Abstract
Objectives
Treatment interruptions (TI) of combination antiretroviral therapy (cART) are known to lead to unfavourable treatment outcomes but do still occur in resource-limited settings. We investigated the effects of TI associated with adverse events (AEs) and non-AE-related reasons, including their durations, on treatment failure after cART resumption in HIV-infected individuals in Asia.
Methods
Patients initiating cART between 2006-2013 were included. TI was defined as stopping cART for >1 day. Treatment failure was defined as confirmed virological, immunological or clinical failure. Time to treatment failure during cART was analysed using Cox regression, not including periods off treatment. Co-variables with p<0.10 in univariable analyses were included in multivariable analyses, where p<0.05 was considered statistically significant.
Results
Of 4549 patients from 13 countries in Asia, 3176 (69.8%) were male and the median age was 34 years. A total of 111 (2.4%) had TIs due to AEs and 135 (3.0%) had TIs for other reasons. Median interruption times were 22 days for AE and 148 days for non-AE TIs. In multivariable analyses, interruptions >30 days were associated with failure (31-180 days HR=2.66, 95%CI (1.70-4.16); 181-365 days HR=6.22, 95%CI (3.26-11.86); and >365 days HR=9.10, 95% CI (4.27-19.38), all p<0.001, compared to 0-14 days). Reasons for previous TI were not statistically significant (p=0.158).
Conclusions
Duration of interruptions of more than 30 days was the key factor associated with large increases in subsequent risk of treatment failure. If TI is unavoidable, its duration should be minimised to reduce the risk of failure after treatment resumption.
Keywords: HIV, treatment interruptions, adverse events, Asia, antiretroviral
Introduction
Interruptions of HIV combination antiretroviral therapy (cART) are associated with unfavourable outcomes. The largest clinical trial investigating the impact of CD4-guided treatment interruptions (TI; SMART study) (1) found the risk for disease progression was significantly greater in the TI group than in the continuous cART group, largely due to low CD4 count and high viral load (VL). Other studies have also reported the link between TI and rapid decline in CD4 count, VL rebound and mortality (2, 3). Duration of TI is a significant predictor of virologic rebound. Longer time off therapy is often associated with increased risk of viral failure after cART has been resumed (4).
Adverse events (AEs), including drug toxicities and side-effects, are commonly seen in HIV-positive individuals receiving cART. AEs can vary from less serious (e.g., headaches, rash, nausea) to more serious toxicities (e.g., severe hepatic toxicity, high-grade anaemia) (5-7). A study in India has found that 79% of patients who developed rash or Stevens-Johnson syndrome (SJS) had interrupted or stopped cART (7). In resource-limited settings where cART normally consists of nucleoside and non-nucleoside reverse transcriptase inhibitors (NRTIs and NNRTIs), the rate of AEs after cART initiation has ranged from 4.6 to 52 per 100 person-years (100PY) (8, 9).
Patients experiencing AEs are more likely to have treatment modifications, interruptions and/or discontinuations (10, 11). Although the prevalence of TIs has fallen over recent years, drug toxicities and side-effects have remained the most common causes of TI (12-14). Unplanned cART interruptions may be unavoidable when there are drug stockouts, in order to manage cART-related AEs, or when there are adherence challenges.
In this study, we investigated the reasons for unplanned TI in patients initiating cART from 2006 (post SMART study) and the effects of different causes of TIs and duration of interruption on subsequent treatment failure after cART resumption. Due to the known high rates of detectable viral load during the absence of cART, the main focus of the study was to examine how well patients responded to treatment after cART had been resumed, rather than their virological responses during periods of TI.
Methods
Study population
Patients enrolled in the TREAT Asia HIV Observational database (TAHOD) who initiated cART between 2006 to 2013, with at least six months of follow-up time were selected. A detailed description of the cohort profile was published elsewhere (15), but briefly TAHOD is a prospective HIV observational cohort which began recruitment in 2003, consisting of 23 sites in 13 countries in Asia. All TAHOD sites are major referral centres in urban cities with data transfers occurring every six months. TAHOD patients are monitored and treated according to local practices, with patients not seen for 180 days being at higher risk of permanent loss to follow-up (LTFU) (16). CD4 testing occurs bi-annually and the overall median CD4 cell count at cART initiation was 150 cells/µl but levels have increased over time from 115 cells/µL in 2008 to 302 cells/µL after 2011. Nevertheless, most patients in TAHOD continued to enter care late and had CD4 levels <350 cells/µL (17). VL testing frequency in TAHOD has been reported to range from <1/year to ≥3/year (18). In this study, we chose not to include patients who started cART before 2006 to avoid potential biases associated with structured TIs. Patients who received prior mono/dual therapy were excluded.
Definition
TI was defined as a period of no cART for >1 day. We used a minimum duration of one day to incorporate all periods of no cART, including non-adherence, in the analyses and to compare the effects of different TI durations. Although this minimal TI duration is not commonly used, it has been reported in previous literature (14, 19). Reasons for TI were categorised into one of two groups: stopped due to AEs and stopped due to other reasons. For the purpose of this study, AEs included stop reasons specified as drug adverse reactions, toxicity and/or side-effects. TAHOD collects cART stop reasons according to the physician’s clinical judgement, possibly in consultation with the patient. If multiple stop reasons were recorded with at least one due to AEs, the reason was then coded AE-related TI. The analysis outcome was treatment failure defined as the earliest date of clinical, immunological or virological failure according to the World Health Organization (WHO) (20). We combined the three types of failure into a common end-point in order to include TAHOD sites that do not routinely perform VL testing in the analyses. Because disease staging in TAHOD is done using U.S. Centers for Disease Control and Prevention (CDC) criteria, new CDC stage C events were used to define clinical failure. Immunological failure was defined as a CD4 count <100 cells/mm3 and confirmed within six months or a CD4 count equal to or below pre-treatment levels. Virological failure was defined as VL ≥1000 copies/mL with a second consecutive VL confirmation within six months. All failures must occur at least six months after cART initiation and while on treatment. Failures occurring during periods of TI were not counted in the analyses because we wanted to measure treatment failures after cART has been resumed. A sensitivity analysis was performed by ignoring the requirement for a second confirmed test for immunological and virological failures.
Statistical analyses
Time to first treatment failure was analysed using a Cox regression model. We stratified our analyses by site to account for any site-level effects, such as differences in VL testing frequency. Analysis risk time began at six months after cART initiation and ended at first documented failure. Patients not experiencing treatment failure were censored at the date of their last visit. Periods when patients were off treatment were not counted as risk time. In other words, if a patient had not experienced treatment failure prior to TI, the patient was removed from the risk set during TI and re-entered after cART had been resumed. Patients who became LTFU defined as those not seen in the previous 12 months of the September 2013 data transfer, or died during TI were censored on the last date of cART prior to TI. Regression models were fitted using backward stepwise procedures. In the univariable analysis, covariates with p-values <0.10 were chosen for inclusion in the multivariable model. After the backward stepwise model selection, covariates with p-values <0.05 were considered statistically significant. Other non-significant covariates are each presented adjusted for the significant variables, however they did not form part of the final model.
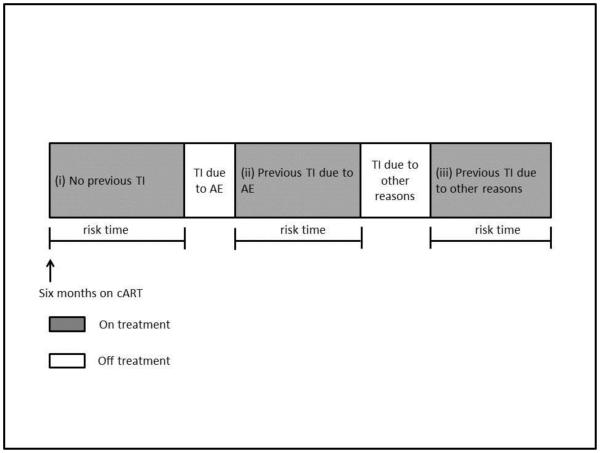
Time-fixed covariates adjusted in the regression models were age, sex, mode of HIV exposure, pre-treatment VL and CD4, initial cART regimen, hepatitis B/C co-infection and prior AIDS diagnosis. Time-updated covariates, where a patient is allowed to change between categories with the effect sizes calculated based on the varying risk time spent in each category, were “Reasons for Previous TI” and “Previous TI duration variables. “Reasons for Previous TI” was coded as (i) no previous TI, (ii) previous TI due to AE, and (iii) previous TI due to other reasons (Figure 1) and “Previous TI Duration” variable was coded as (i) 0-14 days, (ii) 15-30 days, (iii) 31-180 days, (iv) 181-365 days, and (vi) >365 days. We used these categories to approximately aggregate the TI duration according to the period commonly not considered as a TI :0-14 days (21-23); and according to those reported in previous literature :15-30 days (22, 24, 25), 31-180 days (13, 26, 27), 181-365 days (28, 29) and >365 days (30). It was decided a priori that self-reported adherence levels would not be included in the analyses due to the potential collinearity between adherence and the Reasons for TI variable, and that prospective adherence data was only collected in TAHOD from 2011 onwards.
Figure 1. Example of the time-updated reasons for previous treatment interruption variable coding.
Abbreviations: TI – treatment interruption, AE – adverse event, cART - combination antiretroviral therapy.
Ethics approvals were obtained from UNSW Australia Ethics Committee, Western Institutional Review Board, and respective local ethics committees of all TAHOD-participating sites, the data management and biostatistical center (UNSW Australia Ethics Committee), and the coordinating center (TREAT Asia/amfAR). All data management and statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA) and Stata software version 13.1 (Stata Corp., College Station, TX, USA).
Results
Patient characteristics
A total of 4549 patients from Cambodia, China, Hong Kong, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand and Vietnam were included. Table 1 shows that 111/4549 (2.4%) patients experienced TIs as a result of AEs, while 135 (3.0%) had TIs due to other reasons, and 4303 (94.6%) never had an interruption. The median duration of TI caused by AE-related reasons was 22 days (interquartile range (IQR): 12-47) compared to 148 days (IQR: 27-319) for non-AE-related causes. Of the 246 patients who had a TI, 189 (76.8%) resumed cART after the first episode of TI with the same drug classes as the initial regimen, which were mostly NRTI+NNRTI. Others had a drug class change, mainly from NNRTI to a protease inhibitor. 52 (1.1%) patients died and 493 (10.8%) became LTFU prior to experiencing treatment failure. Of the dead and LTFU patients, 5 and 32, respectively, had a TI during follow-up.
Table 1.
Patient demographics
| Total (%) 4549 (100) |
|
|---|---|
|
Reasons for TI
No interruption At least 1 TI due to adverse events All TI due to other reasons |
4303 (94.6) 111 (2.4) 135 (3.0) |
|
Duration of TI (days)
Interruption due to adverse events Interruption due to other reasons |
median = 22, IQR (12-47) median = 148, IQR (27-319) |
|
Age at cART initiation (years)
<=30 31-40 41-50 >50 |
median =34 , IQR (29-41) 1433 (31.5) 1895 (41.7) 860 (18.9) 361 (7.9) |
|
Sex
Male Female |
3176 (69.8) 1373 (30.2) |
|
Mode of HIV exposure
Heterosexual contact Homosexual contact Injecting drug use Other/unknown |
2791 (61.4) 952 (20.9) 496 (10.9) 310 (6.8) |
|
Pre-cART viral load (copies/mL)
≤100000 >100000 Missing |
median =100000, IQR (31697-260000) 1290 (28.4) 1238 (27.2) 2021 (44.4) |
|
Pre-cART CD4 (cells/μL)
<=50 51-100 101-200 >200 Missing |
median =131, IQR (41-226) 1169 (25.7) 564 (12.4) 1015 (22.3) 1290 (28.4) 511 (11.2) |
|
Initial cART regimen
NRTI+NNRTI NRTI+PI Other |
4103 (90.2) 401 (8.8) 45 (1.0) |
|
Hepatitis B co-infection
Negative Positive Not tested |
3363 (73.9) 379 (8.3) 807 (17.7) |
|
Hepatitis C co-infection
Negative Positive Not tested |
2839 (62.4) 616 (13.5) 1094 (24.1) |
|
Previous AIDS
No Yes |
2907 (63.9) 1642 (36.1) |
Abbreviations: TI – treatment interruption, cART - combination antiretroviral therapy, NRTI – nucleoside reverse tran-scriptase inhibitors, NNRTI – non-nucleoside reverse transcriptase inhibitors, PI – protease inhibitors.
Reasons for TI
Taking into account that a patient can have multiple reasons for TI and TI can occur multiple times, we found that skin side effects were the most common reasons for TI due to AEs (55 patients). Liver toxicities (17 patients) included jaundice, hepatitis, raised liver function tests and hyperbilirubinemia. Drug allergies (11 patients) were hypersensitivity reactions and SJS. Gastrointestinal side effects (8 patients) included nausea, vomiting and diarrhoea. When cART was stopped due to reasons other than AEs, the most frequently entered reasons were “other” (70 patients) and “patient decision/request” (50 patients).
Treatment failure
A total of 730 (16.0%) patients experienced at least one type of treatment failure: 89 had virological failure, 396 had CD4 below baseline values, 105 had CD4 below 100 cells/µL and 175 had clinical failure. Combining these totals resulted in 765 failures associated with 730 patients, indicating that some experienced more than one type of failure on the same day. Of the immunological failures, 36 patients continued to have CD4 counts <100 cells/µL well beyond six months from cART initiation, with eventual rise in CD4 levels. These patients were counted as having immunological failure on the first CD4 measurement <100 cells/µL after six months on cART. The crude failure rate was 5.89 per 100PY. The median time from cART initiation up to treatment failure or censoring date was 3.0 years (IQR: 1.8-4.6).
Factors associated with time to treatment failure
From Table 2, the multivariable model shows that the TI duration variable was significant (p<0.001) with interruptions >30 days being associated with treatment failure (31-180 days HR=2.66, 95%CI (1.70-4.16); 181-365 days HR=6.22, 95%CI (3.26-11.86); and >365 days HR=9.10, 95%CI (4.27-19.38); compared to 0-14 days). The Reasons for Previous TI variable, however, was not significant (p=0.158). The interaction between Reasons for Previous TI and TI duration was also not significant (p=0.443). Other factors associated with treatment failure were age >50 years (HR=1.56, 95%CI (1.17-2.08)) compared to age ≤30 years, and higher pre-cART CD4 count of 101-200 cells/µL (HR=1.42, 95%CI (1.42-1.78)) and CD4 counts >200 cells/µL (HR=1.76, 95%CI (1.42-2.18)), compared to CD4 counts ≤50 cells/µL. Factors that showed a protective effect against treatment failure were female sex (HR=0.74, 95%CI (0.61-0.89)) vs. male sex, and homosexual HIV exposure (HR=0.74, 95%CI (0.56-0.97)) and other/unknown exposure group (HR=0.69, 95%CI (0.49-0.99)) vs. heterosexual HIV exposure. Pre-cART VL, initial cART regimen, hepatitis B/C co-infection and previous AIDS diagnosis were not associated with treatment failure.
Table 2.
Time to first treatment failure (clinical, immunological and virological failure)
| Univariable | Multivariable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person-years (pys) |
Number with treatment failure |
Crude rate (/100pys) |
95% CI | HR | 95% CI | *p | HR | 95% CI | *p | |
| Total | 12395.4 | 730 | 5.89 | (5.48, 6.33) | ||||||
|
| ||||||||||
|
~Reasons for Previous Treatment
Interruption |
<0.001 | ^0.158 | ||||||||
| No previous TI | 11827.2 | 668 | 5.65 | (5.24, 6.09) | 1 | 1 | ||||
| Previous TI due to AE | 309.7 | 19 | 6.13 | (3.91, 9.62) | 1.27 | (0.80, 2.01) |
0.318 | 0.86 | (0.43, 1.74) |
0.677 |
| Previous TI due to other reasons | 258.5 | 43 | 16.64 | (12.34, 22.43) |
3.25 | (2.36, 4.49) |
<0.001 | 1.53 | (0.76, 3.08) |
0.228 |
|
| ||||||||||
|
~Previous Treatment Interrup-
tion Duration (days) |
#<0.001 | # <0.001 | ||||||||
| 0-14 | 11996.4 | 678 | 5.65 | (5.24, 6.09) | 1 | 1 | ||||
| 15-30 | 148.3 | 12 | 8.09 | (4.60, 14.25) | 1.57 | (0.88, 2.79) |
0.126 | 1.61 | (0.91, 2.87) |
0.105 |
| 31-180 | 173.1 | 21 | 12.13 | (7.91, 18.61) | 2.47 | (1.58, 3.84) |
<0.001 | 2.66 |
(1.70,
4.16) |
<0.001 |
| 181-365 | 39.3 | 10 | 25.46 | (13.70, 47.32) | 5.43 | (2.86, 10.31) |
<0.001 | 6.22 |
(3.26,
11.86) |
<0.001 |
| >365 | 38.3 | 9 | 23.47 | (12.21, 45.11) |
8.90 | (4.24, 18.68) |
<0.001 | 9.10 | (4.27, 19.38) |
<0.001 |
|
| ||||||||||
| Age at cART initiation (years) | #0.004 | # 0.010 | ||||||||
| <=30 | 3833.4 | 210 | 5.48 | (4.79, 6.27) | 1 | 1 | ||||
| 31-40 | 5205.7 | 309 | 5.94 | (5.31, 6.64) | 1.13 | (0.94, 1.35) |
0.196 | 1.11 | (0.93, 1.34) |
0.247 |
| 41-50 | 2443.1 | 137 | 5.61 | (4.74, 6.63) | 1.13 | (0.90, 1.41) |
0.287 | 1.12 | (0.89, 1.41) |
0.321 |
| >50 | 913.4 | 74 | 8.10 | (6.45, 10.18) | 1.63 | (1.24, 2.15) |
0.001 | 1.56 |
(1.17,
2.08) |
0.002 |
|
| ||||||||||
| Sex | ||||||||||
| Male | 8397.1 | 541 | 6.44 | (5.92, 7.01) | 1 | 1 | ||||
| Female | 3998.4 | 189 | 4.73 | (4.10, 5.45) | 0.80 | (0.67, 0.95) |
0.011 | 0.74 |
(0.61,
0.89) |
0.002 |
|
| ||||||||||
| Mode of HIV Exposure | 0.015 | 0.009 | ||||||||
| Heterosexual contact | 8060.3 | 450 | 5.58 | (5.09, 6.12) | 1 | 1 | ||||
| Homosexual contact | 2414.1 | 139 | 5.76 | (4.88, 6.80) | 0.82 | (0.63, 1.06) |
0.133 | 0.74 |
(0.56,
0.97) |
0.032 |
| Injecting drug use | 1033.4 | 99 | 9.58 | (7.87, 11.67) | 1.32 | (1.02, 1.72) |
0.037 | 1.27 | (0.96, 1.68) |
0.092 |
| Other/unknown | 887.6 | 42 | 4.73 | (3.50, 6.40) | 0.70 | (0.49, 0.99) |
0.044 | 0.69 |
(0.49,
0.99) |
0.042 |
|
| ||||||||||
| Pre-cART viral load (copies/mL) | ||||||||||
| ≤100000 | 3329.2 | 190 | 5.71 | (4.95, 6.58) | 1 | 1 | ||||
| >100000 | 3281.1 | 169 | 5.15 | (4.43, 5.99) | 0.84 | (0.68, 1.04) |
0.107 | 0.93 | (0.75, 1.16) |
^0.543 |
| Missing | 5785.1 | 371 | 6.41 | (5.79, 7.10) | ||||||
|
| ||||||||||
| Pre-cART CD4 (cells/μL) | #<0.001 | # <0.001 | ||||||||
| <=50 | 3237.0 | 151 | 4.66 | (3.98, 5.47) | 1 | 1 | ||||
| 51-100 | 1604.5 | 62 | 3.86 | (3.01, 4.96) | 0.83 | (0.61, 1.11) |
0.206 | 0.85 | (0.63, 1.15) |
0.298 |
| 101-200 | 2951.1 | 179 | 6.07 | (5.24, 7.02) | 1.34 | (1.07, 1.68) |
0.010 | 1.42 |
(1.14,
1.78) |
0.002 |
| >200 | 3114.2 | 269 | 8.64 | (7.66, 9.73) | 1.60 | (1.30, 1.97) |
<0.001 | 1.76 |
(1.42,
2.18) |
<0.001 |
| Missing | 1488.7 | 69 | 4.64 | (3.66, 5.87) | ||||||
|
| ||||||||||
| Initial cART Regimen | 0.208 | ^0.212 | ||||||||
| NRTI+NNRTI | 11159.1 | 664 | 5.95 | (5.51, 6.42) | 1 | 1 | ||||
| NRTI+PI | 1097.4 | 65 | 5.92 | (4.64, 7.55) | 1.01 | (0.71, 1.44) |
0.935 | 1.01 | (0.71, 1.44) |
0.960 |
| Other | 138.9 | 1 | 0.72 | (0.10, 5.11) | 0.17 | (0.02, 1.22) |
0.078 | 0.17 | (0.02, 1.23) |
0.079 |
|
| ||||||||||
| Hepatitis B co-infection | ||||||||||
| Negative | 9257.5 | 550 | 5.94 | (5.46, 6.46) | 1 | 1 | ||||
| Positive | 1057.3 | 61 | 5.77 | (4.49, 7.42) | 1.05 | (0.80, 1.37) |
0.735 | 1.01 | (0.77, 1.32) |
^0.941 |
| Not tested | 2080.6 | 119 | 5.72 | (4.78, 6.85) | ||||||
|
| ||||||||||
| Hepatitis C co-infection | ||||||||||
| Negative | 7945.9 | 452 | 5.69 | (5.19, 6.24) | 1 | 1 | ||||
| Positive | 1417.3 | 122 | 8.61 | (7.21, 10.28) | 1.31 | (1.04, 1.65) |
0.021 | 1.18 | (0.89, 1.56) |
^0.242 |
| Not tested | 3032.2 | 156 | 5.14 | (4.40, 6.02) | ||||||
| Previous AIDS | ||||||||||
| No | 7852.9 | 478 | 6.09 | (5.57, 6.66) | 1 | 1 | ||||
| Yes | 4542.6 | 252 | 5.55 | (4.90, 6.28) | 0.85 | (0.72, 0.99) |
0.042 | 0.96 | (0.80, 1.14) |
^0.625 |
Non-significant variables did not form part of the final model, but each is presented adjusted for the significant variables.
Time-updated covariate where a patient can contribute to more than one categories.
Not tested/missing values were included as a separate category but excluded from test for heterogeneity or test for trend. Individual HR, 95% CI and p-values were calculated by including not tested/missing values.
Test for trend
P-values in bold represent significant covariates in the final multivariable model.
Abbreviations: TI – treatment interruption, cART - combination antiretroviral therapy, NRTI – nucleoside reverse transcriptase inhibitors, NNRTI – non-nucleoside reverse transcriptase inhibitors, PI – protease inhibitors.
Sensitivity analysis
In the sensitivity analysis (Table 3) where a second confirmatory test for CD4 or VL failure was not required, the total patients who experienced treatment failure increased to 1152/4549 (25.3%), with the failure rate of 10.15 per 100PY. Of 1152 patients 348 had VL failure, 376 had CD4 below baseline values, 415 had CD4 below 100 cells/µL and 159 experienced a clinical failure. The Reasons for Previous TI variable was significant in the multivariable model where resuming cART after an interruption due to other reasons was significantly associated with higher hazard of failure (HR=1.91, 95%CI (1.13-3.22)). Resuming after interruption due to AEs showed a slight increase in hazard compared to the no interruption group, but was not statistically significant (HR=1.05, 95%CI (0.63-1.75)). The duration of previous interruption remained significant in the adjusted model with increasing HR for treatment failure for TIs >30 days (31-180 days HR=1.83, 95%CI (1.01-3.29); 181-365 days HR=3.27, 95%CI (1.63-6.57); and >365 days HR=9.97, 95%CI (4.55-21.86); compared to 0-14 days). There was no interaction between Reasons for Previous TI and TI duration (p=0.750). Female sex (HR=0.68, 95%CI (0.58-0.79)) and homosexual HIV exposure (HR=0.78, 95%CI (0.63-0.96)) showed similar effects to the main analysis shown in Table 2. The CD4 variable, however, now showed a protective effect at all levels compared to CD4 counts ≤50 cells/µL (CD4 51-100 cells/µL HR=0.49, 95%CI (0.39-0.61); CD4 101-200 cells/µL HR=0.60, 95%CI (0.51-0.72); CD4 >200 cells/µL HR=0.69, 95%CI (0.59-0.81)). This is in contrast to results derived from Table 2 where higher CD4 count was associated with higher risk of treatment failure. The reversion of the HR in the CD4 count variable reflects the different definitions used to define our treatment failure endpoint in both analyses. In the main analysis, only 89 of the 730 failures (12.2%) were due to virological failures. However, 348/1152 (30.2%) failures in the sensitivity analysis were due to having VL >1000 copies/mL.
Table 3.
Sensitivity analysis: time to first unconfirmed treatment failure (clinical, immunological and virological failure)
| Univariable | Multivariable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person- years (pys) |
Number with treatment failure |
Crude rate (/100pys) |
95% CI | HR | 95% CI | *p | HR | 95% CI | *p | |
| Total | 11351.5 | 1152 | 10.15 | (9.58, 10.75) | ||||||
|
| ||||||||||
|
~Reasons for Previous Treatment
Interruption |
<0.001 | 0.017 | ||||||||
| No previous TI | 10860.4 | 1057 | 9.73 | (9.16, 10.34) | 1 | 1 | ||||
| Previous TI due to AE | 286.9 | 31 | 10.80 | (7.60, 15.36) | 1.33 | (0.92, 1.91) | 0.125 | 1.05 | (0.63, 1.75) | 0.863 |
| Previous TI due to other reasons | 204.2 | 64 | 31.35 | (24.54, 40.05) | 3.79 | (2.91, 4.94) | <0.001 | 1.91 | (1.13, 3.22) | 0.015 |
|
| ||||||||||
|
~Previous Treatment Interrup-
tion Duration (days) |
#<0.001 | #<0.001 | ||||||||
| 0-14 | 11014.2 | 1076 | 9.77 | (9.20, 10.37) | 1 | 1 | ||||
| 15-30 | 136.8 | 15 | 10.97 | (6.61, 18.19) | 1.34 | (0.80, 2.24) | 0.262 | 0.99 | (0.50, 1.96) | 0.976 |
| 31-180 | 145.9 | 29 | 19.88 | (13.81, 28.61) | 2.47 | (1.70, 3.60) | <0.001 | 1.83 | (1.01, 3.29) | 0.045 |
| 181-365 | 30.4 | 17 | 55.87 | (34.73, 89.87) | 6.51 | (3.97, 10.66) | <0.001 | 3.27 | (1.63, 6.57) | 0.001 |
| >365 | 24.2 | 15 | 62.10 | (37.44, 103) | 17.06 | (9.43, 30.87) | <0.001 | 9.97 | (4.55, 21.86) | <0.001 |
|
| ||||||||||
| Age at cART initiation (years) | #0.075 | ^#0.091 | ||||||||
| <=30 | 3521.2 | 354 | 10.05 | (9.06, 11.16) | 1 | 1 | ||||
| 31-40 | 4761.3 | 479 | 10.06 | (9.20, 11.00) | 1.05 | (0.91, 1.20) | 0.534 | 1.02 | (0.89, 1.18) | 0.769 |
| 41-50 | 2213.7 | 219 | 9.89 | (8.67, 11.29) | 1.08 | (0.90, 1.28) | 0.403 | 1.06 | (0.89, 1.27) | 0.497 |
| >50 | 855.2 | 100 | 11.69 | (9.61, 14.23) | 1.26 | (1.00, 1.59) | 0.049 | 1.27 | (1.00, 1.61) | 0.049 |
|
| ||||||||||
| Sex | ||||||||||
| Male | 7549.8 | 874 | 11.58 | (10.83, 12.37) | 1 | 1 | ||||
| Female | 3801.6 | 278 | 7.31 | (6.50, 8.22) | 0.67 | (0.58, 0.77) | <0.001 | 0.68 | (0.58, 0.79) | <0.001 |
|
| ||||||||||
| Mode of HIV Exposure | 0.001 | 0.013 | ||||||||
| Heterosexual contact | 7422.5 | 694 | 9.35 | (8.68, 10.07) | 1 | 1 | ||||
| Homosexual contact | 2237.4 | 217 | 9.70 | (8.49, 11.08) | 0.88 | (0.72, 1.08) | 0.213 | 0.78 | (0.63, 0.96) | 0.020 |
| Injecting drug use | 903.7 | 169 | 18.70 | (16.08, 21.74) | 1.43 | (1.17, 1.75) | 0.001 | 1.19 | (0.96, 1.47) | 0.108 |
| Other/unknown | 787.9 | 72 | 9.14 | (7.25, 11.51) | 0.84 | (0.64, 1.10) | 0.215 | 0.79 | (0.61, 1.04) | 0.098 |
|
| ||||||||||
| Pre-cART viral load (copies/mL) | ||||||||||
| ≤100000 | 3134.5 | 280 | 8.93 | (7.95, 10.04) | 1 | 1 | ||||
| >100000 | 2943.1 | 312 | 10.60 | (9.49, 11.84) | 1.12 | (0.95, 1.32) | 0.175 | 1.04 | (0.88, 1.24) | ^0.631 |
| Missing | 5273.8 | 560 | 10.62 | (9.77, 11.54) | ||||||
|
| ||||||||||
| Pre-cART CD4 (cells/μL) | #<0.001 | #<0.001 | ||||||||
| <=50 | 2613.4 | 384 | 14.69 | (13.30, 16.24) | 1 | 1 | ||||
| 51-100 | 1507.2 | 102 | 6.77 | (5.57, 8.22) | 0.48 | (0.39, 0.60) | <0.001 | 0.49 | (0.39, 0.61) | <0.001 |
| 101-200 | 2864.3 | 221 | 7.72 | (6.76, 8.80) | 0.56 | (0.48, 0.67) | <0.001 | 0.60 | (0.51, 0.72) | <0.001 |
| >200 | 3013.4 | 309 | 10.25 | (9.17, 11.46) | 0.63 | (0.54, 0.74) | <0.001 | 0.69 | (0.59, 0.81) | <0.001 |
| Missing | 1353.2 | 136 | 10.05 | (8.50, 11.89) | ||||||
|
| ||||||||||
| Initial cART Regimen | 0.070 | ^0.111 | ||||||||
| NRTI+NNRTI | 10180.2 | 1055 | 10.36 | (9.76, 11.01) | 1 | 1 | ||||
| NRTI+PI | 1035.3 | 94 | 9.08 | (7.42, 11.11) | 0.86 | (0.64, 1.14) | 0.291 | 0.91 | (0.68, 1.22) | 0.524 |
| Other | 136.0 | 3 | 2.21 | (0.71, 6.84) | 0.29 | (0.09, 0.91) | 0.033 | 0.30 | (0.10, 0.95) | 0.041 |
|
| ||||||||||
| Hepatitis B co-infection | ||||||||||
| Negative | 8495.0 | 851 | 10.02 | (9.37, 10.71) | 1 | 1 | ||||
| Positive | 945.9 | 107 | 11.31 | (9.36, 13.67) | 1.15 | (0.94, 1.41) | 0.179 | 1.10 | (0.90, 1.35) | ^0.347 |
| Not tested | 1910.6 | 194 | 10.15 | (8.82, 11.69) | ||||||
|
| ||||||||||
| Hepatitis C co-infection | ||||||||||
| Negative | 7309.4 | 697 | 9.54 | (8.85, 10.27) | 1 | 1 | ||||
| Positive | 1262.3 | 195 | 15.45 | (13.42, 17.78) | 1.31 | (1.10, 1.57) | 0.003 | 1.06 | (0.85, 1.32) | ^0.612 |
| Not tested | 2779.8 | 260 | 9.35 | (8.28, 10.56) | ||||||
|
| ||||||||||
| Previous AIDS | ||||||||||
| No | 7379.3 | 687 | 9.31 | (8.64, 10.03) | 1 | 1 | ||||
| Yes | 3972.2 | 465 | 11.71 | (10.69, 12.82) | 1.16 | (1.02, 1.31) | 0.021 | 0.95 | (0.83, 1.10) | ^0.515 |
Non-significant variables did not form part of the final model, but each is presented adjusted for the significant variables.
Time-updated covariate where a patient can contribute to more than one categories.
Not tested/missing values were included as a separate category but excluded from test for heterogeneity or test for trend. Individual HR, 95% CI and p-values were calculated by including not tested/missing values.
Test for trend
P-values in bold represent significant covariates in the final multivariable model.
Abbreviations: TI – treatment interruption, cART - combination antiretroviral therapy, NRTI – nucleoside reverse transcriptase inhibitors, NNRTI – non-nucleoside reverse transcriptase inhibitors, PI – protease inhibitors.
Discussion
Our results show that the overall proportion of TAHOD patients having TI after cART initiation was reasonably low at 5.45%, with reasons for interruptions varying greatly from common clinical adverse reactions such as rash and liver toxicities, to interruptions recorded as being due to patient decision/request. Longer time off treatment was associated with treatment failure. TIs caused by AEs remained non-significant in both the main and sensitivity analyses, while patients with non-AE related TIs were almost twice as likely to fail compared to those with no previous TI in the sensitivity analysis.
Studies conducted in the post-SMART era have shown proportions of TI ranging from 12.8% to 53.4%, with TI defined as periods of no cART from 2 to 90 days (26, 31, 32). TI in our study was defined as no cART for >1 day, however we have combined those with TI <14 days to enable comparison with longer TI durations. Our study reports 5.45% of patients with at least one TI. Given the minimal definition of TI used in this study, this proportion is considered to be relatively low compared to other studies. We hypothesise that this may reflect the observational nature of the cohort where some TI episodes may have been missed, and the patient sampling in our TAHOD sites where clinicians are encouraged to enrol patients who are likely to remain in follow-up and adhere to treatment programs.
The results from our study show that TI >30 days increased the chances of treatment failure after cART resumption. These findings are consistent with other studies reporting an increase in virologic rebound with longer intervals of TI (4, 33). Reasons for Previous TI was not significant in the main analysis, however the sensitivity model suggests the risk of treatment failure was higher after interruption from non-AE-related causes, while interruption due to AEs only showed a ~5% increase in the hazard for failure. These associations were similar to those observed in a Spanish study (34), which categorised reasons for TI as (i) due to physician’s advice in response to AEs or toxicities and (ii) due to the patient’s own choice (14). Compared to the no interruption group, the HR for detectable VL for group (i) was 1.36 (p=0.13). The HR for group (ii) was 3.62 (p<0.0001). The findings of the Spanish study convey a similar message to ours in that interruption due to AEs may be a less undesirable consequence than interruption caused by other reasons. A possible explanation to these findings could be that patients experiencing this type of TI are largely managed under medical care and clinicians may switch antiretrovirals in response to AEs thus allowing patients to achieve optimal treatment outcomes once cART has been resumed. Our study has further shown that the short duration of AE-related TI could be another contributing factor. Because TI >30 days showed significant associations in both the main and sensitivity analyses while shorter TIs did not, we believe that the length of TI rather than their reasons was the key factor affecting treatment response.
Age, sex and mode of HIV transmission were shown to be significant predictors of treatment failure, which is consistent with previous studies (15, 35, 36). An interesting phenomenon was observed between the association of CD4 count and treatment failure. By requiring a secondary confirmed VL or CD4 test, the results showed higher CD4 count being associated with treatment failure. In this analysis, a high proportion of treatment failures were attributed to immunological failure. This is because VL testing in resource-limited settings, which includes many TAHOD sites, is recommended every 12 months (20, 37). Therefore, it is most likely that many virological failures would have been missed due to the long lag time between the first and second consecutive VL test. The association seen here is therefore a reflection of a higher proportion of patients who initiated cART with high CD4 count having at some point a CD4 count dropping below their initial pre-treatment level as maintaining CD4 count above the already high baseline level may be difficult to achieve compared to those who started with lower CD4 count. This is supported by a previous TAHOD study which showed that higher CD4 count prior to cART was associated with smaller increases in CD4 count (38). In the sensitivity analysis, however, a second confirmatory testing was not required. The number of virological failures in this sensitivity analysis was increased which resulted in high CD4 count having a protective effect over treatment failure consistent with previous studies (39-41).
This study has several limitations including the lack of HIV drug resistance information after periods of TI. It is known that TI can lead to the development of HIV drug resistance mutations, especially NNRTI-related mutations as drugs belonging to this particular class tend to have longer half-life than other HIV drug classes. It has been shown that NNRTI mutations can occur in high proportions after cART interruption, which could ultimately lead to virological failure after resumption of therapy (4, 42-44). A recent Thai study has shown that the incidence of virological failure and resistance mutations was higher, but not statistically significant, in patients who had treatment interruption of all drugs in a nevirapine-based regimen, than in pateints who continued with NRTIs after discontinuation of nevirapine . As the majority of TAHOD patients initiated on an NNRTI-based regimen, the risk for treatment failure may have been confounded by the development of drug resistance mutations which could not be adjusted for in our study. Another limitation included the free text field used to record stop reasons. It was not possible to completely classify all reasons, and therefore misclassification of the TI variable may have occurred. Immune reconstitution syndrome (IRIS) was not taken into consideration in our clinical failure definition as the information has not been reported consistently in our cohort. Lastly, we did not adjust for LTFU patients in the analyses. However, a previous TAHOD study found no association between LTFU and HIV disease progression indicators suggesting that these patients who are LTFU are not substantially different from patients remaining in care (16).
Conclusions
TIs in our regional cohort were relatively uncommon, with interruptions due to AEs being shorter than TIs due to other reasons. Length of interruption was the key factor in associations with treatment failure and the lack of an association between AE-related interruptions and treatment failure could be due to their shorter time span. These findings should not be interpreted as advocating short term TIs. However, if TI is unavoidable, in situations such as when a patient experiences a drug reaction, the length of time cART is suspended should be minimised.
Acknowledgements
The TREAT Asia HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS. TREAT Asia is also supported by ViiV Healthcare. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia (The University of New South Wales). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Appendix 1. The TREAT Asia HIV Observational Database
| CV Mean, V Saphonn* and V Khol, National Center for HIV/AIDS, Dermatology & STDs, and University of Health Sciences, Phnom Penh, Cambodia; |
| FJ Zhang*, HX Zhao and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China; |
| MP Lee* ‡, PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong, China; |
| N Kumarasamy*, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India; |
| S Pujari*, K Joshi, S Gaikwad and A Chitalikar, Institute of Infectious Diseases, Pune, India; |
| TP Merati*
†, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia; |
| E Yunihastuti*, D Imran and A Widhani, Working Group on AIDS Faculty of Medicine, University of Indone- sia/ Cipto Mangunkusumo Hospital, Jakarta, Indonesia; |
| S Oka*, J Tanuma and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan; |
| JY Choi*, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei Univer- sity College of Medicine, Seoul, South Korea; |
| BLH Sim*, YM Gani and R David, Hospital Sungai Buloh, Sungai Buloh, Malaysia; |
| A Kamarulzaman*, SF Syed Omar, S Ponnampalavanar and I Azwa, University Malaya Medical Centre, Kuala Lumpur, Malaysia; |
| M Mustafa and N Nordin, Hospital Raja Perempuan Zainab II, Kota Bharu, Malaysia; |
| R Ditangco*, E Uy and R Bantique, Research Institute for Tropical Medicine, Manila, Philippines; |
| WW Wong*, WW Ku and PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan; |
| OT Ng*, PL Lim, LS Lee and PS Ohnmar, Tan Tock Seng Hospital, Singapore; |
| P Phanuphak*, K Ruxrungtham, A Avihingsanon and P Chusut, HIV-NAT/Thai Red Cross AIDS Research Cen- tre, Bangkok, Thailand; |
| S Kiertiburanakul*, S Sungkanuparph, L Chumla and N Sanmeema, Faculty of Medicine Ramathibodi Hospi- tal, Mahidol University, Bangkok, Thailand; |
| R Chaiwarith*, T Sirisanthana, W Kotarathititum and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai, Thailand; |
| P Kantipong* and P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; |
| W Ratanasuwan* and R Sriondee, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thai- land; |
| KV Nguyen*, VH Bui, THD Nguyen and TD Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam; |
| TT Pham*, DD Cuong and HL Ha, Bach Mai Hospital, Hanoi, Vietnam; |
| AH Sohn*, N Durier* and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand; |
| DA Cooper, MG Law*, A Jiamsakul* and DC Boettiger, The Kirby Institute, UNSW Australia, Sydney, Austral- ia. |
Steering Committee member;
Steering Committee Chair;
co-Chair
References
- 1.Strategies for Management of Antiretroviral Therapy Study G. El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 2.Sungkanuparph S, Kiertiburanakul S, Apisarnthanarak A, Malathum K, Watcharananan S, Sathapatayavongs B. Rapid CD4 decline after interruption of non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy in a resource-limited setting. AIDS Res Ther. 2007;4:26. doi: 10.1186/1742-6405-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holkmann Olsen C, Mocroft A, Kirk O, Vella S, Blaxhult A, Clumeck N, et al. Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med. 2007;8(2):96–104. doi: 10.1111/j.1468-1293.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 4.Parienti JJ, Das-Douglas M, Massari V, Guzman D, Deeks SG, Verdon R, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3(7):e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdissa SG, Fekade D, Feleke Y, Seboxa T, Diro E. Adverse drug reactions associated with antiretroviral treatment among adult Ethiopian patients in a tertiary hospital. Ethiopian medical journal. 2012;50(2):107–13. [PubMed] [Google Scholar]
- 6.Nuesch R, Srasuebkul P, Ananworanich J, Ruxrungtham K, Phanuphak P, Duncombe C. Monitoring the toxicity of antiretroviral therapy in resource limited settings: a prospective clinical trial cohort in Thailand. J Antimicrob Chemother. 2006;58(3):637–44. doi: 10.1093/jac/dkl313. [DOI] [PubMed] [Google Scholar]
- 7.Kumarasamy N, Venkatesh KK, Cecelia AJ, Devaleenal B, Lai AR, Saghayam S, et al. Spectrum of adverse events after generic HAART in southern Indian HIV-infected patients. AIDS patient care and STDs. 2008;224:337–44. doi: 10.1089/apc.2007.0093. [DOI] [PubMed] [Google Scholar]
- 8.Shet A, Antony J, Arumugam K, Kumar Dodderi S, Rodrigues R, DeCosta A. Influence of adverse drug reactions on treatment success: prospective cohort analysis of HIV-infected individuals initiating first-line antiretroviral therapy in India. PLoS One. 2014;9(3):e91028. doi: 10.1371/journal.pone.0091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eluwa GI, Badru T, Agu KA, Akpoigbe KJ, Chabikuli O, Hamelmann C. Adverse drug reactions to antiretroviral therapy (ARVs): incidence, type and risk factors in Nigeria. BMC clinical pharmacology. 2012;12:7. doi: 10.1186/1472-6904-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumarasamy N, Vallabhaneni S, Cecelia AJ, Yepthomi T, Balakrishnan P, Saghayam S, et al. Reasons for modification of generic highly active antiretroviral therapeutic regimens among patients in southern India. J Acquir Immune Defic Syndr. 2006;41(1):53–8. doi: 10.1097/01.qai.0000188123.15493.43. [DOI] [PubMed] [Google Scholar]
- 11.Cicconi P, Cozzi-Lepri A, Castagna A, Trecarichi EM, Antinori A, Gatti F, et al. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naive patients. HIV Med. 2010;11(2):104–13. doi: 10.1111/j.1468-1293.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- 12.Guy R, Wand H, McManus H, Vonthanak S, Woolley I, Honda M, et al. Antiretroviral treatment interruption and loss to follow-up in two HIV cohorts in Australia and Asia: implications for 'test and treat' prevention strategy. AIDS patient care and STDs. 2013;27(12):681–91. doi: 10.1089/apc.2012.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore DM, Zhang W, Yip B, Genebat M, Lima VD, Montaner JS, et al. Non-medically supervised treatment interruptions among participants in a universally accessible antiretroviral therapy programme. HIV Med. 2010;11(5):299–307. doi: 10.1111/j.1468-1293.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 14.Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Tropical medicine & international health : TM & IH. 2011;16(10):1297–313. doi: 10.1111/j.1365-3156.2011.02828.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38(2):174–9. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Tanuma J, Chaiwarith R, Lee CK, Law MG, Kumarasamy N, et al. Loss to Followup in HIV-Infected Patients from Asia-Pacific Region: Results from TAHOD. AIDS research and treatment. 2012;2012:375217. doi: 10.1155/2012/375217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiertiburanakul S, Boettiger D, Lee MP, Omar SF, Tanuma J, Ng OT, et al. Trends of CD4 cell count levels at the initiation of antiretroviral therapy over time and factors associated with late initiation of antiretroviral therapy among Asian HIV-positive patients. J Int AIDS Soc. 2014;17:18804. doi: 10.7448/IAS.17.1.18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyomopito R, Lee MP, Phanuphak P, Lim PL, Ditangco R, Zhou J, et al. Measures of site resourcing predict virologic suppression, immunologic response and HIV disease progression following highly active antiretroviral therapy (HAART) in the TREAT Asia HIV Observational Database (TAHOD) HIV Med. 2010;11(8):519–29. doi: 10.1111/j.1468-1293.2010.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass TR, De Geest S, Weber R, Vernazza PL, Rickenbach M, Furrer H, et al. Correlates of self-reported nonadherence to antiretroviral therapy in HIV-infected patients: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2006;41(3):385–92. doi: 10.1097/01.qai.0000186371.95301.52. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treatment and preventing HIV infection: Recommendations for a public health approach June 2013. [cited 2014 January 8]. Available from: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf.
- 21.Touloumi G, Pantazis N, Antoniou A, Stirnadel HA, Walker SA, Porter K, et al. Highly active antiretroviral therapy interruption: predictors and virological and immunologic consequences. J Acquir Immune Defic Syndr. 2006;42(5):554–61. doi: 10.1097/01.qai.0000230321.85911.db. [DOI] [PubMed] [Google Scholar]
- 22.Bansi LK, Benzie AA, Phillips AN, Portsmouth S, Hill T, Leen C, et al. Are previous treatment interruptions associated with higher viral rebound rates in patients with viral suppression? AIDS. 2008;22(3):349–56. doi: 10.1097/QAD.0b013e3282f4709a. [DOI] [PubMed] [Google Scholar]
- 23.Srasuebkul P, Calmy A, Zhou J, Kumarasamy N, Law M, Lim PL, et al. Impact of drug classes and treatment availability on the rate of antiretroviral treatment change in the TREAT Asia HIV Observational Database (TAHOD) AIDS Res Ther. 2007;4:18. doi: 10.1186/1742-6405-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, Kityo C, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21(8):965–71. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Moreno JA, Fumaz CR, Prats A, Ferrer MJ, Negredo E, Perez-Alvarez N, et al. Interruptions of antiretroviral therapy in human immunodeficiency virus infection: are they detrimental to neurocognitive functioning? Journal of neurovirology. 2010;16(3):208–18. doi: 10.3109/13550281003767710. [DOI] [PubMed] [Google Scholar]
- 26.Pasquet A, Messou E, Gabillard D, Minga A, Depoulosky A, Deuffic-Burban S, et al. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan, Cote d'Ivoire. PLoS One. 2010;5(10):e13414. doi: 10.1371/journal.pone.0013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallecillo G, Mojal S, Roquer A, Samos P, Luque S, Martinez D, et al. Low Non-structured Antiretroviral Therapy Interruptions in HIV-Infected Persons Who Inject Drugs Receiving Multidisciplinary Comprehensive HIV Care at an Outpatient Drug Abuse Treatment Center. AIDS and behavior. 2015 doi: 10.1007/s10461-015-1211-y. [DOI] [PubMed] [Google Scholar]
- 28.Bedimo R, Chen RY, Westfall AO, Raper JL, Allison JJ, Saag MS. Sustained HIV viral suppression following treatment interruption: an observational study. AIDS Res Hum Retroviruses. 2006;22(1):40–4. doi: 10.1089/aid.2006.22.40. [DOI] [PubMed] [Google Scholar]
- 29.Kavasery R, Galai N, Astemborski J, Lucas GM, Celentano DD, Kirk GD, et al. Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr. 2009;50(4):360–6. doi: 10.1097/QAI.0b013e318198a800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nacher M, El Guedj M, Vaz T, Nasser V, Randrianjohany A, Alvarez F, et al. Risk factors for follow-up interruption of HIV patients in French Guiana. The American journal of tropical medicine and hygiene. 2006;74(5):915–7. [PubMed] [Google Scholar]
- 31.Samji H, Chen Y, Salters K, Montaner JS, Hogg RS. Correlates of Unstructured Antiretroviral Treatment Interruption in a Cohort of HIV-Positive Individuals in British Columbia. AIDS and behavior. 2014 doi: 10.1007/s10461-014-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcellin F, Boyer S, Protopopescu C, Dia A, Ongolo-Zogo P, Koulla-Shiro S, et al. Determinants of unplanned antiretroviral treatment interruptions among people living with HIV in Yaounde, Cameroon (EVAL survey, ANRS 12-116) Tropical medicine & international health : TM & IH. 2008;13(12):1470–8. doi: 10.1111/j.1365-3156.2008.02170.x. [DOI] [PubMed] [Google Scholar]
- 33.Genberg BL, Wilson IB, Bangsberg DR, Arnsten J, Goggin K, Remien RH, et al. Patterns of antiretroviral therapy adherence and impact on HIV RNA among patients in North America. AIDS. 2012;26(11):1415–23. doi: 10.1097/QAD.0b013e328354bed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knobel H, Urbina O, Gonzalez A, Sorli ML, Montero M, Carmona A, et al. Impact of different patterns of nonadherence on the outcome of highly active antiretroviral therapy in patients with long-term follow-up. HIV Med. 2009;10(6):364–9. doi: 10.1111/j.1468-1293.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- 35.Crawford K, Wakabi S, Magala F, Kibuuka H, Liu M, Hamm T. Evaluation of treatment outcomes for patients on first-line regimens in US President's Emergency Plan for AIDS Relief (PEPFAR) clinics in Uganda: predictors of virological and immunological response from RV288 analyses. HIV Med. 2014 doi: 10.1111/hiv.12177. [DOI] [PubMed] [Google Scholar]
- 36.Marconi VC, Wu B, Hampton J, Ordonez CE, Johnson BA, Singh D, et al. Early warning indicators for first-line virologic failure independent of adherence measures in a South African urban clinic. AIDS patient care and STDs. 2013;27(12):657–68. doi: 10.1089/apc.2013.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowley CF. Developments in CD4 and viral load monitoring in resource-limited settings. Clin Infect Dis. 2014;58(3):407–12. doi: 10.1093/cid/cit733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oyomopito R, Lee MP, Phanuphak P, Lim PL, Ditangco R, Zhou J, et al. Measures of site resourcing predict virologic suppression, immunologic response and HIV disease progression following highly active antiretroviral therapy (HAART) in the TREAT Asia HIV Observational Database (TAHOD) HIV Med. 2010;11(8):519–29. doi: 10.1111/j.1468-1293.2010.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phanuphak P, Sirivichayakul S, Jiamsakul A, Sungkanuparph S, Kumarasamy N, Lee MP, et al. Transmitted Drug Resistance and Antiretroviral Treatment Outcomes in Non-Subtype B HIV-1-Infected Patients in South East Asia. J Acquir Immune Defic Syndr. 2014;66(1):74–9. doi: 10.1097/QAI.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, He C, Hsi JH, Xu X, Liu Y, He J, et al. Virological outcomes and drug resistance in Chinese patients after 12 months of 3TC-based first-line antiretroviral treatment, 2011-2012. PLoS One. 2014;9(2):e88305. doi: 10.1371/journal.pone.0088305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran DA, Wilson DP, Shakeshaft A, Ngo AD, Doran C, Zhang L. Determinants of virological failure after 1 year's antiretroviral therapy in Vietnamese people with HIV: findings from a retrospective cohort of 13 outpatient clinics in six provinces. Sexually transmitted infections. 2014 doi: 10.1136/sextrans-2013-051353. [DOI] [PubMed] [Google Scholar]
- 42.Fox Z, Phillips A, Cohen C, Neuhaus J, Baxter J, Emery S, et al. Viral resuppression and detection of drug resistance following interruption of a suppressive non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS. 2008;22(17):2279–89. doi: 10.1097/QAD.0b013e328311d16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luebbert J, Tweya H, Phiri S, Chaweza T, Mwafilaso J, Hosseinipour MC, et al. Virological failure and drug resistance in patients on antiretroviral therapy after treatment interruption in Lilongwe, Malawi. Clin Infect Dis. 2012;55(3):441–8. doi: 10.1093/cid/cis438. [DOI] [PubMed] [Google Scholar]
- 44.Graham SM, Jalalian-Lechak Z, Shafi J, Chohan V, Deya RW, Jaoko W, et al. Antiretroviral treatment interruptions predict female genital shedding of genotypically resistant HIV-1 RNA. J Acquir Immune Defic Syndr. 2012;60(5):511–8. doi: 10.1097/QAI.0b013e31825bd703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siripassorn K, Manosuthi W, Pakdee A, Natprom S, Chaovavanich A, Hengphadpanadamrong N, et al. Virological failure of staggered and simultaneous treatment interruption in HIV patients who began Efavirenz-based regimens after allergic reactions to nevirapine. AIDS Res Ther. 2013;10(1):4. doi: 10.1186/1742-6405-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]