Abstract
Exposure to chlorine (Cl2) gas can occur during accidents and intentional release scenarios. However, biomarkers that specifically indicate Cl2 exposure and Cl2-derived products that mediate postexposure toxicity remain unclear. We hypothesized that chlorinated lipids (Cl-lipids) formed by direct reactions between Cl2 gas and plasmalogens serve as both biomarkers and mediators of post-Cl2 gas exposure toxicities. The 2-chloropalmitaldehyde (2-Cl-Pald), 2-chlorostearaldehyde (2-Cl-Sald), and their oxidized products, free- and esterified 2-chloropalmitic acid (2-Cl-PA) and 2-chlorostearic acid were detected in the lungs and plasma of mouse and rat models of Cl2 gas exposure. Levels of Cl-lipids were highest immediately post-Cl2 gas exposure, and then declined over 72 h with levels remaining 20- to 30-fold higher at 24 h compared with baseline. Glutathione adducts of 2-Cl-Pald and 2-Cl-Sald also increased with levels peaking at 4 h in plasma. Notably, 3-chlorotyrosine also increased after Cl2 gas exposure, but returned to baseline within 24 h. Intranasal administration of 2-Cl-PA or 2-Cl-Pald at doses similar to those formed in the lung after Cl2 gas exposure led to increased distal lung permeability and inflammation and systemic endothelial dysfunction characterized by loss of eNOS-dependent vasodilation. These data suggest that Cl-lipids could serve as biomarkers and mediators for Cl2 gas exposure and toxicity.
Keywords: inflammation, kinetics, lipids/oxidation, lung, nitric oxide
Chlorine (Cl2) is widely used for various industrial and manufacturing applications and is often transported through densely populated areas. Accidental exposure to high doses of these halogens has been reported in mass casualty scenarios worldwide (1–3). In addition, exposure to these halogens remains a concern in the military arena, underscoring the need to understand detailed mechanisms of toxicity and for targeted postexposure therapeutics.
Recent studies have elucidated the temporal toxicity profile resulting from Cl2 gas exposure. Using murine and rodent models, we and others have shown that postexposure injury occurs over hours to weeks, characterized by acute lung injury (ALI) (increased endothelial and epithelial permeability and neutrophilic inflammation), pulmonary and systemic vascular dysfunction (loss of nitric oxide homeostasis), reactive airways, pulmonary fibrosis, bronchiolitis obliterans, and myocardial and dermal injury (4–13). Cl2 and its hydration product, hypochlorous acid, are highly reactive, limiting their direct reactions to either low molecular weight antioxidants present in the epithelial lining fluid or targets on the surfaces of epithelial and inflammatory cells (14). This suggests that Cl2-induced injury to the hyaluronan, present mainly in the lung interstitial matrix, as well as to extrapulmonary tissues and vasculature (13, 15–18) are due to the formation of secondary Cl2-derived intermediates.
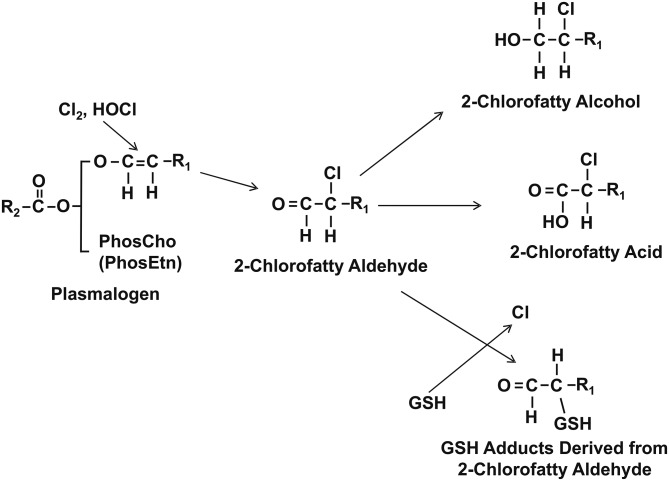
Chlorinated lipids (Cl-lipids) derived from plasmalogen oxidation are likely to be produced during Cl2 exposure because the lung and surfactant are enriched with plasmalogens. Evidence for lipid chlorination is primarily derived from in vitro/ex vivo studies investigating pathogenic mechanisms of PMN-derived reactive Cl2 species (e.g., HOCl). The strongest evidence for in vivo formation of Cl-lipids resides with the detection of 2-chlorofatty aldehydes in biological tissues (19). These species are formed by chlorination of the vinyl ether bond of plasmalogens (20) and have been detected during inflammatory diseases including atherosclerosis and myocardial infarction (21–25). Several different 2-chloro-lipids can be directly formed from plasmalogen chlorination including 2-chloropalmitaldehyde (2-Cl-Pald) or 2-chlorostearaldehyde (2-Cl-Sald), which in turn can be oxidized to the corresponding 2-chloropalmitic acid (2-Cl-PA) and 2-chlorostearic acid (2-Cl-SA) (Fig. 1) or reduced to the respective 2-chlorofatty alcohols. Additionally, recent studies have shown that 2-Cl-Pald and 2-Cl-Sald are targets for nucleophilic attack by GSH leading to glutathionylated adducts of palmitaldehyde and stearaldehyde, respectively (26). Moreover, these fatty acid species have been detected as being free or esterified. Furthermore, emerging studies indicate unique biological effects of Cl-lipids in stimulating inflammation, promoting cell-death, and dysfunction in eNOS-signaling (19, 21, 23, 26–30), which are also all features of post-Cl2 gas toxicity (4, 15). Cl-lipids are predicted to be formed via direct reactions of Cl2 gas with the vinyl ether bond in plasmalogens, leading us to hypothesize that these species may be mediators of post-Cl2 gas toxicity. In this study, we show that Cl-lipids and their GSH adducts are formed after Cl2 gas exposure and provide evidence that these species are potential mediators of postexposure toxicity.
Fig. 1.
Plasmalogen-derived Cl2 and HOCl oxidation products. The vinyl ether bond of plasmalogens is targeted by Cl2 and HOCl resulting in 2-chlorofatty aldehyde production including 2-Cl-Pald and 2-Cl-Sald. The 2-chlorofatty aldehydes are either oxidized to the 2-chlorofatty acids, 2-Cl-PA and 2-Cl-SA, or reduced to the 2-chlorofatty alcohols, 2-chloropalmitoyl alcohol and 2-chlorostearoyl alcohol. Alternatively, nucleophilic attack of 2-chlorofatty aldehydes by GSH results in either palmitaldehyde or stearaldehyde GSH adduct formation. R1 = C14H29 or C16H33.
MATERIALS AND METHODS
Materials
Unless stated otherwise, all reagents were purchased from Sigma (St. Louis, MO). Male (25–27 g, 10–12 weeks of age) C57bl/6 mice and rats were purchased from Harlan (Indianapolis, IN) and kept on 12 h light-dark cycles with access to standard chow and water ad libitum prior to and post-Cl2 gas exposure.
Methods
Mouse or rat exposure to Cl2 gas.
Whole body exposures of male mice or male rats to Cl2 gas were performed as previously described (31–33). Exposures were performed with either two mice or rats in the same chamber at any one time, and all exposures were performed between 8:00 AM and 12:00 PM. Exposure conditions were 400 ppm Cl2 in air for 30 min using either 400 ppm Cl2 tanks or by mixing 1,000 ppm Cl2 gas with compressed air. Flow meters were used to control flow rates to achieve the chamber Cl2 target concentrations. A bubble flow meter was used to validate their performance on a weekly basis. In each case, immediately following exposure, mice were returned to room air. All experiments involving animals were conducted according to protocols approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Food and water were provided ad libitum.
Inducing neutropenia.
C57bl/6 mice were rendered neutropenic, as described previously (34), by ip injection with 200 μg of either anti Ly-6G (clone 1A8) (Bxcel; catalog number BE0075-1) or IgG2a isotype control (Bxcel; catalog number BE0089) 24 h prior to Cl2 gas exposure.
Cl-lipid measurement.
Mice were euthanized at various times post-Cl2 gas exposure using a mixture of ketamine/xylazine (200/10 mg/kg) administered by ip injection. Blood was collected via cardiac puncture and lungs excised. Blood was centrifuged at 6,000 rpm for 5 min to obtain the plasma fraction. Lungs and plasma samples were flash-frozen in liquid nitrogen and stored at −80°C. Samples were shipped overnight on dry ice to Dr. Ford at St. Louis University. Free and total (i.e., free + esterified) Cl-lipids shown in Fig. 1 were measured as previously described by LC/MS following Dole extraction (35). Total lipids were measured by LC/MS after base hydrolysis and esterified Cl-lipids calculated by subtracting free lipids from total lipids. Extractions were performed using 25 μl of plasma spiked with 517 fmol of 2-chloro-[d4-7,7,8,8]palmitic acid (2-[d4]ClPA) as the internal standard, and for lungs, 40–50 mg of tissue was used, spiked with 20 pmol of 2-[d4]ClPA internal standard.
Measurement of glutathione adducts of 2-Cl-Pald and 2-Cl-Sald.
Plasma and lung samples were analyzed as previously described (26) with modifications. Briefly, 25 ul of plasma was spiked with 90 fmol of [d4]HDAGSH and 10 mg of pulverized lung tissue was spiked with 900 fmol [d4]HDAGSH. Both plasma and lung were then extracted according to a similar Bligh and Dyer method as described for the Cl-lipids (35); however, the aqueous layer was saved as the GSH adducts partition to the aqueous layer. The organic layer was subsequently washed with 1 vol methanol:water (1:1 v:v) and combined with the previous aqueous layer. The combined aqueous layers were diluted with 1/3 vol of water and extracted on a Strata-X followed by ESI-LC/MS/MS quantitation, as previously described (26).
3-[13C9]chlorotyrosine internal standard synthesis.
The 3-[13C9]chlorotyrosine internal standard was synthesized and purified as previously reported (36) with slight modification. Briefly, 2 mM L-[13C9]tyrosine was added to 50 mM phosphoric acid (pH 2) supplemented with 100 mM NaCl in water. NaOCl (final concentration 2 mM) was then added dropwise to the constantly stirred solution and then incubated for 60 min at 37°C. Trifluoroacetic acid (TFA) was then added to a final concentration of 0.1% and the 3-[13C9]chlorotyrosine was purified by HPLC on a C18 column (Beckman Ultrasphere; 5 μm resin, 4.6 × 250 mm). Amino acids were applied to the column equilibrated with 0.1% TFA in water and eluted with 0.1% TFA in methanol. Fractions were collected monitoring at 276 nm.
Tissue and plasma hydrolysis for chlorotyrosine measurement.
Plasma and lung samples were collected and prepared as previously described (37) with slight modification. For lung analysis, lung tissue was prepared by homogenizing tissue in 100 μM DTPA and BHT in water. Approximately 0.5 mg of homogenate was then diluted with 100 uM DTPA and BHT in water to 500 ul. For plasma analysis, 25 μl of plasma was diluted with 100 μM DTPA and BHT in water to 500 μl. Both diluted lung homogenate and plasma were delipidated twice with a single phase mixture of water/methanol/water-saturated diethyl ether (1:3:8 v/v/v). The pellet was dried under N2, 10 nmol [13C9,15N1]tyrosine and 10 pmol 3-[13C9]chlorotyrosine were added, and methane sulfonic acid was added to a final volume and concentration of 500 ul of 4 N methane sulfonic acid in water (1% phenol). The samples were capped, purged with argon, and incubated for 20 h at 110°C. Samples were then diluted with 2 ml 0.1% TFA and purified on a C18 SPE column (Supelco Discovery DSC-18LT) equilibrated with 0.1% TFA. After loading, the column was washed with 2 ml 0.1% TFA, and amino acids were eluted with 2 ml water:methanol (7:3, v/v; 30%) supplemented with 0.1% TFA. Samples were then dried by vacuum.
3-Chlorotyrosine MS.
Dried samples were suspended in 200 μl water and analyzed by LC-ESI/MS/MS using a Thermo Fisher TSQ Quantum Ultra mass spectrometer (Thermo Fisher, Waltham, MA). For experiments requiring LC/MS, a Thermo Fisher Surveyor LC system was coupled to the Quantum Ultra. LC/MS data analysis was performed using XCalibur software (Thermo Fisher). A Prodigy C-18 column (150 × 2.0 mm internal diameter, 5 μm particle) was equilibrated with solvent A (0.2% formic acid in water). Twenty microliters of sample was then injected and amino acids were eluted over an 18 min linear gradient from 0% solvent B (0.2% formic acid in 8:2 ACN:water) to 30% solvent B. All analytes were analyzed using selective reaction monitoring in positive ion mode. The analyte ions measured were tyrosine (m/z 182.1→136.1), [13C9,15N1]tyrosine (m/z 192.1→145.1), 3-chlorotyrosine (m/z 216.1→170.1), 3-[13C9]chlorotyrosine (m/z 225.1→178.1), and 3-[13C9,15N1]chlorotyrosine (m/z 226.1→179.1. The analyte 3-[13C9,15N1]chlorotyrosine was used as a marker for experimentally induced chlorination, but no significant experimentally induced chlorination was observed.
Intranasal administration of Cl-lipids.
A 5 mM stock of palmitic acid (PA), palmitaldehyde, 2-Cl-PA, and 2-Cl-Pald in 100% ethanol was diluted 10-fold in PBS prior to administration to mice. C57bl/6 male mice were divided into six groups. The mice were anesthetized using isoflurane and PBS, and 10% ethanol or each fatty acid (50 μl, equivalent to 25 nmol) was administered into the nares (intranasal). Mice were returned to room air and upon waking returned to their cages and then euthanized at 6 or 24 h postadministration. Mice were provided with food and water ad libitum.
Airway hyperresponsiveness.
C57bl/6 male mice were mechanically ventilated and challenged with increasing concentrations of aerosolized methacholine. Mice were sedated with xylazine (10 mg/kg ip and anesthetized with sodium pentobarbital (40 mg/kg ip). After the trachea was cannulated with a 16 gauge cannula, mice were connected to a ventilator (FlexiVent; Scireq, Montreal, PQ, Canada) and paralyzed using pancuronium chloride (1 mg/kg ip). Mice were ventilated at a rate of 90 breaths per minute with a positive end-expiratory pressure of 3 cm water. Increasing concentrations of methacholine (0–20 mg/ml) were administered via aerosolization. From 20 s up to 3 min after each aerosol challenge, resistance (centimeters of water per milliliter per second) and elastance (centimeters of water per milliliter) were recorded continuously, as previously described (38).
ALI measurements.
Mice were euthanized with ip ketamine and xylazine (100 and 10 mg/kg body weight, respectively). An incision was made at the neck to expose the trachea and a 3 mm endotracheal cannula inserted. Lungs were lavaged three times with 1 ml of PBS; ∼1.8–1.9 ml was recovered in all groups. Mice were then exsanguinated by cardiac puncture for collection of blood. Recovered aliquots of lavage fluid were kept on ice and centrifuged immediately at 300 g for 10 min to pellet cells. Supernatants were removed and stored on ice for protein analysis using the Bio-Rad protein assay reagent kit compared with BSA standards. Cells were resuspended in 100 μl PBS and counted using a Neubauer hemocytometer. Cells were then placed on slides using a Cellspin (Tharmac, Germany) and stained using a two stain set consisting of EosinY and a solution of thiazine dyes (Quik-Stain; Siemens, Washington, DC). Differential counts (specifically monocytes, neutrophils, and lymphocytes) were then performed on slides via light microscopy.
Vessel studies.
Mice were euthanized and the aortas excised and sectioned into 2–3 mm segments and then used for vessel bioactivity assays. All vessel bioassay studies were performed in indomethacin (5 μM) pretreated vessel segments and in bicarbonate buffered Krebs Henseleit buffer of the following composition: NaCl, 118 mM; KCl, 4.6 mM; NaHCO3, 27.2 mM; KH2PO4, 1.2 mM; MgSO4, 1.2 mM; CaCl2, 1.75 mM; Na2 EDTA, 0.03 mM; and glucose, 11.1 mM and perfused with 21% O2 and 5% CO2 balanced with N2. A passive load of 2 g was applied to all ring segments and maintained at this level throughout the experiments. At the beginning of each experiment, ring segments were depolarized with KCl (70 mM) to determine the maximal contractile capacity of the vessel. Rings were then washed extensively and allowed to equilibrate and again depolarized with KCl (70 mM). The rings were then washed and allowed to re-equilibrate. Vasoconstrictor responses were tested by cumulative addition of phenylephrine (PE) doses ranging from 1 nM to 3,000 nM. Endothelium-dependent vasodilator responses were tested by administering cumulative doses of acetylcholine, ranging from 1 nM to 3,000 nM after tension development at maximal PE dose. In subsequent experiments, vessels were submaximally contracted (50% of KCl response) with PE (300–1,000 nM). When tension development reached a plateau, endothelium-independent vasodilator responses were induced by administering cumulative doses of the nitric oxide donor, MAHMA NONOate (MNO).
Statistical analysis.
All results are reported as the mean ± SEM; significant differences were calculated as P < 0.05 by one-way ANOVA with Dunnett’s multiple comparison post test or two-way ANOVA with Bonferroni post test using GraphPad Prism 5. One data point from the 72 h group for Cl-lipid time course measurements was excluded based on Grubb’s outlier test (P < 0.05).
RESULTS
Cl2 gas exposure increases lung levels of Cl-lipids
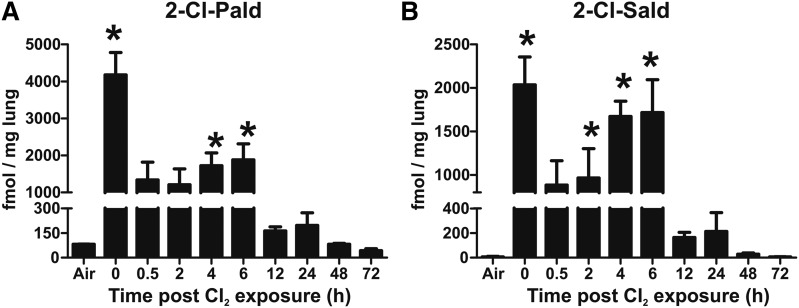
We first tested to determine whether Cl2 gas exposure resulted in increases in Cl-lipids. C57bl/6 mice were exposed to Cl2 gas (400 ppm for 30 min) and then euthanized at various times (0–72 h) thereafter. The predicted initial products of Cl2 reactions with plasmalogens are the 2-chlorofatty aldehydes. Figure 2 shows that both 2-Cl-Pald and 2-Cl-Sald are formed in the lungs after Cl2 gas exposure with the levels being highest immediately postexposure. Both 2-chlorofatty aldehydes decreased by ∼50–75% within 30 min postexposure, which remained at a steady state over the next 6 h, although a trend toward an increase over this time period was noted. For 2-Cl-Pald, levels decreased further at 12 h, but still remained 2-fold higher compared with baseline (P < 0.05 by t-test) at 24 h. Thereafter, levels decreased to basal. Similar temporal changes were observed for 2-Cl-Sald.
Fig. 2.
Cl2 gas exposure increases chlorinated fatty aldehydes in the lung. C57bl/6 mice were exposed to Cl2 gas (400 ppm, 30 min) and then brought back to room air. At the indicated times thereafter, lungs were collected and 2-Cl-Pald (A) and 2-Cl-Sald (B) were measured. Air indicates basal or air-exposed mice. Time point 0 indicates immediately post-Cl2 exposure. Data shown are mean ± SEM, n = 3–4 (except 72 h, n = 2) and expressed relative to lung wet weight. *P < 0.05 by one-way ANOVA with Dunnett’s multiple comparison post test relative to air.
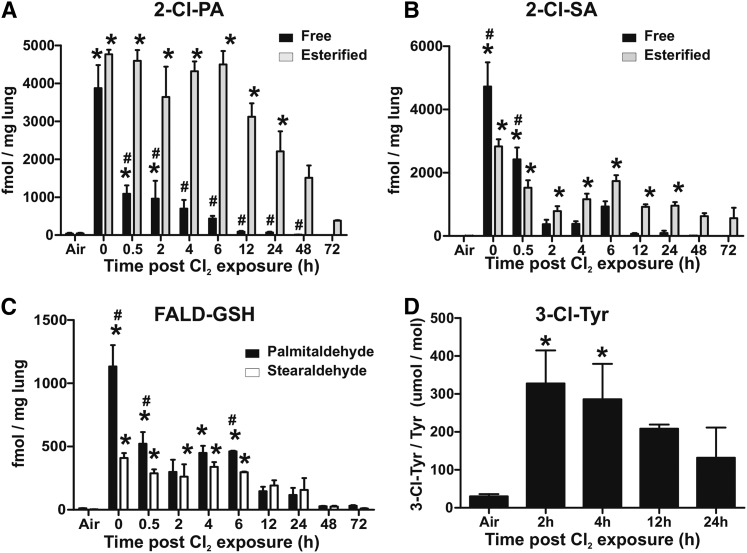
Figure 3A shows changes in free and esterified 2-Cl-PA and 2-Cl-SA in the lungs: esterified levels representing total minus free. Both of these 2-chlorofatty acids are formed via oxidation of aldehydes shown in Fig. 2. Free 2-Cl-PA levels were highest immediately post-Cl2 exposure and then decreased to basal levels over 12 h. Interestingly, esterified 2-Cl-PA levels also increased to a similar extent as free levels immediately post-Cl2 exposure, but remained at this high level for 6 h and then slowly decreased over 72 h; esterified 2-Cl-PAs were still significantly elevated (8-fold) relative to basal at 72 h (P < 0.05 by t-test). Also, at all time points between 0.5 and 72 h, esterified 2-Cl-PA levels were greater compared with free levels. Figure 3B shows changes in free and esterified 2-Cl-SA. Free levels showed a similar temporal profile compared with 2-Cl-PA, with levels decreasing after the cessation of exposure. Esterified 2-Cl-SA also increased initially and then decreased over 2 h and then reaching a steady state that was maintained over 24 h. Notably, levels of 2-Cl-SA were lower compared with 2-Cl-PA.
Fig. 3.
Cl2 gas exposure increases chlorinated fatty acids, GSH-fattyaldehyde adducts, and chlorotyrosine in the lung. C57bl/6 mice were exposed to Cl2 gas (400 ppm, 30 min) and then brought back to room air. At the indicated times thereafter, lungs were collected and free (black bars) and esterified (gray bars) 2-Cl-PA (A) and 2-Cl-SA (B) were measured. Air indicates basal or air-exposed mice. Time point 0 indicates immediately post-Cl2 exposure. Data shown are mean ± SEM, n = 3–9 (except 72 h, n = 2) and expressed relative to lung wet weight. *P < 0.05 by one-way ANOVA with Dunnett’s multiple comparison post test relative to air. #P < 0.05 by two-way repeated measures-ANOVA with Bonferroni post test comparing free and esterified levels at each time. C: GSH adducts with palmitaldehyde (black bars) and stearaldehyde (open bars) in the lung. Data shown are mean ± SEM, n = 3–4 (except 72 h, n = 2) and expressed relative to lung wet weight. *P < 0.05 by one-way ANOVA with Dunnett’s multiple comparison post test relative to air. #P < 0.05 by two-way repeated measures-ANOVA with Bonferroni post test comparing palmitaldehyde- and stearaldehyde-GSH adducts at each time. D: The 3-Cl-Tyr levels normalized to total tyrosine in lungs. Maximum values at 2 h correspond to 6.6 ± 2.8 pmol/mg in lung. All data are mean ± SEM, n = 3–4. *P < 0.05 by one-way ANOVA with Dunnett’s multiple comparison post test relative to air.
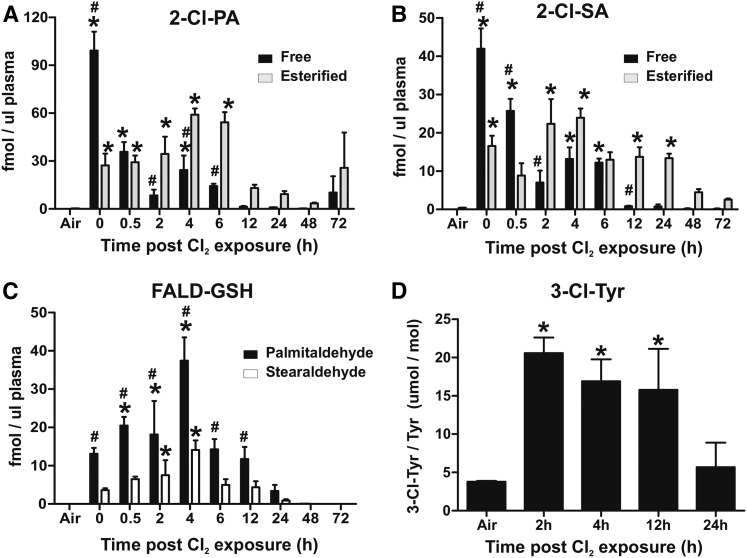
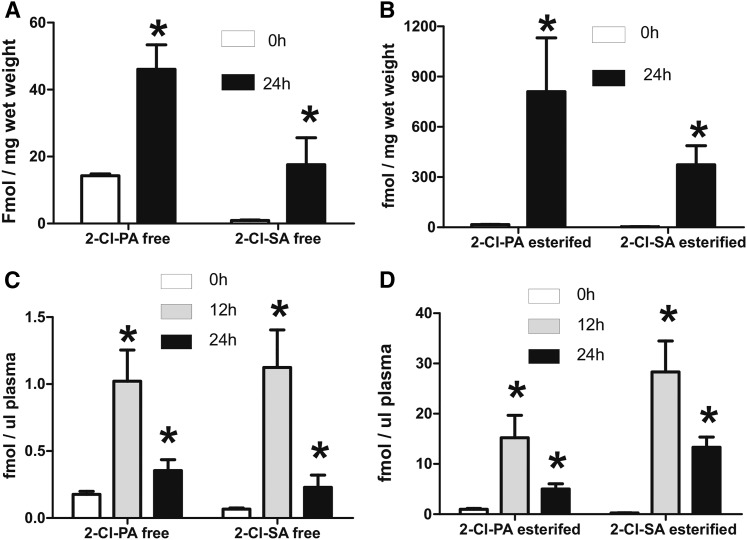
Cl2 gas exposure increases circulating levels of Cl-lipids
Figure 4A, B shows that both free and esterified 2-Cl-PA and 2-Cl-SA increase in the plasma post-Cl2 exposure. Similar to the lung, levels of free forms were highest immediately postexposure, decreasing over 6–12 h thereafter. Esterified Cl-lipid levels immediately postexposure trended to be higher than air (P < 0.05 by t-test for t = 0 and 0.5 h postexposure), and increased between 2 and 6 h (reaching significance by one-way ANOVA), and then decreasing thereafter. Levels at 48 and 72 h remained elevated (5- to 20-fold) compared with air (by t-test). Also, between 2 and 48 h, esterified forms of both 2-Cl-PA and 2-Cl-SA were higher than the respective free forms, and 2-Cl-PA was typically higher than 2-Cl-SA.
Fig. 4.
Cl2 gas exposure increases chlorinated fatty acids, GSH-fattyaldehyde adducts, and chlorotyrosine in the circulation. C57bl/6 mice were exposed to Cl2 gas (400 ppm, 30 min) and then brought back to room air. At the indicated times thereafter, plasma was collected and free (black bars) and esterified (gray bars) 2-Cl-PA (A) and 2-Cl-SA (B) were measured. Air indicates basal or air-exposed mice. Time point 0 indicates immediately post-Cl2 exposure. Data shown are mean ± SEM, n = 3–4 (except 72 h, n = 2). *P < 0.05 by one-way ANOVA with Dunnett’s multiple comparison post test relative to air. #P < 0.05 by two-way repeated measures-ANOVA with Bonferroni post test comparing free and esterified levels at each time. C: GSH adducts with palmitaldehyde (black bars) and stearaldehyde (open bars) in the plasma. Data shown are mean ± SEM, n = 3–4 (except 72 h, n = 2). *P < 0.05 by one-way ANOVA with Dunnett’s multiple comparison post test relative to air. #P < 0.05 by two-way repeated measures-ANOVA with Bonferroni post test comparing palmitaldehyde- and stearaldehyde-GSH adducts at each time. D: The 3-Cl-Tyr levels normalized to total tyrosine in plasma. Maximum values at 2 h correspond to 0.1 ± 0.01 pmol/μl plasma. All data are mean ± SEM, n = 3–4. *P < 0.05 by one-way ANOVA with Dunnett’s multiple comparison post test relative to air.
Cl2 gas exposure increases glutathione-aldehyde adducts
Our recent studies demonstrate that 2-chlorofatty aldehydes react with GSH to form aldehyde conjugates with the elimination of Cl2 (Fig. 1) (26). Figures 3C, 4C show that GSH-adducts with Pald and Sald increase in the lung and circulation, respectively. Temporal changes of these adducts are similar to changes in free 2-chloro-lipids in the lung. In the plasma, however, formation is slower, peaking at 4 h with levels declining thereafter.
Effects of Cl2 gas on Cl-Tyr formation
Figures 3D, 4D show that Cl2 exposure also increased lung and plasma 3-chlorotyrosine, respectively, with levels being highest early postexposure and then declining toward basal 12–24 h thereafter.
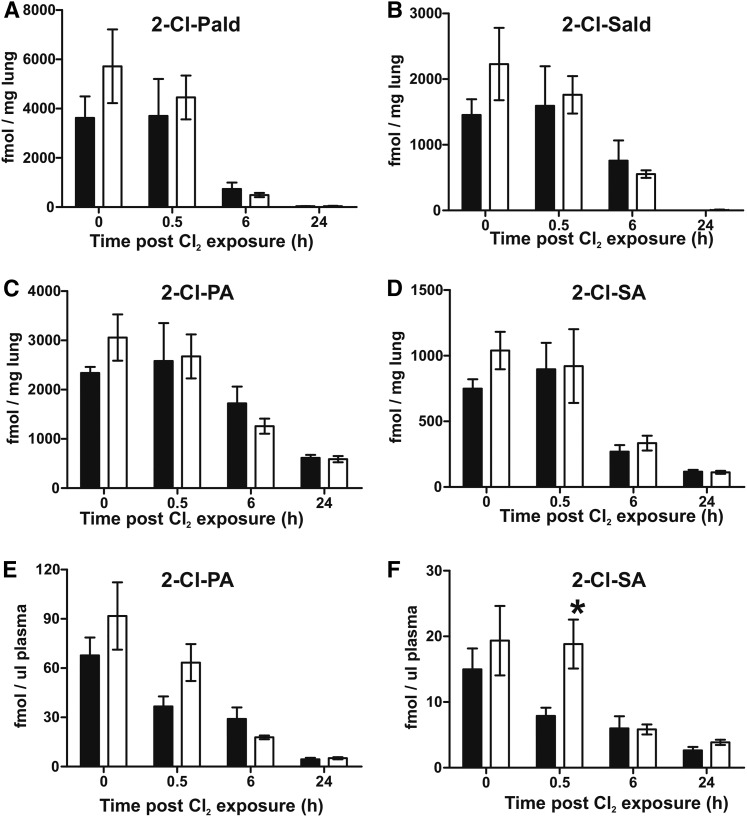
Cl2 gas exposure increases lung and circulating Cl-lipids in rats
Figure 5 shows that Cl-lipids were also increased in rats after similar regimens of Cl2 gas exposure both in the lungs (Fig. 5A, B) and the plasma (Fig. 5C, D), with significant increases still evident 24 h after exposure. These data underscore the possibility that Cl-lipid formation is uniform across different animal species after Cl2-gas exposure.
Fig. 5.
Cl2 gas exposure increases chlorinated fatty acids in rats. Male Sprague Dawley rats were exposed to Cl2 gas (400 ppm, 30 min) and then brought back to room air. At the indicated times thereafter, lungs (A, B) and plasma (C, D) were collected and free and esterified forms of 2-Cl-PA and 2-Cl-SA were measured. Data are mean ± SEM (n = 3). *P < 0.05 relative to air by t-test.
Effects of neutropenia on Cl-lipid formation
To delineate the relative contribution of Cl2 gas versus endogenous PMN (MPO)-dependent formation of chlorinated fatty acids, mice were rendered neutropenic and then exposed to Cl2 gas. Figure 6 shows that neutropenia had no effect on esterified 2-Cl-fatty acid, 2-Cl-fatty acid aldehydes in the lung, or on 2-Cl-fatty acids in the plasma. This is most likely due to the fact that levels of chlorinated fatty acids formed by activated neutrophils are orders of magnitude less than those formed by the reaction of inhaled Cl2 and HOCl with plasmalogens.
Fig. 6.
Effects of neutropenia on Cl2 gas-dependent formation of Cl fatty acids. C57bl/6 mice were treated with anti Ly-6G (clone 1A8) to induce neutropenia or IgG2a isotype control antibody 24 h prior to Cl2 gas (400 ppm, 30 min) exposure. Mice were then brought back to room air and 2-chlorofatty aldehydes and 2-chlorofatty acids were measured in the lung and plasma at the indicated times. A, B: Lung 2-chlorofatty aldehyde levels. C–F: Lung and plasma esterified levels of 2-chlorofatty acids. Control (black bars), neutropenia (open bars). Data shown are mean ± SEM, n = 3–4. *P < 0.05 relative to the respective control by two-way ANOVA with Bonferroni post test.
Effects of Cl-lipids on ALI indices in vivo
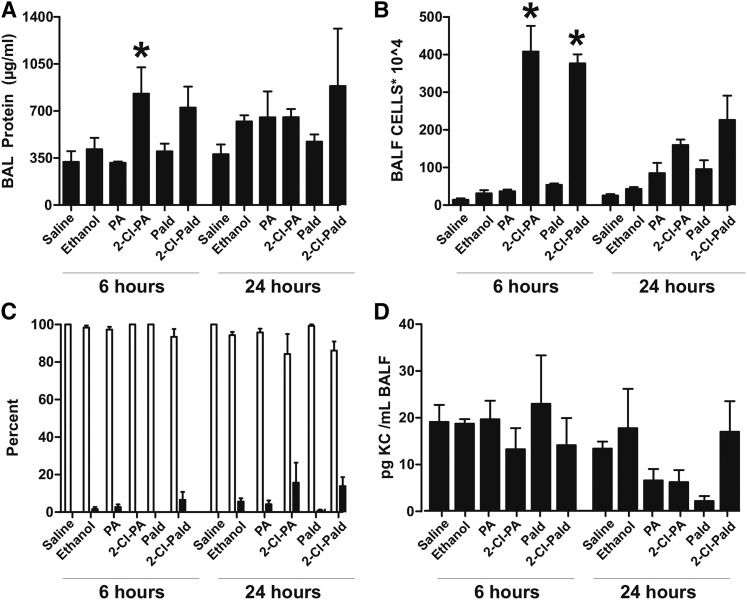
To test to determine whether Cl-lipids could mediate ALI in vivo, 2-Cl-PA and 2-Cl-Pald or their respective native (nonchlorinated) fatty acids/aldehydes were administered intranasally to mice and ALI assessed 6–24 h thereafter. The final nominal administered dose of Cl-lipids was 12 pmol/mg lung, which is comparable to maximal levels of 2-Cl-PA and 2-Cl-Pald measured in the lung after Cl2 gas exposure (5 pmol/mg lung), and we note that the actual concentration of applied Cl-lipids that reach the distal airways is likely to be less than 5% of the intranasal dose. Figure 7A shows that 6 h after administration, 2-Cl-PA significantly increased BAL protein compared with PA alone, with trends toward increased protein for 2-Cl-Pald also noted. No differences between Cl-lipids and respective fatty acid controls or vehicle controls were observed 24 h after administration following this single intranasal administration. Figure 7B shows that both 2-Cl-PA and 2-Cl-Pald significantly increased levels of inflammatory cells in the BAL compared with nonchlorinated fatty acids at 6 h that comprised both macrophages and neutrophils (Fig. 6C). No significant differences were observed at 24 h, although trends toward increased inflammatory cells were noted. Figure 6D shows that there were no significant changes in the CXCL1 chemokine in the BAL 24 h after 2-Cl-PA administration.
Fig. 7.
Effects of Cl-lipids on lung permeability and inflammation. C57bl/6 male mice were exposed to saline, ethanol, PA, Pald, 2-Cl-PA, or 2-Cl-Pald by intranasal administration and lung injury was assessed by measuring BAL levels of protein (A) and inflammatory cells (B) 6–24 h thereafter. C: Percent of total cells that were macrophages (open bars) or neutrophils (black bars). Data are mean ± SEM (n = 4–6). *P < 0.05 relative to corresponding native fatty acid by one-way ANOVA with Tukey post test. D: BALF levels of CXCL1 chemokine (KC) at 6 and 24 h post-Cl-lipid administration.
Effects of Cl-lipids on airway reactivity
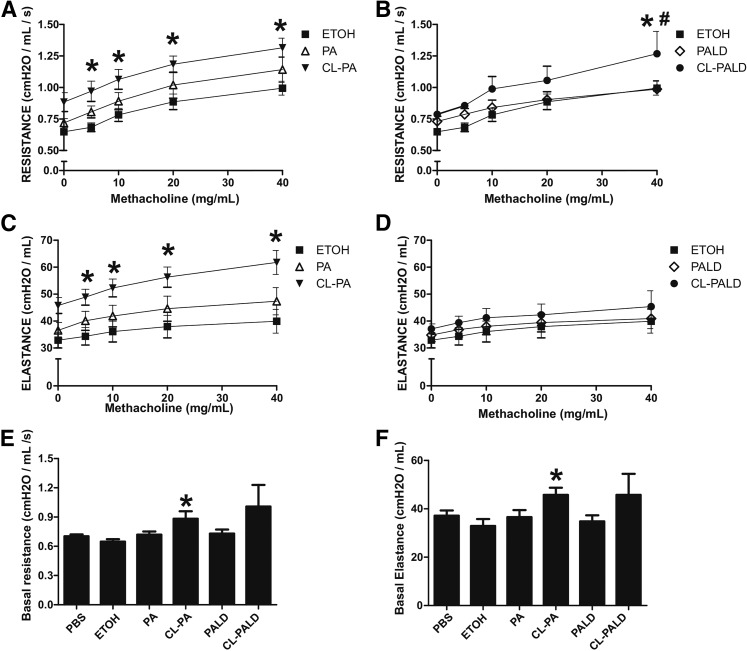
Airway hypersensitivity is a feature of post-Cl2 gas exposure toxicity. To test whether Cl-lipids play a role in this process, Cl-lipids were administered as described above and basal- and methacholine-induced airway resistance and elastance was measured 24 h thereafter. Figure 8A, B shows that methacholine induced a dose-dependent increase in resistance. This was exacerbated with 2-Cl-PA at all methacholine doses, and with 2-Cl-Pald also, albeit only at the highest methacholine dose tested. Figure 8C, D shows changes in elastance. Similar to resistance, 2-Cl-PA exaggerated methacholine effects; however, 2-Cl-Pald had no effect. Finally, Figure 8E, F shows basal effects of Cl-lipid treatment of reactive airways. Small but significant increases in basal resistance and elastance were observed only with 2-Cl-PA.
Fig. 8.
Effects of Cl-lipids on airway hyperresponsiveness. C57bl/6 male mice were exposed to ethanol (ETOH) (filled square), PA (open triangle), Pald (open diamond), 2-Cl-PA (filled inverted triangle) or 2-Cl-Pald (filled circle) by intranasal administration and basal and methacholine-induced airway resistance (A, B, E) and elastance (C, D, F) determined by Flexivent. For clarity, 2-Cl-PA (A, C, E) and 2-Cl-Pald (B, D, F) are shown on separate panels; ethanol control group is the same on each panel. Data are mean ± SEM (n = 3–4). For (A–D), *P < 0.05 relative to ethanol or #P < 0.05 relative to parent fatty acid by two-way repeated measures-ANOVA with Bonferroni post test. For (E, F), *P < 0.05 by one-way ANOVA with Tukey post test.
Effects of Cl-lipids of systemic vascular dilation
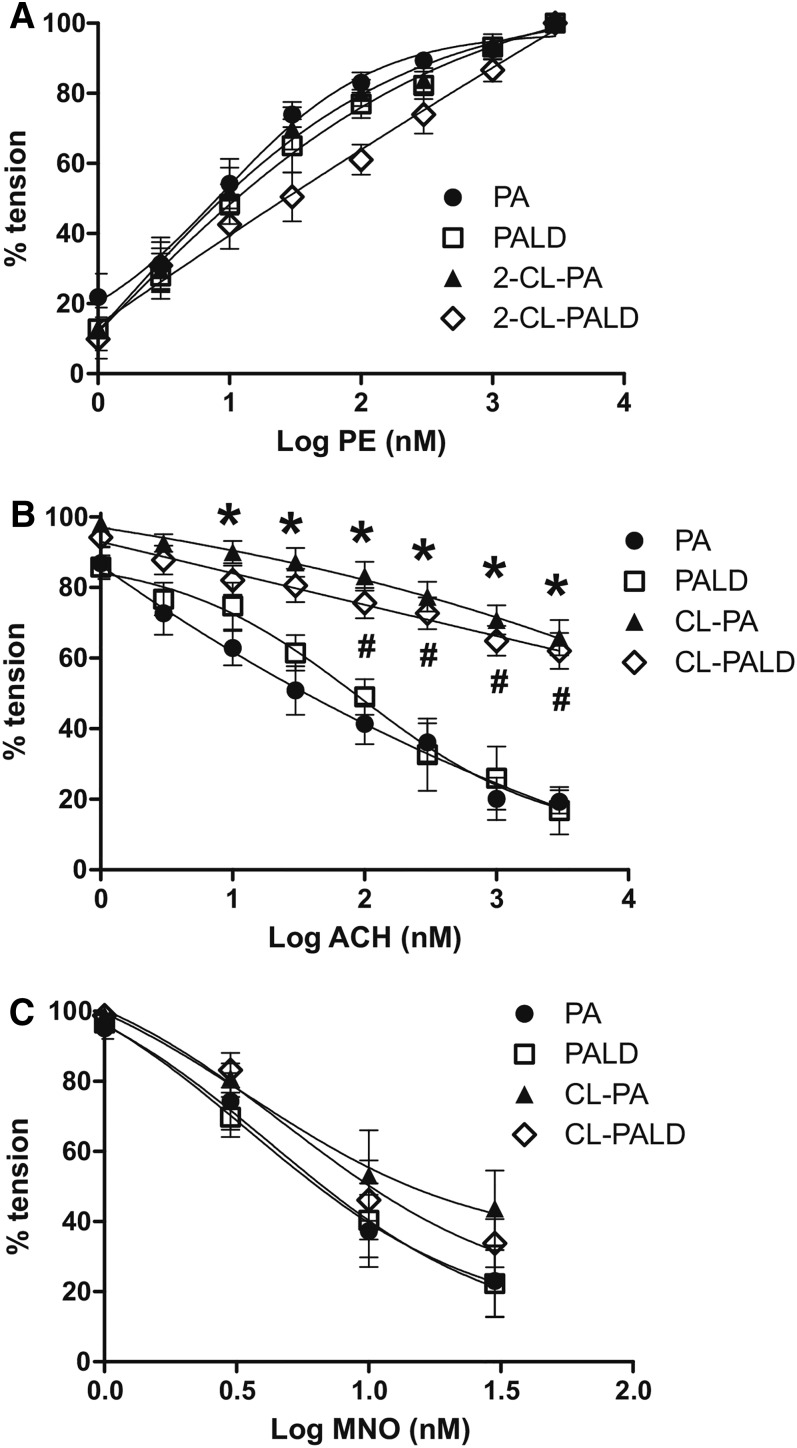
Our previous studies have shown that eNOS-dependent vasodilation becomes inhibited post-Cl2 gas exposure (15, 16) and other groups have shown that Cl-lipid addition to cultured endothelial cells inhibits eNOS activity (27). We therefore tested eNOS-dependent vasodilation of aortas isolated 24 h after intranasal Cl-lipid administration. Figure 9A shows that PE induced contraction in a dose-dependent manner that did not differ among experimental groups. Figure 9B shows that the acetylcholine-induced vasodilation, which is eNOS-dependent, was similar in aortas isolated from native fatty acid- or aldehyde-treated mice. This response was significantly blunted (indicated by right shifts in dose-curves), however, in aorta isolated from either 2-Cl-PA- or 2-Cl-Pald-treated mice. Figure 9C shows that MNO-dependent vasodilation was similar in all groups, indicating that smooth muscle responsiveness to NO-donors was not altered.
Fig. 9.
Effects of Cl-lipids on aortic vasodilation. C57bl/6 male mice were exposed to PA (filled circle), Pald (open square), 2-Cl-PA (filled triangle), or 2-Cl-Pald (open diamond) by intranasal administration. Twenty-four hours thereafter, the aortas were isolated and ex vivo responsiveness to PE-dependent contraction (A), acetylcholine-dependent relaxation (B), and MNO-dependent relaxation (C) were assessed. Data show mean ± SEM (n = 3–5). *P < 0.05 by two-way repeated measures-ANOVA with Bonferroni post test for PA versus 2-Cl-PA. #P < 0.05 by two-way repeated measures-ANOVA with Bonferroni post test for Pald versus 2-Cl-Pald.
DISCUSSION
During exposure, Cl2 gas reacts with the airways to cause acute toxicity characterized by airway cell death and activation of pathways leading to postexposure pulmonary inflammation and increased permeability of the blood gas barrier to plasma proteins. It is likely that the postexposure phase reflects both tissue responses to the initial insult and ongoing injury to secondary derived species. The mediators and mechanisms remain unclear, underscoring the need to understand Cl2 gas-derived products. Cl2 gas exposure also leads to tissue inflammation and microcirculatory dysfunction in extrapulmonary tissues. For example, animals exposed to Cl2 and returned to room air develop endothelial and myocardial dysfunction and failure (13, 16). This pattern of injury is observed with many chemically distinct inhaled irritants and pollutants (e.g., ozone, particulate matter) (39–42). A key question is how is the primary reactivity of the inhaled species in the lungs transduced to the periphery: with one model proposed being that the initial insult forms a secondary longer-lived species that can egress into the circulation. There are several Cl2-specific products that may be formed, including chloramines (18, 43); here we tested a role for Cl-lipids.
We used mouse and rat models of Cl2 gas exposure that were sublethal but which resulted in postexposure ALI, reactive airway syndrome, and systemic inflammation. Both 16 carbon and 18 carbon chlorinated fatty acids and aldehydes were formed following Cl2 gas exposure. For all Cl-lipid species, the levels were greatest immediately postexposure, suggesting direct formation during the Cl2 gas exposure. The levels of Cl-lipid decreased after exposure. The kinetics of this decay was not a simple first or second order process however, and was unique to the specific Cl-fatty acid/aldehyde species. That said, three distinct phases for the postexposure decay could be generalized. The first was a rapid phase in which ∼50–70% of the Cl-lipids formed decreased within 30 min postexposure. This was followed by a second phase where levels did not change, or changed very slightly over 6 h. Whether this reflects Cl-lipid stability (lack of metabolism) or a steady-state (where formation equals decay and/or wash out) is not known. We speculate that it is lack of metabolism, because eliminating neutrophils, the major source of endogenous chlorinating species, did not change Cl-lipid levels. The final phase was a slow (>12 h) decay back to basal levels. An additional consideration is the relationship of free and esterified pools of chlorinated fatty acids. The source of 2-chlorofatty acids is 2-chlorofatty aldehyde (the product of Cl2 oxidation of plasmalogens). The biological half-life of the esterified pool is longer than that of the free pool. The longer biological half-life of esterified 2-chlorofatty acids in plasma made this a better biomarker of Cl2 gas exposure compared with free 2-chlorofatty acids because it was detectable 72 h after exposure. One question that remains is to determine the complex lipid pool esterifying 2-chlorofatty acids. This requires further study, but we note that esterified levels were higher than free forms, likely reflecting esterified pools as more stable, perhaps biologically inert, pools to store bioactive 2-chlorofatty acids.
Importantly, 2-chlorofatty acids also increased in the plasma. For the free forms, levels were highest immediately postexposure, whereas esterified forms increased postexposure. This suggests an active esterification of free Cl-lipids and/or egress of esterified Cl-lipids from the lungs to the circulation. As with the lung levels, neutropenia had no effect on plasma Cl-lipid levels, suggesting that the contribution of PMN-derived myeloperoxidase activity to circulating Cl-lipid levels is minimal. This is consistent with our prior experiences, where the levels of Cl-lipids formed in models of PMN-inflammation (21) are still much lower compared with levels observed here with Cl2 gas formation. Collectively, these data show that plasmalogens are significant targets for Cl2 gas, resulting ultimately in 2-chlorofatty acid production, and also highlight our lack of understanding of how these lipid species are metabolized. In this regard, fatty aldehyde adducts with GSH increased post-Cl2 exposure, likely due to reactions between 2-chloroaldehydes and GSH, as recently described, and confirming the concept that the redox milieu is key in controlling Cl-lipid levels and metabolism (19, 26). We also recognize that there may be a host of other Cl-lipid species formed either directly from Cl2 gas or after reaction of formed Cl-lipids with biomolecules or chlorinated amino acids, which have been shown to damage SERCA and lung ion channels (13, 43); measurement of these is required to fully understand metabolism and fate of formed Cl-lipids.
In addition, circulating levels of Cl-lipids over the first 6 h postexposure were several orders of magnitude higher compared with basal, and remained at least 2-fold higher at 24–48 h postexposure. Notably, levels of Cl-lipids only increase by 2- to 3-fold in response to inflammation [e.g., after Sendai virus exposure or LPS treatment (21) via myeloperoxidase-dependent mechanisms]. Formation of high concentrations, ability to detect differences several hours after exposure in a readily accessible compartment (i.e., plasma), stability of Cl-lipids to sample freezing, storage, and transport, and the high sensitivity of LC/MS to detect Cl-lipids all suggest that Cl-lipids should be considered as selective biomarkers for Cl2 gas exposure. A consideration in proposing Cl-lipids as biomarkers is how the levels and mechanisms of formation compare with endogenous MPO/HOCl-dependent processes. Cl2 and HOCl are in equilibrium, and both direct reactivity of Cl2 (in aprotic solvents) and HOCl can lead to Cl-lipid formation (20). While there are Cl-lipids and other oxidation products that are unique to Cl2 and not HOCl reactions (44, 45), it is likely that both Cl2 and Cl2-derived HOCl mediate formation of Cl-lipids during Cl2 gas exposure. A significant role for MPO-derived HOCl can be excluded, however, based on similar Cl-lipid levels in neutropenic mice exposed to Cl2 gas. The key point for considering Cl-lipids as biomarkers for Cl2 gas exposure is that the levels of Cl-lipids formed after Cl2 gas exposure will be significantly higher than those formed during endogenous inflammation. This concept could also be applied for other halogens. Indeed, Br-fatty acids are increased in bromine-exposed mice (Matalon et al., unpublished observations). We also showed formation of 3-Cl-tyrosine in the lungs and plasma of mice exposed to Cl2 gas consistent with a recent study showing formation of both 3-Cl-Tyr and di-chloro-Tyr after Cl2 exposure of blood ex vivo (46). Similar to Cl-lipids, 3-Cl-Tyr levels declined 12–24 h postexposure, although at 24 h, 3-Cl-Tyr levels returned to baseline, whereas Cl-lipids remained 10- to 20-fold higher, suggesting a greater sensitivity for the latter in detecting Cl2 gas exposure. We also appreciate that there are a myriad of other Cl2-dependent products that are likely formed after Cl2 gas exposure that could be used as biomarkers. While some, such as chloramines, may mediate Cl2 gas toxicity (18, 43), their relatively short lifetimes in vivo preclude them as useful biomarkers. Future studies need to more extensively compare Cl-lipids with other chlorinated modifications for biomarker assessments.
Beyond a biomarker role, we also evaluated the potential for Cl-lipids to mediate post-Cl2 gas exposure toxicity. Previous studies have shown that Cl-lipids can elicit cell death in endothelial and neuronal cells, promote cell permeability leading to compromised blood-brain barrier, inhibit endothelial function by inhibiting eNOS-dependent signaling, and promote the inflammatory potential of endothelial cells, neutrophils, and macrophages (27, 28, 30, 47, 48). The precise mechanisms linking Cl-lipids to these responses remains under investigation, but modulation of MAPKs and perturbation of redox-signaling are likely candidates. Indeed, Cl-lipids are more electrophilic compared with parent fatty acids (49), and fatty acid electrophiles are known to change redox signaling and alter cell function in various inflammatory disease settings. We therefore conducted proof-of-concept studies to test whether intranasal administration (into the lungs) of 2-Cl-PA or 2-Cl-Pald could elicit effects similar to those observed after Cl2 gas exposure. Changes in lung endothelial and/or epithelial permeability were indicated by increased BAL protein levels, consistent with the pro-permeability effects of Cl-lipids. Also, similar to Cl2 gas, Cl-lipids increased levels of inflammatory cells; however, these were predominantly macrophages, with smaller increases in neutrophils. Cl2 gas increases both macrophages and neutrophils, with neutrophils being predominant. Consistent with a minimal pro-neutrophil effect, neither 2-Cl-PA nor 2-Cl-Pald had any effect on pro-neutrophilic chemokines. Ongoing studies are exploring the mechanisms by which Cl-lipids increase airway macrophage numbers and suggest regulatory roles on airway immunity. With respect to reactive airways, 2-Cl-16:0 fatty acid had a modest effect on basal airway resistance and increased methacholine sensitivity compared with vehicle or nonchlorinated fatty acid. 2-Cl-Pald had minimal effects on reactive airways, however, suggesting that distinct 2-Cl-PA may elicit unique responses. The extrapulmonary effects of intranasally administered Cl-lipids were more compelling. Both 2-Cl-PA and 2-Cl-Pald inhibited eNOS-dependent vasodilation in the aorta. This effect appears to be endothelial dependent, as NO (generated by the donor, MNO)-dependent vasodilation of the smooth muscle was not affected. These data are consistent with previous studies showing loss of eNOS activity in cultured endothelial cells treated with Cl-lipids and with studies showing a similar loss of eNOS-dependent vasodilation in aortas isolated from Cl2 gas-exposed animals. These data suggest that Cl-lipids can move from the lung to the periphery to elicit extrapulmonary effects characterized by eNOS inhibition, which leads to the hypothesis that plasma Cl-lipids formed after Cl2 gas exposure may mediate extrapulmonary toxicities to the vasculature and heart (13, 15, 16).
We do note several limitations, however, and appreciate that Cl-lipids alone do not duplicate all Cl2 gas exposure effects. For example, mice exposed to Cl2 gas develop hyper responsiveness to methacholine, which is not the case with Cl-lipid administration. The dose of Cl-lipids administered was different to that formed endogenously and other experimental limitations precluded testing of 16 and 18 carbon chlorinated species. Whether there are antagonistic, additive, or synergistic interactions between 16 and 18 carbon chlorofatty acids is not known, but testing of the combination is required to better assess the potential role of Cl-lipids in Cl2 gas exposure toxicity. Also, intranasal administration does not only lead to airway deposition and it is possible that some effects could be mediated by direct absorption of Cl-lipids into the blood via vessels perfusing the nares and upper airways. Notwithstanding these limitations, collectively these data suggest that a single instillation of chlorinated palmitate results in an increase of airway resistance and alveolar permeability, which are also seen in mice exposed to Cl2 and returned to room air, and suggest that targeting of Cl-lipid metabolism to facilitate clearance or limit reactivity may provide a new perspective on therapeutics to limit postexposure toxicities.
Footnotes
Abbreviations:
- ALI
- acute lung injury
- Cl-lipid
- chlorinated lipid
- Cl2
- chlorine
- 2-Cl-PA
- 2-chloropalmitic acid
- 2-Cl-Pald
- 2-chloropalmitaldehyde
- 2-Cl-SA
- 2-chlorostearic acid
- 2-Cl-Sald
- 2-chlorostearaldehyde
- KC
- CXCL1 chemokine
- MNO
- MAHMA NONOate
- PA
- palmitic acid
- PE
- phenylephrine
- TFA
- trifluoroacetic acid
This research was supported by the CounterACT Program, National Institutes of Health, Office of the Director, and the National Institute of Environmental Health Sciences, Grants U01ES023759 (R.P.P.), and 1 U01 ES02645801A1, 1R21 ES02682901, and 5R21ES025423 02 (S.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Evans R. B. 2005. Chlorine: state of the art. Lung. 183: 151–167. [DOI] [PubMed] [Google Scholar]
- 2.Matalon S., and Maull E. A.. 2010. Understanding and treating chlorine-induced lung injury. Proc. Am. Thorac. Soc. 7: 253. [DOI] [PubMed] [Google Scholar]
- 3.Van Sickle D., Wenck M. A., Belflower A., Drociuk D., Ferdinands J., Holguin F., Svendsen E., Bretous L., Jankelevich S., Gibson J. J., et al. . 2009. Acute health effects after exposure to chlorine gas released after a train derailment. Am. J. Emerg. Med. 27: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honavar J., Samal A. A., Bradley K. M., Brandon A., Balanay J., Squadrito G. L., Mohankumar K., Maheshwari A., Postlethwait E. M., Matalon S., et al. . 2011. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am. J. Respir. Cell Mol. Biol. 45: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C., Weng Z., Doran S. F., Srivastava R. K., Afaq F., Matalon S., and Athar M.. 2013. Chlorine induces the unfolded protein response in murine lungs and skin. Am. J. Respir. Cell Mol. Biol. 49: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin J. G., Campbell H. R., Iijima H., Gautrin D., Malo J. L., Eidelman D. H., Hamid Q., and Maghni K.. 2003. Chlorine-induced injury to the airways in mice. Am. J. Respir. Crit. Care Med. 168: 568–574. [DOI] [PubMed] [Google Scholar]
- 7.McGovern T., Day B. J., White C. W., Powell W. S., and Martin J. G.. 2011. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic. Biol. Med. 50: 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musah S., Chen J., and Hoyle G. W.. 2012. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir. Res. 13: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Koren E. G., Hogan B. L., and Gunn M. D.. 2013. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am. J. Respir. Cell Mol. Biol. 49: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samal A., Honovar J., White C. R., and Patel R. P.. 2010. Potential for chlorine gas-induced injury in the extrapulmonary vasculature. Proc. Am. Thorac. Soc. 7: 290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White C. W., and Martin J. G.. 2010. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc. Am. Thorac. Soc. 7: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav A. K., Doran S. F., Samal A. A., Sharma R., Vedagiri K., Postlethwait E. M., Squadrito G. L., Fanucchi M. V., Roberts L. J. II, Patel R. P., et al. . 2011. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am. J. Physiol. Lung Cell. Mol. Physiol. 300: L362–L369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaky A., Bradley W. E., Lazrak A., Zafar I., Doran S., Ahmad A., White C. W., Dell’Italia L. J., Matalon S., and Ahmad S.. 2015. Chlorine inhalation-induced myocardial depression and failure. Physiol. Rep. 3: e12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Squadrito G. L., Postlethwait E. M., and Matalon S.. 2010. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am. J. Physiol. Lung Cell. Mol. Physiol. 299: L289–L300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honavar J., Bradley E., Bradley K., Oh J. Y., Vallejo M. O., Kelley E. E., Cantu-Medellin N., Doran S., Dell’italia L. J., Matalon S., et al. . 2014. Chlorine gas exposure disrupts nitric oxide homeostasis in the pulmonary vasculature. Toxicology. 321: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honavar J., Samal A. A., Bradley K. M., Brandon A., Balanay J., Squadrito G. L., MohanKumar K., Maheshwari A., Postlethwait E. M., Matalon S., et al. . 2011. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am. J. Respir. Cell Mol. Biol. 45: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazrak A., Creighton J., Yu Z., Komarova S., Doran S. F., Aggarwal S., Emala C. W. Sr., Stober V. P., Trempus C. S., Garantziotis S., et al. . 2015. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 308: L891–L903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad S., Ahmad A., Hendry-Hofer T. B., Loader J. E., Claycomb W. C., Mozziconacci O., Schoneich C., Reisdorph N., Powell R. L., Chandler J. D., et al. . 2015. Sarcoendoplasmic reticulum Ca(2+) ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am. J. Respir. Cell Mol. Biol. 52: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spickett C. M. 2007. Chlorinated lipids and fatty acids: an emerging role in pathology. Pharmacol. Ther. 115: 400–409. [DOI] [PubMed] [Google Scholar]
- 20.Albert C. J., Crowley J. R., Hsu F. F., Thukkani A. K., and Ford D. A.. 2001. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J. Biol. Chem. 276: 23733–23741. [DOI] [PubMed] [Google Scholar]
- 21.Anbukumar D. S., Shornick L. P., Albert C. J., Steward M. M., Zoeller R. A., Neumann W. L., and Ford D. A.. 2010. Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal. J. Lipid Res. 51: 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thukkani A. K., Albert C. J., Wildsmith K. R., Messner M. C., Martinson B. D., Hsu F. F., and Ford D. A.. 2003. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J. Biol. Chem. 278: 36365–36372. [DOI] [PubMed] [Google Scholar]
- 23.Thukkani A. K., Martinson B. D., Albert C. J., Vogler G. A., and Ford D. A.. 2005. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 288: H2955–H2964. [DOI] [PubMed] [Google Scholar]
- 24.Thukkani A. K., McHowat J., Hsu F. F., Brennan M. L., Hazen S. L., and Ford D. A.. 2003. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 108: 3128–3133. [DOI] [PubMed] [Google Scholar]
- 25.Wildsmith K. R., Albert C. J., Anbukumar D. S., and Ford D. A.. 2006. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J. Biol. Chem. 281: 16849–16860. [DOI] [PubMed] [Google Scholar]
- 26.Duerr M. A., Aurora R., and Ford D. A.. 2015. Identification of glutathione adducts of alpha-chlorofatty aldehydes produced in activated neutrophils. J. Lipid Res. 56: 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsche G., Heller R., Fauler G., Kovacevic A., Nuszkowski A., Graier W., Sattler W., and Malle E.. 2004. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler. Thromb. Vasc. Biol. 24: 2302–2306. [DOI] [PubMed] [Google Scholar]
- 28.Nusshold C., Kollroser M., Kofeler H., Rechberger G., Reicher H., Ullen A., Bernhart E., Waltl S., Kratzer I., Hermetter A., et al. . 2010. Hypochlorite modification of sphingomyelin generates chlorinated lipid species that induce apoptosis and proteome alterations in dopaminergic PC12 neurons in vitro. Free Radic. Biol. Med. 48: 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Üllen A., Nusshold C., Glasnov T., Saf R., Cantillo D., Eibinger G., Reicher H., Fauler G., Bernhart E., Hallstrom S., et al. . 2015. Covalent adduct formation between the plasmalogen-derived modification product 2-chlorohexadecanal and phloretin. Biochem. Pharmacol. 93: 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W. Y., Albert C. J., and Ford D. A.. 2014. Alpha-chlorofatty acid accumulates in activated monocytes and causes apoptosis through reactive oxygen species production and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 34: 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leustik M., Doran S., Bracher A., Williams S., Squadrito G. L., Schoeb T. R., Postlethwait E., and Matalon S.. 2008. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am. J. Physiol. Lung Cell. Mol. Physiol. 295: L733–L743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarogiannis S. G., Jurkuvenaite A., Fernandez S., Doran S. F., Yadav A. K., Squadrito G. L., Postlethwait E. M., Bowen L., and Matalon S.. 2011. Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice. Am. J. Respir. Cell Mol. Biol. 45: 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fanucchi M. V., Bracher A., Doran S. F., Squadrito G. L., Fernandez S., Postlethwait E. M., Bowen L., and Matalon S.. 2012. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol. 46: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honavar J., Doran S., Oh J. Y., Steele C., Matalon S., and Patel R. P.. 2014. Nitrite therapy improves survival postexposure to chlorine gas. Am. J. Physiol. Lung Cell. Mol. Physiol. 307: L888–L894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wacker B. K., Albert C. J., Ford B. A., and Ford D. A.. 2013. Strategies for the analysis of chlorinated lipids in biological systems. Free Radic. Biol. Med. 59: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hazen S. L., Crowley J. R., Mueller D. M., and Heinecke J. W.. 1997. Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: a sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation. Free Radic. Biol. Med. 23: 909–916. [DOI] [PubMed] [Google Scholar]
- 37.Brennan M. L., Wu W., Fu X., Shen Z., Song W., Frost H., Vadseth C., Narine L., Lenkiewicz E., Borchers M. T., et al. . 2002. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 277: 17415–17427. [DOI] [PubMed] [Google Scholar]
- 38.Samal A. A., Honavar J., Brandon A., Bradley K. M., Doran S., Liu Y., Dunaway C., Steele C., Postlethwait E. M., Squadrito G. L., et al. . 2012. Administration of nitrite after chlorine gas exposure prevents lung injury: effect of administration modality. Free Radic. Biol. Med. 53: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuang G. C., Yang Z., Westbrook D. G., Pompilius M., Ballinger C. A., White C. R., Krzywanski D. M., Postlethwait E. M., and Ballinger S. W.. 2009. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 297: L209–L216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newby D. E., Mannucci P. M., Tell G. S., Baccarelli A. A., Brook R. D., Donaldson K., Forastiere F., Franchini M., Franco O. H., Graham I., et al. . 2015. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 36: 83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penn A., and Snyder C. A.. 1996. 1,3 Butadiene, a vapor phase component of environmental tobacco smoke, accelerates arteriosclerotic plaque development. Circulation. 93: 552–557. [DOI] [PubMed] [Google Scholar]
- 42.Ying Z., Kampfrath T., Thurston G., Farrar B., Lippmann M., Wang A., Sun Q., Chen L. C., and Rajagopalan S.. 2009. Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol. Sci. 111: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song W., Wei S., Zhou Y., Lazrak A., Liu G., Londino J. D., Squadrito G. L., and Matalon S.. 2010. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J. Biol. Chem. 285: 9716–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazen S. A., Waheed A., Sly W. S., LaNoue K. F., and Lynch C. J.. 1996. Differentiation-dependent expression of CA V and the role of carbonic anhydrase isozymes in pyruvate carboxylation in adipocytes. FASEB J. 10: 481–490. [DOI] [PubMed] [Google Scholar]
- 45.Hazen S. L., Hsu F. F., Duffin K., and Heinecke J. W.. 1996. Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J. Biol. Chem. 271: 23080–23088. [DOI] [PubMed] [Google Scholar]
- 46.Crow B. S., Quinones-Gonzalez J., Pantazides B. G., Perez J. W., Winkeljohn W. R., Garton J. W., Thomas J. D., Blake T. A., and Johnson R. C.. 2016. Simultaneous measurement of 3-chlorotyrosine and 3,5-dichlorotyrosine in whole blood, serum and plasma by isotope dilution HPLC-MS-MS. J. Anal. Toxicol. 40: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messner M. C., Albert C. J., and Ford D. A.. 2008. 2-Chlorohexadecanal and 2-chlorohexadecanoic acid induce COX-2 expression in human coronary artery endothelial cells. Lipids. 43: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Üllen A., Singewald E., Konya V., Fauler G., Reicher H., Nusshold C., Hammer A., Kratky D., Heinemann A., Holzer P., et al. . 2013. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo. PLoS One. 8: e64034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wildsmith K. R., Albert C. J., Hsu F. F., Kao J. L., and Ford D. A.. 2006. Myeloperoxidase-derived 2-chlorohexadecanal forms Schiff bases with primary amines of ethanolamine glycerophospholipids and lysine. Chem. Phys. Lipids. 139: 157–170. [DOI] [PubMed] [Google Scholar]