Abstract
The impact of alpha linolenic acid (ALA), EPA, and DHA on obesity and metabolic complications was studied in mice fed a high-fat, high-sucrose (HF) diet. HF diets were supplemented with ALA, EPA, or DHA (1% w/w) and given to C57BL/6J mice for 16 weeks and to Ob/Ob mice for 6 weeks. In C57BL/6J mice, EPA reduced plasma cholesterol (−20%), limited fat mass accumulation (−23%) and adipose cell hypertrophy (−50%), and reduced plasma leptin concentration (−60%) compared with HF-fed mice. Furthermore, mice supplemented with EPA exhibited a higher insulin sensitivity (+24%) and glucose tolerance (+20%) compared with HF-fed mice. Similar effects were observed in EPA-supplemented Ob/Ob mice, although fat mass accumulation was not prevented. By contrast, in comparison with HF-fed mice, DHA did not prevent fat mass accumulation, increased plasma leptin concentration (+128%) in C57BL/6J mice, and did not improve glucose homeostasis in C57BL/6J and Ob/Ob mice. In 3T3-L1 adipocytes, DHA stimulated leptin expression whereas EPA induced adiponectin expression, suggesting that improved leptin/adiponectin balance may contribute to the protective effect of EPA. In conclusion, supplementation with EPA, but not ALA and DHA, could preserve glucose homeostasis in an obesogenic environment and limit fat mass accumulation in the early stage of weight gain.
Keywords: omega-3 polyunsaturated fatty acid, insulin resistance, obesity, adipose tissue, eicosapentaenoic acid
Obesity is a complex disorder involving an excessive amount of body fat. It has several health consequences, which are frequently linked to metabolic syndrome (MetS). Metabolic syndrome (MetS) is defined as a cluster of several risk factors for type 2 diabetes and cardiovascular diseases (1, 2). MetS is closely related to the progression of insulin resistance (IR) and the accumulation of fat mass, notably in the visceral area (2). Thus, alteration in the cross-talk between key metabolic tissues, such as the liver, adipose tissue (AT), skeletal muscle, and intrinsic dysfunctions in these organs probably represent a key step in the progression of MetS (3, 4). The impairment of lipid storage capacity in subcutaneous AT has been related to the alteration of the endocrine function of the tissue, the increased free FA release in the circulation, visceral fat accumulation (5), and the deposition of ectopic fat in other organs contributing to IR (6). Lifestyle changes are recommended for the prevention and the management of MetS. Several reports from the literature have suggested that metabolic abnormalities and alterations of AT biology could be prevented by increasing the intake of n-3 PUFAs (7). n-3 PUFAs, especially those of marine origin (i.e., C20:5n-3, EPA and C22:6n-3, DHA), were identified as potent positive regulators of insulin sensitivity in vitro (8) and in animal models (9). However, epidemiological studies aiming at determining the beneficial effects of those n-3 PUFAs and their common precursor alpha linolenic acid (C18:3n-3, ALA), led to inconclusive results (10). This may be mainly due to the fact that different experimental protocols were used for these studies. Furthermore, most of the interventional studies in humans or animal models examined the impact of a mixture of EPA and DHA on IR (11, 12), and the impact of ALA has been poorly investigated. Finally, the specific roles of each n-3 PUFA on IR during obesity still remain poorly known because a mixture of EPA and DHA was generally used to study the effects of n-3 PUFAs on adiposity and MetS. Overall, nutritional supplementations with EPA and DHA had a beneficial effect on adiposity and fat cell production of adipokines or FA metabolites (13–15). Only one study examined the impact of EPA-, ALA-, or DHA-enriched oils in rats fed with a high-fat, high-carbohydrate diet for 8 weeks (16). This study suggested that EPA and DHA, but not ALA, could partially protect animals from whole body IR and limit abdominal adiposity, but without any exploration in AT. Therefore, the present study aimed at analyzing the specific effects of supplementing the diet with pure ALA, EPA, or DHA preparations on AT biology and whole body and tissue IR in a murine model of diet-induced obesity.
MATERIALS AND METHODS
Chemicals
Diets.
Diet preparations were purchased as powder form from Brogaarden (Denmark). Free FAs were used for dietary supplementation; ALA and DHA were purchased from Nu-Chek-Prep Inc. (MN), and EPA and a mixture of oleic acid, palmitic acid, stearic acid, and linoleic acid (50:25:15:10) were purchased from Larodan (Malmö, Sweden). Primary antibodies were obtained from Cell Signaling Technology (Leiden, Netherlands) and Sigma Aldrich (Saint-Quentin Fallavier, France). Secondary antibodies were from Bethyl Laboratories (Montgomery, TX); ECL and PierceTM BCA protein assay kit were purchased from Thermo Scientific (Villebon sur Yvette, France). DMEM was from Sigma Aldrich (Saint-Quentin Fallavier, France). Calf and FBS, FA-free BSA, PBS, and penicillin/streptomycin mix were from PAA (Velizy-Villacoublay, France). For cell culture experiments, ALA, EPA, and DHA (catalog numbers 90210, 90110, and 90310, respectively) were from Cayman Chemicals (Ann Arbor, MI). The [1-14C] palmitate (catalog number NEC075H001MC) was from Perkin Elmer (Courtaboeuf, France). When not specified, chemicals were from Sigma Aldrich.
Animals
Five-week-old WT male C57BL/6J mice and Ob/Ob male mice were obtained from Janvier Laboratories (Le Genest Saint Isle, France). WT and Ob/Ob mice were housed four/five and one per cage, respectively, in a room maintained at 22°C–24°C with an alternating 12 h light/dark cycle with free access to food and water. Mice were weighed, and food consumption was measured every 2 weeks. Body composition of WT mice was measured during the 16th week of dietary experiment and during the 6th week for Ob/Ob mice, using EchoMRITM (EchoMRI®, Houston, TX). All protocols followed animal care guidelines of the European Union and were approved by the local research ethics committee (CEMEAA, CE91-12 and 00845.02).
Diets
After 1 week of acclimation, five groups of WT mice and Ob/Ob mice of similar body weight (BW) were randomly constituted. Animals were then assigned to receive five diets as follows: 1) CTRL, low-fat (LF) diet (Research Diet D12450H); 2) HF, high-fat, high-sucrose (HF) diet (Research diet D12451, providing 45 kcal% and 17 kcal% from fat and sucrose, respectively) supplemented with 1% w/w of an FA mixture of the four main FAs in plasma triglycerides (oleic acid, palmitic acid, stearic acid, and linoleic acid; 50:25:15:10%, respectively); 3) HF-A, HF diet supplemented with 1% w/w ALA; 4) HF-E, HF diet supplemented with 1% w/w EPA; 5) HF-D, HF diet supplemented with 1% w/w DHA. Each FA and FA mix were dissolved in a minimal volume of high oleic sunflower oil before incorporation in diet powder. Diets were stored at −20°C and provided fresh every 2 days for 16 weeks (WT mice) or 6 weeks (Ob/Ob mice). Food intake per cage was evaluated every 2 weeks, averaged, and expressed as grams per mouse per day or kilojoules per mouse per day.
Indirect calorimetry
Dioxygen consumption (VO2), carbon dioxide production (VCO2), and activity of mice were measured during 24 h using a four-cage TSE System Pheno-Master/LabMaster (Bad Homburg, Germany). Energy expenditure was calculated using Weir’s equation (17). The respiratory quotient (RQ) was calculated as the ratio of VCO2 to VO2. Spontaneous activity was measured using a three dimensions meshing of light beams. Ambient temperature was maintained at 22°C, the light was on from 8 AM to 8 PM, and mice had free access to food and water. Data were collected after 24 h of acclimation, and the O2 and CO2 analysers were calibrated before each measurement period.
Intraperitoneal insulin and glucose tolerance tests
Insulin tolerance test (ITT) and glucose tolerance test (GTT) were performed on 10 WT mice per group during the 16th week of diet. All Ob/Ob mice were submitted to ITT and GTT, with a 3 day recovery between them. After 6 h of fasting, animals received an intraperitoneal injection of insulin (1.2 mIU/g) or glucose (2 mg/g for WT and 1.5 mg/g for Ob/Ob mice), and blood samples were collected from the tail vein 0, 15, 30, 45, 60, and 120 min later. Blood glucose levels were determined using a commercial glucometer (One Touch®Vita®, Issy les Moulineaux, France) for the calculation of the area under the curve.
Euthanizing and sampling
Sixteen-hour fasted mice were anesthetized by intraperitoneal administration of ketamine-xylazine mix (20 mg/kg:4 mg/kg). To explore insulin sensitivity, eight WT mice per group and all Ob/Ob mice were subjected to insulin injection (1.2 mIU/g) 30 min before anesthesia. Cardiac puncture was performed to collect blood samples, and cervical dislocation was then done to euthanize mice. Blood was sampled in EDTA-coated tubes to avoid coagulation and was processed for plasma collection. Red blood cells (RBCs) were separately collected from WT mice for FA composition analyses. Blood samples, heart, epididymal white adipose tissue (eWAT), gastrocnemius, and quadriceps were removed, weighed, frozen in liquid nitrogen, and stored at −80°C until analyses.
Lipid profiles
Lipid extracts from RBCs or other tissues were prepared using 4 ml chloroform-methanol (2:1, v/v; Sigma Aldrich) and 1 ml 0.9% NaCl. Extracts were centrifuged to separate lipid phase to aqueous phase. Methylation was then performed before FA methyl ester separation by GC as previously described (18).
Quantification of protein content by Western blot
One hundred to 150 mg of gastrocnemius, liver, or eWAT were ground three times in a mini-bead beater in the presence of 0.7 ml lysis buffer (50 mM HEPES, 150 mM sodium chloride, 10 mM EDTA, 10 mM sodium pyrophosphate tetrabasic anhydrous, 25 mM β-glycerophosphate, 100 mM sodium fluoride, 10% glycerol anhydrous) supplemented with phosphatase inhibitors cocktail (Sigma Aldrich) respecting 2 min timeout between each session. Successive centrifugations were done to collect supernatant. Protein quantification was performed using a BCA protein assay kit (Pierce). BSA standard curve and sample preparation and analysis were realized according to the manufacturer’s instructions. For protein immunoblotting, 20 µg of proteins loaded for separation by SDS-PAGE electrophoresis. Proteins were transferred on polyvinylidene difluoride membranes. These membranes were then immunoblotted with the appropriate antibody to detect GAPDH, serine 473 phosphorylated Akt (also called protein kinase B), and total Akt. Antibody binding was detected using HRP-conjugated secondary antibodies and ECL Western blotting substrate (Thermo Scientific). Immunoblots were visualized by chemiluminescence imaging system (MF ChemiBIS 2020; DNR Bio-Imaging Systems, Jerusalem, Israel) and quantified using MultiGauge V3.2 software.
ELISA
ELISA on plasma samples were performed according to the manufacturer’s instructions (BioRad, Marnes-la-Coquette, France). Leptin, insulin, monocyte chemoattractant protein-1 (MCP-1), resistin, and hormones of appetite [ghrelin, glucagon-like peptide-1 (GLP-1), gastric inhibitory polypeptide (GIP), and glucagon] were quantified using the Luminex® technology (Bioplex® 200, BioRad). Adiponectin was quantified using an ELISA assay (Eurobio, Courtaboeuf, France).
Glucose level and lipid profile in plasma
Plasma levels of glucose, nonesterified FA, glycerol, triglyceride, and total cholesterol were measured using KonelabTM 20 analyzer (Thermo Electron SA, Cergy-Pontoise, France), according to the manufacturer’s instruction of each assay.
Gene expression
For tissue or cell culture gene expression assays, RNA extraction was performed using TRIzol® (Thermo Scientific) according to the manufacturer’s instructions. Chloroform was added (0.2 ml/ml of TRIzol®), and samples were mixed and centrifuged for 15 min at 12,000 g and 4°C. Aqueous phase containing RNA was collected, mixed with isopropanol to precipitate RNA, and centrifuged (12,000 g, 4°C, 15 min). After centrifugation, the pellet was washed with ethanol 70% (v/v), dried, and suspended in water. RNA quantification and integrity were verified by measuring the ratio of optical density at 260 nm and 280 nm and by agarose gel migration, respectively. Two micrograms of total RNA was used to realize reverse transcription. The products of reverse transcription were used for reverse transcription quantitative polymerase chain reaction (RT-qPCR) to evaluate gene expression. TaqMan low density array was used for liver and skeletal muscle samples using 384-well format plates on a 7900HT Fast Real Time PCR system (Applied Biosystems). AT gene expression was performed using specific primers (sequences available on request) and Rotor-Gene SYBR Green PCR master mix on a Rotor-Gene Q system (Qiagen, Courtaboeuf, France). mRNA quantification was assayed using the ddCT method. Hypoxanthine guanine phosphoribosyltransferase (Hprt) or non-POU-domain-containing octamer binding protein (NoNo) gene were used as the housekeeping gene in the liver/skeletal muscle and AT, respectively.
DNA extraction and cellularity
DNA was extracted using TRIzol® after separation of aqueous phase containing RNA and organic phase. Five hundred microliters of back extraction buffer (guanidine thiocyanate 4 M, sodium citrate 50 mM, and Tris base 1 M) were added to the organic phase before centrifugation at 16,000 g for 30 min. Superior phase was then used for DNA precipitation by addition 400 µl of isopropanol (Sigma Aldrich) and centrifugation at 16,000 g for 15 min. DNA pellet was then washed twice with ethanol 75% (v/v) and dissolved in DNA RNA-free water. DNA content was quantified by measuring the ratio of optical density at 260 nm and 280 nm. Ratio between total DNA content and tissue sample weight was used to calculate cellularity, as DNA content is proportional to the number of cells.
Adipocyte cell culture
3T3-L1 cells were purchased from ATCC (LGC Standards, Molsheim, France) and grown in DMEM supplemented with 10% calf serum and 100 U/ml penicillin and 100 mg/ml streptomycin in 5% CO2/humidified atmosphere at 37°C. Differentiation to adipocytes was induced 2 days post confluency (day 0) by incubating the cells in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 1 µM dexamethasone, and 10 µg/ml insulin for 48 h. Cells were maintained in the same medium without IBMX and dexamethasone for an additional 48 h. Insulin was removed, and cells were maintained until day 8 (medium was replaced at day 6). FAs were added to cell culture medium at 50 µM from days 0 to 8. Stock solutions of FAs were prepared in ethanol and further diluted at 1:1,000 in DMEM containing 2% of FA-free BSA. Control cells were also exposed to 0.1% ethanol. Cells were harvested every 2 days from day 0 (undifferentiated-untreated and confluent cells) up to day 8. Before RNA isolation and purification, cells were washed in ice-cold PBS and harvested by scrapping in TRIzol®.
Statistical analysis
All data are presented as mean ± SEM. One-way ANOVA (ANOVA) was used to compare each treatment. If significant (P < 0.05), one-way ANOVA was followed by Fisher’s least significant difference post hoc test with the Benjamini-Hochberg multiple testing correction. All statistical analyses were performed using R (Bioconductor). In some cases, a Student’s t-test was performed.
RESULTS
EPA reduces fat mass accumulation in C57BL/6J mice after an HF diet challenge
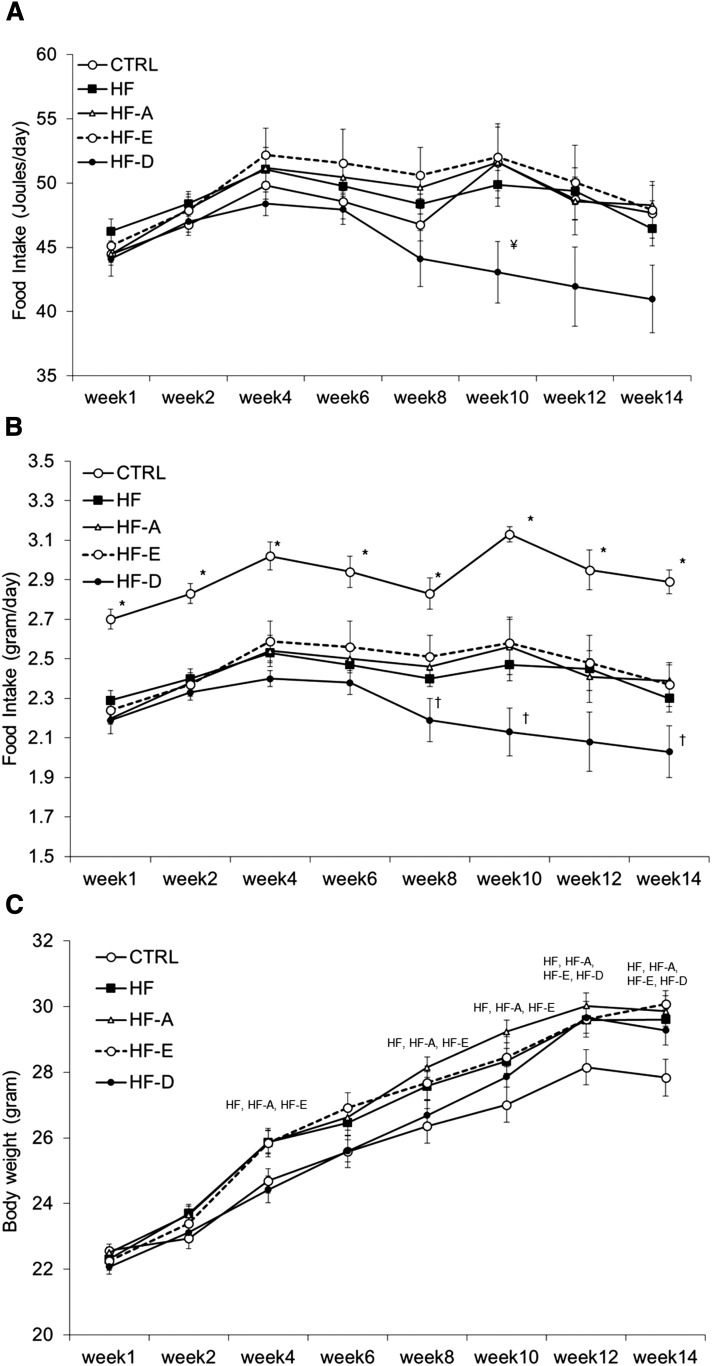
Daily energy intake (J/day) was not statistically different between groups from weeks 1 to 14 (ANOVA, P = NS; Fig. 1A). However, energy intake at week 10 tended to be lower in mice from the HF-D group compared with control, HF-A, or HF-E groups (ANOVA, P = 0.05; post hoc analysis showed a significant difference between HF-D vs. LF, HF-A, and HF-E groups at P < 0.05). Food intake expressed in grams per day was similar in the four groups receiving an HF diet (i.e., HF, HF-A, HF-E, and HF-D) during the first 8 weeks of the intervention. Thereafter, mice consuming the HF-D diet ate significantly less food from weeks 8 to 14 (Fig. 1B), compared with HF-A and HF-E groups. Unsurprisingly and because of a lower caloric density of the diet, mice fed with the control diet ate more food than mice receiving the four HF diets with or without n-3 PUFA. No changes in fasting GLP-1, GIP, or ghrelin plasma levels were detected between groups (data not shown). Despite this, in animals fed with the HF-D diet, BW gain between weeks 6 and 14 was comparable to mice from the HF, HF-A, and HF-E groups (Fig. 1C).
Fig. 1.
Evolution of food consumption in C57BL/6J mice. Five-week-old mice were placed on an HF or n-3 PUFA-supplemented HF (HF-A, HF-E, and HF-D) diets and compared with mice fed with a standard LF diet (CTRL) for 16 weeks. Food consumption was recorded every 2 weeks between week 0 and week 14 and expressed in joules per day (A) or grams per day (B). Total food consumption was measured for each cage (n = 5 mice per cage); the measured value was divided by the number of animals in the corresponding cage. BW was recorded every 2 weeks at the animal facility throughout the intervention and expressed in grams (C). Data represent mean consumption per animal or mean BW ± SEM (n = 20). A, B: * P < 0.05 versus all other groups; † P < 0.05 versus HF-A and HF-E; ¥ P < 0.05 versus control, HF-A, and HF-E. C: Groups mentioned on the graph significantly differed from controls.
At the end of the dietary intervention, animals fed with the HF diet gained significantly more weight than control mice (Table 1). Final BW of HF, HF-A, HF-E, and HF-D mice was not statistically different. Supplementation with EPA significantly limited fat mass accumulation compared with HF and HF-D diets. Due to a higher variability in the HF-A group, fat mass accumulation only tended to be lower in the HF-E group compared with HF-A animals (P = 0.1; Table 1). Although absolute lean mass was similar between controls and all HF groups (P = NS; Table 1), lean body mass expressed in % of BW was ∼4% higher in HF-E mice compared with the other HF groups (P < 0.05) and was similar to controls (Table 1). By contrast, lean body mass in % BW was ∼3.5% lower in the HF, HF-A, and HF-D groups compared with controls (P < 0.05; Table 1).
TABLE 1.
Anthropometric and calorimetric parameters of C57BL/6J mice
| Parameters | Control | HF | HF-A | HF-E | HF-D |
| BW, g | 27.0 ± 0.4b | 29.6 ± 0.3a | 29.8 ± 0.5a | 29.8 ± 0.5a | 29.2 ± 0.4a |
| Fat mass, g | 2.66 ± 0.13b | 3.86 ± 0.38a | 3.82 ± 0.38a | 2.74 ± 0.28b | 3.77 ± 0.31a |
| Lean mass, g | 23.13 ± 0.49 | 22.85 ± 0.44 | 23.13 ± 0.51 | 23.81 ± 0.74 | 22.65 ± 0.48 |
| Fat mass, % | 9.5 ± 0.4c | 13.4 ± 1.2a | 13.0 ± 1.2ab | 10.3 ± 1.1bc | 13.3 ± 1.1a |
| Lean mass, % | 82.6 ± 0.4a | 79.6 ± 1.2b | 79.5 ± 1.1b | 82.8 ± 1.1a | 79.8 ± 1.0b |
| eWAT, %BW | 1.3 ± 0.1b | 2.1 ± 0.3a | 2.4 ± 0.4a | 1.3 ± 0.2b | 2.3 ± 0.2a |
| Gastrocnemius, %BW | 0.49 ± 0.01 | 0.47 ± 0.01 | 0.49 ± 0.01 | 0.49 ± 0.01 | 0.48 ± 0.01 |
| Liver, %BW | 3.85 ± 0.12 | 3.52 ± 0.13 | 3.65 ± 0.09 | 3.63 ± 0.08 | 3.41 ± 0.13 |
| Heart, %BW | 0.49 ± 0.01ab | 0.47 ± 0.01abc | 0.46 ± 0.01bc | 0.5 ± 0.02a | 0.45 ± 0.01c |
| LM-EE (kJ/g) | 1.95 ± 0.02 | 1.87 ± 0.04 | 1.89 ± 0.02 | 1.95 ± 0.03 | 1.90 ± 0.04 |
| Locomotor activity | 178 ± 12 | 174 ± 19 | 139 ± 14 | 179 ± 19 | 119 ± 18a |
| RQ | 0.95 ± 0.01a | 0.81 ± 0.01b | 0.79 ± 0.01b | 0.80 ± 0.01b | 0.8 ± 0.01b |
Five-week-old C57BL/6J mice were placed on an HF or n-3 PUFA-supplemented HF (HF-A, HF-E, and HF-D) diets and compared with mice fed with a standard LF diet (control) for 16 weeks. Body composition, 24 h LM-EE, RQ, and locomotor activity were evaluated during weeks 14 and 15. Tissue weights were evaluated at euthanizing after 16 weeks on the diets. Data are means ± SEM (n = 13–19 and 4–6 for anthropometric and calorimetric parameters, respectively). Means within a row with different superscripted letters differ at P < 0.05 (ANOVA).
Individual comparison versus LF, HF, and HF-E found a difference at P = 0.1 (t-test).
As expected, RQ of HF, HF-A, HF-E, and HF-D mice was lower than in the LF group (P < 0.05; Table 1). Energy expenditure adjusted for differences in lean mass (LM-EE) was not different between groups, but locomotor activity of HF-E mice was higher compared with HF-D and HF-A animals (P < 0.05, Student’s t-test).
Effect of the dietary interventions on the FA profile of erythrocyte phospholipids from C57BL/6J mice
The FA composition of erythrocyte phospholipids (PLs) was used to validate n-3 PUFA dietary supplementations. Consumption of the HF-A, HF-E, and HF-D diets induced a significant enrichment of the corresponding FAs in erythrocyte PLs (Table 2) compared with the HF diet. Mice consuming the HF-E diet presented the highest percentage of n-3 PUFAs incorporated into erythrocyte PLs (P < 0.05 vs. the other groups), notably due to a high accumulation of docosapentaenoic acid (DPA, 22:5n-3). Supplementation with ALA and EPA similarly increased the percentage of DHA incorporation. As a consequence, the proportion of C18, C22, C20:4 n-6, C22:4 n-6, and C22:5 n-6 FA in erythrocyte PLs was reduced in mice fed the HF-E and HF-D diets compared with both HF and control diets. Only EPA supplementation significantly decreased total n-6 PUFA content compared with the HF diet (P < 0.05; Table 2). As compared with HF group, the n-6 to n-3 ratio was improved (Table 2) in HF-E and HF-D groups (−72% and −63%, respectively, vs. HF; P < 0.05) and to a lesser extent in HF-A group (−35% vs. HF, P < 0.05). A significant increase in EPA incorporation in erythrocyte PLs from HF-D mice was detected compared with HF mice. FA profiling in skeletal muscle and liver PL extracts showed very similar effects with some exceptions, regarding n-6 to n-3 ratio and DHA retroconversion, which were respectively lower and nonexistent in skeletal muscle compared with the liver (supplemental Tables S1, S2). Although the percentage of DHA in muscle PLs was high in control and HF mice, it was further increased by EPA and DHA supplementation. Then, the total amount of n-3 PUFA in muscle PLs was strongly increased (+56% and +86%, respectively, vs. HF) following EPA and DHA supplementation compared with mice receiving the HF diet alone leading to a marked change in the relative content of n-3 and n-6 PUFAs (supplemental Table S1).
TABLE 2.
FA composition of erythrocytes from C57BL/6J mice
| Fatty Acid | Control | HF | HF-A | HF-E | HF-D |
| C14.0 | 0.50 ± 0.06 | 0.51 ± 0.07 | 0.44 ± 0.07 | 0.45 ± 0.07 | 0.37 ± 0.05 |
| C15.0 | 0.36 ± 0.03 | 0.40 ± 0.06 | 0.33 ± 0.05 | 0.33 ± 0.05 | 0.28 ± 0.04 |
| C16.0 | 33.22 ± 0.69 | 31.81 ± 0.94 | 30.13 ± 0.65 | 30.37 ± 0.46 | 30.93 ± 1.13 |
| C16.1n.9 | 0.75 ± 0.09 | 0.89 ± 0.17 | 0.74 ± 0.14 | 0.70 ± 0.13 | 0.58 ± 0.1 |
| C16.1n.7 | 0.67 ± 0.04a | 0.30 ± 0.02b | 0.27 ± 0.03b | 0.23 ± 0.03b | 0.29 ± 0.04b |
| C17.0 | 0.40 ± 0.01c | 0.57 ± 0.03a | 0.53 ± 0.02ab | 0.51 ± 0.02ab | 0.50 ± 0.03b |
| C18.0 | 12.24 ± 0.37c | 16.55 ± 0.43a | 15.41 ± 0.46ab | 14.83 ± 0.33b | 14.75 ± 0.51b |
| C18.19cis | 14.31 ± 0.30 | 15.33 ± 0.57 | 14.70 ± 0.23 | 14.66 ± 0.19 | 14.00 ± 0.38 |
| C18.1n.7 | 2.69 ± 0.09a | 1.66 ± 0.07b | 1.56 ± 0.05b | 1.48 ± 0.05bc | 1.24 ± 0.18c |
| C18.2n6cis | 9.34 ± 0.23d | 10.97 ± 0.25c | 12.09 ± 0.40b | 11.61 ± 0.30bc | 13.59 ± 0.47a |
| C20.1n.9 | 0.42 ± 0.01 | 0.44 ± 0.02 | 0.43 ± 0.01 | 0.34 ± 0.01 | 0.32 ± 0.01 |
| C18.3n.3 | 0.26 ± 0.01a | 0.15 ± 0.00b | 0.29 ± 0.01a | 0.13 ± 0.00bc | 0.12 ± 0.01c |
| C20.2 | 0.32 ± 0.01d | 0.62 ± 0.02b | 0.68 ± 0.02a | 0.52 ± 0.01bc | 0.54 ± 0.01a |
| C22.0 | 0.40 ± 0.04b | 0.63 ± 0.04a | 0.48 ± 0.05ab | 0.45 ± 0.06b | 0.41 ± 0.09b |
| C20.3n.6 | 1.26 ± 0.04 | 0.79 ± 0.10 | 1.35 ± 0.48 | 0.97 ± 0.12 | 1.38 ± 0.37 |
| C20.4n.6 | 14.49 ± 0.69a | 12.00 ± 1.18b | 12.42 ± 0.31b | 8.80 ± 0.33c | 7.99 ± 0.54c |
| C20.5n.3 | 0.90 ± 0.07c | 0.93 ± 0.09c | 1.08 ± 0.07c | 4.92 ± 0.18a | 1.63 ± 0.14b |
| C22.4n.6 | 1.45 ± 0.09a | 1.36 ± 0.16ab | 1.14 ± 0.06b | 0.58 ± 0.03c | 0.33 ± 0.03c |
| C22.5n.6 | 0.81 ± 0.07a | 0.61 ± 0.09b | 0.25 ± 0.02c | 0.08 ± 0.01d | 0.05 ± 0.01d |
| C22.5n.3 | 0.46 ± 0.02c | 0.40 ± 0.05c | 0.86 ± 0.04b | 2.38 ± 0.09a | 0.36 ± 0.04c |
| C22.6n.3 | 4.74 ± 0.40b | 3.11 ± 0.48c | 4.85 ± 0.29b | 5.63 ± 0.27b | 8.98 ± 0.88a |
| Total n-3 | 6.37 ± 0.47cd | 4.59 ± 0.57d | 7.08 ± 0.39c | 13.06 ± 0.49a | 11.09 ± 1.02b |
| Total n-6 | 27.35 ± 0.77a | 25.73 ± 1.43ab | 27.25 ± 0.75a | 22.04 ± 0.61c | 23.34 ± 0.68bc |
| n-6:n-3 | 4.43 ± 0.29b | 5.98 ± 0.45a | 3.92 ± 0.23b | 1.70 ± 0.05c | 2.22 ± 0.19c |
Data are means ± SEM (n = 8). Variables with a significant ANOVA at P < 0.05 appear in bold. Means within a row with different superscripted letters differ at P < 0.05 (ANOVA).
EPA improved fasting metabolic parameters in C57BL/6J mice
Plasma cholesterol was significantly lower in HF-E and HF-D mice compared with HF animals (P < 0.05; Table 3). By contrast, HF-A and HF-D groups exhibited significant higher plasma nonesterified FA concentrations compared with the HF-E group (P < 0.05; Table 3). Fasting plasma insulin was similar between groups. Yet, fasting plasma insulin tended to be lower in HF-E mice compared with HF group (P < 0.1; Table 3).
TABLE 3.
Plasma parameters in fasted C57BL/6J mice fed after 16 weeks with LF (control), HF, or n-3 PUFA-supplemented HF (HF-A, HF-E, and HF-D) diets
| Parameters | Control | HF | HF-A | HF-E | HF-D |
| Insulin (ng/ml) | 5.54 ± 0.19 | 7.04 ± 0.68 | 5.86 ± 0.64 | 4.64 ± 0.53a | 6.69 ± 0.76 |
| NEFA (mM) | 0.30 ± 0.01ab | 0.27 ± 0.01bc | 0.37 ± 0.02a | 0.22 ± 0.01c | 0.31 ± 0.01ab |
| Glucose (g/l) | 0.98 ± 0.02b | 1.50 ± 0.05a | 1.24 ± 0.05a | 1.36 ± 0.02a | 1.30 ± 0.04a |
| Triglyceride (g/l) | 0.37 ± 0.04 | 0.38 ± 0.03 | 0.41 ± 0.05 | 0.33 ± 0.02 | 0.42 ± 0.03 |
| Glycerol (µM) | 231 ± 16 | 231 ± 24 | 191 ± 12 | 183 ± 7 | 208 ± 18 |
| Total cholesterol (g/l) | 0.72 ± 0.03c | 0.92 ± 0.03a | 0.85 ± 0.07ab | 0.73 ± 0.03c | 0.74 ± 0.02bc |
| Leptin (ng/ml) | 2.39 ± 0.24b | 2.30 ± 0.53b | 1.82 ± 0.33b | 0.85 ± 0.07c | 5.24 ± 0.55a |
| Adiponectin (µg/ml) | 9.13 ± 0.55a | 6.14 ± 0.49b | 5.73 ± 0.37b | 5.57 ± 0.31b | 5.68 ± 0.57b |
Data are means ± SEM (n = 8–11). Means within a row with different superscripted letters differ at P < 0.05 (ANOVA).
P < 0.1 versus HF (ANOVA: P = 0.07).
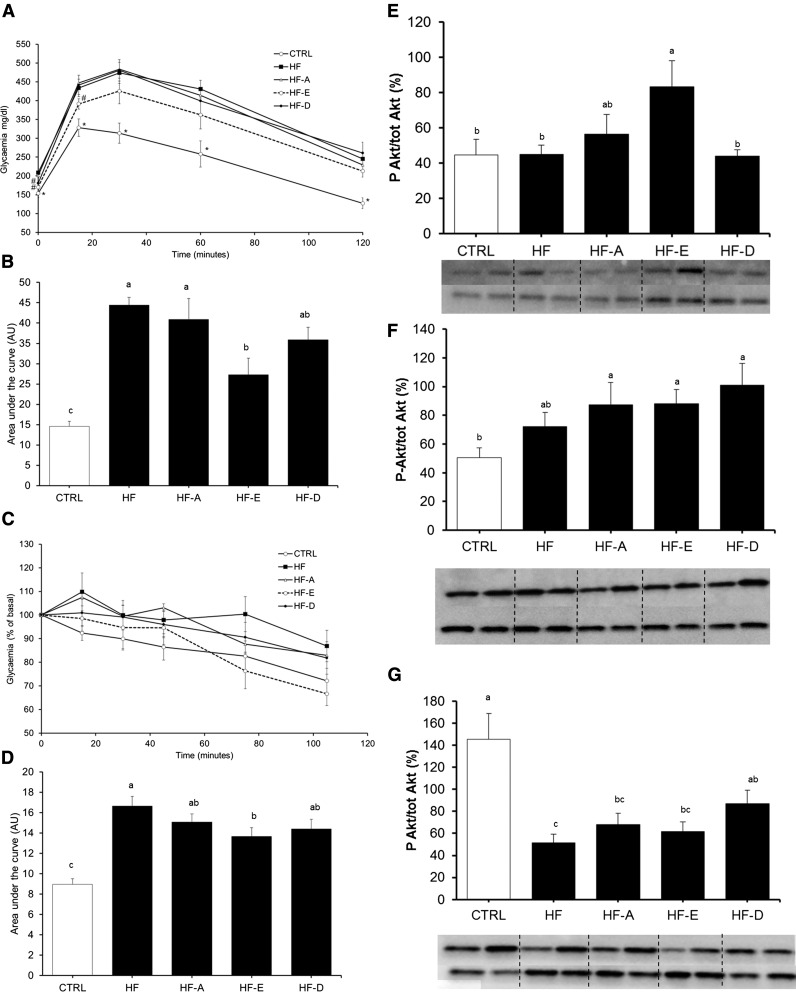
Effect of the dietary intervention on glucose tolerance and IR in C57BL/6J mice
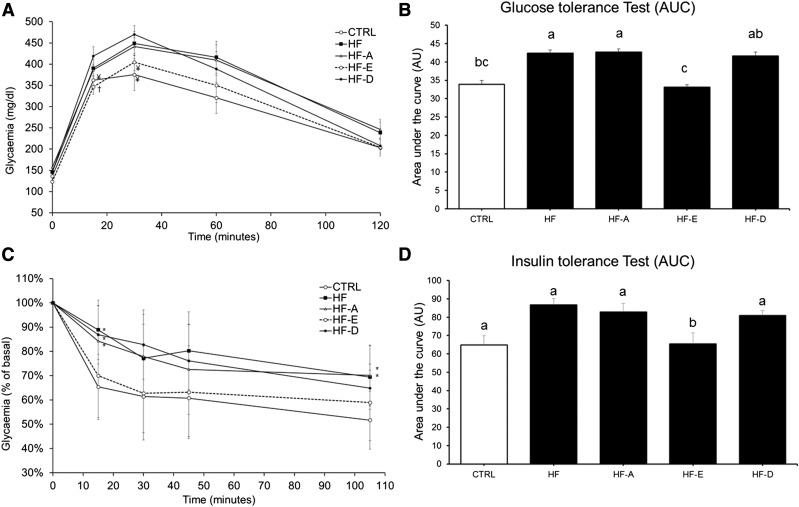
Glucose tolerance was significantly lower in mice fed the HF and HF-A diets compared with those fed the control diet (P < 0.05; Fig. 2A, B). By contrast, glucose homeostasis was significantly improved in mice receiving the HF-E diet compared with the HF and HF-A diets, notably due to a lower increase in glycemia in the first 30 min of the GTT (Fig. 2A). Glucose tolerance of HF-E mice was similar to controls. DHA only slightly improved glucose tolerance by decreasing blood glucose concentration to control and HF-E values after 2 h.
Fig. 2.
GTT and ITT in C57BL/6J mice. GTT (A, B) or insulin sensitivity (C, D) were performed in two separate sets of animals. A: Plasma glucose concentrations following intraperitoneal glucose injection to fasted mice after 16 weeks of intervention (same groups of mice as in Fig. 1). B: Area under the glycemic curve values from GTT. C: Plasma glycemia following intraperitoneal insulin injection to fasted mice. D: Area under the glycemic curve values from insulin sensitivity test. Data are means ± SEM (n = 8–10 and n = 7–9 for A, B and C, D, respectively). In A: † P < 0.05 versus HF-D; ¥ P ≤ 0.1 versus HF-D. In C: * P < 0.05 versus control. B, D: Means with different superscripts differ at P < 0.05 (ANOVA).
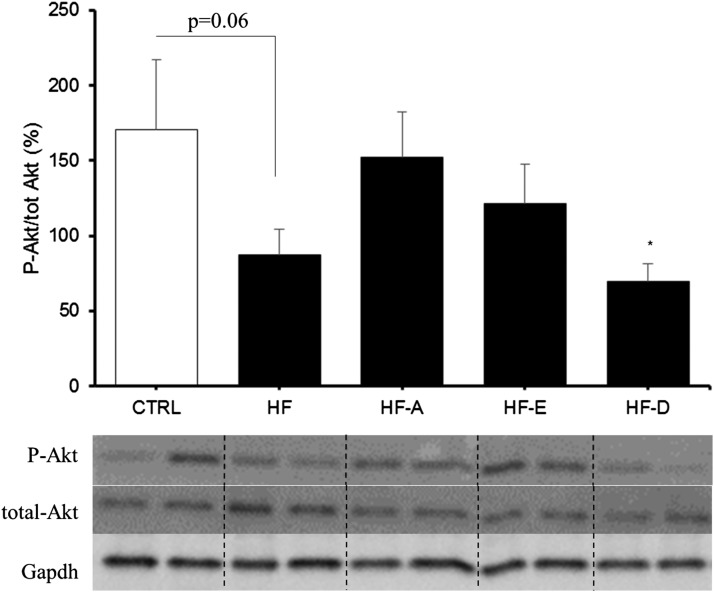
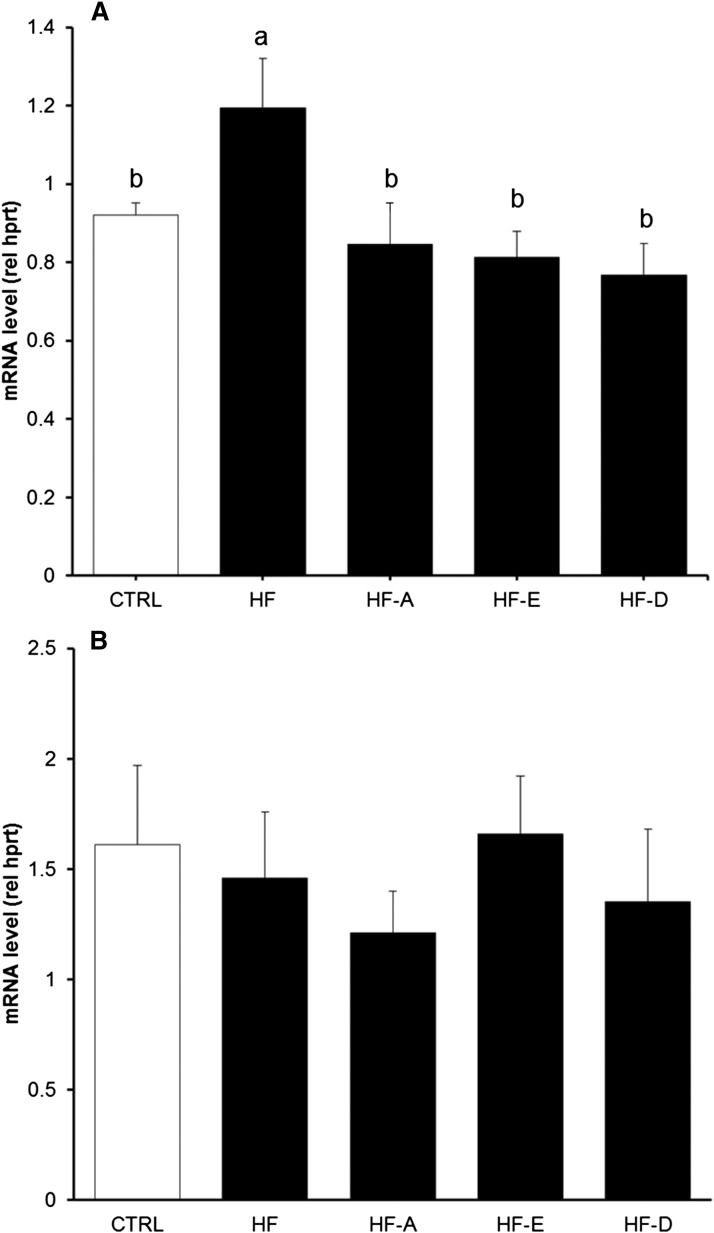
Insulin sensitivity was similar between HF-E mice and controls and significantly higher compared with the other HF groups (Fig. 2C, D). Insulin sensitivity was further investigated by quantifying Akt protein phosphorylation in AT, skeletal muscle, and the liver. Akt phosphorylation was not affected by the different diets in skeletal muscle and AT (data not shown). In the liver, the result of the ANOVA did not reach the significance level (P = 0.18) probably because we administered a physiological dose of insulin (1.2 mIU/g). Interestingly, we observed a lower Akt protein phosphorylation in the liver from HF-D and HF animals compared with controls (P < 0.05 and P = 0.06, respectively; Fig. 3). The expression level of genes related to glucose homeostasis were evaluated in the liver and skeletal muscle of C57BL/6J mice (supplemental Table S3A, B). G6pc mRNA level was decreased in the liver of HF-D, HF-A, and HF-E mice (P < 0.05, P < 0.05, and P = 0.13 vs. HF, respectively; supplemental Table S3A). Pck1 mRNA level was increased in HF and HF-E mice compared with control (P < 0.05). Hepatic Pdk4 mRNA levels were decreased in all HF groups compared with control mice (P < 0.05; supplemental Table S3A). In skeletal muscle, Pck1 mRNA level was decreased in HF-A, HF-E, and HF-D mice. Glycerol kinase (Gyk) mRNA level was elevated in skeletal muscle of HF and HF-D mice compared with controls (P < 0.05; supplemental Table S3B). Contrasting with the effect observed in the liver, Pdk4 expression remained unchanged in skeletal muscle (supplemental Table S3B). We also evaluated gene expression level of Trb3, a protein involved in the inhibition of insulin response. Trb3 mRNA level was significantly higher in skeletal muscle from HF mice compared with controls and other HF groups (Fig. 4; P < 0.05), but unchanged in the liver (Fig. 4).
Fig. 3.
Insulin-dependent Akt phosphorylation in the liver from C57BL/6J mice. Histogram represents means of Akt phosphorylation in skeletal muscle from C57Bl6J mice (same groups of mice as in Fig. 1). The abundance of Akt phosphorylated at Ser 473 was normalized to total Akt abundance using specific antibodies. Data are means ± SEM (n = 7–8 experiments per group). Representative pictures of samples from each group are reported. * P < 0.05 versus control group (t-test).
Fig. 4.
Modulation of Trb3 gene expression in C57BL/6J mice. Trb3 mRNA levels were determined in skeletal muscle (A) and the liver (B) from C57BL/6J mice after 16 weeks of intervention (same groups of mice as in Fig. 1). Quantification of mRNA was measured by RT-qPCR and normalized to Hprt mRNA level. Data are expressed as mean ± SEM of the relative abundance of each mRNA (n = 5–8 and n = 5–6 in skeletal muscle and the liver, respectively). Means with different superscripts differ at P < 0.05 (ANOVA).
Effect of the dietary intervention on AT adipokines and metabolism
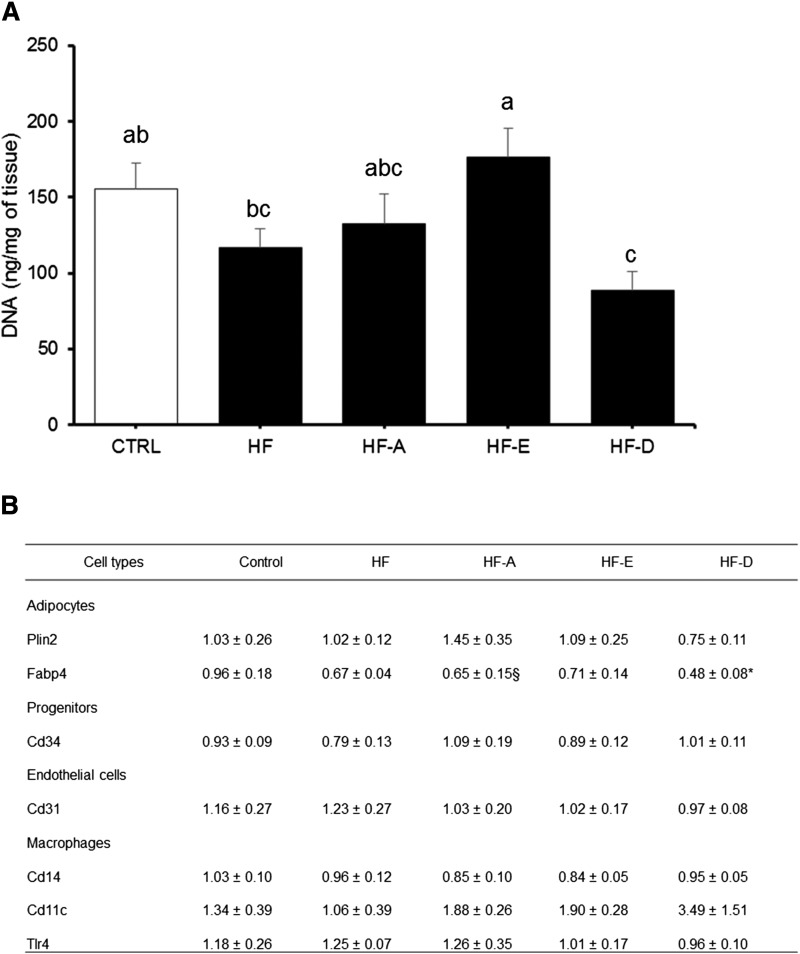
In eWAT, significant accumulation of ALA, EPA, and DHA was observed following the consumption of HF-A, HF-E, and HF-D diets, respectively (supplemental Table S4). The n-6 to n-3 ratio in eWAT was elevated in mice consuming the HF diet compared with control diet (32.7 ± 2.3 vs. 18.3 ± 0.6 in HF and control groups, respectively; P < 0.05). Supplementation with n-3 PUFA significantly reduced n-6 to n-3 ratio in HF-A, HF-D, and HF-E mice compared with HF mice (10.1 ± 0.5, 14.0 ± 0.9, and 17.3 ± 0.6 vs. 32.71 ± 0.6, respectively, all significant at P < 0.05 vs. HF). The percentage of ALA in AT following supplementation with this FA was higher compared with our observations in hepatic or skeletal muscle PLs (supplemental Tables S1, S2). Accumulation of DPA was detectable in HF-A, HF-E, and HF-D mice but was significantly higher in HF-E group compared with the two others (+ 200%, P < 0.05; supplemental Table S4). DNA content per gram of eWAT from HF-E mice was similar to controls but significantly higher compared with other HF animals, notably HF and HF-D (Fig. 5A), indicating a lower adipose cell size in HF-E mice compared with other HF mice. DNA content in eWAT of HF-D animals was the lowest of the HF groups (Fig. 5A), suggesting adipocyte hypertrophy in HF-D mice. Variations in cell type representation within eWAT were evaluated by the quantification of specific mRNA of adipocytes, progenitors, and endothelial and immune cells. The measurement of mRNA levels of adipocyte markers showed a decreased Fabp4 expression in HF-D mice compared with control mice (Fig. 5B) but similar Plin2 mRNA level between all groups. The expression level of markers of progenitor cells (Cd34), endothelial cells (Cd31), and macrophages (Cd14, Cd11c, Tlr4) was not significantly affected by the interventions (Fig. 5B).
Fig. 5.
eWAT DNA concentration and marker mRNA levels in C57BL/6J mice. DNA concentration (A) and mRNA levels (B) were determined in eWAT depot from mice after 16 weeks of intervention (same groups of mice as in Fig. 1). A: DNA concentration in eWAT (n = 13–15). B: mRNA level of AT cell type markers in eWAT depot (n = 5–6); mRNA levels were measured by RT-qPCR and normalized to non-POU domain-containing, octamer-binding (NoNo) mRNA level. Data are expressed as mean ± SEM. Means with different letters differ at P < 0.05 (ANOVA). *, § P < 0.05 and P < 0.1 versus control, respectively.
In direct line with the differences in fat mass accumulation, plasma leptin was greater in HF-D mice compared with all other groups. Although HF and HF-A mice exhibited a significant accumulation of fat mass (P < 0.05 vs. control; Table 1), these animals did not present an increase in plasma leptin level compared with control mice (Table 3). On the contrary, circulating leptin concentration was decreased in mice receiving EPA compared with the other HF groups (P < 0.05; Table 3). By contrast, plasma adiponectin was similar between all HF groups but reduced compared with controls (P < 0.05 vs. controls; Table 3). Consequently, the adiponectin to leptin ratio was significantly higher following EPA supplementation compared with other HF groups (data not shown).
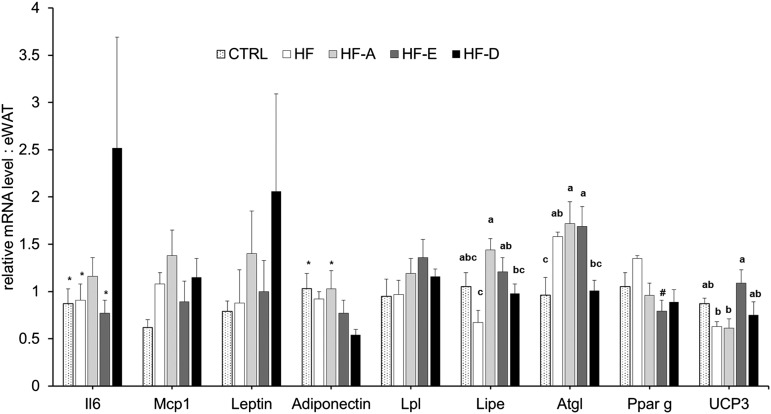
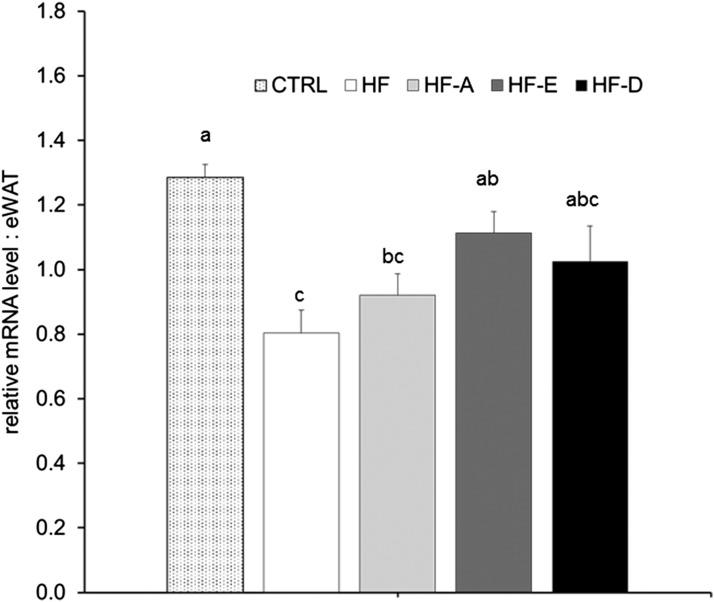
Transcriptomic analyses were performed in eWAT to explore the modulation of AT adipokine and inflammation-related gene expression (Fig. 6). Significant differences were observed in gene expression of lipolytic enzymes. Indeed eWAT Lipe mRNA level was significantly higher in HF-A and HF-E mice compared with HF mice (Fig. 6; P < 0.05). Atgl expression was also higher in HF-A and HF-E mice compared with both control and HF-D mice (Fig. 6; P < 0.05). Furthermore, Ucp3 mRNA level was significantly higher in HF-E mice compared with HF and HF-A groups (Fig. 6; P < 0.05). We did not found any significant effect of diets on Mcp1, leptin, or Pparg mRNA level. Despite a nonsignificant ANOVA, interleukin-6 and adiponectin mRNA levels were respectively higher and lower in HF-D mice compared with control group (Fig. 6; P < 0.05). Leptin mRNA level tended to be increased in the HF-D group compared with controls (Fig. 6; P < 0.01). Pparg expression was reduced in HF-E mice compared with HF animals (P < 0.05; Fig. 6).
Fig. 6.
Gene expression in eWAT from C57BL/6J mice. Quantification of mRNA was measured by RT-qPCR and normalized to non-POU domain-containing, octamer-binding (NoNo) mRNA level in eWAT after 16 weeks of intervention (same groups of mice as in Fig. 1). Data are expressed as mean ± SEM of the relative abundance of each mRNA (n = 5–6 per group). Means with different letters were significantly different (ANOVA); * P < 0.05 versus HF-D; # P < 0.05 versus HF (t-test).
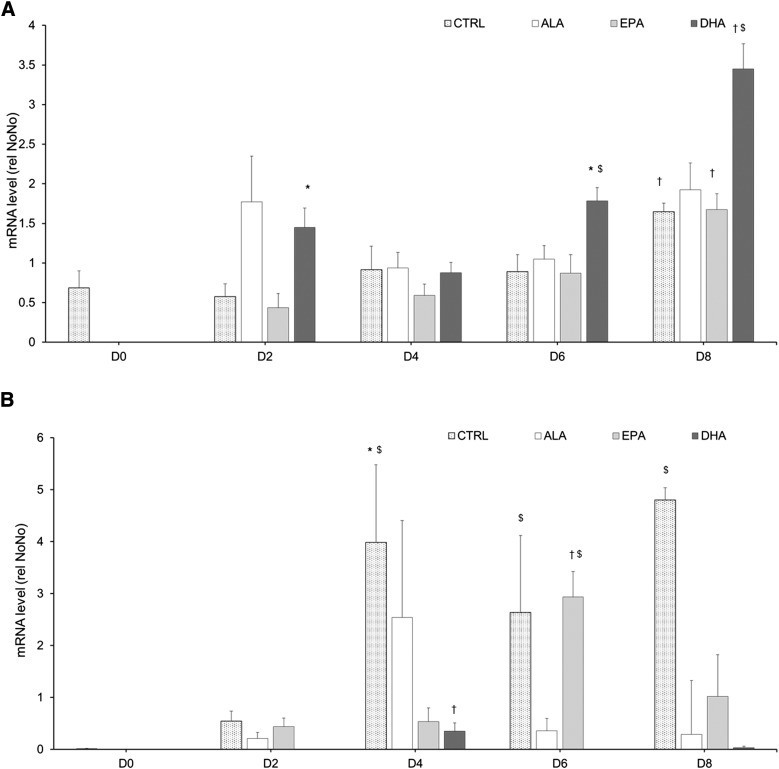
DHA hastened the induction of leptin expression during 3T3-L1 differentiation into adipocytes
To better characterize the effect of n-3 PUFA on AT development and its secretory function, 3T3-L1 cells were differentiated into adipocytes in the presence of 50 µM of ALA or EPA or DHA throughout 8 days of differentiation. Leptin and adiponectin expression kinetics were analyzed by the quantification of their mRNA levels every 2 days. mRNA levels of leptin and adiponectin were compared with day 0 and between treatments at days 2, 4, 6, and 8 (Fig. 7). In control cells, adiponectin and leptin expression profiles during differentiation were similar. In these cells, 8 days of differentiation were necessary to detect a significant increase of leptin gene expression compared with day 0. Adipocytes exposed to EPA responded similarly to control because leptin mRNA level differed from baseline only at day 8 (Fig. 7A). DHA treatment, but not ALA and EPA, increased leptin expression above control values on days 2, 6, and 8 (P < 0.05; Fig. 7A). In control cells, adiponectin was significantly expressed from day 4 and remained elevated throughout the differentiation process (P < 0.05 vs. D0; Fig. 7B). Cells exposed to EPA significantly expressed adiponectin mRNA at day 6 (Fig. 7B) and exhibited a higher adiponectin gene expression compared with cells exposed to ALA or DHA at this differentiation stage (P < 0.05; Fig. 7B). By contrast, treatment with ALA and DHA significantly reduced the effect of the differentiation process on adiponectin mRNA levels, which were significantly lower than in control cells on day 8 (Fig. 7B). Adiponectin expression level was very low in cells after 6 and 8 days of differentiation in the presence of DHA (Fig. 7B).
Fig. 7.
Leptin and adiponectin gene expression in 3T3-L1 adipocytes. Leptin (A) and adiponectin (B) mRNA levels were measured by RT-qPCR and normalized to non-POU domain-containing, octamer-binding (NoNo) mRNA level. Measurements were performed in cells exposed to 0 (CTRL) or 50 µM of ALA, EPA, or DHA during the differentiation process at day 0 (before FA treatment) and days 2, 4, 6, and 8. Data are expressed as mean ± SEM of the relative abundance of each mRNA (n = 4–6 per group). * P < 0.05 versus D0; † P < 0.05 versus all other days in the same treatment (ANOVA); $ P < 0.05 versus other treatments at the same day.
Prevention of glucose intolerance by EPA during severe obesity
After the identification of the differential effect of n-3 PUFA on metabolic disturbances during the dynamic phase of the development of obesity in C57BL/6J mice, we examined whether these effects were similar in Ob/Ob mice. These mice are hyperphagic because of the lack of leptin secretion and develop obesity and metabolic disorders more rapidly than C57BL/6J mice.
As expected after 5 weeks under each experimental diet (control, HF, HF-A, HF-E, and HF-D), BW and fat mass percentage were significantly elevated in Ob/Ob mice fed with the HF, HF-A, HF-E, and HF-D diets compared with Ob/Ob mice fed with the control diet (Table 4). Ob/Ob mice fed with the control diet ate 17% more calories than C57BL/6J WT mice fed the same diet (58.5 ± 1.9 vs. 47.8 ± 1. 0 J/day evaluated 2 weeks before euthanizing, respectively; P < 0.05) leading to a higher fat mass gain (Table 4) compared with C57BL/6J (Table 1). When consuming the HF diet, Ob/Ob mice ate more calories than animals fed with the control diet (Table 4) and gained significantly more weight. Supplementation with ALA, EPA, or DHA did not affect food intake expressed in grams or joules per day (Table 4) compared with the HF diet. Fat and lean relative mass were not different between groups (Table 4). Despite this, the HF-E diet significantly improved glucose tolerance compared with the other HF groups (Fig. 8A, B) and insulin sensitivity compared with HF group (Fig. 8C, D). However, these parameters were significantly altered compared with the control group (P < 0.05; Fig. 8B, D). As described for C57BL/6J mice, peripheral insulin sensitivity was evaluated in the liver, skeletal muscle, and eWAT after administration of a physiological dose of insulin. Akt protein phosphorylation following insulin injection was higher in the liver from HF-E-fed animals compared with control, HF, and HF-D groups (Fig. 8E). In skeletal muscle, consumption of the HF diet tended to increase Akt phosphorylation compared with the control diet (Fig. 8F). Supplementation with ALA, EPA, and DHA further increased Akt phosphorylation, leading to a significant improvement compared with controls in this tissue. Measurement of insulin-dependent Akt phosphorylation in the AT showed a decrease in all groups of Ob/Ob mice fed the HF diets compared with the control group (Fig. 8G). Finally, adiponectin gene expression was determined in eWAT from these mice; its mRNA content was significantly higher in eWAT of mice supplemented with EPA compared with HF mice (P < 0.05; Fig. 9). It was intermediate in the eWAT of mice supplemented with DHA compared with HF mice (P = 0.09 vs. HF; Fig. 9).
TABLE 4.
Anthropometric and food consumption measurements in fasted Ob/Ob mice after 6 weeks with LF (control), HF, or n-3 PUFA-supplemented HF (HF-A, HF-E, and HF-D) diets
| Parameters | Control | HF | HF-A | HF-E | HF-D |
| BW, g | 40.16 ± 0.68b | 48.67 ± 1.00a | 47.80 ± 0.77a | 47.76 ± 1.04a | 48.90 ± 0.58a |
| Fat mass, g | 20.45 ± 0.52b | 25.45 ± 0.87a | 24.81 ± 0.63a | 24.81 ± 1.02a | 25.69 ± 0.75a |
| Lean mass, g | 17.88 ± 0.48b | 21.3 ± 0.43a | 21.2 ± 0.53a | 21.05 ± 0.49a | 21.38 ± 0.46a |
| Fat % | 51.67 ± 0.96 | 53.05 ± 0.84 | 52.32 ± 0.91 | 52.74 ± 1.15 | 53.20 ± 0.89 |
| Lean % | 45.16 ± 0.87 | 44.53 ± 0.88 | 44.74 ± 0.87 | 44.94 ± 1.13 | 44.35 ± 0.75 |
| Food consumption (g/day) | 3.54 ± 0.11 | 3.7 ± 0.05 | 3.68 ± 0.04 | 3.77 ± 0.06 | 3.7 ± 0.05 |
| Food consumption (J/day) | 58.48 ± 1.76b | 74.63 ± 1.04a | 74.28 ± 0.69a | 76.05 ± 1.26a | 74.70 ± 0.91a |
Data are means ± SEM (n = 8). Means within a row with different superscripted letters differ at P < 0.05 (ANOVA).
Fig. 8.
Exploration of glucose tolerance and insulin sensitivity in Ob/Ob mice. A–D: Experiments were performed 5 weeks after the beginning of dietary treatments with LF (control), HF, or n-3 PUFA-supplemented HF (HF-A, HF-E, and HF-D) diets. Glucose tolerance (A, B) or insulin sensitivity (C, D) tests were performed in the same animals. A: Plasma glucose concentrations following intraperitoneal glucose injection to fasted mice. B: Area under the glycemic curve values from GTT in A. C: Plasma glucose concentrations following intraperitoneal insulin injection to fasted mice. D: Area under the glycemic curve values from insulin sensitivity test in C. Data are means ± SEM (n = 7–8). In A: *, significantly different from the other groups; #, value did not differ from control. Means with different superscripts differ at P < 0.05 (ANOVA). E–G: Insulin-dependent Akt phosphorylation in the liver skeletal muscle and eWAT from Ob/Ob mice. Histograms represent means of Akt phosphorylation in the liver (E), skeletal muscle (F), and eWAT (G) from Ob/Ob mice. The abundance of Akt phosphorylated at Ser 473 was normalized to total Akt abundance using specific antibodies. Data are means ± SEM (n = 6–8 experiments per group). Representative pictures of samples from each group are reported. Means with different letters were significantly different (ANOVA).
Fig. 9.
Adiponectin gene expression in eWAT from Ob/Ob mice. Quantification of mRNA was measured by RT-qPCR and normalized to non-POU domain-containing, octamer-binding (NoNo) mRNA level in eWAT after 6 weeks of intervention (same groups of mice as in Fig. 1). Data are expressed as mean ± SEM of the relative abundance of adiponectin mRNA (n = 5–7 per group). Means with different letters were significantly different (ANOVA); * P < 0.05 versus HF-D; # P < 0.05 versus HF (t-test).
DISCUSSION
The roles of dietary n-3 PUFAs against MetS and cardiovascular diseases were investigated in numerous studies, leading frequently to controversial results (7, 11). Furthermore, the specific effects of the three most important n-3 PUFAs retrieved in food products (ALA, EPA, and DHA) are partially characterized. We compared here the effect of these FAs, given at a nutritional dose (1% w/w of the diet) in the prevention of MetS and AT expansion in C57BL/6J mice fed with an HF diet. The experimental diet was enriched with fat and sugars (236 and 200 g/kg of fat and sucrose, respectively), mimicking a typical Western diet. C57BL/6J mice are frequently used as a relevant model for the study of human metabolic disorders (19). This strain develops obesity and IR within 16–20 weeks under an HF diet, but cautions should be taken regarding housing to limit the interindividual variability in terms of BW gain (20, 21). Each n-3 PUFA-supplemented diet allowed a significant incorporation of the corresponding FA in different tissues. The total amount of n-3 PUFAs incorporated into erythrocyte PLs was higher in mice receiving EPA and DHA supplementation compared with ALA, confirming the good incorporation of n-3 PUFAs as observed in rat tissues after the consumption of n-3 PUFA-enriched oils (16). All tissues that were analyzed from control and HF mice accumulated similar amounts of ALA and EPA. However, their respective increases following dietary supplementation were higher for EPA. ALA was probably preferentially stored in neutral lipids because absolute percentages of ALA were higher in AT and in hepatic neutral lipids (supplemental Table S5) compared with PL fractions. We also detected significant percentages of EPA, DPA, and DHA in AT (which contains mainly neutral lipids) and hepatic neutral lipids, showing that long chain n-3 PUFAs could also be significantly stored in neutral lipids from these tissues. Nutritional supplementation with ALA was not sufficient to induce long chain n-3 PUFA accumulation, probably because of its high rate of β-oxidation (22) or a reduced ability for elongation and desaturation (23). As expected, we observed that EPA supplementation induced a significant accumulation of DPA in PL from different tissues. It confirms that the final conversion of DPA into DHA is rate limiting (23) but suggests that DPA may participate to the beneficial effects of EPA supplementation on fat mass and glucose homeostasis. Supporting this, an increased EPA content in different tissues from mice receiving DHA due to the retroconversion of DHA into EPA did not lead to the similar physiological adaptations compared with mice fed with the HF-E diet. Beneficial effects of fish oil supplementation against AT expansion or metabolic disturbances were previously identified under various experimental conditions. Hence, very high doses of DHA and EPA (15% to 40% of n-3 PUFAs) prevented BW, fat mass gain, and AT hyperplasia or hypertrophy in mice fed a high-fat diet (13). Furthermore, a mixture of n-3 PUFAs stimulated lipid mobilization in AT from rats fed a normolipidic diet containing 60% of sucrose (24).
In the present study, ALA, EPA, or DHA supplementations did not prevent BW gain in mice receiving a Western diet. Despite a lower BW gain during the beginning of the intervention, mice receiving DHA had a similar weight compared with the other HF-fed groups at the end of the experiment. This observation could be explained by a slightly reduced locomotor activity in mice receiving DHA supplementation compared with other HF-fed groups. We demonstrated that adding 1% of EPA in the diet could prevent AT expansion, glucose intolerance, and IR and reduce plasma total cholesterol. Hence, supplementation with EPA had a beneficial effect on insulin response and plasma level in mice exposed to an obesogenic environment. This effect was not related to modifications of food intake, energy expenditure, or significant modulation of incretin plasma levels. Hence, energy expenditure expressed per gram of lean mass was similar between control and the four HF groups, suggesting that supplementation with n-3 PUFAs did not alter the metabolic activity of lean tissues in mice receiving a Western diet. Furthermore, variability in locomotor activity was not related to modification in fat mass or lean mass percentages or metabolic parameters.
The increased DNA concentration in eWAT from mice receiving the HF-E diet suggests that the adipose cell size was lower in these animals in comparison with other HF-fed groups. Similarly, the low DNA concentration in eWAT of HF-D-fed mice evokes adipose cell hypertrophy in response to DHA supplementation. Supporting these assumptions, we did not observe significant differences between groups in mRNA level of markers of nonadipocyte cells, suggesting no interference of other cell types with the DNA quantification procedure. Furthermore, the decreased Fabp4 expression in mice receiving the HF-D diet is in agreement with an IR state, fat cell hypertrophy, and changes in body composition as described in humans (25, 26).
In agreement with these latter observations and the differences in fat mass accretion, plasma leptin concentration was significantly higher in mice receiving the HF-D diet than in the other HF-fed groups. Also, leptin mRNA level in eWAT tended to be higher (P < 0.1) whereas adiponectin mRNA level was lower (P < 0.05) in HF-D group compared with control animals. Despite this, it had no impact on the adiponectin circulating level. Modifications of adipokine expression in other fat depots may have contributed to alter or regulate their plasma level. Hence, subcutaneous AT, which contains larger cells than visceral AT, is an important source of leptin (27). Nevertheless, plasma adiponectin to leptin ratio was significantly different between diets. It was higher in HF-E mice in comparison with other HF-fed groups. It indicates an improvement in AT function and insulin sensitivity in animals receiving EPA (28, 29). Supporting this, adiponectin expression or plasma level were positively associated with AT lipolysis and inversely related to MetS (30). Kinetics of leptin and adiponectin mRNA expression in 3T3-L1 adipocytes exposed to n-3 PUFAs supported the differential effect of EPA and DHA on leptin and adiponectin expression. When administrated chronically at a low dose, EPA stimulated adiponectin expression whereas DHA increased leptin expression in 3T3-L1 adipocytes during their differentiation process. These observations were obtained in cells that were undifferentiated at the beginning of FA exposure. The use of in vitro experiments to explore the effect of DHA and EPA on adipokine expression in adipocytes led to conflicting observations (31), indicating that the present in vitro effects cannot be fully extrapolated to an in vivo situation. Although we cannot directly relate the beneficial effect of EPA to change in adiponectin concentration, the present work strongly suggest that n-3 PUFAs may alter adipokine expression and/or secretion to regulate glucose homeostasis.
EPA and surprisingly ALA, but not DHA, increased eWAT Atgl and Lipe mRNA levels, suggesting a better capacity for triglyceride mobilization in the adipose depots of EPA- and ALA-supplemented animals. It was previously shown that EPA could reduce adipogenesis in 3T3-L1 adipocytes through the inhibition of PPARγ and CCAAT/enhancer-binding protein (CEBP)α, two key transcriptional regulators of adipocyte differentiation (32, 33). We also observed an effect of EPA on Pparg expression in eWAT from mice receiving a Western diet. By contrast, we can postulate from our combined results that DHA did not promote lipolytic pathways in eWAT (34, 35). This is in agreement with in vitro experiments (33), which demonstrated that DHA promotes adipogenesis in 3T3-L1 adipocytes.
Our experiment with Ob/Ob mice supports the notion that a preventive role of nutrients against fat mass accumulation could be mostly observed in the initial stage of weight gain. In Ob/Ob mice, EPA supplementation was not able to modulate fat mass gain. Neither ALA nor EPA or DHA counteracted the effect of overfeeding on body composition. However, despite this, EPA was still able to partially protect Ob/Ob mice against glucose intolerance and IR.
These differential effects were related to modifications of the insulin sensitive activation of Akt protein in the liver, but not in skeletal muscle. Insulin-dependent Akt phosphorylation was decreased in C57BL/6J mice receiving the HF and HF-D diets compared with mice fed with the standard diet. On the contrary, supplementation with ALA or EPA had a protective effect. In direct line with these observations, only EPA supplementation increased the phosphorylation of Akt upon insulin stimulation in the liver of Ob/Ob obese mice. ALA had only a limited stimulatory effect, which was not significant. No such improvement was reported in the skeletal muscle and AT. Although no significant changes were reported in WT mice, EPA significantly restored adiponectin mRNA level in eWAT from Ob/Ob mice, suggesting that this adipokine could partly mediate the beneficial effect of EPA supplementation on glucose homeostasis.
It was previously identified that Akt activation could be repressed by TRB3 protein in the liver (36). We recently confirmed the hypothesis in C2C12 myotubes that exhibited resistance to insulin-dependent Akt phosphorylation and increased Trb3 expression (8). The present study further validated a potential contribution of this protein in IR. Hence, Trb3 mRNA levels were reduced in skeletal muscle from WT mice receiving n-3 PUFAs compared with HF-fed animals. However, no significant changes were detected in the liver from the same animals. These data are the first to evidence in vivo the impact of n-3 PUFAs on Trb3 gene expression. However, further studies are required to understand the links with muscle insulin response.
Gene expression level of genes involved in glucose homeostasis could not fully explain the differences observed between n-3-supplemented groups. Gene expression of key regulators of hepatic glucose production was not consistently affected among the different types of n-3 PUFA supplementation. We observed that the mRNA levels of G6pc and Pck1 were reduced in the liver from HF-A and HF-D mice. G6PC and PCK proteins are involved in hepatic glucose production, and unexpectedly, HF-A and HF-D mice exhibited an impaired glucose tolerance compared with control and EPA-supplemented mice. However, it was previously described that PCK1 was not crucial for hepatic glucose production, but its blockage could affect FA β-oxidation (37). Despite our observation that Pck genes were not regulated by n-3 PUFAs in skeletal muscle, ALA and to a lesser extent EPA reduced glycerol kinase gene expression. It was previously found that FA esterification and glucose production could be affected when glycerol kinase activity is diminished (38). Further studies are necessary to decipher the connections between all the regulators of glucose metabolism and supplementation with n-3 PUFAs.
In conclusion, EPA dietary supplementation, but not ALA and DHA, could prevent the deterioration of glucose homeostasis and excessive accumulation of fat mass induced by an obesogenic environment. EPA directly affected tissue metabolism, inhibiting fat cell hypertrophy in visceral AT, improving the adiponectin and leptin expression profile, and enhancing the insulin signaling pathway in the liver. By contrast, DHA promoted fat mass accumulation and did not improve glucose homeostasis or IR. In agreement with a previous study using ALA-rich oils to decrease the dietary omega 6 to omega 3 ratio (39), ALA did not exhibit a beneficial effect on body composition or glucose homeostasis when used at a dose fitting with the nutritional recommendations.
Supplementary Material
Acknowledgments
The authors thank the staff from Auvergne University Experimental Animal Laboratory and Christophe Del’Homme, Philippe Denis, Anne Terisse-Lottier, and Alexandre Teynie from the Experimental Animal Facility of the Human Nutrition Unit (INRA of Clermont-Ferrand) for their assistance throughout the animal protocol. The authors also thank Celine Bobby for her help with TaqMan gene expression assays.
Footnotes
Abbreviations:
- ALA
- alpha linolenic acid
- AT
- adipose tissue
- BW
- body weight
- DPA
- docosapentaenoic acid
- eWAT
- epididymal white adipose tissue
- GTT
- glucose tolerance test
- HF diet
- high-fat, high-sucrose diet
- HF-A
- HF diet supplemented with 1% w/w ALA
- HF-D
- HF diet supplemented with 1% w/w DHA
- HF-E
- HF diet supplemented with 1% w/w EPA
- IR
- insulin resistance
- ITT
- insulin tolerance test
- LF diet
- low-fat diet
- LM-EE
- energy expenditure adjusted for differences in lean mass
- MetS
- metabolic syndrome
- PL
- phospholipid
- RQ
- respiratory quotient
- RT-qPCR
- reverse transcription quantitative polymerase chain reaction
This work was supported by Avril in partnership with Lesieur. We gratefully acknowledge financial support and a doctoral fellowship (A.P.) from Lesieur and Avril; A. Pinel was funded by a grant from Avril. A. Huertas is employed by Lesieur.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Eckel R. H., Grundy S. M., and Zimmet P. Z.. 2005. The metabolic syndrome. Lancet. 365: 1415–1428. [DOI] [PubMed] [Google Scholar]
- 2.Reaven G. M. 1988. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 37: 1595–1607. [DOI] [PubMed] [Google Scholar]
- 3.Cusi K. 2010. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr. Diab. Rep. 10: 306–315. [DOI] [PubMed] [Google Scholar]
- 4.Argilés J. M., López-Soriano J., Almendro V., Busquets S., and López-Soriano F. J.. 2005. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med. Res. Rev. 25: 49–65. [DOI] [PubMed] [Google Scholar]
- 5.Alligier M., Gabert L., Meugnier E., Lambert-Porcheron S., Chanseaume E., Pilleul F., Debard C., Sauvinet V., Morio B., Vidal-Puig A., et al. 2013. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. J. Clin. Endocrinol. Metab. 98: 802–810. [DOI] [PubMed] [Google Scholar]
- 6.Després J. P., Lemieux I., Bergeron J., Pibarot P., Mathieu P., Larose E., Rodés-Cabau J., Bertrand O. F., and Poirier P.. 2008. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 28: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 7.Pinel A., Morio-Liondore B., and Capel F.. 2014. n-3 Polyunsaturated fatty acids modulate metabolism of insulin-sensitive tissues: implication for the prevention of type 2 diabetes. J. Physiol. Biochem. 70: 647–658. [DOI] [PubMed] [Google Scholar]
- 8.Pinel A., Rigaudière J. P., Laillet B., Pouyet C., Malpuech-Brugère C., Prip-Buus C., Morio B., and Capel F.. 2016. N-3PUFA differentially modulate palmitate-induced lipotoxicity through alterations of its metabolism in C2C12 muscle cells. Biochim. Biophys. Acta. 1861: 12–20. [DOI] [PubMed] [Google Scholar]
- 9.Storlien L. H., Kraegen E. W., Chisholm D. J., Ford G. L., Bruce D. G., and Pascoe W. S.. 1987. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 237: 885–888. [DOI] [PubMed] [Google Scholar]
- 10.Wu J. H., Micha R., Imamura F., Pan A., Biggs M. L., Ajaz O., Djousse L., Hu F. B., and Mozaffarian D.. 2012. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br. J. Nutr. 107 (Suppl. 2): S214–S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorente-Cebrián S., Costa A. G., Navas-Carretero S., Zabala M., Martínez J. A., and Moreno-Aliaga M. J.. 2013. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J. Physiol. Biochem. 69: 633–651. [DOI] [PubMed] [Google Scholar]
- 12.Fedor D., and Kelley D. S.. 2009. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr, Metab. Care. 12: 138–146. [DOI] [PubMed] [Google Scholar]
- 13.Ruzickova J., Rossmeisl M., Prazak T., Flachs P., Sponarova J., Veck M., Tvrzicka E., Bryhn M., and Kopecky J.. 2004. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 39: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 14.Flachs P., Mohamed-Ali V., Horakova O., Rossmeisl M., Hosseinzadeh-Attar M. J., Hensler M., Ruzickova J., and Kopecky J.. 2006. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 49: 394–397. [DOI] [PubMed] [Google Scholar]
- 15.Kuda O., Rombaldova M., Janovska P., Flachs P., and Kopecky J.. 2016. Cell type-specific modulation of lipid mediator’s formation in murine adipose tissue by omega-3 fatty acids. Biochem. Biophys. Res. Commun. 469: 731–736. [DOI] [PubMed] [Google Scholar]
- 16.Poudyal H., Panchal S. K., Ward L. C., and Brown L.. 2013. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 24: 1041–1052. [DOI] [PubMed] [Google Scholar]
- 17.Weir J. B. 1990. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition. 6: 213–221. [PubMed] [Google Scholar]
- 18.Capel F., Acquaviva C., Pitois E., Laillet B., Rigaudiere J. P., Jouve C., Pouyet C., Gladine C., Comte B., Vianey Saban C., et al. 2015. DHA at nutritional doses restores insulin sensitivity in skeletal muscle by preventing lipotoxicity and inflammation. J. Nutr. Biochem. 26: 949–959. [DOI] [PubMed] [Google Scholar]
- 19.Fraulob J. C., Ogg-Diamantino R., Fernandes-Santos C., Aguila M. B., and Mandarim-de-Lacerda C. A.. 2010. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J. Clin. Biochem. Nutr. 46: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Heek M., Compton D. S., France C. F., Tedesco R. P., Fawzi A. B., Graziano M. P., Sybertz E. J., Strader C. D., and Davis H. R. Jr. 1997. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J. Clin. Invest. 99: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C. Y., and Liao J. K.. 2012. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 821: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunnane S. C., and Anderson M. J.. 1997. The majority of dietary linoleate in growing rats is beta-oxidized or stored in visceral fat. J. Nutr. 127: 146–152. [DOI] [PubMed] [Google Scholar]
- 23.Arterburn L. M., Hall E. B., and Oken H.. 2006. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 83: 1467S–1476S. [DOI] [PubMed] [Google Scholar]
- 24.Peyron-Caso E., Quignard-Boulange A., Laromiguiere M., Feing-Kwong-Chan S., Veronese A., Ardouin B., Slama G., and Rizkalla S. W.. 2003. Dietary fish oil increases lipid mobilization but does not decrease lipid storage-related enzyme activities in adipose tissue of insulin-resistant, sucrose-fed rats. J. Nutr. 133: 2239–2243. [DOI] [PubMed] [Google Scholar]
- 25.Queipo-Ortuño M. I., Escoté X., Ceperuelo-Mallafré V., Garrido-Sanchez L., Miranda M., Clemente-Postigo M., Pérez-Pérez R., Peral B., Cardona F., Fernández-Real J. M., et al. 2012. FABP4 dynamics in obesity: discrepancies in adipose tissue and liver expression regarding circulating plasma levels. PLoS One. 7: e48605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clemente-Postigo M., Queipo-Ortuno M. I., Fernandez-Garcia D., Gomez-Huelgas R., Tinahones F. J., and Cardona F.. 2011. Adipose tissue gene expression of factors related to lipid processing in obesity. PLoS One. 6: e24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Harmelen V., Reynisdottir S., Eriksson P., Thorne A., Hoffstedt J., Lonnqvist F., and Arner P.. 1998. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 47: 913–917. [DOI] [PubMed] [Google Scholar]
- 28.Inoue M., Yano M., Yamakado M., Maehata E., and Suzuki S.. 2006. Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism. 55: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 29.Finucane F. M., Luan J., Wareham N. J., Sharp S. J., O’Rahilly S., Balkau B., Flyvbjerg A., Walker M., Hojlund K., Nolan J. J., et al. 2009. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 52: 2345–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lara-Castro C., Fu Y., Chung B. H., and Garvey W. T.. 2007. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol. 18: 263–270. [DOI] [PubMed] [Google Scholar]
- 31.Todorčević M., and Hodson L.. 2015. The effect of marine derived n-3 fatty acids on adipose tissue metabolism and function. J. Clin. Med. 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanabe Y., Matsunaga Y., Saito M., and Nakayama K.. 2008. Involvement of cyclooxygenase-2 in synergistic effect of cyclic stretching and eicosapentaenoic acid on adipocyte differentiation. J. Pharmacol. Sci. 106: 478–484. [DOI] [PubMed] [Google Scholar]
- 33.Murali G., Desouza C. V., Clevenger M. E., Ramalingam R., and Saraswathi V.. 2014. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3T3–L1 preadipocytes. Prostaglandins Leukot. Essent. Fatty Acids. 90: 13–21. [DOI] [PubMed] [Google Scholar]
- 34.Bulló M., Salas-Salvadó J., and García-Lorda P.. 2005. Adiponectin expression and adipose tissue lipolytic activity in lean and obese women. Obes. Surg. 15: 382–386. [DOI] [PubMed] [Google Scholar]
- 35.You T., Yang R., Lyles M. F., Gong D., and Nicklas B. J.. 2005. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am. J. Physiol. Endocrinol. Metab. 288: E741–E747. [DOI] [PubMed] [Google Scholar]
- 36.Du K., Herzig S., Kulkarni R. N., and Montminy M.. 2003. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 300: 1574–1577. [DOI] [PubMed] [Google Scholar]
- 37.Burgess S. C., Hausler N., Merritt M., Jeffrey F. M., Storey C., Milde A., Koshy S., Lindner J., Magnuson M. A., Malloy C. R., et al. 2004. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 279: 48941–48949. [DOI] [PubMed] [Google Scholar]
- 38.Watford M. 2000. Functional glycerol kinase activity and the possibility of a major role for glyceroneogenesis in mammalian skeletal muscle. Nutr. Rev. 58: 145–148. [DOI] [PubMed] [Google Scholar]
- 39.Enos R. T., Velazquez K. T., McClellan J. L., Cranford T. L., Walla M. D., and Murphy E. A.. 2014. Reducing the dietary omega-6:omega-3 utilizing alpha-linolenic acid; not a sufficient therapy for attenuating high-fat-diet-induced obesity development nor related detrimental metabolic and adipose tissue inflammatory outcomes. PLoS One. 9: e94897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.