Abstract
The endoplasmic reticulum (ER) is a cellular organelle important for regulating calcium homeostasis, lipid metabolism, protein synthesis, and posttranslational modification and trafficking. Numerous environmental, physiological, and pathological insults disturb ER homeostasis, referred to as ER stress, in which a collection of conserved intracellular signaling pathways, termed the unfolded protein response (UPR), are activated to maintain ER function for cell survival. However, excessive and/or prolonged UPR activation leads to initiation of self-destruction through apoptosis. Excessive accumulation of lipids and their intermediate products causes metabolic abnormalities and cell death, called lipotoxicity, in peripheral organs, including the pancreatic islets, liver, muscle, and heart. Because accumulating evidence links chronic ER stress and defects in UPR signaling to lipotoxicity in peripheral tissues, understanding the role of ER stress in cell physiology is a topic under intense investigation. In this review, we highlight recent findings that link ER stress and UPR signaling to the pathogenesis of peripheral organs due to lipotoxicity.
Keywords: endoplasmic reticulum, cell signaling, diabetes, fatty acid, lipids
The endoplasmic reticulum (ER) is a central organelle where proteins destined for the cell surface and the endomembrane system enter the secretory pathway. Once inside the ER lumen, newly synthesized polypeptides fold into their three-dimensional structures, assemble into higher order multimeric complexes, and are subject to posttranslational modifications such as glycosylation, hydroxylation, lipidation, and disulfide formation. The ER contains an extremely high Ca2+ concentration (1), which is maintained by the active transport function of the sarco/ER calcium ATPase (SERCA) (2). Many ER chaperone proteins and enzymes depend on higher Ca2+ levels to facilitate protein folding and maturation (3). Therefore, maintaining ER homeostasis is essential for proper protein production and cell function. Homeostasis in the ER is disrupted by a number of insults, including pharmacological perturbation, genetic mutation of ER chaperones or their client proteins, elevated expression of proteins that transit the endomembrane system, viral infection, alterations in Ca2+ or redox status, and limited or excessive available nutrients such as lipids. The accumulation of unfolded or misfolded proteins in the ER lumen activates a set of intracellular signaling pathways known as the unfolded protein response (UPR) (4, 5). The UPR regulates the quantity of ER driven by synthesis of lipids (6) and protein components of the ER to accommodate fluctuating demands on protein folding and other ER functions in response to different physiological and pathological conditions.
The ER is also the major site for the synthesis of sterols and phospholipids that constitute the bulk of the lipid components of all biological membranes. In addition, many enzymes and regulatory proteins involved in lipid metabolism reside in the ER. The ER, therefore, plays an essential role in controlling membrane lipid composition (7) and membrane lipid homeostasis in all cell types. Fat accumulates in the presence of excessive caloric uptake to serve as an energy store. Adipocytes are specialized cells that store excess lipids. However, lipid overloading of adipocytes causes lipids to accumulate in nonadipose tissues, including the pancreas, liver, muscle, and heart, causing cell dysfunction and associated pathologies. This lipotoxicity is a fundamental contributor to metabolic disease and insulin resistance (8, 9). Given the pivotal role of the ER in lipid metabolism, it is important to understand the linkage between ER homeostasis and lipotoxicity.
ER STRESS AND THE UPR
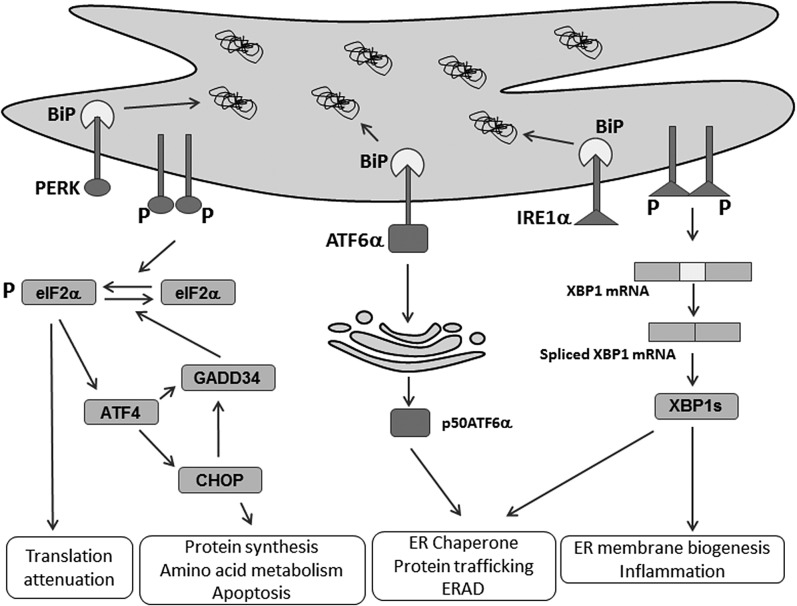
In mammals, the UPR is signaled through activation of three ER transmembrane ER stress sensors (Fig. 1): 1) inositol-requiring enzyme 1α (IRE1α); 2) the double-stranded RNA-activated protein kinase-like eukaryotic initiation factor 2α kinase (PERK); and 3) activating transcription factor 6α (ATF6α) (10). The UPR sensors are all maintained in an inactive state through interaction between their ER luminal domains with the protein chaperone, immunoglobulin heavy-chain binding protein (BiP; also known as GRP78 and HSP5A). When unfolded/misfolded proteins accumulate in the ER lumen upon ER stress, they bind to and sequester BiP, thereby promoting BiP dissociation and initiating downstream signaling (11–13).
Fig. 1.
ER stress and the UPR. Numerous environmental, physiological, and pathological insults cause ER stress and activate the UPR. The UPR is signaled by three ER membrane-bound proteins, PERK, IRE1α, and ATF6α, to resolve ER homeostasis through translational and transcriptional changes in response to ER stress. PERK phosphorylates eIF2α to attenuate protein synthesis, which preferentially activates ATF4 mRNA translation to induce its target genes, CHOP and GADD34. Activated IRE1α cleaves XBP1 mRNA to produce a spliced form that translates a novel polypeptide, XBP1s, to upregulate genes involved in ER membrane biogenesis, ER folding and trafficking, and ERAD. ATF6α traffics to Golgi apparatus for cleavage by S1P and S2P, which release p50ATF6α that transcriptionally induces its target genes encoding ER chaperone and ERAD functions.
Once released from BiP, PERK is activated through homodimerization and trans-autophosphorylation (14). Activated PERK phosphorylates the α subunit of the eukaryotic translation initiation factor 2α (eIF2α) at Ser51, leading to rapid and transient inhibition of protein synthesis (14, 15). While attenuating global mRNA translation, eIF2α phosphorylation paradoxically enhances the translation of several mRNAs, including the transcription factor, activating transcription factor 4 (ATF4) (16). ATF4 promotes expression of its target genes, including the growth arrest and DNA damage-inducible protein 34 (GADD34; also known as PPP1R5A) to dephosphorylate eIF2α and restore global mRNA translation (17). ATF4 also activates transcription of the C/EBP homologous protein (CHOP; also known as DDIT3 and GADD153), which is essential for the apoptotic response to ER stress (18–20).
IRE1α, the most conserved branch of the UPR, is a type I transmembrane protein where the ER luminal dimerization domain is structurally related to PERK and the cytosolic domain contains dual catalytic functions of a serine/threonine kinase and an endoRNase (RNase). Once released from BiP, IRE1α oligomerizes, autophosphorylates, and activates its kinase and RNase activities. Activated IRE1α cleaves X-box binding protein 1 (XBP1) mRNA to initiate removal of a 26-base intron in the cytoplasm to produce a translational frame-shift, producing a transcriptionally active form (Xbp1s) (21). Xbp1s, as a transcription factor, enters the nucleus to regulate expression of its target genes that encode functions in cotranslational translocation into the ER, ER protein folding and trafficking, ER membrane biogenesis, ER-associated degradation (ERAD), and protein secretion from the cell (22–25).
ATF6α is a type II transmembrane protein that contains a cytosolic cAMP-responsive element-binding protein/ATF basic leucine zipper domain. Once released from BiP upon accumulation of misfolded proteins, ATF6α traffics to the Golgi complex where it is cleaved by the serine protease site-1 (S1P) and metalloprotease site-2 (S2P) to produce a cytosolic fragment (p50ATF6α), an active transcription factor (26). p50ATF6α can act independently or synergistically with XBP1s to mediate the adaptive response to ER protein misfolding by increasing the transcription of genes that increase ER capacity and the expression of Xbp1. Although deletion of Atf6α in the mouse does not cause a significant phenotype, when these mice are challenged by ER protein misfolding, they exhibit many deficiencies (27).
ER STRESS AND LIPID METABOLISM
The ER is the major site of lipid metabolism, as many enzymes involved in lipid metabolism are located in the ER. Although the UPR was originally identified to maintain the protein homeostasis in the ER, a number of studies suggest that the UPR plays essential roles in maintaining the metabolic and lipid homeostasis (Fig. 2). For example, forced expression of BiP in the liver attenuates hepatic steatosis and improves glucose homeostasis by inhibiting activation of the central lipogenic regulator, sterol regulatory element-binding protein (SREBP)-1c, suggesting that ER stress has a pivotal role in hepatic lipid metabolism (28).
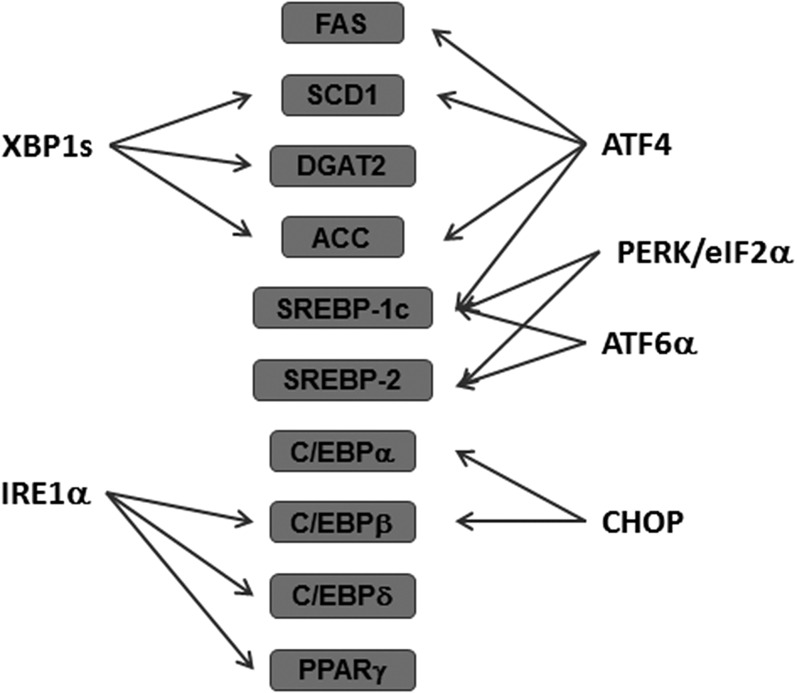
Fig. 2.
ER stress and lipid metabolism. ER stress and UPR induction alters expression of genes in lipid metabolism. PERK/eIF2α phosphorylation activates SREBP-1c and SREBP-2. ATF4 upregulates expression of SCD1, FAS, ACC, and SREBP-1c. CHOP also negatively regulates the activity of C/EBPα and C/EBPβ. IRE1α is involved in lipid metabolism by suppressing the expression of C/EBPβ, C/EBPδ, and PPARγ. XBP1s regulated expression of SCD1, DGAT2, and ACC. ATF6α interacts with the nuclear form of SREBP-2 to antagonize SREBP-2-mediated transcription of lipogenic genes. ATF6α also upregulates SREBP-1c expression.
Cross-talk between ER stress and lipid metabolism is evident from the finding that S1P and S2P processing enzymes also cleave and activate the SREBPs, SREBP-1c and SREBP-2, that regulate biosynthesis of cholesterol and other lipids. It should also be noted that protein misfolding in the ER causes oxidative stress that can lead to apoptosis (29). In addition, antioxidant treatment can prevent oxidative stress, reduce apoptosis, and even improve protein folding (30, 31). Although the mechanism that connects protein misfolding with oxidative stress is unknown, it might involve Ca2+ flux to regulate mitochondrial oxidative phosphorylation, increased fatty acid oxidation, oxidation of free sulfhydryls during disulfide bond formation in the ER, the induction of oxidases, such as NO synthase, or inhibition of antioxidant responses (32).
Several reports indicate that the PERK-eIF2α pathway regulates lipogenesis and hepatic steatosis. For example, PERK and eIF2α phosphorylation are induced by antipsychotic drugs, resulting in increased lipid accumulation in hepatocytes through activation of SREBP-1c and SREBP-2 (33). Mice with compromised eIF2α phosphorylation by overexpression of GADD34 in the liver, displayed reduced high-fat diet (HFD)-induced hepatosteatosis (34). ATF4, the downstream gene of the PERK/eIF2α pathway, was also reported to be involved in lipid metabolism. Livers from Atf4−/− mice fed a high-carbohydrate diet displayed markedly lower levels of stearoyl-CoA desaturase 1 (SCD1) expression with less accumulation of TG compared with livers from wild-type mice (35). Lipid accumulation caused by a high-fructose diet (HFrD) was also attenuated in Atf4−/− mice due to decreased levels of SREBP-1c, acetyl-CoA carboxylase (ACC), and FAS, suggesting a role of ATF4 in regulating hepatic lipid metabolism in response to nutritional stimuli (36). CHOP also seems to be involved in the lipid metabolism through suppression of genes encoding C/EBPα and other proteins related with lipid metabolism (37). Therefore, liver from Chop−/− mice, upon ER stress challenges, showed less suppressed expression of transcriptional regulators, including C/EBPα, Pparα, Pgc1α, and Srebp1, compared with wild-type mice. However, Chop−/− mice displayed reduced expression of lipogenic genes with less lipid accumulation than wild-type mice upon human immunodeficiency virus protease inhibitor (38). Therefore, the exact molecular and cellular mechanisms underlying CHOP-mediated dysregulation of hepatic lipid metabolism remain to be elucidated. CHOP is also involved in adipocyte differentiation. As a dominant-negative inhibitor of C/EBPα and C/EBPβ (39), CHOP expression inhibited adipocyte differentiation under ER stress conditions (40, 41).
The IRE1α/XBP1 pathway was identified as a critical regulator of hepatic lipid metabolism. Hepatocyte-specific Ire1α deletion increased hepatic lipid levels and reduced plasma lipids by modulating several genes involved in lipid metabolism, including C/ebpβ, C/ebpδ, Pparγ, and enzymes involved in TG biosynthesis (42). In addition, XBP1s was shown to directly regulate lipogenic genes in the liver, including Dgat2, Scd1, and Acc2 (43). Specific deletion of Xbp1 in the liver compromised de novo hepatic lipogenesis, leading to reduced serum TG, cholesterol, and FFAs (43). Interestingly, hepatocyte-specific Ire1α deletion did not alter lipogenesis, but rather, specifically impaired VLDL assembly and secretion, leading to hepatosteatosis and hypolipidemia (42, 44). In the absence of IRE1α, there was reduced protein disulfide isomerase, which acts with microsomal triglyceride transfer protein to promote the delivery of neutral lipids to the smooth ER lumen for VLDL assembly (45). Therefore, there is some controversy as to how ER stress affects lipid accumulation and/or secretion through the IRE1α/XBP1 pathway. This discrepancy can be explained by the finding that IRE1α is hyperactivated in liver-specific Xbp1−/− mice (46). IRE1α induces degradation of certain mRNAs, a process known as regulated IRE1-dependent decay (47, 48). Hyperactivated IRE1α in liver-specific Xbp1−/− mice promoted degradation of mRNAs encoding lipid metabolism functions, thereby reducing plasma TG and cholesterol in these mice (46). In addition to lipid metabolism, IRE1α/XBP1 also induces expression of many inflammatory cytokines, and Xbp1 deletion or small molecule inhibition of IRE1α has demonstrated anti-inflammatory effects (49–51).
The ATF6α pathway also plays a role in stress-induced lipid accumulation. p50ATF6α interacts with the nuclear form of SREBP-2, thereby antagonizing SREBP-2-regulated transcription of lipogenic genes and lipid accumulation in cultured hepatocytes and kidney cells (52). Moreover, Atf6α-deleted mice displayed hepatic dysfunction and steatosis much longer than wild-type mice in response to pharmacological induction of ER stress (37, 53). This could be explained by chronic expression of CHOP and sustained suppression of C/EBPα (37) and/or a failure of ATF6α-mediated induction of genes encoding protein chaperone, trafficking, and ERAD functions (53). When fed a HFD, Atf6α−/− mice developed hepatic steatosis and glucose intolerance in association with increased expression of SREBP-1c (54). On the other hand, overexpression of a functionally active nuclear fragment of ATF6 in zebrafish caused fatty liver (55), suggesting that fine-tuning of ATF6α may be important to prevent liver steatosis. Alternatively, different species may have evolved to utilize these pathways for different functions to cope with their specific environmental challenges.
ER STRESS AND LIPOTOXICITY IN PERIPHERAL ORGANS
Pancreatic β cells
Chronically elevated levels of FFA in vitro or in vivo are detrimental to pancreatic β cell function. Saturated fatty acids (SFAs), such as palmitate, activate the UPR with subsequent induction of apoptosis compared with unsaturated fatty acids, such as oleate, suggesting a harmful effect of SFAs (56). Damage driven by prolonged exposure to SFAs in pancreatic β cells was believed to be a main cause of type 2 diabetes (57–59). Their cytotoxic effect was attributed to an increased lipogenesis in β cells (60) or upregulation of inducible NO synthase followed by NO-mediated cell death (61). However, exposure of FFAs to INS-1E insulinoma cells or primary rat islets did not induce inducible NO synthase or NF-κB activation, which is usually caused by TNFα or treatment with other cytokines (62), suggesting that another mechanism governs the death of β cells upon FFA exposure. SFAs induced ER stress and the UPR, with increased expression of mRNAs for CHOP, ATF4, and ATF6α, and splicing of XBP1 in pancreatic β cells (62–65). When ER stress was reduced by a small molecule (66) or chemical chaperones that buffer proteins to prevent misfolding, such as 4-phenylbutyric acid (4-PBA) (67) or tauroursodeoxycholic acid (TUDCA) (68), β cells were protected from palmitate-induced lipotoxicity. These results suggest that ER stress and the UPR mediate SFA-induced lipotoxicity in β cells.
The mechanisms by which SFAs directly or indirectly impair β cell function through ER stress have been the subject of several recent studies (Fig. 3). Protein palmitoylation is a major posttranslational modification that was induced by palmitate treatment of insulinoma cells with associated cell death (69). When palmitoylation was inhibited by 2-bromopalmitate, a nonmetabolizable form of palmitic acid, the induction of ER stress and the activation of caspase activity were attenuated in insulinoma cells and isolated islets (69), suggesting a role of protein palmitoylation, ceramide production, or fatty acid oxidation in SFA-induced ER stress. On the other hand, a lipidomic screen of palmitate-treated insulinoma cells found that altered sphingolipid metabolism may be implicated in the defective protein trafficking from ER to Golgi and disruption of ER lipid rafts, causing ER stress and subsequent β cell death (70–72). In addition, palmitate treatment of insulinoma cells impaired trafficking of ceramide from the ER to the Golgi apparatus, thus promoting the accumulation of ceramide in the ER and lipotoxicity through ER stress (73). Palmitate-induced lipotoxicity can also be caused by altered phospholipid composition and consequent changes in membrane rigidity and fluidity (74), which are known to induce UPR signaling (75, 76). Palmitate might also deplete ER Ca2+ stores in insulinoma cells (56), which, in turn, cause protein misfolding and ER stress (77–79).
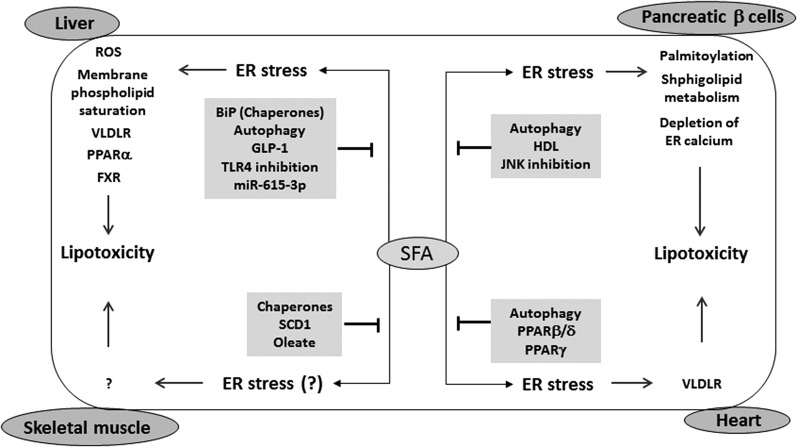
Fig. 3.
ER stress and lipotoxicity. SFAs cause ER stress in numerous peripheral tissues, including pancreatic β cells, hepatocytes, skeletal myocytes, and cardiomyocytes, leading to cell dysfunction or death through lipotoxicity. In pancreatic β cells, SFA-mediated ER stress induces protein palmitoylation, alteration in sphingolipid metabolism, and depletion of ER Ca2+. In the liver, ER stress induces ROS production, saturation of membrane phospholipid, induction of VLDLR expression, and reduction of PPARα and FXR expression. In skeletal muscle, it is not clear whether ER stress is directly involved in SFA-mediated lipotoxicity. In the heart, ER stress induces expression of VLDLR to cause lipotoxicity. In each organ, inhibition of ER stress can prevent SFA-mediated lipotoxicity.
On the other hand, palmitate treatment activated autophagy in insulinoma cells to prevent lipotoxicity (80). An islet transcriptome study using RNA sequencing in palmitate-treated human islets identified that palmitate modulates expression of genes involved in autophagic flux and lysosome function (81). When autophagy was inhibited by chloroquine, ER stress was induced in isolated islets (82). In addition, autophagy induced by interleukin-22 alleviated palmitate-induced ER stress in insulinoma cells (83). These results suggest that activation of autophagy by palmitate or HFD feeding prevents ER stress-mediated pancreatic β cell death. Interestingly, JNK activity, which is associated with ER stress, also appears to be involved in β cell death upon palmitate-induced ER stress. When JNK1 activity was attenuated using JNK1 shRNA in insulinoma cells, apoptosis was decreased with reduced levels of CHOP protein upon palmitate treatment (84). In addition, palmitate-induced β cell death was attenuated by metformin, an insulin sensitizer, by suppressing ER stress (85).
HDL is a potential inhibitor of ER stress-mediated cell death, especially in pancreatic β cells. Thapsigargin, a SERCA inhibitor, caused disruption of the Ca2+ gradient between the ER and the cytosol and thus induced ER stress-mediated apoptosis, which was attenuated by HDL treatment (86). HDLs also prevented palmitate-induced UPR induction and ER stress and β cell death through restoration of ER functionality in terms of protein folding and trafficking (86). Interestingly, HDLs can inhibit cell death by tunicamycin, an N-glycosylation inhibitor, without modulating the UPR response (87). HDLs may be able to restore ER function upon Ca2+ depletion by thapsigargin or palmitate, whereas HDLs may not prevent ER stress induced by accumulation of misfolded proteins, such as nonglycosylated proteins, upon tunicamycin treatment. It also is possible that HDL-mediated protection of β cells from lipotoxicity and subsequent ER stress might be mediated through modulation of lipid metabolism that was observed in other tissues, including the liver (88).
Of note, TUDCA was reported to reduce symptoms of type 1 and type 2 diabetes in murine models (89, 90). However, intriguingly, this protection in type 1 diabetes required ATF6α function and was lost in mice with β cell-specific deletion of Atf6α (89). This is curious because inasmuch as TUDCA should prevent UPR activation, why would this response require activation of ATF6α. Nevertheless, this data provides an in vivo correlate for effects in cultured β cell studies.
Liver
Several lines of evidence suggest that ER stress is one important contributing factor for SFA-mediated lipotoxicity in the liver (Fig. 3). Exposure of L02 immortal liver cells and HepG2 hepatoma cells to SFAs reduced viability through increased levels of ER stress accompanied with upregulation of PERK phosphorylation and downstream genes, including ATF4 and CHOP (91). Consistently, knock-down of PERK significantly reduced palmitate-induced cell death (91). Attenuation of ER stress by BiP overexpression (92), by resveratrol treatment (93), glucagon-like peptide-1 (GLP-1) treatment (94), or TLR4 knockout (95) suppressed palmitate-induced cell death. In addition, palmitate-induced hepatocyte death was attenuated by miR-615-3p, which suppressed the expression of the apoptotic gene, CHOP (96). These results suggest the pivotal role of ER stress in hepatic lipotoxicity.
Palmitate-induced lipotoxicity may occur through generation of reactive oxygen species (ROS) (97, 98). Elevated levels of palmitate compromised the ER’s capability to maintain Ca2+ stores, resulting in the stimulation of mitochondrial oxidative metabolism, ROS production, and ER stress-mediated cellular dysfunction (98). It is well-known that protein misfolding in the ER and subsequent induction of ER stress contribute to ROS production and cellular dysfunction, which can be prevented by antioxidant treatment (29, 31, 32).
On the other hand, SFAs may decrease membrane phospholipid saturation, which in turn activates the UPR transducers, including IRE1α and PERK, via their transmembrane domains (75, 76). Enhancing incorporation of PUFAs into membrane phospholipid by liver X receptors-Lpcat3, prevented ER stress and cell death induced by palmitate treatment in vitro or by hepatic lipid accumulation in vivo (99). Palmitate also increased de novo biosynthesis of saturated phospholipids, resulting in ER stress, suggesting that aberrant phospholipid metabolism may largely contribute to palmitate-induced ER stress and its downstream lipotoxic effect (100). Consistently, mice compromised in their ability to synthesize monounsaturated fatty acids due to a deletion of Scd1 displayed activation of the UPR in their livers due to insufficiency of unsaturated fatty acids (101). In addition, an increased level of phosphatidylcholine in obese hepatic ER inhibited SERCA activity, resulting in depletion of ER Ca2+ (102). These results suggest that preservation of membrane homeostasis during SFA stress is important to prevent ER stress-mediated lipotoxicity.
Pharmacologically induced ER stress in HepG2 cells increased the expression of SREBP-1c via cap-independent internal translation of SREBP-1c mRNA by hnRNP-A1 (103, 104), which in turn activated fatty acid synthesis in L02 cells (105). ER stress also induced hepatic steatosis via increased expression of hepatic VLDL receptor (VLDLR) through direct binding of the ATF4 transcription factor to the VLDLR promoter region leading to accumulation of TG (106). In addition, HFrD-induced upregulation or excessive expression of hepatic apoB100 in transgenic mice increased ER stress and insulin resistance by decreasing expression of SERCA (107). The increase of apoB100 expression by HFrD was mediated by reduced mRNA expression of hepatic PPARα (108). Increasing PPARα activity by agonist WY-14643 reduced apoB100 accumulation and ER stress in the liver from HFrD-fed mice (108). Interestingly, another PPARα agonist, fenofibrate, also improved hepatic insulin resistance and steatosis in HFrD-fed mice; but in this case, ER stress was still upregulated (109). Therefore, the role of PPARα in ER stress-mediated lipotoxicity remains to be elucidated. In old mice, TG was markedly accumulated and lipogenic genes were deregulated by ER stress-mediated downregulation of farnesoid X receptor (FXR) (110) that plays a pivotal role in the regulation of hepatic TG metabolism (111). TUDCA and 4-PBA treatment in old mice reduced ER stress marker expression, but increased FXR expression with reduced hepatic TG content, suggesting a protective role for FXR in ER stress-mediated lipotoxicity. Further studies are needed to understand the relationship between ER stress and orphan nuclear receptors.
Alteration in autophagic flux may also contribute to palmitate-induced ER stress. In livers from patients with nonalcoholic fatty liver disease or nonalcoholic steatohepatitis, there was increased ER stress gene expression with decreased autophagic flux (112). Consistently, palmitate treatment in Huh7 cells increased ER stress associated with decreased autophagy, which was restored with an autophagy inducer, rapamycin (112).
Skeletal muscle
Lipotoxicity in muscle may also be mediated by ER stress (Fig. 3), evidenced by a range of studies from in vitro to human studies. For in vitro studies, palmitate treatment in cultured human myotubes induced ER stress markers and decreased cell viability, which was attenuated by overexpression of SCD1 (113). Interestingly, SCD1 inducibility upon palmitate treatment was different in individual human myotubes. Higher SCD1 induction was associated with a reduced ER stress response and better insulin sensitivity, whereas myotubes with lower SCD1 induction showed more ER stress marker gene expression (113), suggesting SFA-mediated ER stress causes muscle cell dysfunction. The relationship between ER stress and lipotoxicity was also demonstrated in skeletal muscle. When mice were fed HFD, the expression levels of ER stress marker genes, including BiP, the spliced form of XBP1, and ATF4/CHOP, were markedly increased in skeletal muscle (114). In human studies, TUDCA (115) and 4-PBA (116), chemical chaperones that relieve ER stress, improved muscle insulin sensitivity in obese patients, strongly suggesting that lipotoxicity in skeletal muscle is mediated by ER stress. In addition to chemical chaperones, palmitate-induced lipotoxicity was also prevented by attenuation of ER stress using oleate treatment (116, 117) or PPARβ/δ activation through AMPK activation (118). These results suggest a pivotal role for ER stress in palmitate-induced lipotoxicity in skeletal muscle and that attenuating ER stress might ameliorate lipotoxicity in skeletal muscle.
However, there are several contradictory reports indicating that ER stress has little association with lipid-induced skeletal myocyte dysfunction. No significant change in ER stress marker gene expression resulting from TUDCA treatment was observed despite improved liver and skeletal muscle function compared with placebo-treated patients (115). In addition, muscle tissues from HFD-fed patients did not show significant induction of ER stress (119). In other reports, it was found that ER stress was moderately induced in response to palmitate compared with treatment with pharmacological ER stress inducers (120). Treatment with chemical chaperones did not improve palmitate-induced insulin sensitivity in myotubes (121). Therefore, the role of ER stress in palmitate-induced lipotoxicity in skeletal muscle remains unclear and further studies are required.
Heart
Unlike other tissues, cardiomyocytes in the adult heart preferentially use fatty acids to meet their high energy requirement, suggesting an importance of lipid metabolism in the heart. On the other hand, myocardial TG accumulation is often associated with impaired cardiac function. Intracellular TG accumulation was observed in stressed hearts and this was regarded as a hallmark of cardiac lipotoxicity that eventually led to heart failure (122, 123). It is of note that specific cardiac conditions, such as pressure overload (124) or ischemia (125), cause ER stress that likely contributes to cardiomyocyte dysfunction upon stress-mediated lipid accumulation (Fig. 3). Recent studies support this hypothesis, in which hypoxia/ischemia-induced lipid accumulation in HL-1 cardiomyocytes and mouse hearts was dependent on expression of the VLDLR (126). It was found that ischemia-induced ER stress and subsequent apoptosis in mouse heart were reduced in Vldlr−/− mice and in mice treated with antibodies against VLDLR (126). These results suggest that VLDLR-induced lipid accumulation in the ischemic heart causes cell death through activation of ER stress. In another report, myocardial ischemia in a pig model increased intracellular cholesteryl ester levels, which in turn activated the UPR and ER stress accompanied by myocardial dysfunction (127).
In addition to ischemic injury, SFAs also induce ER stress and apoptosis in cardiomyocytes in vitro and in vivo. Palmitate induced ER stress in AC16 cells, a cardiomyocyte cell line of human origin (128). Activation of PPARβ/δ by the agonist, GW501516, in AC16 cells, prevented palmitate-induced ER stress. In addition, HFD-fed Pparβ/δ knockout mice displayed ER stress, suggesting a protective role of PPARβ/δ in ER stress-mediated lipotoxicity (128). Interestingly, autophagy was reduced by HFD or PPARβ/δ suppression, but increased by the PPARβ/δ agonist in murine hearts, suggesting that PPARβ/δ-induced autophagy is beneficial toward SFA-mediated ER stress and lipotoxicity. Palmitate also induced ER stress-mediated lipotoxicity in cardiomyocytes by inhibiting sequestration of fatty acids as a less harmful form of TG in lipid droplets (129). When neutral lipid storage was induced by PPARγ or acyl-CoA synthetase in human cardiomyocytes, expression levels of ER stress marker genes were significantly reduced upon palmitate treatment. All of these results suggest that ER stress is one of the potential reasons for lipotoxicity in heart failure.
CONCLUSION
As discussed above, ER stress and the UPR pathways are involved in lipid metabolism and lipotoxicity in peripheral tissues. ER stress, caused by various stimuli, affects lipid metabolism through modulating the expression levels of key enzymes involved in lipid synthesis or modification. On the other hand, ER stress mediates lipotoxicity in several peripheral organs through ER stress-induced apoptosis or modulation of membrane composition of phospholipids. Although extensively studied, it is still not completely understood how ER stress mediates lipotoxicity in each peripheral organ. For example, ceramide accumulation by ER stress seems to contribute to pancreatic β cell death, but its role in hepatocytes is not clear. In addition, the role of ER stress is yet controversial in SFA-mediated lipotoxicity in the skeletal muscle. However, it is likely that relieving ER stress can prevent lipotoxicity in several organs, suggesting that ER stress might potentially be an untapped therapeutic target for diseases associated with lipid accumulation. Therefore, further studies on identifying these mechanisms should yield approaches to modulate UPR activity to reach a desired therapeutic benefit and minimize tissue damage.
Acknowledgments
The authors apologize to those who were not referenced due to space limitations.
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- ATF4
- activating transcription factor 4
- ATF6α
- activating transcription factor 6α BiP, immunoglobulin heavy-chain binding protein
- CHOP
- C/EBP homologous protein
- eIF2α
- eukaryotic translation initiation factor 2α ER, endoplasmic reticulum
- ERAD
- ER-associated degradation
- FXR
- farnesoid X receptor
- GADD34
- growth arrest and DNA damage-inducible protein 34
- HFD
- high-fat diet
- HFrD
- high-fructose diet
- IRE1α
- inositol-requiring enzyme 1α 4-PBA, 4-phenylbutyric acid
- PERK
- the double-stranded RNA-activated protein kinase-like eukaryotic initiation factor 2α kinase
- ROS
- reactive oxygen species
- SCD1
- stearoyl-CoA desaturase 1
- SERCA
- sarco/endoplasmic reticulum calcium ATPase
- SFA
- saturated fatty acid
- S1P
- serine protease site-1
- S2P
- metalloprotease site-2
- SREBP
- sterol regulatory element-binding protein
- TUDCA
- tauroursodeoxycholic acid
- UPR
- unfolded protein response
- VLDLR
- VLDL receptor
- XBP1
- X-box binding protein 1
- Xbp1s
- transcriptionally active form of X-box binding protein 1
This work was supported by Office of Extramural Research, National Institutes of Health Grants DK042394, DK088227, DK103183, and CA128814 (R.J.K.), by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2015R1D1A1A01058846) funded by the Ministry of Education, and by the Soonchunhyang University Research Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Clapham D. E. 2007. Calcium signaling. Cell. 131: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 2.Moore L., Chen T., Knapp H. R. Jr., and Landon E. J.. 1975. Energy-dependent calcium sequestration activity in rat liver microsomes. J. Biol. Chem. 250: 4562–4568. [PubMed] [Google Scholar]
- 3.Michalak M., Robert Parker J. M., and Opas M.. 2002. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 32: 269–278. [DOI] [PubMed] [Google Scholar]
- 4.Schröder M., and Kaufman R. J.. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74: 739–789. [DOI] [PubMed] [Google Scholar]
- 5.Wang M., and Kaufman R. J.. 2016. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 529: 326–335. [DOI] [PubMed] [Google Scholar]
- 6.Schuck S., Prinz W. A., Thorn K. S., Voss C., and Walter P.. 2009. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J. Cell Biol. 187: 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagone P., and Jackowski S.. 2009. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 50(Suppl): S311–S316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unger R. H., and Scherer P. E.. 2010. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol. Metab. 21: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S., and Kaufman R. J.. 2014. How does protein misfolding in the endoplasmic reticulum affect lipid metabolism in the liver? Curr. Opin. Lipidol. 25: 125–132. [DOI] [PubMed] [Google Scholar]
- 10.Rutkowski D. T., and Kaufman R. J.. 2004. A trip to the ER: coping with stress. Trends Cell Biol. 14: 20–28. [DOI] [PubMed] [Google Scholar]
- 11.Shen J., Chen X., Hendershot L., and Prywes R.. 2002. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell. 3: 99–111. [DOI] [PubMed] [Google Scholar]
- 12.Bertolotti A., Zhang Y., Hendershot L. M., Harding H. P., and Ron D.. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2: 326–332. [DOI] [PubMed] [Google Scholar]
- 13.Ma K., Vattem K. M., and Wek R. C.. 2002. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J. Biol. Chem. 277: 18728–18735. [DOI] [PubMed] [Google Scholar]
- 14.Harding H. P., Zhang Y., and Ron D.. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 397: 271–274. [DOI] [PubMed] [Google Scholar]
- 15.Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., and Kaufman R. J.. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 7: 1165–1176. [DOI] [PubMed] [Google Scholar]
- 16.Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., and Ron D.. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 6: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 17.Novoa I., Zeng H., Harding H. P., and Ron D.. 2001. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 153: 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva R. M., Ries V., Oo T. F., Yarygina O., Jackson-Lewis V., Ryu E. J., Lu P. D., Marciniak S. J., Ron D., Przedborski S., et al. 2005. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J. Neurochem. 95: 974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi H., and Wang H. G.. 2004. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 279: 45495–45502. [DOI] [PubMed] [Google Scholar]
- 20.Oyadomari S., and Mori M.. 2004. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11: 381–389. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H., Matsui T., Yamamoto A., Okada T., and Mori K.. 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 107: 881–891. [DOI] [PubMed] [Google Scholar]
- 22.Casagrande R., Stern P., Diehn M., Shamu C., Osario M., Zuniga M., Brown P. O., and Ploegh H.. 2000. Degradation of proteins from the ER of S. cerevisiae requires an intact unfolded protein response pathway. Mol. Cell. 5: 729–735. [DOI] [PubMed] [Google Scholar]
- 23.Lee A. H., Iwakoshi N. N., and Glimcher L. H.. 2003. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23: 7448–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acosta-Alvear D., Zhou Y., Blais A., Tsikitis M., Lents N. H., Arias C., Lennon C. J., Kluger Y., and Dynlacht B. D.. 2007. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell. 27: 53–66. [DOI] [PubMed] [Google Scholar]
- 25.Hassler J. R., Scheuner D. L., Wang S., Han J., Kodali V. K., Li P., Nguyen J., George J. S., Davis C., Wu S. P., et al. 2015. The IRE1alpha/XBP1s pathway is essential for the glucose response and protection of β cells. PLoS Biol. 13: e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haze K., Yoshida H., Yanagi H., Yura T., and Mori K.. 1999. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 10: 3787–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J., Rutkowski D. T., Dubois M., Swathirajan J., Saunders T., Wang J., Song B., Yau G. D., and Kaufman R. J.. 2007. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell. 13: 351–364. [DOI] [PubMed] [Google Scholar]
- 28.Kammoun H. L., Chabanon H., Hainault I., Luquet S., Magnan C., Koike T., Ferre P., and Foufelle F.. 2009. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 119: 1201–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song B., Scheuner D., Ron D., Pennathur S., and Kaufman R. J.. 2008. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118: 3378–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra J. D., Miao H., Zhang K., Wolfson A., Pennathur S., Pipe S. W., and Kaufman R. J.. 2008. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. USA. 105: 18525–18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J., Song B., Kim J., Kodali V. K., Pottekat A., Wang M., Hassler J., Wang S., Pennathur S., Back S. H., et al. 2015. Antioxidants complement the requirement for protein chaperone function to maintain β-cell function and glucose homeostasis. Diabetes. 64: 2892–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhotra J. D., and Kaufman R. J.. 2007. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9: 2277–2293. [DOI] [PubMed] [Google Scholar]
- 33.Lauressergues E., Bert E., Duriez P., Hum D., Majd Z., Staels B., and Cussac D.. 2012. Does endoplasmic reticulum stress participate in APD-induced hepatic metabolic dysregulation? Neuropharmacology. 62: 784–796. [DOI] [PubMed] [Google Scholar]
- 34.Oyadomari S., Harding H. P., Zhang Y., Oyadomari M., and Ron D.. 2008. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 7: 520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H., Meng Q., Xiao F., Chen S., Du Y., Yu J., Wang C., and Guo F.. 2011. ATF4 deficiency protects mice from high-carbohydrate-diet-induced liver steatosis. Biochem. J. 438: 283–289. [DOI] [PubMed] [Google Scholar]
- 36.Xiao G., Zhang T., Yu S., Lee S., Calabuig-Navarro V., Yamauchi J., Ringquist S., and Dong H. H.. 2013. ATF4 protein deficiency protects against high fructose-induced hypertriglyceridemia in mice. J. Biol. Chem. 288: 25350–25361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutkowski D. T., Wu J., Back S. H., Callaghan M. U., Ferris S. P., Iqbal J., Clark R., Miao H., Hassler J. R., Fornek J., et al. 2008. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell. 15: 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Zhang L., Wu X., Gurley E. C., Kennedy E., Hylemon P. B., Pandak W. M., Sanyal A. J., and Zhou H.. 2013. The role of CCAAT enhancer-binding protein homologous protein in human immunodeficiency virus protease-inhibitor-induced hepatic lipotoxicity in mice. Hepatology. 57: 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ron D., and Habener J. F.. 1992. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 6: 439–453. [DOI] [PubMed] [Google Scholar]
- 40.Batchvarova N., Wang X. Z., and Ron D.. 1995. Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153). EMBO J. 14: 4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J., Murthy R., Wood B., Song B., Wang S., Sun B., Malhi H., and Kaufman R. J.. 2013. ER stress signalling through eIF2alpha and CHOP, but not IRE1alpha, attenuates adipogenesis in mice. Diabetologia. 56: 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang K., Wang S., Malhotra J., Hassler J. R., Back S. H., Wang G., Chang L., Xu W., Miao H., Leonardi R., et al. 2011. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J. 30: 1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee A. H., Scapa E. F., Cohen D. E., and Glimcher L. H.. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 320: 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S., Chen Z., Lam V., Han J., Hassler J., Finck B. N., Davidson N. O., and Kaufman R. J.. 2012. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab. 16: 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S., Park S., Kodali V. K., Han J., Yip T., Chen Z., Davidson N. O., and Kaufman R. J.. 2015. Identification of protein disulfide isomerase 1 as a key isomerase for disulfide bond formation in apolipoprotein B100. Mol. Biol. Cell. 26: 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.So J. S., Hur K. Y., Tarrio M., Ruda V., Frank-Kamenetsky M., Fitzgerald K., Koteliansky V., Lichtman A. H., Iwawaki T., Glimcher L. H., et al. 2012. Silencing of lipid metabolism genes through IRE1alpha-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 16: 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollien J., Lin J. H., Li H., Stevens N., Walter P., and Weissman J. S.. 2009. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 186: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han D., Lerner A. G., Vande Walle L., Upton J. P., Xu W., Hagen A., Backes B. J., Oakes S. A., and Papa F. R.. 2009. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 138: 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinon F., and Glimcher L. H.. 2011. Regulation of innate immunity by signaling pathways emerging from the endoplasmic reticulum. Curr. Opin. Immunol. 23: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinon F., Chen X., Lee A. H., and Glimcher L. H.. 2010. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu Q., Zheng Z., Chang L., Zhao Y. S., Tan C., Dandekar A., Zhang Z., Lin Z., Gui M., Li X., et al. 2013. Toll-like receptor-mediated IRE1alpha activation as a therapeutic target for inflammatory arthritis. EMBO J. 32: 2477–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng L., Lu M., Mori K., Luo S., Lee A. S., Zhu Y., and Shyy J. Y.. 2004. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 23: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto K., Takahara K., Oyadomari S., Okada T., Sato T., Harada A., and Mori K.. 2010. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol. Biol. Cell. 21: 2975–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Usui M., Yamaguchi S., Tanji Y., Tominaga R., Ishigaki Y., Fukumoto M., Katagiri H., Mori K., Oka Y., and Ishihara H.. 2012. Atf6α-null mice are glucose intolerant due to pancreatic β-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metabolism. 61: 1118–1128. [DOI] [PubMed] [Google Scholar]
- 55.Howarth D. L., Lindtner C., Vacaru A. M., Sachidanandam R., Tsedensodnom O., Vasilkova T., Buettner C., and Sadler K. C.. 2014. Activating transcription factor 6 is necessary and sufficient for alcoholic fatty liver disease in zebrafish. PLoS Genet. 10: e1004335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunha D. A., Hekerman P., Ladriere L., Bazarra-Castro A., Ortis F., Wakeham M. C., Moore F., Rasschaert J., Cardozo A. K., Bellomo E., et al. 2008. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J. Cell Sci. 121: 2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bollheimer L. C., Skelly R. H., Chester M. W., McGarry J. D., and Rhodes C. J.. 1998. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J. Clin. Invest. 101: 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y. P., and Grill V. E.. 1994. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J. Clin. Invest. 93: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee Y., Hirose H., Ohneda M., Johnson J. H., McGarry J. D., and Unger R. H.. 1994. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. USA. 91: 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee Y., Hirose H., Zhou Y. T., Esser V., McGarry J. D., and Unger R. H.. 1997. Increased lipogenic capacity of the islets of obese rats: a role in the pathogenesis of NIDDM. Diabetes. 46: 408–413. [DOI] [PubMed] [Google Scholar]
- 61.Shimabukuro M., Ohneda M., Lee Y., and Unger R. H.. 1997. Role of nitric oxide in obesity-induced beta cell disease. J. Clin. Invest. 100: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kharroubi I., Ladriere L., Cardozo A. K., Dogusan Z., Cnop M., and Eizirik D. L.. 2004. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 145: 5087–5096. [DOI] [PubMed] [Google Scholar]
- 63.Cnop M., Ladriere L., Hekerman P., Ortis F., Cardozo A. K., Dogusan Z., Flamez D., Boyce M., Yuan J., and Eizirik D. L.. 2007. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J. Biol. Chem. 282: 3989–3997. [DOI] [PubMed] [Google Scholar]
- 64.Karaskov E., Scott C., Zhang L., Teodoro T., Ravazzola M., and Volchuk A.. 2006. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 147: 3398–3407. [DOI] [PubMed] [Google Scholar]
- 65.Laybutt D. R., Preston A. M., Akerfeldt M. C., Kench J. G., Busch A. K., Biankin A. V., and Biden T. J.. 2007. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 50: 752–763. [DOI] [PubMed] [Google Scholar]
- 66.Tran K., Li Y., Duan H., Arora D., Lim H. Y., and Wang W.. 2014. Identification of small molecules that protect pancreatic beta cells against endoplasmic reticulum stress-induced cell death. ACS Chem. Biol. 9: 2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi S. E., Lee Y. J., Jang H. J., Lee K. W., Kim Y. S., Jun H. S., Kang S. S., Chun J., and Kang Y.. 2008. A chemical chaperone 4-PBA ameliorates palmitate-induced inhibition of glucose-stimulated insulin secretion (GSIS). Arch. Biochem. Biophys. 475: 109–114. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y. Y., Sun L. Q., Wang B. A., Zou X. M., Mu Y. M., and Lu J. M.. 2013. Palmitate induces autophagy in pancreatic beta-cells via endoplasmic reticulum stress and its downstream JNK pathway. Int. J. Mol. Med. 32: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 69.Baldwin A. C., Green C. D., Olson L. K., Moxley M. A., and Corbett J. A.. 2012. A role for aberrant protein palmitoylation in FFA-induced ER stress and beta-cell death. Am. J. Physiol. Endocrinol. Metab. 302: E1390–E1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boslem E., MacIntosh G., Preston A. M., Bartley C., Busch A. K., Fuller M., Laybutt D. R., Meikle P. J., and Biden T. J.. 2011. A lipidomic screen of palmitate-treated MIN6 beta-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem. J. 435: 267–276. [DOI] [PubMed] [Google Scholar]
- 71.Véret J., Coant N., Gorshkova I. A., Giussani P., Fradet M., Riccitelli E., Skobeleva A., Goya J., Kassis N., Natarajan V., et al. 2013. Role of palmitate-induced sphingoid base-1-phosphate biosynthesis in INS-1 beta-cell survival. Biochim. Biophys. Acta. 1831: 251–262. [DOI] [PubMed] [Google Scholar]
- 72.Boslem E., Weir J. M., MacIntosh G., Sue N., Cantley J., Meikle P. J., and Biden T. J.. 2013. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic beta-cells. J. Biol. Chem. 288: 26569–26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gjoni E., Brioschi L., Cinque A., Coant N., Islam M. N., Ng C. K., Verderio C., Magnan C., Riboni L., Viani P., et al. 2014. Glucolipotoxicity impairs ceramide flow from the endoplasmic reticulum to the Golgi apparatus in INS-1 beta-cells. PLoS One. 9: e110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moffitt J. H., Fielding B. A., Evershed R., Berstan R., Currie J. M., and Clark A.. 2005. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 48: 1819–1829. [DOI] [PubMed] [Google Scholar]
- 75.Ariyama H., Kono N., Matsuda S., Inoue T., and Arai H.. 2010. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J. Biol. Chem. 285: 22027–22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Volmer R., van der Ploeg K., and Ron D.. 2013. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl. Acad. Sci. USA. 110: 4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuznetsov G., Brostrom M. A., and Brostrom C. O.. 1992. Demonstration of a calcium requirement for secretory protein processing and export. Differential effects of calcium and dithiothreitol. J. Biol. Chem. 267: 3932–3939. [PubMed] [Google Scholar]
- 78.Lodish H. F., and Kong N.. 1990. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J. Biol. Chem. 265: 10893–10899. [PubMed] [Google Scholar]
- 79.Borradaile N. M., Han X., Harp J. D., Gale S. E., Ory D. S., and Schaffer J. E.. 2006. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47: 2726–2737. [DOI] [PubMed] [Google Scholar]
- 80.Choi S. E., Lee S. M., Lee Y. J., Li L. J., Lee S. J., Lee J. H., Kim Y., Jun H. S., Lee K. W., and Kang Y.. 2009. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology. 150: 126–134. [DOI] [PubMed] [Google Scholar]
- 81.Cnop M., Abdulkarim B., Bottu G., Cunha D. A., Igoillo-Esteve M., Masini M., Turatsinze J. V., Griebel T., Villate O., Santin I., et al. 2014. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes. 63: 1978–1993. [DOI] [PubMed] [Google Scholar]
- 82.Chu K. Y., O’Reilly L., Ramm G., and Biden T. J.. 2015. High-fat diet increases autophagic flux in pancreatic beta cells in vivo and ex vivo in mice. Diabetologia. 58: 2074–2078. [DOI] [PubMed] [Google Scholar]
- 83.Hu M., Yang S., Yang L., Cheng Y., and Zhang H.. 2016. Interleukin-22 alleviated palmitate-induced endoplasmic reticulum stress in INS-1 cells through activation of autophagy. PLoS One. 11: e0146818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prause M., Christensen D. P., Billestrup N., and Mandrup-Poulsen T.. 2014. JNK1 protects against glucolipotoxicity-mediated β-cell apoptosis. PLoS One. 9: e87067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simon-Szabó L., Kokas M., Mandl J., Kéri G., and Csala M.. 2014. Metformin attenuates palmitate-induced endoplasmic reticulum stress, serine phosphorylation of IRS-1 and apoptosis in rat insulinoma cells. PLoS One. 9: e97868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pétremand J., Puyal J., Chatton J. Y., Duprez J., Allagnat F., Frias M., James R. W., Waeber G., Jonas J. C., and Widmann C.. 2012. HDLs protect pancreatic beta-cells against ER stress by restoring protein folding and trafficking. Diabetes. 61: 1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puyal J., Petremand J., Dubuis G., Rummel C., and Widmann C.. 2013. HDLs protect the MIN6 insulinoma cell line against tunicamycin-induced apoptosis without inhibiting ER stress and without restoring ER functionality. Mol. Cell. Endocrinol. 381: 291–301. [DOI] [PubMed] [Google Scholar]
- 88.Hong D., Li L. F., Gao H. C., Wang X., Li C. C., Luo Y., Bai Y. P., and Zhang G. G.. 2015. High-density lipoprotein prevents endoplasmic reticulum stress-induced downregulation of liver LOX-1 expression. PLoS One. 10: e0124285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Engin F., Yermalovich A., Nguyen T., Hummasti S., Fu W., Eizirik D. L., Mathis D., and Hotamisligil G. S.. 2013. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci. Transl. Med. 5: 211ra156 [Erratum. 2013. Sci. Transl. Med 5: 214er11.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Engin F., and Hotamisligil G. S.. 2010. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab. 12(Suppl 2): 108–115. [DOI] [PubMed] [Google Scholar]
- 91.Cao J., Dai D. L., Yao L., Yu H. H., Ning B., Zhang Q., Chen J., Cheng W. H., Shen W., and Yang Z. X.. 2012. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 364: 115–129. [DOI] [PubMed] [Google Scholar]
- 92.Gu X., Li K., Laybutt D. R., He M. L., Zhao H. L., Chan J. C., and Xu G.. 2010. Bip overexpression, but not CHOP inhibition, attenuates fatty-acid-induced endoplasmic reticulum stress and apoptosis in HepG2 liver cells. Life Sci. 87: 724–732. [DOI] [PubMed] [Google Scholar]
- 93.Pan Q. R., Ren Y. L., Liu W. X., Hu Y. J., Zheng J. S., Xu Y., and Wang G.. 2015. Resveratrol prevents hepatic steatosis and endoplasmic reticulum stress and regulates the expression of genes involved in lipid metabolism, insulin resistance, and inflammation in rats. Nutr. Res. 35: 576–584. [DOI] [PubMed] [Google Scholar]
- 94.Ao N., Yang J., Wang X., and Du J.. 2016. Glucagon-like peptide-1 preserves non-alcoholic fatty liver disease through inhibition of the endoplasmic reticulum stress-associated pathway. Hepatol. Res. 46: 343–353. [DOI] [PubMed] [Google Scholar]
- 95.Pierre N., Deldicque L., Barbe C., Naslain D., Cani P. D., and Francaux M.. 2013. Toll-like receptor 4 knockout mice are protected against endoplasmic reticulum stress induced by a high-fat diet. PLoS One. 8: e65061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyamoto Y., Mauer A. S., Kumar S., Mott J. L., and Malhi H.. 2014. Mmu-miR-615-3p regulates lipoapoptosis by inhibiting C/EBP homologous protein. PLoS One. 9: e109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Listenberger L. L., Ory D. S., and Schaffer J. E.. 2001. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 276: 14890–14895. [DOI] [PubMed] [Google Scholar]
- 98.Egnatchik R. A., Leamy A. K., Jacobson D. A., Shiota M., and Young J. D.. 2014. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol. Metab. 3: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rong X., Albert C. J., Hong C., Duerr M. A., Chamberlain B. T., Tarling E. J., Ito A., Gao J., Wang B., Edwards P. A., et al. 2013. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 18: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leamy A. K., Egnatchik R. A., Shiota M., Ivanova P. T., Myers D. S., Brown H. A., and Young J. D.. 2014. Enhanced synthesis of saturated phospholipids is associated with ER stress and lipotoxicity in palmitate treated hepatic cells. J. Lipid Res. 55: 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flowers M. T., Keller M. P., Choi Y., Lan H., Kendziorski C., Ntambi J. M., and Attie A. D.. 2008. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol. Genomics. 33: 361–372. [DOI] [PubMed] [Google Scholar]
- 102.Fu S., Yang L., Li P., Hofmann O., Dicker L., Hide W., Lin X., Watkins S. M., Ivanov A. R., and Hotamisligil G. S.. 2011. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 473: 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Damiano F., Alemanno S., Gnoni G. V., and Siculella L.. 2010. Translational control of the sterol-regulatory transcription factor SREBP-1 mRNA in response to serum starvation or ER stress is mediated by an internal ribosome entry site. Biochem. J. 429: 603–612. [DOI] [PubMed] [Google Scholar]
- 104.Damiano F., Rochira A., Tocci R., Alemanno S., Gnoni A., and Siculella L.. 2013. hnRNP A1 mediates the activation of the IRES-dependent SREBP-1a mRNA translation in response to endoplasmic reticulum stress. Biochem. J. 449: 543–553. [DOI] [PubMed] [Google Scholar]
- 105.Fang D. L., Wan Y., Shen W., Cao J., Sun Z. X., Yu H. H., Zhang Q., Cheng W. H., Chen J., and Ning B.. 2013. Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells. Mol. Cell. Biochem. 381: 127–137. [DOI] [PubMed] [Google Scholar]
- 106.Jo H., Choe S. S., Shin K. C., Jang H., Lee J. H., Seong J. K., Back S. H., and Kim J. B.. 2013. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 57: 1366–1377. [DOI] [PubMed] [Google Scholar]
- 107.Su Q., Tsai J., Xu E., Qiu W., Bereczki E., Santha M., and Adeli K.. 2009. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 50: 77–84. [DOI] [PubMed] [Google Scholar]
- 108.Su Q., Baker C., Christian P., Naples M., Tong X., Zhang K., Santha M., and Adeli K.. 2014. Hepatic mitochondrial and ER stress induced by defective PPARalpha signaling in the pathogenesis of hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 306: E1264–E1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chan S. M., Sun R. Q., Zeng X. Y., Choong Z. H., Wang H., Watt M. J., and Ye J. M.. 2013. Activation of PPARalpha ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes. 62: 2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiong X., Wang X., Lu Y., Wang E., Zhang Z., Yang J., Zhang H., and Li X.. 2014. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J. Hepatol. 60: 847–854. [DOI] [PubMed] [Google Scholar]
- 111.Watanabe M., Houten S. M., Wang L., Moschetta A., Mangelsdorf D. J., Heyman R. A., Moore D. D., and Auwerx J.. 2004. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113: 1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.González-Rodríguez A., Mayoral R., Agra N., Valdecantos M. P., Pardo V., Miquilena-Colina M. E., Vargas-Castrillón J., Lo Iacono O., Corazzari M., Fimia G. M., et al. 2014. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 5: e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peter A., Weigert C., Staiger H., Machicao F., Schick F., Machann J., Stefan N., Thamer C., Haring H. U., and Schleicher E.. 2009. Individual stearoyl-CoA desaturase 1 expression modulates endoplasmic reticulum stress and inflammation in human myotubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes. 58: 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deldicque L., Cani P. D., Philp A., Raymackers J. M., Meakin P. J., Ashford M. L., Delzenne N. M., Francaux M., and Baar K.. 2010. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 299: E695–E705. [DOI] [PubMed] [Google Scholar]
- 115.Kars M., Yang L., Gregor M. F., Mohammed B. S., Pietka T. A., Finck B. N., Patterson B. W., Horton J. D., Mittendorfer B., Hotamisligil G. S., et al. 2010. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 59: 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang H., Wang Y., Li J., Yu J., Pu J., Li L., Zhang H., Zhang S., Peng G., Yang F., et al. 2011. Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein a-I. J. Proteome Res. 10: 4757–4768. [DOI] [PubMed] [Google Scholar]
- 117.Salvadó L., Coll T., Gómez-Foix A. M., Salmerón E., Barroso E., Palomer X., and Vázquez-Carrera M.. 2013. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. 56: 1372–1382. [DOI] [PubMed] [Google Scholar]
- 118.Salvadó L., Barroso E., Gómez-Foix A. M., Palomer X., Michalik L., Wahli W., and Vázquez-Carrera M.. 2014. PPARbeta/delta prevents endoplasmic reticulum stress-associated inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. 57: 2126–2135. [DOI] [PubMed] [Google Scholar]
- 119.Deldicque L., Van Proeyen K., Francaux M., and Hespel P.. 2011. The unfolded protein response in human skeletal muscle is not involved in the onset of glucose tolerance impairment induced by a fat-rich diet. Eur. J. Appl. Physiol. 111: 1553–1558. [DOI] [PubMed] [Google Scholar]
- 120.Hage Hassan R., Hainault I., Vilquin J. T., Samama C., Lasnier F., Ferre P., Foufelle F., and Hajduch E.. 2012. Endoplasmic reticulum stress does not mediate palmitate-induced insulin resistance in mouse and human muscle cells. Diabetologia. 55: 204–214. [DOI] [PubMed] [Google Scholar]
- 121.Rieusset J., Chauvin M. A., Durand A., Bravard A., Laugerette F., Michalski M. C., and Vidal H.. 2012. Reduction of endoplasmic reticulum stress using chemical chaperones or Grp78 overexpression does not protect muscle cells from palmitate-induced insulin resistance. Biochem. Biophys. Res. Commun. 417: 439–445. [DOI] [PubMed] [Google Scholar]
- 122.Nielsen L. B., Perko M., Arendrup H., and Andersen C. B.. 2002. Microsomal triglyceride transfer protein gene expression and triglyceride accumulation in hypoxic human hearts. Arterioscler. Thromb. Vasc. Biol. 22: 1489–1494. [DOI] [PubMed] [Google Scholar]
- 123.Marfella R., Di Filippo C., Portoghese M., Barbieri M., Ferraraccio F., Siniscalchi M., Cacciapuoti F., Rossi F., D’Amico M., and Paolisso G.. 2009. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J. Lipid Res. 50: 2314–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Okada K., Minamino T., Tsukamoto Y., Liao Y., Tsukamoto O., Takashima S., Hirata A., Fujita M., Nagamachi Y., Nakatani T., et al. 2004. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 110: 705–712. [DOI] [PubMed] [Google Scholar]
- 125.Azfer A., Niu J., Rogers L. M., Adamski F. M., and Kolattukudy P. E.. 2006. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am. J. Physiol. Heart Circ. Physiol. 291: H1411–H1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Perman J. C., Bostrom P., Lindbom M., Lidberg U., StAhlman M., Hagg D., Lindskog H., Scharin Tang M., Omerovic E., Mattsson Hulten L., et al. 2011. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J. Clin. Invest. 121: 2625–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drevinge C., Karlsson L. O., Stahlman M., Larsson T., Perman Sundelin J., Grip L., Andersson L., Boren J., and Levin M. C.. 2013. Cholesteryl esters accumulate in the heart in a porcine model of ischemia and reperfusion. PLoS One. 8: e61942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palomer X., Capdevila-Busquets E., Botteri G., Salvado L., Barroso E., Davidson M. M., Michalik L., Wahli W., and Vazquez-Carrera M.. 2014. PPARbeta/delta attenuates palmitate-induced endoplasmic reticulum stress and induces autophagic markers in human cardiac cells. Int. J. Cardiol. 174: 110–118. [DOI] [PubMed] [Google Scholar]
- 129.Bosma M., Dapito D. H., Drosatos-Tampakaki Z., Huiping-Son N., Huang L. S., Kersten S., Drosatos K., and Goldberg I. J.. 2014. Sequestration of fatty acids in triglycerides prevents endoplasmic reticulum stress in an in vitro model of cardiomyocyte lipotoxicity. Biochim. Biophys. Acta. 1841: 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]