Abstract
The past decade has witnessed multiple advances in our understanding of magnesium (Mg2+) homeostasis. The discovery that mutations in claudin-16/paracellin-1 or claudin-19 are responsible for familial hypomagnesemia with hypercalciuria and nephrocalcinosis provided insight into the molecular mechanisms governing paracellular transport of Mg2+. Our understanding of the transcellular movement of Mg2+ was similarly enhanced by the realization that defects in transient receptor potential melastatin 6 (TRPM6) cause hypomagnesemia with secondary hypocalcemia. This channel regulates the apical entry of Mg2+ into epithelia. In so doing, TRPM6 alters whole-body Mg2+ homeostasis by controlling urinary excretion. Consequently, investigation into the regulation of TRPM6 has increased. Acid-base status, 17β estradiol, and the immunosuppressive agents FK506 and cyclosporine affect plasma Mg2+ levels by altering TRPM6 expression. A mutation in epithelial growth factor is responsible for isolated autosomal recessive hypomagnesemia, and epithelial growth factor activates TRPM6. A defect in the γ-subunit of the Na,K-ATPase causes isolated dominant hypomagnesemia by altering TRPM6 activity through a decrease in the driving force for apical Mg2+ influx. We anticipate that the next decade will provide further detail into the control of the gatekeeper TRPM6 and, therefore, overall whole-body Mg2+ balance.
Magnesium (Mg2+) is the second most common intracellular cation.1 Its abundance facilitates multiple roles that it plays in common, essential intracellular processes. It is a co-factor in multiple enzymatic reactions, including those involving energy metabolism and DNA and protein synthesis, and it participates in the regulation of ion channels.2 Mg2+ homeostasis is therefore fundamental to the existence of life. Mg2+ balance in the body is controlled by a dynamic interplay among intestinal absorption, exchange with bone, and renal excretion.3 This last process is where the greatest regulation occurs and consequently is the major focus of this review.
The consequence of altered Mg2+ homeostasis is multifold. Hypermagnesemia can cause neurologic and cardiac sequelae, including lethargy, confusion, coma, a prolongation in the PR interval, widened QRS, complete heart block, and cardiac arrest.4 Hypomagnesemia results in similar clinical manifestations that include tetany, seizures, and cardiac arrhythmias.4,5 Altered plasma Mg2+ levels can in turn affect calcium (Ca2+) and potassium (K+) levels.4–7 Thus, understanding Mg2+homeostasis is important not only for the treatment of these disorders but also for the understanding and management of other electrolyte abnormalities. Although Mg2+ homeostasis has been studied for decades, it is only recently that the molecular determinants of this process have become clearer. Much of this insight derives from the study of patients, often children, with disorders of Mg2+ wasting. From these studies, the molecular determinants of Mg2+ homeostasis have started to be unraveled.
INTESTINAL UPTAKE
Typically, 300 mg of Mg2+ is ingested daily, 24 to 75% of which is absorbed, a process dependent on body stores and dietary content.8–10 The entire length of the bowel is capable of absorbing Mg2+. As occurs in the nephron, intestinal absorption proceeds in both a passive paracellular and an active transcellular manner.8,11 Mg2+ absorption from the small bowel occurs predominately in a paracellular manner.12,13 Given the appropriate driving force, significant paracellular absorption can also take place in the colon.12 The kinetics of this movement are governed by active absorption of sodium (Na+) followed by water.14 Mg2+ and other ions flow down their concentration gradient from bowel lumen to peri-intestinal capillary. It is noteworthy that under conditions producing a luminal driving force, such as diarrheal states, Mg2+ can be secreted into the lumen of the gut along with water and electrolytes.11
Active transcellular absorption of Mg2+ occurs almost exclusively in the colon.5,8 The rare monogenetic disorder hypomagnesemia with secondary hypocalcemia (HSH) provides molecular insight into this process. Children with this disease have seizures and tetany, secondary to extremely low Mg2+levels.15,16 Their hypomagnesemia is due to a failure in the active transcellular (re)absorption of Mg2+ from both the gut and the kidney.17–20 A mutation in the transient receptor potential (TRP) channel transient receptor potential melastatin 6 (TRPM6) was found to be responsible for this disease.21,22 Localization studies demonstrated this channel in the colon and distal convoluted tubule (DCT), which is the site of active renal transcellular reabsorption.23,24 At the subcellular level, TRPM6 is predominantly expressed apically.23 Hence, elucidation of this rare genetic disorder resulted in the discovery of the apical entry mechanism for Mg2+ into epithelia. The molecular identity of the protein responsible for the basolateral exit of Mg2+ from the epithelial cell remains unidentified. Furthermore, whether there exists an intracellular chaperone that facilitates transcellular diffusion of Mg2+, as occurs for Ca2+, is also not known.
BONE: A MG2+ RESERVOIR
The majority of the body’s Mg2+ (>50%) resides within the skeleton, as part of the hydroxyapatite crystalline structure.1,9,25 As is the case for Ca2+, bone is thought to provide a buffer for plasma Mg2+, leaching Mg2+ when plasma levels drop and facilitating the synthesis of new bone when the circulating level is plentiful.26 Consistent with this notion, animals fed Mg2+-deficient diets have a bone Mg2+ content that is reduced by 30 to 40% and a reduced bone mineral density.27–29 This is mediated by a decrease in the number of osteoblasts, with inhibited function,9,27,28 and an increase in both the number and function of osteoclasts.9,27–29 Finally, current data, although by no means conclusive, suggest that Mg2+ deficiency predisposes an individual to osteoporosis.29–32 Unfortunately, our understanding of the molecular details governing the incorporation of Mg2+ into bone by osteoblasts and its retrieval by osteoclasts is minimal.
RENAL REGULATION OF MG2+ EXCRETION
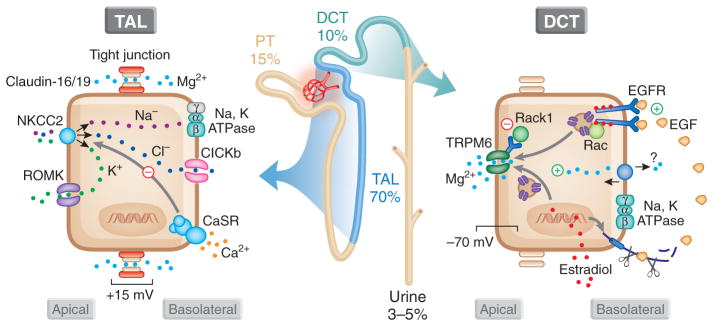
Approximately 80% of total plasma Mg2+ is filtered by the glomeruli,33,34 the vast majority of which is absorbed along the course of the nephron.6 On a normal diet, fractional excretion of Mg2+ is between 3 and 5%35; however, in the presence of hypomagnesemia, this can be decreased to 0.5 to 1% to conserve Mg2+ stores.36 The majority of filtered Mg2+ is absorbed in the proximal tubule (approximately 20%) and thick ascending limb (TAL) of the loop of Henle (approximately 70%) by a passive paracellular mechanism (Figure 1).37,38 That two thirds of filtered Mg2+is absorbed in the TAL and not the proximal tubule is unique for renal tubular ion transport. All ions studied to date, except for Mg2+, are reabsorbed to a greater extent in the proximal tubule. The remaining 10% of filtered Mg2+ is absorbed by an active transcellular mechanism in DCT.6 This latter process ultimately controls the amount of Mg2+ excreted in the urine, because no Mg2+ reabsorption occurs distal to this segment.6 The DCT is therefore the predominant site of specifically regulated Mg2+excretion. Insights gleaned from the delineation of monogenetic Mg2+ wasting disorders have proved highly valuable in deciphering the molecular events governing renal regulated excretion (Figure 1, Table 1).39,40
Figure 1.
Renal regulation of Mg2+ homeostasis. A total of 80% of Mg2+ is filtered at the glomeruli, 15% of which is absorbed proximally, 70% in the TAL, and 15% in the DCT, leaving 3 to 5% to be excreted in the urine. The TAL is the main site of passive paracellular reabsorption of Mg2+, a process mediated by claudin-16 and -19. This paracellular reabsorption depends on the active reabsorption of Na+, which is mediated by apical entry through sodium potassium chloride cotransporter (NKCC) and efflux via the Na+,K+-ATPase. Efflux of chloride (Cl−) occurs through CLCKb, and K+ is recycled back into the lumen via ROMK. The Ca2+-sensing receptor (CaSR) acts to inhibit this process and prevent both paracellular Ca2+ and Mg2+ reabsorption. In the DCT, luminal Mg2+ enters via TRPM6. The mediator of its efflux is unknown. Intracellular Mg2+ and RACK1 inhibit TRPM6. EGF, cleaved from the basolateral membrane, activates TRPM6, and its expression is increased by estradiol. EGFR, EGF receptor; PT, proximal tubule.
Table 1.
Proteins implicated in the molecular control of Mg2+ homeostasisa
| Protein | Gene | Localization | Function | Associated Human Disease | Reference |
|---|---|---|---|---|---|
| Claudin-16/paracellin-1 | CLDN16 | TAL, tight junction | Permissive of paracellular permeability | FHHNC | 41–44,53 |
| Claudin-19 | CLDN19 | TAL, tight junction | Permissive of paracellular permeability | FHHNC | 49,50,53 |
| NCC | SLC12A3 | DCT, apical membrane | Sodium chloride co-transporter | Gitelman syndrome | 54,55 |
| TRPM6 | TRPM6 | DCT, apical membrane, colon, lung | Selective Mg2+ channel, apical entry in transcellular transport | HSH | 17–23 |
| Na,K-ATPase γ-subunit | FXYD2 | PT, MD/DCT, ?TAL, medulla | Alters kinetics of Na+ and K+ exchange | Isolated dominant hypomagnesemia | 76–80,82 |
| EGF | EGF | Adrenal, brain, heart, kidney (DCT), salivary gland, spleen, thymus, intestine, thyroid, and uterus | Increases TRPM6 activity | Isolated recessive hypomagnesemia | 87 |
The gene name, renal localization, function, and associated human disease of proteins known to be involved in the molecular control of Mg2+ homeostasis.
PT, proximal tubule.
Paracellular Transport: The Proximal Tubule and Loop of Henle
Reabsorption of Mg2+ from the lumen of the proximal tubule and TAL occurs in a passive, paracellular manner.38 Mg2+ flows between the epithelial cells down its electrochemical gradient.39 The exact determinants that govern paracellular Mg2+ movement are, as yet, unknown. Surprising, whereas >60% of filtered Na+ and water is absorbed in the proximal tubule, only approximately 15% of Mg2+ is reabsorbed in this segment.37,38 This is in direct contrast to Ca2+, another divalent cation reabsorbed by the paracellular route, which is absorbed in a similar ratio to Na+ and water.40 The majority of Mg2+is absorbed in the TAL, approximately 70%.39 This observation suggests that something facilitates paracellular Mg2+absorption in the TAL that is absent in the proximal tubule.37
It was through study of a rare disorder of Mg2+ wasting, familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC), that the molecular identity of this paracellular mediator was identified. Patients with FHHNC have mutations in claudin-16/paracellin-1,41– 44 a protein localized exclusively to the tight junction of epithelia in the TAL.43,44 Claudins are tetraspanning, transmembrane proteins localized to the tight junction by zona occludens proteins.45,46 Their localization and both intracellular and intercellular interactions are postulated to form pores that regulate paracellular movement of ions.47,48 Absent or faulty claudin-16 activity could therefore prevent the reabsorption of Mg2+ (as well as Ca2+) and result in FHHNC. Consistent with this hypothesis is the recent observation that a similar phenotype is observed in individuals with mutations in claudin-19, which is also located at the tight junction of the TAL.49,50 That claudin-16 affects paracellular permeability is supported by heterologous expression studies demonstrating increased paracellular flux of cations.51,52 A recent study extended this observation to demonstrate a functional interaction between claudin-16 and -19 in renal epithelia at the tight junction, which increases cation selectivity above that of claudin-16 in isolation.53
A molecular enigma is presented by the most common monogenetic disorder resulting in Mg2+ wasting, Gitelman syndrome. Patients with this disorder have a mutation in the thiazide-sensitive Na+/Cl– co-transporter (NCC).54,55 Their disease is characterized by a hypokalemic metabolic alkalosis with hypomagnesemia and hypocalciuria.56 Inhibition of NCC with thiazide diuretics results in a similar phenotype.57 Mice genetically engineered with null alleles of the gene encoding NCC or those treated with thiazide diuretics have decreased expression of TRPM6.58 This channel is the protein responsible for apical entry of luminal Mg2+and provides an explanation for the Mg2+ wasting observed in this disease23; however what signals the decrease in abundance of TRPM6 in the absence of NCC activity is unclear.
Transcellular Transport: The DCT
The DCT is the last site of Mg2+ reabsorption in the nephron and the only site where it occurs in an active transcellular manner.6 There has been significant recent progress in understanding the molecular details governing this process. Study of the affected protein, TRPM6, in patients with HSH reveals its localization to the DCT.23 Moreover, Mg2+ loading experiments demonstrated that these individuals not only failed to absorb Mg2+ from the gut but also had a renal leak.17 As with intestinal epithelia, subcellular localization of TRPM6 in DCT is apical, supporting a role for this channel in luminal Mg2+ influx.23 In parallel to the gut, the mechanism of basolateral efflux of Mg2+is unknown, although it is speculated this occurs through exchange with Na+ in a secondarily active process (Figure 1).59 Because the apical membrane potential in the DCT is approximately –70 mV or greater,60,61 the Nernst potential favors Mg2+ influx. In fact, because intracellular and extracellular Mg2+ concentrations are comparable,62,63 membrane potential is likely the major determinant of apical Mg2+ entry.26 Consequently, energy must be expended to effect its efflux at the basolateral membrane.6 As such, it is possible that Mg2+efflux is mediated by an ATP-dependent Mg2+ pump.6
It is unclear whether intracellular Mg2+ is buffered by a chaperone in the kidney; if so, then the identity of such a chaperone is also a matter of speculation.64 In DCT, parvalbumin and, to a lesser extent, calbindin-D28K have overlapping expression with TRPM6.23,65 Both proteins have an affinity for Mg2+ that favors binding under physiologic conditions.66 Recently, investigators made a parvalbumin null mouse.65 The null animal has polydipsia and polyuria as a result of a decrease in NCC expression. Ca2+ excretion is somehow decreased, whereas renal Mg2+excretion is unaltered, neither confirming nor excluding parvalbumin from a role in DCT Mg2+ reabsorption. Whether these animals will inappropriately excrete Mg2+ when deprived remains unclear. Regardless, intracellular concentrations of Mg2+ in the millimolar range are not detrimental to a cell (as would be the case for Ca2+), and, unlike Ca2+ that is used as a dynamic signaling molecule, the intracellular concentration of Mg2+ is not known to fluctuate. This obviates the necessity for a Mg2+ chaperone; however such a chaperone may increase the rate at which Mg2+ can diffuse from the apical to basolateral cell surface. Alternatively, because intracellular Mg2+ is known to inhibit TRPM6,23 such a chaperone would relieve the Mg2+-dependent inhibition of TRPM6. Both of these possibilities would increase the efficiency of transcellular Mg2+ transport. This option remains speculative, and further research will elucidate whether this mechanism exists in vivo.
TRPM6
TRPM6 has only recently been confirmed as the channel responsible for the apical entry of Mg2+ into epithelia; consequently, the amount of information with respect to its regulation is limited (Table 2). Its location and function position it to be a key modulator of Mg2+ homeostasis. As such, this is an area of active research with several recent, interesting results.
Table 2.
Effectors of Mg2+ homeostasisa
| Effector | Effect on TRPM6 | Effect on Mg2+ Homeostasis | Reference |
|---|---|---|---|
| EGF | ↑ Activity, mechanism unknown | Hypomagnesuria (±hypermagnesemia) | 87–89 |
| RACK1 | ↑ Activity, via association and phosphorylation of the α kinase domain | nd | 75 |
| Mg2+ (intracellular) | ↓ Activity | ↑ Urinary Mg2+ excretion | 23,24 |
| Mg2+ (extracellular) | ↓ TRPM6 expression | ↑ Urinary Mg2+ excretion | 24 |
| Acidosis | ↓ TRPM6 expression/↓ activity | ↑ Urinary Mg2+ excretion (±hypomagnesemia) | 71,94 |
| Alkalosis | ↑ TRPM6 expression | ↓ Urinary Mg2+ excretion (±hypermagnesemia) | 94 |
| 17β-estradiol | ↑ TRPM6 expression | ↓ Urinary Mg2+ excretion | 24 |
| FK506/cyclosporine | ↓ TRPM6 expression | ↑ Urinary Mg2+ excretion (±hypomagnesemia) | 95,96 |
| Thiazide diuretic | ↓ TRPM6 expression | ↑ Urinary Mg2+ excretion (±hypomagnesemia) | 58 |
The known effectors of TRPM6 and their effect on TRPM6 activity and the mechanism (if known) and their affect on magnesium (Mg2+) homeostasis.
nd, not determined.
Structure of TRPM6
TRPM6 is predicted to share structural homology to other TRP channels. It is composed of a large intracellular amino-terminus, six membrane-spanning domains that make up the channel pore, and a large intracellular carboxy-terminal domain. Fused to the carboxy-terminus is an α-kinase domain.67 The functional unit is thought to be a homo- or heterotetramer with TRPM7.68,69 The exact composition is debated in the literature. We have been able to express TRPM6 successfully in mammalian cells and characterize the electrophysiologic properties of the channel without coexpressing TRPM7.23 Consistent with this, Li et al.68 detected TRPM6-specific currents from the plasma membrane of cells in the presence and absence of TRPM7. Furthermore, two separate studies demonstrated by heterologous expression (without coexpressing TRPM7) that mutation of a single residue (E1024) in the pore region of TRPM6 alters cation selectivity of the channel70,71 and its sensitivity to extracellular pH.71 Together, these studies suggested that TRPM6 can function as a homotetramer. This is in contrast to findings in Xenopus oocytes and in inducible mammalian cell culture systems, where coexpression of TRPM7 is required for plasma membrane localization and TRPM6-specific currents.69,72,73 These authors used electrochemical, biochemical, and immunofluorescent techniques to show that a direct interaction between TRPM6 and TRPM7 is required for plasma membrane localization of TRPM6.69,72,73 What the actual composition of the functional unit of channels is in native epithelia remains unclear.
Function of TRPM6
TRPM6 and TRPM7 are unique channels. They conduct Mg2+ preferentially over Ca2+ 23,68 and contain a functional α-kinase domain. This has dubbed them chanzymes.67 The α-kinase domain plays a role in regulating channel activity. This domain is not necessary for basal function; however, as with TRPM7, auto-phosphorylation is a mechanism regulating channel activity.74,75 TRPM6 is a cation-selective channel with strong outward rectification. It is inhibited by ruthenium red in a voltage-dependent manner, and intracellular Mg2+ acts as a negative regulator of channel activity.23 Furthermore, an acidic extracellular pH inhibits the conductance of TRPM6, a characteristic dependent on specific residues in the pore region.71 Together these properties provide a means to regulate the apical entry of Mg2+ from tubular fluid and ultimately regulate whole-body Mg2+ homeostasis.
Recently, a new TRPM6-interacting protein, receptor for activated C-kinase 1 (RACK1), has been described.75 This protein interacts directly with the α-kinase domain inhibiting channel activity. The interaction itself is independent of the phosphorylation state of the kinase domain; however, RACK1-mediated inhibition requires autophosphorylation of residue T1851 in the kinase domain.75 Furthermore, the inhibition of TRPM6 activity by intracellular Mg2+ depends on this auto-phosphorylation. It is possible, therefore, that TRPM6-mediated Mg2+ influx induces phosphorylation of T1851 located in the α-kinase domain, a process that activates the inhibitory effect of RACK1. This last step may act as an intracellular feedback mechanism controlling TRPM6-mediated Mg2+ influx and preventing Mg2+ overload during renal epithelial Mg2+ transport.
The notion that transcellular Mg2+ transport, specifically Mg2+ flux through TRPM6, is dependent on membrane potential is supported by the finding that a defect in the γ-subunit of the Na,K-ATPase causes isolated dominant hypomagnesemia.76 The exact location of this kidney-specific subunit of the Na,K-ATPase is debated, although it has been localized to the same part of the nephron as TRPM6, the DCT,77–80 as well as the renal medulla.81 The null mouse has nearly a 50% increase in Mg2+ excretion,78 and although not significantly different from wild-type mice, it certainly suggests a role for this protein in renal Mg2+ handling. Expression with the other subunits alters the kinetics of the pump such that it has an increased affinity for K+at negative membrane potentials,77,82 a decreased affinity for Na+,77,83 and altered affinity for ATP.84 Coexpression of the mutant subunit with wild-type protein prevented trafficking to the plasma membrane,76,85 although association between wild-type and mutant subunits is not observed with synthetic peptides.86 Regardless, decreased or absent γ-subunits affect pump activity and consequently alter intracellular K+ concentration. This could, in turn, inhibit transcellular transport of Mg2+ because of an altered membrane potential. The exact mechanism causing increased urinary Mg2+ excretion and hypomagnesemia has yet to be determined but is an area of active research.
Local Regulation of TRPM6
Recently, the identification of the causative mechanism for another Mg2+-wasting disease, isolated autosomal recessive hypomagnesemia (IRH), provided further insight into the regulation of TRPM6 and identified the first magnesiotropic hormone.87 A family with hypomagnesemia was found to have a mutation in the gene encoding epithelial growth factor (EGF) that is expressed in DCT along with TRPM6. The mutation was in the cytosolic carboxy-terminus, within a conserved basolateral-sorting motif (PXXP), prompting us to suggest that trafficking to the basolateral membrane and consequent release into the extracellular space are inhibited. Consistent with our hypothesis, the application of EGF to cells expressing TRPM6 increased the activity of this channel. Furthermore, when culture medium from cells expressing wild-type EGF was applied to TRPM6, channel activity increased, whereas medium from cells expressing mutant EGF did not stimulate TRPM6.87 Several other observations support a role for EGF in regulating TRPM6 activity. Lactating ewes, when administered EGF, have a decrease in fractional excretion of Mg2+ and develop hypermagnesemia.88 Patients treated with the anticancer agent cetuximab, a mAb that blocks the EGF receptor, develop hypomagnesemia secondary to increased renal wasting.89 Taken together, these findings suggest that renal EGF acts in an autocrine or a paracrine manner to increase TRPM6 activity. This stimulates the reabsorption of Mg2+from DCT and consequently decreases the fractional excretion of Mg2+ (Figure 1).90
Systemic Regulation of TRPM6
Reduction in dietary Mg2+ results in hypomagnesemia. This in turn stimulates Mg2+ reabsorption along the DCT.35,91 Rodents fed a diet deficient in Mg2+ demonstrated an increase in colonic, cecal, and DCT expression of TRPM6.24 Coincident with this increase in TRPM6, their urinary excretion of Mg2+ (and Ca2+) diminished. Conversely, animals fed a diet high in Mg2+ paradoxically up-regulated colonic TRPM6 yet remained eumagnesemic secondary to an increased renal excretion of Mg2+.24
17β-Estradiol also increased the expression of TRPM6.24 Indeed, ovariectomized rats showed a decrease in levels of TRPM6 (and magnesuria) that was normalized by administration of the hormone.24 This is in contrast to vitamin D and parathyroid hormone, which are unable to alter TRPM6 expression in vivo; however, they both increased Mg2+ influx in a DCT cell culture model as measured by radiometric imaging using Mag-fura.3,6 These results can be reconciled as an effect mediated by altered activity, not by expression level of the channel. Given the presumed clarity of these later studies characterizing the affect of hormones on Mg2+ influx in cell culture and the available micropuncture and microperfusion data,6,92 it is likely that our understanding of the hormonal regulation of TRPM6 activity will grow even further.
The acid-base status of an individual affects the body’s handling of Mg2+.92,93 This occurs through an alteration in levels of TRPM6. Mice with chronic metabolic acidosis display a reduced renal expression of TRPM6, increased excretion of Mg2+, and decreased plasma Mg2+,94 whereas metabolic alkalosis results in the opposite effect.94 Finally, long-term administration of the immunosuppressive agent FK506 commonly causes hypomagnesemia. Both this compound and cyclosporine mediate this effect by decreasing the expression of TRPM6, explaining this common clinical complication of kidney transplantation.95,96
CONCLUSIONS
Studies on monogenetic disorders of Mg2+ wasting have unraveled the molecular details of renal and intestinal Mg2+ absorption and consequently whole-body Mg2+ homeostasis. Renal paracellular transport of Mg2+ in the TAL occurs in the presence of claudin-16/19, and loss of functioning claudin-16/19 causes FHHNC as a result of Mg2+ wasting. Gitelman syndrome, a disease characterized by renal salt loss because of a defect in NCC, results in hypokalemic metabolic alkalosis and hypomagnesemia. The exact mechanism of this latter finding remains unknown; however, absent or inhibited NCC activity leads to the downregulation of TRPM6, the DCT channel responsible for apical entry of Mg2+. This channel plays a central role in Mg2+ homeostasis by regulating the luminal entry of Mg2+ in both the DCT and the intestine. The absence or malfunction of TRPM6 results in severe hypomagnesemia and is responsible for HSH. TRPM6 is regulated at the transcriptional level by acid-base status, 17β-estradiol, and both FK506 and cyclosporine. We are just beginning to understand how its activity is regulated in a shorter time scale through trafficking to the plasma membrane or from alterations in channel kinetics. To this end, both EGF and RACK1 are implicated in its acute regulation. Indeed, mutations in the former protein have been found to cause IRH.
Acknowledgments
The laboratory of R.J.B. and J.G.H. is supported by the Netherlands Organization for Scientific Research (Zon-Mw 016.006.001, Zon-Mw 9120.6110), a EURYI award from the European Science Foundation, Human Frontiers Science Program (RGP32/2004), and the Dutch Kidney foundation (C02.2030, C03.6017). R.T.A. is supported by a phase I, CIHR Clinician Scientist award and a KRESCENT postdoctoral award from the Kidney Foundation of Canada.
Footnotes
DISCLOSURES
None.
References
- 1.Elin RJ. Magnesium: The fifth but forgotten electrolyte. Am J Clin Pathol. 1994;102:616–622. doi: 10.1093/ajcp/102.5.616. [DOI] [PubMed] [Google Scholar]
- 2.Flatman PW. Magnesium transport across cell membranes. J Membr Biol. 1984;80:1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- 3.Quamme GA. Renal handling of magnesium: Drug and hormone interactions. Magnesium. 1986;5:248–272. [PubMed] [Google Scholar]
- 4.Topf JM, Murray PT. Hypomagnesemia and hypermagnesemia. Rev Endocr Metab Disord. 2003;4:195–206. doi: 10.1023/a:1022950321817. [DOI] [PubMed] [Google Scholar]
- 5.Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med. 2005;20:3–17. doi: 10.1177/0885066604271539. [DOI] [PubMed] [Google Scholar]
- 6.Dai LJ, Ritchie G, Kerstan D, Kang HS, Cole DE, Quamme GA. Magnesium transport in the renal distal convoluted tubule. Physiol Rev. 2001;81:51–84. doi: 10.1152/physrev.2001.81.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007;18:2649–2652. doi: 10.1681/ASN.2007070792. [DOI] [PubMed] [Google Scholar]
- 8.Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci. 2000;5:D666–D677. doi: 10.2741/schweigel. [DOI] [PubMed] [Google Scholar]
- 9.Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: Animal and human observations. J Nutr Biochem. 2004;15:710–716. doi: 10.1016/j.jnutbio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Kayne LH, Lee DB. Intestinal magnesium absorption. Miner Electrolyte Metab. 1993;19:210–217. [PubMed] [Google Scholar]
- 11.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest. 1991;88:396–402. doi: 10.1172/JCI115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behar J. Magnesium absorption by the rat ileum and colon. Am J Physiol. 1974;227:334–340. doi: 10.1152/ajplegacy.1974.227.2.334. [DOI] [PubMed] [Google Scholar]
- 13.Brannan PG, Vergne-Marini P, Pak CY, Hull AR, Fordtran JS. Magnesium absorption in the human small intestine: Results in normal subjects, patients with chronic renal disease, and patients with absorptive hypercalciuria. J Clin Invest. 1976;57:1412–1418. doi: 10.1172/JCI108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frizzell RA, Schultz SG. Ionic conductances of extracellular shunt pathway in rabbit ileum: Influence of shunt on transmural sodium transport and electrical potential differences. J Gen Physiol. 1972;59:318–346. doi: 10.1085/jgp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paunier L, Radde IC, Kooh SW, Conen PE, Fraser D. Primary hypomagnesemia with secondary hypocalcemia in an infant. Pediatrics. 1968;41:385–402. [PubMed] [Google Scholar]
- 16.Dudin KI, Teebi AS. Primary hypomagnesaemia: A case report and literature review. Eur J Pediatr. 1987;146:303–305. doi: 10.1007/BF00716481. [DOI] [PubMed] [Google Scholar]
- 17.Matzkin H, Lotan D, Boichis H. Primary hypomagnesemia with a probable double magnesium transport defect. Nephron. 1989;52:83–86. doi: 10.1159/000185588. [DOI] [PubMed] [Google Scholar]
- 18.Skyberg D, Stromme JH, Nesbakken R, Harnaes K. Neonatal hypomagnesemia with selective malabsorption of magnesium: A clinical entity. Scand J Clin Lab Invest. 1968;21:355–363. doi: 10.3109/00365516809077007. [DOI] [PubMed] [Google Scholar]
- 19.Stromme JH, Skyberg D, Nesbakken R. A selective intestinal malabsorption of magnesium in an infant. Scand J Clin Lab Invest Suppl. 1967;100:103. [PubMed] [Google Scholar]
- 20.Milla PJ, Aggett PJ, Wolff OH, Harries JT. Studies in primary hypomagnesaemia: Evidence for defective carrier-mediated small intestinal transport of magnesium. Gut. 1979;20:1028–1033. doi: 10.1136/gut.20.11.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 22.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 23.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 24.Groenestege WM, Hoenderop JG, van den Heuvel L, Knoers N, Bindels RJ. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J Am Soc Nephrol. 2006;17:1035–1043. doi: 10.1681/ASN.2005070700. [DOI] [PubMed] [Google Scholar]
- 25.Wallach S. Effects of magnesium on skeletal metabolism. Magnes Trace Elem. 1990;9:1–14. [PubMed] [Google Scholar]
- 26.van de Graaf SF, Bindels RJ, Hoenderop JG. Physiology of epithelial Ca2+ and Mg2+ transport. Rev Physiol Biochem Pharmacol. 2007;158:77–160. doi: 10.1007/112_2006_0607. [DOI] [PubMed] [Google Scholar]
- 27.Gruber HE, Rude RK, Wei L, Frausto A, Mills BG, Norton HJ. Magnesium deficiency: Effect on bone mineral density in the mouse appendicular skeleton. BMC Musculoskelet Disord. 2003;4:7. doi: 10.1186/1471-2474-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rude RK, Gruber HE, Wei LY, Frausto A, Mills BG. Magnesium deficiency: Effect on bone and mineral metabolism in the mouse. Calcif Tissue Int. 2003;72:32–41. doi: 10.1007/s00223-001-1091-1. [DOI] [PubMed] [Google Scholar]
- 29.Rude RK, Kirchen ME, Gruber HE, Meyer MH, Luck JS, Crawford DL. Magnesium deficiency-induced osteoporosis in the rat: Uncoupling of bone formation and bone resorption. Magnes Res. 1999;12:257–267. [PubMed] [Google Scholar]
- 30.Rude RK. Magnesium deficiency: A cause of heterogeneous disease in humans. J Bone Miner Res. 1998;13:749–758. doi: 10.1359/jbmr.1998.13.4.749. [DOI] [PubMed] [Google Scholar]
- 31.Gur A, Colpan L, Nas K, Cevik R, Sarac J, Erdogan F, Duz MZ. The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and a new effect of calcitonin. J Bone Miner Metab. 2002;20:39–43. doi: 10.1007/s774-002-8445-y. [DOI] [PubMed] [Google Scholar]
- 32.Brodowski J. Levels of ionized magnesium in women with various stages of postmenopausal osteoporosis progression evaluated on the basis of densitometric examinations [in Polish] Przegl Lek. 2000;57:714–716. [PubMed] [Google Scholar]
- 33.Brunette MG, Crochet ME. Fluormetric method for the determination of magnesium in renal tubular fluid. Anal Biochem. 1975;65:79–88. doi: 10.1016/0003-2697(75)90493-5. [DOI] [PubMed] [Google Scholar]
- 34.Grimellec CL, Poujeol P, Rouffignia C. 3H-inulin and electrolyte concentrations in Bowman’s capsule in rat kidney: Comparison with artificial ultrafiltration. Pflugers Arch. 1975;354:117–131. doi: 10.1007/BF00579943. [DOI] [PubMed] [Google Scholar]
- 35.Quamme GA. Laboratory evaluation of magnesium status: Renal function and free intracellular magnesium concentration. Clin Lab Med. 1993;13:209–223. [PubMed] [Google Scholar]
- 36.Sutton RA, Domrongkitchaiporn S. Abnormal renal magnesium handling. Miner Electrolyte Metab. 1993;19:232–240. [PubMed] [Google Scholar]
- 37.Murayama Y, Morel F, Le Grimellec C. Phosphate, calcium and magnesium transfers in proximal tubules and loops of Henle, as measured by single nephron microperfusion experiments in the rat. Pflugers Arch. 1972;333:1–16. doi: 10.1007/BF00586037. [DOI] [PubMed] [Google Scholar]
- 38.Quamme GA. Control of magnesium transport in the thick ascending limb. Am J Physiol. 1989;256:F197–F210. doi: 10.1152/ajprenal.1989.256.2.F197. [DOI] [PubMed] [Google Scholar]
- 39.de Rouffignac C, Quamme G. Renal magnesium handling and its hormonal control. Physiol Rev. 1994;74:305–322. doi: 10.1152/physrev.1994.74.2.305. [DOI] [PubMed] [Google Scholar]
- 40.Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 41.Sanjad SA, Hariri A, Habbal ZM, Lifton RP. A novel PCLN-1 gene mutation in familial hypomagnesemia with hypercalciuria and atypical phenotype. Pediatr Nephrol. 2007;22:503–508. doi: 10.1007/s00467-006-0354-5. [DOI] [PubMed] [Google Scholar]
- 42.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 43.Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Sulakova T, Kuwertz-Broking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Scharer K, Seyberth HW, Konrad M. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 44.Weber S, Hoffmann K, Jeck N, Saar K, Boeswald M, Kuwertz-Broeking E, Meij II, Knoers NV, Cochat P, Sulakova T, Bonzel KE, Soergel M, Manz F, Schaerer K, Seyberth HW, Reis A, Konrad M. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur J Hum Genet. 2000;8:414–422. doi: 10.1038/sj.ejhg.5200475. [DOI] [PubMed] [Google Scholar]
- 45.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 46.Balkovetz DF. Claudins at the gate: Determinants of renal epithelial tight junction paracellular permeability. Am J Physiol Renal Physiol. 2006;290:F572–F579. doi: 10.1152/ajprenal.00135.2005. [DOI] [PubMed] [Google Scholar]
- 47.Van Itallie CM, Anderson JM. The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc. 2004;1:38–41. doi: 10.1513/pats.2306013. [DOI] [PubMed] [Google Scholar]
- 48.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 49.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol. 2007;293:F166–F177. doi: 10.1152/ajprenal.00087.2007. [DOI] [PubMed] [Google Scholar]
- 51.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 52.Ikari A, Hirai N, Shiroma M, Harada H, Sakai H, Hayashi H, Suzuki Y, Degawa M, Takagi K. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J Biol Chem. 2004;279:54826–54832. doi: 10.1074/jbc.M406331200. [DOI] [PubMed] [Google Scholar]
- 53.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 55.Mastroianni N, Bettinelli A, Bianchetti M, Colussi G, De Fusco M, Sereni F, Ballabio A, Casari G. Novel molecular variants of the Na-Cl cotransporter gene are responsible for Gitelman syndrome. Am J Hum Genet. 1996;59:1019–1026. [PMC free article] [PubMed] [Google Scholar]
- 56.Simon DB, Lifton RP. The molecular basis of inherited hypokalemic alkalosis: Bartter’s and Gitelman’s syndromes. Am J Physiol. 1996;271:F961–F966. doi: 10.1152/ajprenal.1996.271.5.F961. [DOI] [PubMed] [Google Scholar]
- 57.Moore MJ. Thiazide-induced hypomagnesemia. JAMA. 1978;240:1241. doi: 10.1001/jama.1978.03290120035018. [DOI] [PubMed] [Google Scholar]
- 58.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gunther T. Mechanisms and regulation of Mg2+ efflux and Mg2+ influx. Miner Electrolyte Metab. 1993;19:259–265. [PubMed] [Google Scholar]
- 60.Cohen B, Giebisch G, Hansen LL, Teuscher U, Wiederholt M. Relationship between peritubular membrane potential and net fluid reabsorption in the distal renal tubule of Amphiuma. J Physiol. 1984;348:115–134. doi: 10.1113/jphysiol.1984.sp015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen LL, Schilling AR, Wiederholt M. Effect of calcium, furosemide and chlorothiazide on net volume reabsorption and basolateral membrane potential of the distal tubule. Pflugers Arch. 1981;389:121–126. doi: 10.1007/BF00582101. [DOI] [PubMed] [Google Scholar]
- 62.Grubbs RD. Intracellular magnesium and magnesium buffering. Biometals. 2002;15:251–259. doi: 10.1023/a:1016026831789. [DOI] [PubMed] [Google Scholar]
- 63.Romani AM, Maguire ME. Hormonal regulation of Mg2+ transport and homeostasis in eukaryotic cells. Biometals. 2002;15:271–283. doi: 10.1023/a:1016082900838. [DOI] [PubMed] [Google Scholar]
- 64.Hoenderop JG, Bindels RJ. Epithelial Ca2+ and Mg2+ channels in health and disease. J Am Soc Nephrol. 2005;16:15–26. doi: 10.1681/ASN.2004070523. [DOI] [PubMed] [Google Scholar]
- 65.Belge H, Gailly P, Schwaller B, Loffing J, Debaix H, Riveira-Munoz E, Beauwens R, Devogelaer JP, Hoenderop JG, Bindels RJ, Devuyst O. Renal expression of parvalbumin is critical for NaCl handling and response to diuretics. Proc Natl Acad Sci U S A. 2007;104:14849–14854. doi: 10.1073/pnas.0702810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang W, Lee HW, Hellinga H, Yang JJ. Structural analysis, identification, and design of calcium-binding sites in proteins. Proteins. 2002;47:344–356. doi: 10.1002/prot.10093. [DOI] [PubMed] [Google Scholar]
- 67.Montell C. Mg2+ homeostasis: The Mg2+ nificent TRPM chanzymes. Curr Biol. 2003;13:R799–R801. doi: 10.1016/j.cub.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 68.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Topala CN, Groenestege WT, Thebault S, van den Berg D, Nilius B, Hoenderop JG, Bindels RJ. Molecular determinants of permeation through the cation channel TRPM6. Cell Calcium. 2007;41:513–523. doi: 10.1016/j.ceca.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–25830. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schlingmann KP, Gudermann T. A critical role of TRPM channel-kinase for human magnesium transport. J Physiol. 2005;566:301–308. doi: 10.1113/jphysiol.2004.080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chubanov V, Schlingmann KP, Waring J, Heinzinger J, Kaske S, Waldegger S, Schnitzler MM, Gudermann T. Hypomagnesemia with secondary hypocalcemia due to a mis-sense mutation in the putative pore-forming region of TRPM6. J Biol Chem. 2007;282:7656–7667. doi: 10.1074/jbc.M611117200. [DOI] [PubMed] [Google Scholar]
- 74.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 75.Cao G, Thébault S, van der Wijst J, van der Kemp A, Lasonder E, Bindels RJ, Hoenderop JG. RACK1 inhibits TRPM6 activity Via phosphorylation of the fused alpha-kinase domain. Curr Biol. 2008;18:168–176. doi: 10.1016/j.cub.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 76.Meij IC, Koenderink JB, van Bokhoven H, Assink KF, Groenestege WT, de Pont JJ, Bindels RJ, Monnens LA, van den Heuvel LP, Knoers NV. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase gamma-subunit. Nat Genet. 2000;26:265–266. doi: 10.1038/81543. [DOI] [PubMed] [Google Scholar]
- 77.Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The gamma subunit modulates Na(+) and K(+) affinity of the renal Na,K-ATPase. J Biol Chem. 1999;274:33183–33185. doi: 10.1074/jbc.274.47.33183. [DOI] [PubMed] [Google Scholar]
- 78.Jones DH, Li TY, Arystarkhova E, Barr KJ, Wetzel RK, Peng J, Markham K, Sweadner KJ, Fong GH, Kidder GM. Na,K-ATPase from mice lacking the gamma subunit (FXYD2) exhibits altered Na+ affinity and decreased thermal stability. J Biol Chem. 2005;280:19003–19011. doi: 10.1074/jbc.M500697200. [DOI] [PubMed] [Google Scholar]
- 79.Arystarkhova E, Wetzel RK, Sweadner KJ. Distribution and oligomeric association of splice forms of Na(+)-K(+)-ATPase regulatory gamma-subunit in rat kidney. Am J Physiol Renal Physiol. 2002;282:F393–F407. doi: 10.1152/ajprenal.00146.2001. [DOI] [PubMed] [Google Scholar]
- 80.Wetzel RK, Sweadner KJ. Immunocytochemical localization of Na-K-ATPase alpha- and gamma-subunits in rat kidney. Am J Physiol Renal Physiol. 2001;281:F531–F545. doi: 10.1152/ajprenal.2001.281.3.F531. [DOI] [PubMed] [Google Scholar]
- 81.Pihakaski-Maunsbach K, Vorum H, Honore B, Tokonabe S, Frokiaer J, Garty H, Karlish SJ, Maunsbach AB. Locations, abundances, and possible functions of FXYD ion transport regulators in rat renal medulla. Am J Physiol Renal Physiol. 2006;291:F1033–F1044. doi: 10.1152/ajprenal.00086.2006. [DOI] [PubMed] [Google Scholar]
- 82.Beguin P, Wang X, Firsov D, Puoti A, Claeys D, Horisberger JD, Geering K. The gamma subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J. 1997;16:4250–4260. doi: 10.1093/emboj/16.14.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beguin P, Crambert G, Guennoun S, Garty H, Horisberger JD, Geering K. CHIF, a member of the FXYD protein family, is a regulator of Na,K-ATPase distinct from the gamma-subunit. EMBO J. 2001;20:3993–4002. doi: 10.1093/emboj/20.15.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Therien AG, Karlish SJ, Blostein R. Expression and functional role of the gamma subunit of the Na, K-ATPase in mammalian cells. J Biol Chem. 1999;274:12252–12256. doi: 10.1074/jbc.274.18.12252. [DOI] [PubMed] [Google Scholar]
- 85.Cairo ER, Friedrich T, Swarts HG, Knoers NV, Bindels RJ, Monnens LA, Willems PH, De Pont JJ, Koenderink JB. Impaired routing of wild type FXYD2 after oligomerisation with FXYD2–G41R might explain the dominant nature of renal hypomagnesemia. Biochim Biophys Acta. 2007;1778:398–404. doi: 10.1016/j.bbamem.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 86.Pu HX, Scanzano R, Blostein R. Distinct regulatory effects of the Na,K-ATPase gamma subunit. J Biol Chem. 2002;277:20270–20276. doi: 10.1074/jbc.M201009200. [DOI] [PubMed] [Google Scholar]
- 87.Groenestege WM, Thebault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van Cutsem E, Hoenderop JG, Knoers NV, Bindels RJ. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest. 2007;117:2260–2267. doi: 10.1172/JCI31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gow CB, Silvapulle MJ, Moore GP. Epidermal growth factor alters the electrolyte profile of lactating ewes (Ovis aries) Comp Biochem Physiol Comp Physiol. 1992;103:687–693. doi: 10.1016/0300-9629(92)90167-o. [DOI] [PubMed] [Google Scholar]
- 89.Tejpar S, Piessevaux H, Claes K, Piront P, Hoenderop JG, Verslype C, Van Cutsem E. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: A prospective study. Lancet Oncol. 2007;8:387–394. doi: 10.1016/S1470-2045(07)70108-0. [DOI] [PubMed] [Google Scholar]
- 90.Muallem S, Moe OW. When EGF is offside, magnesium is wasted. J Clin Invest. 2007;117:2086–2089. doi: 10.1172/JCI33004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shils ME. Experimental human magnesium depletion. Medicine (Baltimore) 1969;48:61–85. doi: 10.1097/00005792-196901000-00003. [DOI] [PubMed] [Google Scholar]
- 92.Martin HE, Jones R. The effect of ammonium chloride and sodium bicarbonate on the urinary excretion of magnesium, calcium, and phosphate. Am Heart J. 1961;62:206–210. doi: 10.1016/0002-8703(61)90319-2. [DOI] [PubMed] [Google Scholar]
- 93.Wong NL, Quamme GA, Dirks JH. Effects of acid-base disturbances on renal handling of magnesium in the dog. Clin Sci (Lond) 1986;70:277–284. doi: 10.1042/cs0700277. [DOI] [PubMed] [Google Scholar]
- 94.Nijenhuis T, Renkema KY, Hoenderop JG, Bindels RJ. Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J Am Soc Nephrol. 2006;17:617–626. doi: 10.1681/ASN.2005070732. [DOI] [PubMed] [Google Scholar]
- 95.Nijenhuis T, Hoenderop JG, Bindels RJ. Downregulation of Ca(2+) and Mg(2+) transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol. 2004;15:549–557. doi: 10.1097/01.asn.0000113318.56023.b6. [DOI] [PubMed] [Google Scholar]
- 96.Ikari A, Okude C, Sawada H, Takahashi T, Sugatani J, Miwa M. Down-regulation of TRPM6-mediated magnesium influx by cyclosporin A. Naunyn Schmiedebergs Arch Pharmacol. 2007 Nov 17; doi: 10.1007/s00210-007-0212-4. epub ahead of print. [DOI] [PubMed] [Google Scholar]