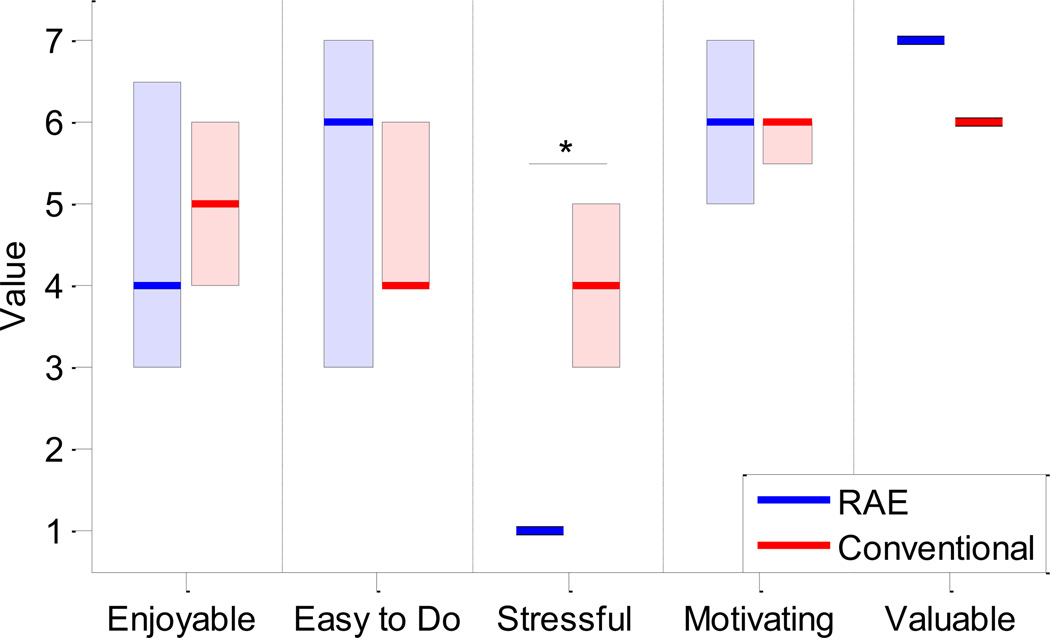
Based on the data from the smartphones, subjects performed an average of 383 repetitions per exercise session with RAE and about 6000 repetitions during the entire study. However, the participants did not use the smartphone for every exercise session, either because they found the interface confusing or they forgot to turn on or charge the smartphone. Based on the self-recorded exercise logs, the participants in the conventional therapy group performed about 10 hours of exercise on average during the 3 week exercise period. This translates to about 500 total repetitions using a rough estimate of 50 repetitions per hour, which is based on the types of exercises and durations prescribed in the booklet of exercises these participants followed. Of the five qualities of the exercises assessed by the IMI, subjects found exercise with RAE to be significantly less stressful than the conventional exercises (p < 0.001, Figure 4). One subject in the conventional group did not provide responses to the IMI.
Figure 4.
Results from IMI assessment for the RAE group (n = 8, left bars) and conventional group (n = 7, right bars). Solid lines denote the median values and the shaded boxes show the interquartile range. Participants in the RAE group found the exercise to be significantly less stressful than conventional exercises (denoted by the ‘*’, p < 0.001).
We also recruited four participants with sub-acute stroke (3 male) to participate in the clinical pilot study of RAE for this dynamic population. The average time since stroke was 3.3 ± 2.1 months. The participants completed thousands of repetitions with RAE without an increase in arm pain or spasticity (Table 3).
Table 3. Results of Pilot Feasibility Study with Individuals with Subacute Stroke*.
| Initial FM |
# of Reps |
Δ FM Score | Pain Score | Δ Ashworth Score | ||||
|---|---|---|---|---|---|---|---|---|
| Post | 1 Month | Post | 1 Month | Post | 1 Month | |||
| Subject 1 | 45 | 7672 | 0 | 1 | 5 | 0 | 0 | −1 |
| Subject 2 | 29 | 18,676 | 12 | 13 | 0 | 0 | −2 | −1 |
| Subject 3 | 11 | 5528 | 15 | 30 | 0 | 0 | 0 | 1 |
| Subject 4 | 18 | 11,628 | 3 | 8 | 0 | 0 | 1 | 0 |
Since subject baselines were not stable, changes were compared to the evaluation performed immediately before exercise with RAE began.
Discussion
Our first key question was whether exercise with RAE is a safe, motivating, and effective method of home-based rehabilitation. RAE did not significantly increase arm pain after exercise, and only one participant reported a moderate level of arm pain after exercise with RAE. Further, no adverse events occurred during the study. The IMI indicated that the participants found exercise with RAE to be easy to do, valuable, and significantly less stressful than conventional exercises. When we analyzed all participants before and after RAE therapy, we observed a significant increase in FM and MAL scores compared to baseline at both the post-therapy and the one-month post-therapy evaluations. These results suggest that self-guided, home-based exercise with RAE is safe, motivating, and effective at reducing arm impairment for severely impaired individuals, although the magnitude of that reduction was moderate.
Our second question was whether home-based, assisted exercise with RAE would lead to greater reductions in arm impairment than conventional exercises. Contrary to our initial hypothesis, exercise with RAE did not improve outcomes compared to conventional exercises, except for distal impairment reduction. We first discuss these results as they relate to home-based therapy for individuals with severe impairments, then with respect to the current idea of task-specific rehabilitation. We conclude by addressing limitations and suggesting several directions for future research.
Machine-Based Home Therapy for Individuals with Severe Impairment
Few studies have explored home-based therapy for individuals with severe upper extremity impairment, presumably under the assumption that these individuals have limited potential for future recovery or would not be able to perform the exercises. Yet, the gains we observed in the present study are similar to those achieved by moderately impaired individuals after intensive exercises24,43. Further, participants in the conventional therapy group performed their exercises at home without human assistance, suggesting conventional home-based therapy is feasible for this population. However, machine-assisted exercise has numerous potential advantages, including motivational aspects, the ability to measure changes, tele-rehabilitation capabilities, and the addition of video games, a possibility we have begun to explore with RAE44. Furthermore, the RAE group in the present study reported their therapy to be significantly less stressful than the conventional therapy group, which suggests that a machine-based approach may also reduce patient anxiety during exercise. In a separate study with non-impaired users, users perceived time as moving more quickly when exercising with RAE, highlighting again possible beneficial psychophysical effects of resonance entrainment45.
Machine-based approaches also allow for large numbers of practice repetitions. Based on the smartphone data, the RAE group performed an average of 383 exercise repetitions per session during the study. This compares favorably with the 400–600 repetitions per day used in animal studies to induce plasticity12,46,47, and is an order of magnitude greater than the normal amount of repetitions performed in conventional, supervised table top therapy11. Given the dose-dependent nature of rehabilitation outcomes5,37,48,49, the ability to both perform and track such a large number of movement repetitions is notable and may help prevent learned non-use (and a subsequent decline in mobility) after rehabilitation is complete48,50,51. Indeed, once spontaneous recovery plateaus after three months52–56, a large number of repetitions is likely required to elicit further recovery through motor learning or other plasticity mechanisms48,49,57. The observation that the participants in the conventional therapy group likely performed an order of magnitude fewer repetitions than the participants in the RAE group during the study may then explain the significant difference in distal impairment reduction between the two groups at the one month follow up. With respect to proximal recovery, the present results suggest that a large number of repetitions of a single, stereotyped shoulder/elbow movement may produce comparable therapeutic benefit as fewer repetitions of more varied movements when the interventions are approximately time matched.
Task-Specific Rehabilitation
The results of this study also have scientific interest with respect to the idea of task-specific rehabilitation, which suggests that patients must practice the specific tasks they want to relearn for rehabilitation to be effective. While there is substantial evidence supporting this claim11,58–62, it may be an oversimplification since it fails to take into account neural plasticity that could generalize to a wide array of tasks. Indeed, the movements performed during exercise with RAE are arguably non-functional and stereotypical (i.e. users only practiced a single movement pattern, repeatedly). Yet, this stereotypical movement practice led to a reduction in impairment across multiple movements as assessed by the FM scale and to a significant improvement in ability to perform activities of daily living as assessed by the MAL score, which is a valid measure of functional arm use63. RAE would likely function best as part of a comprehensive, task-specific rehabilitation program that included repetitive single movement training as well as practice of whole tasks58, but the present results suggest that a sole focus on whole task training is incomplete, a possibility other recent studies have also begun to note29,64–67. A key remaining question is what types of single movement training will maximize transfer to real-world tasks. Or perhaps the kinematics of the machine are less important than the dose of movement it provides.
Exercise with RAE also led to distal gains in addition to proximal gains. From the task-specific rehabilitation perspective, this result is again unexpected, since exercise with RAE primarily involves the shoulder and elbow. However, the positioning of the wrist and hand that RAE provides is out of the flexor synergistic movement pattern common after stroke, and participants may have sometimes tried to actively grip RAE as they exercised, which may have contributed to the observed distal recovery. A similar result was also found with BATRAC: participants who performed 6 weeks of bilateral arm training showed significant improvements in a finger tapping task that was not explicitly trained68. The authors of that study suggest that this was due to the rhythmic nature of the training, which could have affected a central neural control mechanism. Since exercise with RAE is rhythmically cued via resonance entrainment, this could also explain the result observed here. In any case, this result should be studied further since distal function is crucial for many activities of daily living, yet is difficult to practice directly after injury.
Limitations and Future Directions
While the observed benefits of at-home exercise with RAE are encouraging, there are limitations to the device. First, RAE requires a wheelchair to use, which may prevent individuals who no longer rely on a wheelchair from using the device; using a chair again might also have negative psychological ramifications. However, there are many stroke survivors who do regularly use wheelchairs (e.g. >70% of subacute patients69). If a potential user does not own a wheelchair, they must purchase one, but basic chairs only cost about $100 and, in our experience, the individuals who did not regularly use wheelchairs did not mind using a wheelchair as an arm exercise device. Second, the type of floor the wheelchair was placed on affected the operation of the device by either increasing friction or causing the wheels to slip; this was a solvable problem for all our subjects, but it did lead to some initial frustration. Third, clinicians and users desired the ability to move the wheelchair while RAE was attached, which is not possible with the present design. Fourth, while participants did not get motion sickness, some reported being annoyed by the movement of the chair.
We have recently addressed these issues by coupling the lever to the wheel through a custom transmission that allows the lever to be rotated independently of the wheel (thus eliminating movement of the wheelchair and floor-surface dependence). By weighting the lever, we still achieved similar resonance-based assistance without rolling the chair. This transmission also includes a one-way bearing that, when engaged, allows individuals to ambulate overground with the device. The resonance of the lever reduces the average force needed to roll forward by about 40%44. In pilot studies, we found even severely impaired individuals were able to ambulate at least 100 feet in a straight line using this lever drive70.
Other limitations of the study include use of self-report to measure the dose of the therapeutic interventions, a relatively small sample size, and limited improvement in functional ability. Finally, further studies are needed to fully understand the motivational aspects of RAE.
The present study also explored whether RAE could be an appropriate tool for providing arm therapy for individuals with sub-acute stroke (i.e. < 6 months post-stroke), who may have a greater potential for use-dependent plasticity55,71,72. RAE was indeed a safe tool for the participants in this population, as none reported increased arm pain, experienced motion sickness, or exhibited increased spasticity after thousands of repetitions with the device. A larger study is needed to examine the therapeutic benefits of exercise with RAE for this population, but the ability to use it with minimal therapist supervision makes it an attractive tool for providing an early stage, high-repetition, quantifiable therapeutic intervention, similar as to was done with a rocking chair and air splint to positive benefit29,30. Future studies should also directly examine the potential negative psychological ramifications of asking individuals who are recovering from stroke to return to a wheelchair if they no longer use one for mobility.
Conclusion
RAE was a safe and effective device for individuals with severe impairments to use at home that in this study led to a reduction of arm impairment comparable to conventional exercise. Due to its simplicity, exercise with RAE is limited to the practice of a single movement pattern, but this movement pattern is an important one, and repeating it a large number of times led not only to proximal impairment reduction but also to greater improvements in distal movement ability than conventional exercises. RAE would perhaps best be used as an add-on to existing home-therapy programs, rather than as a replacement for them, as a method of increasing the dose of a particular exercise. Indeed, for individuals with severe impairments, the options for rehabilitation are limited and any additional tool they can use to exercise will increase their opportunities for recovery.
Acknowledgments
Funding was provided by the Department of Education through NIDRR grant H133S120032. This study is registered at ClinicalTrials.gov under identifier NCT01769326. Thank you to the UC Irvine Institute for Clinical and Translational Science for their support in the study.
Footnotes
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Daniel Zondervan, Nizan Friedman, and David Reinkensmeyer have a financial interest in Flint Rehabilitation Devices, LLC, a company that develops rehabilitation devices. The terms of this arrangement have been reviewed and approved by the University of California, Irvine, in accordance with its conflict of interest policies. The remaining authors declare that they have no competing interest.
References Cited
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobkin BH. Neurologic Rehabilitation. Philadelphia: F.A. Davis Company; 1996. [Google Scholar]
- 3.Parker VM, Wade DT, Langton Hewer R. Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehabil Med. 1986;8(2):69–73. doi: 10.3109/03790798609166178. [DOI] [PubMed] [Google Scholar]
- 4.Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. J Neurol Neurosurg Psychiatry. 1987;50(6):714–719. doi: 10.1136/jnnp.50.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawaki L. Use-dependent plasticity of the human motor cortex in health and disease. IEEE Eng Med Biol Mag. 2005;24(1):36–39. doi: 10.1109/memb.2005.1384098. [DOI] [PubMed] [Google Scholar]
- 6.Ada L, Dorsch S, Canning CG. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust J Physiother. 2006;52(4):241–248. doi: 10.1016/s0004-9514(06)70003-4. [DOI] [PubMed] [Google Scholar]
- 7.Van der Lee J, Snels I, Beckerman H, Lankhorst G, Wagenaar R, Bouter L. Exercise therapy for arm function in stroke patients: a systematic review of randomized controlled trial. Clin Rehabil. 2001;15:20–31. doi: 10.1191/026921501677557755. [DOI] [PubMed] [Google Scholar]
- 8.Kloosterman MG, Snoek GJ, Jannink MJ. Systematic review of the effects of exercise therapy on the upper extremity of patients with spinal-cord injury. Spinal cord Off J Int Med Soc Paraplegia. 2008 doi: 10.1038/sc.2008.113. [DOI] [PubMed] [Google Scholar]
- 9.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38(2 Suppl):840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 10.MacLellan CL, Keough MB, Granter-Button S, Chernenko GA, Butt S, Corbett D. A critical threshold of rehabilitation involving brain-derived neurotrophic factor is required for poststroke recovery. Neurorehabil Neural Repair. 2011;25(8):740–748. doi: 10.1177/1545968311407517. [DOI] [PubMed] [Google Scholar]
- 11.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692–1698. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-Dependent Alterations of Movement Representations Cortex of Adult Squirrel Monkeys. J Neurosci. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair. 2010;24(7):620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coupar F, Pollock A, Legg LA, Sackley C, van Vliet P. Home-based therapy programmes for upper limb functional recovery following stroke. Cochrane database Syst Rev. 2012;5 doi: 10.1002/14651858.CD006755.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008;131(Pt 2):425–437. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 16.Jurkiewicz MT, Marzolini S, Oh P. Adherence to a home-based exercise program for individuals after stroke. Top Stroke Rehabil. 2011;18(3):277–284. doi: 10.1310/tsr1803-277. [DOI] [PubMed] [Google Scholar]
- 17.Whitall J, Waller SM, Sorkin JD, et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair. 2011;25(2):118–129. doi: 10.1177/1545968310380685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31(10):2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 19.Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA J Am Med Assoc. 2004;292(15):1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johannsen L, Wing AM, Pelton T, et al. Seated bilateral leg exercise effects on hemiparetic lower extremity function in chronic stroke. Neurorehabil Neural Repair. 2010;24(3):243–253. doi: 10.1177/1545968309347679. [DOI] [PubMed] [Google Scholar]
- 21.Hijmans JM, Hale La, Satherley Ja, McMillan NJ, King MJ. Bilateral upper-limb rehabilitation after stroke using a movement-based game controller. J Rehabil Res Dev. 2011;48(8):1005. doi: 10.1682/jrrd.2010.06.0109. [DOI] [PubMed] [Google Scholar]
- 22.King M, Hijmans J, Sampson M, Satherley J, Hale L. Home-based stroke rehabilitation using computer gaming. [Accessed December 6, 2013];New Zeal J Physiother. 2012 40(3):128–134. Available at: http://physiotherapy.org.nz/assets/Professional-dev/Journal/2012-November/NZJP-Nov-2012.pdf#page=24. [Google Scholar]
- 23.Van Delden aLEQ, Peper CLE, Kwakkel G, Beek PJ. A systematic review of bilateral upper limb training devices for poststroke rehabilitation. Stroke Res Treat. 2012;2012:972069. doi: 10.1155/2012/972069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchal-Crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J Neuroeng Rehabil. 2009;6:20. doi: 10.1186/1743-0003-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu XL, Tong KY, Song R, Zheng XJ, Leung WW. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabil Neural Repair. 2009;23(8):837–846. doi: 10.1177/1545968309338191. [DOI] [PubMed] [Google Scholar]
- 27.Turton AJ, Cunningham P, Heron E, et al. Home-based reach-to-grasp training for people after stroke: study protocol for a feasibility randomized controlled trial. Trials. 2013;14(1):109. doi: 10.1186/1745-6215-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zondervan DK, Palafox L, Hernandez J, Reinkensmeyer DJ. The Resonating Arm Exerciser: design and pilot testing of a mechanically passive rehabilitation device that mimics robotic active assistance. J Neuroeng Rehabil. 2013;10(39) doi: 10.1186/1743-0003-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feys H, De Weerdt W, Verbeke G, et al. Early and repetitive stimulation of the arm can substantially improve the long-term outcome after stroke: a 5-year follow-up study of a randomized trial. Stroke. 2004;35(4):924–929. doi: 10.1161/01.STR.0000121645.44752.f7. [DOI] [PubMed] [Google Scholar]
- 30.Feys HM, De Weerdt WJ, Selz BE, et al. Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke: a single-blind, randomized, controlled multicenter trial. Stroke. 1998;29(4):785–792. doi: 10.1161/01.str.29.4.785. [DOI] [PubMed] [Google Scholar]
- 31.See J, Dodakian L, Chou C, et al. A standardized approach to the fugl-meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27(8):732–741. doi: 10.1177/1545968313491000. [DOI] [PubMed] [Google Scholar]
- 32.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 33.Van der Lee JH, Beckerman H, Knol DL, de Vet HCW, Bouter LM. Clinimetric properties of the Motor Activity Log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35(6):1404–1410. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 34.Platz T, Pinkowski C, van Wijck F, Kim I-H, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin Rehabil. 2005;19(4):404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- 35.Meinert CL. Clinical Trials: Design, Conduct, and Analysis. New York: Oxford Press; 1986. [Google Scholar]
- 36.Page SJ, Fulk GD, Boyne P. Clinically Important Differences for the Upper-Extremity Fugl-Meyer Scale in People With Minimal to Moderate Impairment Due to Chronic Stroke. Phys Ther. 2012;92(6) doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 37.Byl NN, Pitsch EA, Abrams GM. Functional outcomes can vary by dose: learning-based sensorimotor training for patients stable poststroke. Neurorehabil Neural Repair. 2008;22(5):494–504. doi: 10.1177/1545968308317431. [DOI] [PubMed] [Google Scholar]
- 38.Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354(9174):191–196. doi: 10.1016/S0140-6736(98)09477-X. [DOI] [PubMed] [Google Scholar]
- 39.Van der Ploeg HP, Streppel KRM, van der Beek AJ, van der Woude LHV, Vollenbroek-Hutten M, van Mechelen W. The Physical Activity Scale for Individuals with Physical Disabilities: test-retest reliability and comparison with an accelerometer. [Accessed May 13, 2014];J Phys Act Health. 2007 4(1):96–100. doi: 10.1123/jpah.4.1.96. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17489011. [DOI] [PubMed] [Google Scholar]
- 40.Ryan RM. Control and Information in the Intrapersonal Sphere: An Extension of Cognitive Evaluation Theory. J Pers Soc Psychol. 1982;43(3):450–461. [Google Scholar]
- 41.Price CIM, Curless RH, Rodgers H. Can Stroke Patients Use Visual Analogue Scales? Stroke. 1999;30(7):1357–1361. doi: 10.1161/01.str.30.7.1357. [DOI] [PubMed] [Google Scholar]
- 42.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 43.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 44.Zondervan DK, Smith B, Reinkensmeyer DJ. Lever-actuated resonance assistance (LARA): A wheelchair-based method for upper extremity therapy and overground ambulation for people with severe arm impairment. IEEE Int Conf Rehabil Robot. 2013;2013:1–6. doi: 10.1109/ICORR.2013.6650400. [DOI] [PubMed] [Google Scholar]
- 45.Zondervan DK, Duarte JE, Rowe JB, Reinkensmeyer DJ. Time flies when you are in a groove: using entrainment to mechanical resonance to teach a desired movement distorts the perception of the movement’s timing. Exp brain Res. 2014 doi: 10.1007/s00221-013-3819-3. to appear. [DOI] [PubMed] [Google Scholar]
- 46.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural Substrates for the Effects of Rehabilitative Training on Motor Recovery After Ischemic Infarct. Science (80- ) 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 47.Kleim JA, Barbay S, Nudo RJ. Functional Reorganization of the Rat Motor Cortex Following Motor Skill Learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 48.Reinkensmeyer DJ, Guigon E, Maier Ma. A computational model of use-dependent motor recovery following a stroke: Optimizing corticospinal activations via reinforcement learning can explain residual capacity and other strength recovery dynamics. Neural networks. 2012;29–30C:60–69. doi: 10.1016/j.neunet.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45(7):2053–2058. doi: 10.1161/STROKEAHA.114.004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schweighofer N, Han CE, Wolf SL, Arbib MA, Winstein CJ. A functional threshold for long-term use of hand and arm function can be determined: predictions from a computational model and supporting data from the Extremity Constraint-Induced Therapy Evaluation (EXCITE) Trial. Phys Ther. 2009;89(12):1327–1336. doi: 10.2522/ptj.20080402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taub E, Uswatte G, Mark V, Morris D. The learned nonuse phenomenon: implications for rehabilitation. [Accessed December 23, 2013];Eura Medicophys. 2006 42(3):241–255. Available at: http://www.researchgate.net/publication/6756661_The_learned_nonuse_phenomenon_implications_for_rehabilitation/file/9fcfd508968a0eee13.pdf. [PubMed] [Google Scholar]
- 52.Skilbeck CE, Wade DT, Hewer RL, Wood VA. Recovery after stroke. [Accessed July 23, 2014];J Neurol Neurosurg Psychiatry. 1983 46(1):5–8. doi: 10.1136/jnnp.46.1.5. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1027255&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 54.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. [Accessed July 23, 2014];Phys Med Rehabil Clin N Am. 1999 10(4):887–906. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10573714. [PubMed] [Google Scholar]
- 55.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24(5):1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 22(1):64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 57.Page SJ, Gater DR, Bach-Y-Rita P. Reconsidering the motor recovery plateau in stroke rehabilitation. Arch Phys Med Rehabil. 2004;85(8):1377–1381. doi: 10.1016/j.apmr.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 58.Hubbard IJ, Parsons MW, Neilson C, Carey LM. Task-specific training: evidence for and translation to clinical practice. Occup Ther Int. 2009;16(3–4):175–189. doi: 10.1002/oti.275. [DOI] [PubMed] [Google Scholar]
- 59.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74(1):27–55. doi: 10.1006/nlme.1999.3934. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10873519. [DOI] [PubMed] [Google Scholar]
- 60.Dean CM, Shepherd RB. Task-related training improves performance of seated reaching tasks after stroke: A randomized controlled trial. Stroke. 1997;28(4):722–728. doi: 10.1161/01.str.28.4.722. [DOI] [PubMed] [Google Scholar]
- 61.French B, Thomas LH, Leathley MJ, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2007;4(4):CD006073. doi: 10.1002/14651858.CD006073.pub2. [DOI] [PubMed] [Google Scholar]
- 62.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 63.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44(4):1111–1116. doi: 10.1161/STROKEAHA.111.674671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hogan N, Krebs HI, Rohrer B, et al. Motions or muscles? Some behavioral factors underlying robotic assistance of motor recovery. J Rehabil Res Dev. 2006;43(5):605–618. doi: 10.1682/jrrd.2005.06.0103. [DOI] [PubMed] [Google Scholar]
- 65.Schaefer SY, Patterson CB, Lang CE. Transfer of Training Between Distinct Motor Tasks After Stroke: Implications for Task-Specific Approaches to Upper-Extremity Neurorehabilitation. Neurorehabil Neural Repair. 2013 doi: 10.1177/1545968313481279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein J, Spencer SJ, Reinkensmeyer DJ. Breaking it down is better: haptic decomposition of complex movements aids in robot-assisted motor learning. IEEE Trans neural Syst Rehabil Eng. 2012;20(3):268–275. doi: 10.1109/TNSRE.2012.2195202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Milot M-H, Spencer SJ, Chan V, et al. A crossover pilot study evaluating the functional outcomes of two different types of robotic movement training in chronic stroke survivors using the arm exoskeleton BONES. J Neuroeng Rehabil. 2013;10:112. doi: 10.1186/1743-0003-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCombe Waller S, Whitall J. Fine motor control in adults with and without chronic hemiparesis: baseline comparison to nondisabled adults and effects of bilateral arm training. Arch Phys Med Rehabil. 2004;85(7):1076–1083. doi: 10.1016/j.apmr.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 69.Barrett Ja, Watkins C, Plant R, et al. The COSTAR wheelchair study: a two-centre pilot study of self-propulsion in a wheelchair in early stroke rehabilitation. Clin Rehabil. 2001;15(1):32–41. doi: 10.1191/026921501672264719. [DOI] [PubMed] [Google Scholar]
- 70.Smith BW, Zondervan DK, Reinkensmeyer DJ. Feasibility of a bimanual, lever-driven wheelchair for people with severe arm impairment after stroke; Intl. Conf. of the IEEE Engineering in Medicine and Biology Society; 2014. [DOI] [PubMed] [Google Scholar]
- 71.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol. 2013;26(6):609–616. doi: 10.1097/WCO.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stinear C, Ackerley S, Byblow W. Rehabilitation is Initiated Early After Stroke, but Most Motor Rehabilitation Trials Are Not: A Systematic Review. Stroke. 2013;44(7):2039–2045. doi: 10.1161/STROKEAHA.113.000968. [DOI] [PubMed] [Google Scholar]