Abstract
Na+/H+ exchange activity in the apical membrane of the proximal tubule is fundamental to the reabsorption of Na+ and water from the filtrate. The role of this exchange process in bicarbonate reclamation and, consequently, the maintenance of acid-base homeostasis has been appreciated for at least half a century and remains a pillar of renal tubular physiology. More recently, apical Na+/H+ exchange, mediated by Na+/H+ exchanger isoform 3 (NHE3), has been implicated in proximal tubular reabsorption of Ca2+ and Ca2+ homeostasis in general. Overexpression of NHE3 increased paracellular Ca2+ flux in a proximal tubular cell model. Consistent with this observation, mice with genetic deletion of Nhe3 have a noticable renal Ca2+ leak. These mice also display decreased intestinal Ca2+ uptake and osteopenia. This review highlights the traditional roles of proximal tubular Na+/H+ exchange and summarizes recent novel findings implicating the predominant isoform, NHE3, in Ca2+ homeostasis.
Keywords: calcium, sodium-hydrogen exchanger isoform 3, paracellular, proximal tubule, parathyroid hormone
The Na+/H+ exchanger (NHE) family of proteins contains at least nine isoforms (23, 95). The family can be divided into plasma membrane isoforms (NHE1–NHE5) and endomembrane isoforms (NHE6–NHE9) (23). The plasma membrane isoforms function to exchange extracellular Na+ for intracellular H+, given typical physiological concentrations of substrates. Much less is known about the function of the endomembrane isoforms, although they likely secrete H+ in exchange for Na+, thereby acidifying the lumen of the endomembrane compartment in which they are expressed (29, 58, 89). NHE1 is ubiquitously expressed and serves a role in several housekeeping functions, including the maintenance of intracellular pH and cell volume regulation (95, 102). The expression pattern of the remaining plasma membrane NHE isoforms is more restricted, and their functional roles are consequently more specialized (Table 1).
Table 1.
NHE expression, localization, and (proposed) function
| Isoform | Location
|
Proposed Function(s) | References | |
|---|---|---|---|---|
| Tissue* | Cellular | |||
| NHE1 | Ubiquitous | Plasma membrane (basolateral membrane in epithelia) | Cell volume and intracellular pH regulation | 5, 92, 95, 104, 105 |
| NHE2 | Gastrointestinal tract, kidney, brain, uterus/testis | Plasma membrane (apical membrane in epithelia) | Glandular secretory processes, minor role in intestinal Na+ and water absorption? | 25, 28, 98, 108 |
| NHE3 | Gastrointestinal tract, kidney, gallbladder, epididymis | Plasma membrane (apical), recycling endosomes | Intravascular volume homeostasis, pH regulation, Ca2+ homeostasis | 17, 18, 96, 97, 109 |
| NHE4 | Stomach (kidney and brain) | Plasma membrane (basolateral membrane in epithelia) | Acid extrusion into the stomach, renal ammonia handling | 21, 45, 96, 99 |
| NHE5 | Brain (neurons) | Plasma membrane and recycling endosomes/synaptic vesicles | Regulation of dentritic spine growth? | 11, 33 |
| NHE6 | Ubiquitous | Recycling endosomes | Regulation of luminal pH? | 24, 81, 89, 95 |
| NHE7 | Ubiquitous | trans-Golgi network and endosomes | Regulation of luminal pH in endosomes and/or Golgi? | 89, 94, 95 |
| NHE8† | Ubiquitous | Endomembrane (and apical plasma membrane) | Regulation of luminal pH? Role in intravascular pH and volume regulation? | 12, 14, 53, 89, 126 |
| NHE9 | Ubiquitous | Late endosomes | Regulation of luminal pH? | 89 |
NHE, Na+/H+ exchanger.
The predominant tissue expression is listed only, i.e., this is not an exhaustive list.
The NHE family can be divided into plasma membrane isoforms (NHE1–NHE5) and endomembrane isoforms (NHE6–NHE9). NHE8, although predominantly endomembrane, localizes to the plasma membrane in some cell types.
The proximal tubule expresses NHE1, NHE3, NHE4, and the so-called endomembrane isoforms (17, 18, 54, 99, 104). NHE3 is the predominant brush-border Na+/H+ exchanger in adults (14, 18, 53). NHE1 is expressed in the basolateral membrane of the proximal tubule, where it likely participates in the housekeeping functions mentioned above (104). NHE4 is also expressed basolaterally in this nephron segment, however, to a lesser extent than NHE1 (99). Its function in the proximal tubule remains unknown. The endomembrane NHEs (NHE6, NHE7, and NHE9) are expressed in the proximal tubule as well. The function of these isoforms in the proximal tubule remains to be determined. When expressed in yeast, COS-7, or HeLa cells, NHE8 predominantly localizes to the Golgi (14, 89), although in the proximal tubule it is situated in the apical plasma membrane (14, 53, 54). It may participate in Na+ and bicarbonate reabsorption when expressed in the apical plasmalema, a role primarily served by NHE3 after development (12, 65, 77, 93, 109, 118, 120, 129). In fact, a recent study (12) using NHE3/NHE8 double-knockout mice has provided evidence consistent with NHE8, at least partially, compensating for the loss of luminal Na+/H+ exchange activity after genetic ablation of NHE3 (12). The apical exchange activity of NHEs in the proximal tubule serves multiple roles beyond simple reabsorption of Na+ and acid/H+ secretion (20). The purpose of this review is to highlight traditional functions and to discuss the recently described role for NHE3 in Ca2+ homeostasis (82, 97).
Luminal Na+/H+ Exchange Is Responsible for the Majority of Na+ and Water Reabsorption From the Proximal Tubule
One of the primary functions of the proximal tubule is to reabsorb more than 2/3rd of the ~180 liters of water and ~27.5 mol Na+ filtered by the glomerulus on a daily basis. In adults, NHE3 plays the predominant role in this. In exchange for a luminal H+, NHE3 mediates the influx of Na+ into the proximal tubular cell. This exchange is driven by a concentration gradient for Na+, generated by basolateral Na+-K+-ATPase effluxing the apically absorbed Na+ (Fig. 1). The massive reclamation of Na+ from the proximal tubule via NHE3 plays a key role in preserving extracellular fluid volume. Given the importance of proximal tubular Na+ transport in blood pressure maintenance, it is not surprising that the reduction of Na+/H+ exhange activity in NHE3−/− mice causes hypotension (109). In addition, a myriad of processes and hormones implicit to blood pressure regulation affect NHE3 localization, expression, and activity (80).
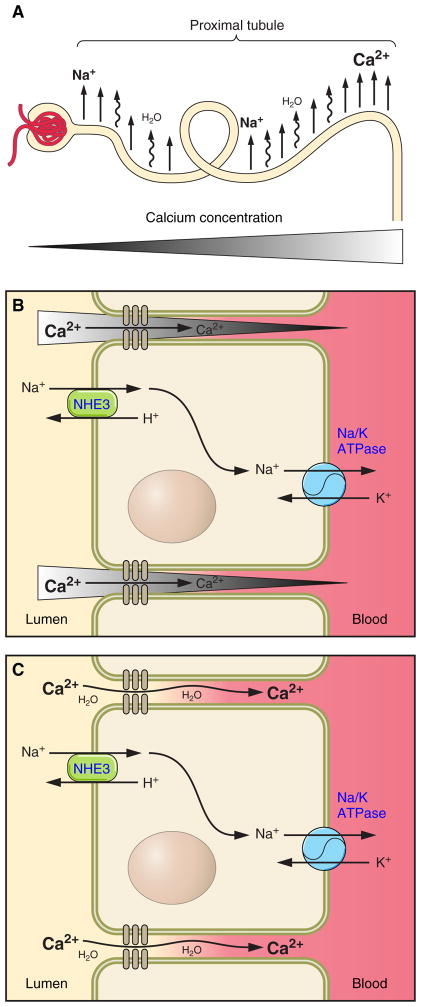
Fig. 1.
Diagrammatic representation of proximal tubular Ca2+ reabsorption. Filtered Na+ is reabsorbed from the proximal tubule (A), creating the osmotic driving force for water reabsorption, which, in turn, drives paracellular Ca2+ reabsorption either by creating a concentration gradient for Ca2+ (water removal increases the luminal concentration of Ca2+; B) or by convection/solvent drag (C). NHE3, Na+/H+ exchanger isoform 3.
The vectorial movement of Na+ across the proximal tubule creates an osmotic gradient, which provides the driving force for water reabsorption (101). Consistent with this, microperfusion studies (109, 120) have demonstrate that fluid uptake from the proximal tubule of NHE3−/− mice is reduced by ~2/3. Similarly, a micropuncture study (77) found an ~50% reduction in proximal fluid reabsorption in the absence of NHE3. There is a significant amount of Na+-coupled solute reabsorption across the proximal tubule, including that of phosphate, glucose, amino acids, etc. Given the relatively small concentration of these solutes in the filtrate, it is unlikely that these other Na+-coupled processes account entirely for the remaining water reabsorption from the proximal tubule of NHE3−/− mice. The residual Na+/H+ exchange activity may be attributable to NHE8.
NHE-dependent Na+/H+ exchange is coupled to apical Cl−/base exchange, permitting some Cl− reabsorption (9, 10), whereas the remainder of Cl− is reabsorbed via the paracellular junction. Cl− absorption, however, does not occur uniformly along the proximal tubule (101). The initial part of the proximal tubule supports significant bicarbonate reabsorption, such that the luminal Cl− concentration rises in latter parts of this segment (76). This provides a significant driving force for paracellular Cl− reabsorption from the latter part of the proximal tubule, where the majority of Cl− reabsorption occurs (43, 101).
NHE3 and NHE8 Play a Role in Acid-Base Homeostasis
The luminal secretion of a H+ in exchange for a Na+ permits the reabsorption of bicarbonate from the proximal tubule (62, 68, 85, 100). This is evinced by the development of renal tubular acidosis in NHE3−/− mice (73, 109) and by microperfusion studies (109, 120) that demonstrated decreased bicarbonate reabsorption from the proximal tubule of these animals. Bicarbonate reclamation is not the only role that luminal NHEs play in acid-base homeostasis. H+ excretion may serve to trap luminal NH3 (44). In addition, the NHE3-dependent exchange of Na+ for would also facilitate the luminal excretion of after its generation in the proximal tubule in response to acidosis (67, 87, 88). Furthermore, H+ extruded by NHE3 titrate citrate to its bivalent form, facilitating its reabsorption (22, 110).
Examination of proximal tubular H+ transport in NHE3−/− mice revealed significant Na+-dependent, amiloride-sensitive, H+ extrusion, implying the presence of another NHE isoform in the brush-border membrane (26). This is likely due to NHE8, as a recent study (12) examining NHE3/NHE8 double-knockout mice found a more pronounced acidosis than in NHE3−/− mice and a near absence of amiloride-dependent H+ extrusion in NHE3/NH8 double-knockout mice compared with significant residual activity in NHE3−/− mice. However, direct measurements of bicarbonate fluxes from perfused tubules of NHE3−/− mice argue against a role for NHE8 in bicarbonate reabsorption from the proximal tubule, as significant residual flux insensitive to the amiloride analog ethylisopropylamiloride has been demonstrated (NHE8 is amiloride sensitive) (126).
Proximal Tubule Ca2+ Reabsorption
There is extensive literature describing Ca2+ reabsorption from the proximal nephron in a number of species (2, 30). Fifty to sixty percent of total plasma Ca2+ is freely filtered by the glomerulus, whereas the remainder is complexed to plasma proteins and consequently retained in the plasma (41, 42, 55). Detailed physiological measurements using micropuncture have estimated that the convoluted part of the proximal tubule reabsorbs ~55–60% of Ca2+ from the filtrate (36, 111, 112). These studies suggested that an additional 10% of the filtered Ca2+ is reabsorbed from the straight proximal segment (pars recta). Therefore, 2/3rd of filtered Ca2+ or ~214 mmol Ca2+ are reabsorbed from the proximal tubule daily. Not surprisingly, even a slight reduction in proximal tubular Ca2+ reabsorption can lead to increased urinary Ca2+ excretion.
Micropuncture studies (36, 83, 115) on the rat, dog, and psammomys proximal tubule have consistently observed a proximal tubule fluid-to-ultrafiltrate Ca2+ ratio [(TF/UF)Ca] between 1.0 and 1.2. The (TF/UF)Ca follows that of Na+ during the administration of parathyroid hormone (PTH), acetazolamide, furosemide, hydrochlorothiazide, or acute and chronic metabolic acidosis (2, 13, 37, 115). Therefore, under the majority of situations studied, proximal tubular Ca2+ reabsorption parallels that of Na+ reabsorption. This finding has been interpreted as Ca2+ reabsorption from the proximal tubule occurs predominantly via a passive paracellular process (32, 112, 115). The proximal tubule is highly permeable to Ca2+, which would facilitate passive paracellular transport (84, 115). Moreover, a perfusion study (90) performed in the absence of a transepithelial potential difference and volume fluxes confirmed that the majority, but not all, Ca2+ transport across the proximal convoluted tubule was passive and likely paracellular.
Proximal tubular (TF/UF)Ca has been reported outside the range of 1.0–1.2. Induction of an osmotic diuresis via administration of saline or mannitol reduces (TF/UF)Ca to <1.0 (36, 72, 113). This finding can be interpreted as the proximal tubule has a capacity for active Ca2+ reabsorption. Consistent with this, a tubular perfusion study (103) performed on isolated pars recta from the rabbit found significant active Ca2+ flux, which was not inhibited by ouabain but was by a colder temperature. Together, the results of these reports suggest that the majority of Ca2+ transport is passive and paracellular in the proximal tubule. However, there may also be a small amount of active Ca2+ transport occurring via an as of yet poorly delineated mechanism.
Conversely, acute Ca2+ loading and immediately postparathyroidectomy are situations where proximal tubular (TF/UF)Ca have been reported to be above 1.2 (38, 71). Both of these experimental perturbations caused hypercalcemia and thus an increased proximal tubular load of Ca2+. These results can be interpreted to mean that the significant capacity for paracellular Ca2+ flux across the proximal tubule is finite and potentially limiting to Ca2+ reabsorption under some circumstances.
What permits paracellular Ca2+ flux across the proximal tubule? Claudins are four membrane-spanning proteins that localize to the tight junction (8). The family consists of at least 24 isoforms in humans. Interactions between claudins in the same membrane and across the tight junction control the paracellular flux of ions (8, 39). For example, the unique paracellular permeability properties of the thick ascending limb of Henle’s loop (TAL) are conferred by a dynamic interplay between claudins. Claudin-16 and claudin-19 form a cationic pore in the TAL, which permits Ca2+ reabsorption and, importantly, Na+ backflux (60, 61). This latter process contributes to the lumen-positive potential difference across the TAL, a driving force for Ca2+ reabsorption. In the presence of increased plasma Ca2+, claudin-14 is expressed in the TAL, where it functions to block Ca2+ absorption, thereby inducing calciuresis (34, 52).
To date, there has been very little published on the molecules permitting Ca2+ flux across the proximal tubule. The proximal tubule expresses claudin-1, claudin-2, claudin-10a, and claudin-12 (1, 69). Claudin-11 has been reported in this nephron segment by immunohistochemistry (117), although this was later found to be nonspecific (86). The claudin-2 knockout mouse displays decreased proximal tubular paracellular Na+, Cl−, and water fluxes as well as an increase in paracellular shunt resistance, consistent with a role for claudin-2 in forming an integral part of the proximal tubular paracellular junction (86). Although the authors reported that these animals have hypercalciuria, extensive evaluation of Ca2+ homeostasis was not described. The role of claudins-1, claudin-10a, and claudin-12 in proximal tubular ion transport is not known.
NHE3 Plays a Role in Ca2+ Homeostasis
The large majority of proximal tubular Ca2+ reabsorption is passive and paracellular, driven by active transcellular Na+ flux (112, 115). NHE3 is responsible for the majority of proximal tubular Na+ and, consequently, for osmotically driven water reabsorption (77, 109). It follows, therefore, that NHE3 activity would drive the passive paracellular flux of Ca2+ (Fig. 1). We (97) recently reported a role for NHE3 in maintaining Ca2+ homeostasis using a proximal tubular cell culture model and NHE3−/− mice. Opossum kidney cells are a well-characterized proximal tubular cell culture model known to express NHE3 in the apical membrane (4, 7). By overexpressing NHE3 in this model system and measuring paracellular Ca2+ flux across confluent monolayers, we observed increased Ca2+ flux when NHE3 was overexpressed, an effect that was eliminated by the omission of Na+ from the medium (97). Investigation of Ca2+ homeostasis in NHE3−/− mice revealed profound abnormalities (97). The fractional excretion of Ca2+ was double that of wild-type mice, consistent with significant urinary Ca2+ wasting. Plasma Ca2+ was not different between genotypes, nor was plasma PTH. However, 1,25[OH]2D3 was dramatically elevated in NHE3−/− mice. Surprisingly, despite this, intestinal Ca2+ absorption was reduced in NHE3−/− animals. Ultimately, reduced intestinal Ca2+ absorption and increased urinary Ca2+ excretion occur at the expense of bone health. NHE3−/− mice were found to have significantly reduced cortical and trabecular bone mass relative to wild-type animals of the same age, sex, and blood pH.
NHE3−/− mice have increased 1,25[OH]2D3 levels, a hormonal response that should increase intestinal Ca2+ absorption. However, contrary to this expectation, they do not. Why is this the case? NHE3 is expressed throughout the small intestine (96) and is responsible for a large amount of Na+ and osmotically driven water reabsorption (46). Ca2+ absorption from the intestine largely occurs in a passive paracellular fashion similar to the proximal tubule. One could hypothesize that the same mechanism is at play in the bowel as in the kidney. Further research will be needed to support this.
The localization of NHE3 in the proximal tubule, the experimental data described above, and the route of Ca2+ absorption from the proximal tubule strongly support that NHE3 drives paracellular proximal tubular Ca2+ absorption (18, 97, 112). It is worth considering the mechanistic details of this process briefly. The transepithelial movement of Na+ provides an osmotic driving force for the reabsorption of water. The movement of water, in turn, drives paracellular Ca2+ flux (Fig. 1). There are at least two potential mechanisms we can envisage whereby water movement causes Ca2+ flux. There is a significant amount of water flux across the proximal tubule, 25% of which is estimated to move via the paracellular pathway (106). This may be sufficient to drive Ca2+ flux by convection, a process referred to as solvent drag. Alternatively, the removal of water will increase the concentration of solutes, including Ca2+, in the proximal tubular lumen, even more so in unstirred layers at the tight junction. This Ca2+ gradient may be sufficient to provide the driving force for diffusive Ca2+ reabsorption. Current experimental approaches are not capable of distinguishing between these possibilities, and, consequently, more sophisticated techniques will be required to differentiate between them.
The amount of Na+ ingested alters urinary Ca2+ excretion (70). Consistent with this, volume depletion decreases urinary Ca2+ excretion (114), and the hypocalciuria induced by thiazide diuretics has been attributed to mild volume contraction and increased proximal tubular transport (91). Such changes are likely dependent on increased NHE activity in the proximal segment. In fact, given the role of NHE3 in maintaining proximal tubular transport, it is likely that increased NHE activity contributes importantly to both Na+ and Ca2+ reabsorption in volume-contracted states. Volume expansion decreases Ca2+ reabsorption from the proximal tubule (114) and causes redistribution of NHE3 from the brush-border membrane to the base of the microvilli (80). However, whether decreased NHE3 activity causes the decrease in Ca2+ reabsorption during volume expansion remains to be determined.
PTH Inhibits NHE3
PTH is a calciophosphoregulatory hormone released by the parathyroid gland in response to a reduction in ionized Ca2+ levels in the blood. Its ability to reduce urinary Ca2+ excretion has been appreciated for decades (31). This effect is largely mediated by increasing active transcellular Ca2+ reabsorption from the distal nephron (2). PTH also induces natriuresis (2, 3). This is the result of decreased proximal tubular Na+ reabsorption. PTH acutely and chronically inhibits NHE3 activity via a variety of mechanisms (15, 27, 51, 74, 122, 127, 130). Given the above discussion implicating NHE3 in proximal tubular Ca2+ reabsorption, it seems contradictory that PTH, a calciotropic hormone that decreases urinary Ca2+ excretion, decreases NHE3 activity. The reasons for this are unclear. A micropuncture study (75) found that the acute administration of PTH increases the distal delivery of bicarbonate, most likely via inhibition of NHE3. An alkaline lumen in the distal nephron increases active transcellular Ca2+ absorption (79, 116). Consistent with this, the transient receptor potential V5 channel, the major influx pathway for Ca2+ in the distal nephron, is activated by an alkaline pH (128). Together, these data have led some authors to speculate that PTH inhibits NHE3 to increase the distal delivery of bicarbonate, which alkalinizes the lumen of the distal nephron, in turn increasing active distal Ca2+ uptake (27, 51).
Other explanations are worth considering. High doses of PTH decrease the glomerular filtration rate (74, 107). This may conserve whole body Ca2+ as less Ca2+ will be filtered and therefore need to be reabsorbed. However, lower doses of PTH fail to alter the glomerular filtration rate and still inhibit NHE3 (74). PTH administration can induce hypercalcemia and increase Ca2+-sensing receptor (CaSR) expression (51, 122), although hypercalcemia and/or CaSR activation are insufficient to alter NHE3 expression (Ref. 121; R. T. Alexander, H. Dimke, and E. Cordat, unpublished observations). Instead, PTH-induced hypercalcemia promotes urinary water loss, leading to volume contraction. This effect, coupled to PTH-mediated decreased Ca2+ excretion, may serve to increase the free plasma Ca2+ concentration. This explanation implies that the maintenance of plasma Ca2+ levels is of greater importance than safeguarding intravascular volume, a huge assumption with little supporting evidence. Further research using novel model systems will be required to understand the role of PTH-mediated NHE3 inhibition in the maintenance of Ca2+ homeostasis.
Administration of PTH or a PTH-related peptide to humans does not lower blood pressure, as one might predict based on the above studies. Instead, an increase in blood pressure has been reported, especially with higher doses (40, 63). This is consistent with the frequent association of hypertension in persons with hyperparathyroidism. PTH administration causes natriuresis in humans, similar to rodents (56, 59), although the effect of PTH on NHE3 activity in humans remains to be determined.
The effect of active vitamin D on NHE3 activity is less clear. A renal cell culture study (19) found an increase in NHE3 activity, whereas intestinal cell culture and brush-border membrane preparation experiments found an inhibition (50, 119). The intestinal effect was attributed to decreased NHE3 expression (50), although the expression of NHE3 is unaltered in the kidneys of vitamin D-treated rats (121). Further studies will be required to clarify these seemingly contradictory effects.
Other Roles for Luminal Na+/H+ Exchange
NHE3 has been implicated in other transport processes. It associates with megalin and participates in receptor-mediated endocytosis and, consequently, the reabsorption of filtered proteins (16, 47–49). The extrusion of a H+ across the brush-border membrane also permits H+-coupled amino acid and oligopeptide influx from the proximal tubular lumen (123). Given the number of transport processes that NHE3 activity is required for, it is not surprising that there is a myriad of factors that regulate NHE3 activity, both acutely and chronically. Many of these have been described in detail and include hormones, posttranslational modifications, altered membrane recycling, protein-protein interactions, and, more recently, protein-lipid interactions (6, 20, 35, 57, 64, 80). Given the important role of NHE activity in secondary reabsorption of Ca2+, it will be important to test whether some of these factors are regulated by extracellular Ca2+ or calciotropic hormones.
Future Directions
NHE3 participates in the reabsorption of Na+, bicarbonate, and water from the proximal tubule, contributing to the maintenance of intravascular volume, blood pressure, and acid-base homeostasis. Emerging work has also implicated NHE3 in proximal tubular Ca2+ reabsorption. The extent to which NHE8 participates in these processes remains to be fully elucidated. Given these findings, several questions arise. For instance, do calciotropic hormones such as PTH and 1,25[OH]2D3 alter NHE8 expression or activity, and could this be a possible mechanism altering renal Ca2+ transport? Moreover, can the divergent developmental expression of NHE3 and NHE8 help explain the increased fractional excretion of Ca2+ observed in neonates and infants (66)? Tubular perfusion and micropuncture studies have indicated significant paracellular permeability of the proximal tubule to Ca2+. It will be critical to establish which components determine the permeability characteristics of the proximal tubule. Following this line of inquiry, it will be important to elucidate whether permeation of Ca2+ in the proximal tubule is regulated as has been observed in the TAL, where the permeability of the paracellular junction is modulated by PTH and Ca2+ itself (34, 52, 78, 124). An elegant translational study (125) on individuals with hypercalciuria and kidney stones has pointed to a defect in proximal tubular Ca2+ absorption. This fact emphasizes the need to dissect out these molecular pathways to better understand the causative factors and improve therapy for the large number of people with kidney stones. The use of genetically modified animals in combination with detailed physiological characterization techniques such as micropuncture and tubular perfusion will hopefully provide a platform to answer the above questions and many more.
Acknowledgments
The authors thank Dr. O. Moe for critically reading the manuscript.
GRANTS
The laboratories of R. T. Alexander and E. Cordat are supported by the Kidney Foundation of Canada and the Canadian Institutes of Health Research (CIHR). R. T. Alexander is a recipient of an Alberta Innovates Health Solutions Clinical Investigator Award and a CIHR Clinician Scientist Award. E. Cordat is supported by a KRESCENT New Investigator Award. H. Dimke is supported by the Danish Medical Research Council (Forskningsrådet for Sundhed og Sygdom).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.T.A. prepared figures; R.T.A. drafted manuscript; R.T.A., H.D., and E.C. edited and revised manuscript; R.T.A., H.D., and E.C. approved final version of manuscript.
References
- 1.Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol. 2006;291:F1132–F1141. doi: 10.1152/ajprenal.00063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agus ZS, Gardner LB, Beck LH, Goldberg M. Effects of parathyroid hormone on renal tubular reabsorption of calcium, sodium, and phosphate. Am J Physiol. 1973;224:1143–1148. doi: 10.1152/ajplegacy.1973.224.5.1143. [DOI] [PubMed] [Google Scholar]
- 3.Agus ZS, Puschett JB, Senesky D, Goldberg M. Mode of action of parathyroid hormone and cyclic adenosine 3′,5′-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest. 1971;50:617–626. doi: 10.1172/JCI106532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akhter S, Kovbasnjuk O, Li X, Cavet M, Noel J, Arpin M, Hubbard AL, Donowitz M. Na+/H+ exchanger 3 is in large complexes in the center of the apical surface of proximal tubule-derived OK cells. Am J Physiol Cell Physiol. 2002;283:C927–C940. doi: 10.1152/ajpcell.00613.2001. [DOI] [PubMed] [Google Scholar]
- 5.Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol (Oxf) 2006;187:159–167. doi: 10.1111/j.1748-1716.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- 6.Alexander RT, Jaumouille V, Yeung T, Furuya W, Peltekova I, Boucher A, Zasloff M, Orlowski J, Grinstein S. Membrane surface charge dictates the structure and function of the epithelial Na+/H+ exchanger. EMBO J. 2011;30:679–691. doi: 10.1038/emboj.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amemiya M, Yamaji Y, Cano A, Moe OW, Alpern RJ. Acid incubation increases NHE-3 mRNA abundance in OKP cells. Am J Physiol Cell Physiol. 1995;269:C126–C133. doi: 10.1152/ajpcell.1995.269.1.C126. [DOI] [PubMed] [Google Scholar]
- 8.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–F876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aronson PS. Ion exchangers mediating Na+, and Cl− transport in the renal proximal tubule. J Nephrol. 2006;19(Suppl 9):S3–S10. [PubMed] [Google Scholar]
- 10.Aronson PS. Ion exchangers mediating NaCl transport in the renal proximal tubule. Cell Biochem Biophys. 2002;36:147–153. doi: 10.1385/CBB:36:2-3:147. [DOI] [PubMed] [Google Scholar]
- 11.Baird NR, Orlowski J, Szabo EZ, Zaun HC, Schultheis PJ, Menon AG, Shull GE. Molecular cloning, genomic organization, and functional expression of Na+/H+ exchanger isoform 5 (NHE5) from human brain. J Biol Chem. 1999;274:4377–4382. doi: 10.1074/jbc.274.7.4377. [DOI] [PubMed] [Google Scholar]
- 12.Baum M, Twombley K, Gattineni J, Joseph C, Wang L, Zhang Q, Dwarakanath V, Moe OW. Proximal tubule Na+/H+ exchanger activity in adult NHE8−/−, NHE3−/−, and NHE3−/−/NHE8−/− mice. Am J Physiol Renal Physiol. 2012;303:F1495–F1502. doi: 10.1152/ajprenal.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck LH, Goldberg M. Effects of acetazolamide and parathyroidectomy on renal transport of sodium, calcium, and phosphate. Am J Physiol. 1973;224:1136–1142. doi: 10.1152/ajplegacy.1973.224.5.1136. [DOI] [PubMed] [Google Scholar]
- 14.Becker AM, Zhang J, Goyal S, Dwarakanath V, Aronson PS, Moe OW, Baum M. Ontogeny of NHE8 in the rat proximal tubule. Am J Physiol Renal Physiol. 2007;293:F255–F261. doi: 10.1152/ajprenal.00400.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bezerra CN, Girardi AC, Carraro-Lacroix LR, Reboucas NA. Mechanisms underlying the long-term regulation of NHE3 by parathyroid hormone. Am J Physiol Renal Physiol. 2008;294:F1232–F1237. doi: 10.1152/ajprenal.00025.2007. [DOI] [PubMed] [Google Scholar]
- 16.Biemesderfer D, Nagy T, DeGray B, Aronson PS. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem. 1999;274:17518–17524. doi: 10.1074/jbc.274.25.17518. [DOI] [PubMed] [Google Scholar]
- 17.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;265:F736–F742. doi: 10.1152/ajprenal.1993.265.5.F736. [DOI] [PubMed] [Google Scholar]
- 18.Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfa AK, Aronson PS. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol Renal Physiol. 1997;273:F289–F299. doi: 10.1152/ajprenal.1997.273.2.F289. [DOI] [PubMed] [Google Scholar]
- 19.Binswanger U, Helmle-Kolb C, Forgo J, Mrkic B, Murer H. Rapid stimulation of Na+/H+ exchange by 1,25-dihydroxyvitamin D3; interaction with parathyroid-hormone-dependent inhibition. Pflügers Arch. 1993;424:391–397. doi: 10.1007/BF00374899. [DOI] [PubMed] [Google Scholar]
- 20.Bobulescu IA, Moe OW. Luminal Na+/H+ exchange in the proximal tubule. Pflügers Arch. 2009;458:5–21. doi: 10.1007/s00424-008-0595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourgeois S, Meer LV, Wootla B, Bloch-Faure M, Chambrey R, Shull GE, Gawenis LR, Houillier P. NHE4 is critical for the renal handling of ammonia in rodents. J Clin Invest. 2010;120:1895–1904. doi: 10.1172/JCI36581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1988;255:F301–F306. doi: 10.1152/ajprenal.1988.255.2.F301. [DOI] [PubMed] [Google Scholar]
- 23.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 24.Brett CL, Wei Y, Donowitz M, Rao R. Human Na+/H+ exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol. 2002;282:C1031–C1041. doi: 10.1152/ajpcell.00420.2001. [DOI] [PubMed] [Google Scholar]
- 25.Chambrey R, Warnock DG, Podevin RA, Bruneval P, Mandet C, Belair MF, Bariety J, Paillard M. Immunolocalization of the Na+/H+ exchanger isoform NHE2 in rat kidney. Am J Physiol Renal Physiol. 1998;275:F379–F386. doi: 10.1152/ajprenal.1998.275.3.F379. [DOI] [PubMed] [Google Scholar]
- 26.Choi JY, Shah M, Lee MG, Schultheis PJ, Shull GE, Muallem S, Baum M. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest. 2000;105:1141–1146. doi: 10.1172/JCI9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collazo R, Fan L, Hu MC, Zhao H, Wiederkehr MR, Moe OW. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem. 2000;275:31601–31608. doi: 10.1074/jbc.M000600200. [DOI] [PubMed] [Google Scholar]
- 28.Collins JF, Honda T, Knobel S, Bulus NM, Conary J, DuBois R, Ghishan FK. Molecular cloning, sequencing, tissue distribution, and functional expression of a Na+/H+ exchanger (NHE-2) Proc Natl Acad Sci USA. 1993;90:3938–3942. doi: 10.1073/pnas.90.9.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Souza S, Garcia-Cabado A, Yu F, Teter K, Lukacs G, Skorecki K, Moore HP, Orlowski J, Grinstein S. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem. 1998;273:2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- 30.de Rouffignac C, Morel F, Moss N, Roinel N. Micropuncture study of water and electrolyte movements along the loop of Henle in psammomys with special reference to magnesium, calcium and phosphorus. Pflügers Arch. 1973;344:309–326. doi: 10.1007/BF00592784. [DOI] [PubMed] [Google Scholar]
- 31.Dennis VW. Actions of parathyroid hormone on isolated renal tubules. Ann NY Acad Sci. 1981;372:552–557. doi: 10.1111/j.1749-6632.1981.tb15505.x. [DOI] [PubMed] [Google Scholar]
- 32.Dennis VW, Stead WW, Myers JL. Renal handling of phosphate and calcium. Annu Rev Physiol. 1979;41:257–271. doi: 10.1146/annurev.ph.41.030179.001353. [DOI] [PubMed] [Google Scholar]
- 33.Diering GH, Mills F, Bamji SX, Numata M. Regulation of dendritic spine growth through activity-dependent recruitment of the brain-enriched Na+/H+ exchanger NHE5. Mol Biol Cell. 2011;22:2246–2257. doi: 10.1091/mbc.E11-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT. Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am J Physiol Renal Physiol. 2013;304:F761–F769. doi: 10.1152/ajprenal.00263.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X. NHE3 regulatory complexes. J Exp Biol. 2009;212:1638–1646. doi: 10.1242/jeb.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duarte CG, Watson JF. Calcium reabsorption in proximal tubule of the dog nephron. Am J Physiol. 1967;212:1355–1360. doi: 10.1152/ajplegacy.1967.212.6.1355. [DOI] [PubMed] [Google Scholar]
- 37.Edwards BR, Baer PG, Sutton RA, Dirks JH. Micropuncture study of diuretic effects on sodium and calcium reabsorption in the dog nephron. J Clin Invest. 1973;52:2418–2427. doi: 10.1172/JCI107432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards BR, Sutton RA, Dirks JH. Effect of calcium infusion on renal tubular reabsorption in the dog. Am J Physiol. 1974;227:13–18. doi: 10.1152/ajplegacy.1974.227.1.13. [DOI] [PubMed] [Google Scholar]
- 39.Elkouby-Naor L, Ben-Yosef T. Functions of claudin tight junction proteins and their complex interactions in various physiological systems. Int Rev Cell Mol Biol. 2010;279:1–32. doi: 10.1016/S1937-6448(10)79001-8. [DOI] [PubMed] [Google Scholar]
- 40.Fliser D, Franek E, Fode P, Stefanski A, Schmitt CP, Lyons M, Ritz E. Subacute infusion of physiological doses of parathyroid hormone raises blood pressure in humans. Nephrol Dial Transplant. 1997;12:933–938. doi: 10.1093/ndt/12.5.933. [DOI] [PubMed] [Google Scholar]
- 41.Friedman PA. Basal and hormone-activated calcium absorption in mouse renal thick ascending limbs. Am J Physiol Renal Fluid Electrolyte Physiol. 1988;254:F62–F70. doi: 10.1152/ajprenal.1988.254.1.F62. [DOI] [PubMed] [Google Scholar]
- 42.Friedman PA, Gesek FA. Cellular calcium transport in renal epithelia: measurement, mechanisms, and regulation. Physiol Rev. 1995;75:429–471. doi: 10.1152/physrev.1995.75.3.429. [DOI] [PubMed] [Google Scholar]
- 43.Fromter E, Rumrich G, Ullrich KJ. Phenomenologic description of Na+, Cl− and absorption from proximal tubules of rat kidney. Pflügers Arch. 1973;343:189–220. doi: 10.1007/BF00586045. [DOI] [PubMed] [Google Scholar]
- 44.Garvin JL, Knepper MA. Bicarbonate and ammonia transport in isolated perfused rat proximal straight tubules. Am J Physiol Renal Fluid Electrolyte Physiol. 1987;253:F277–F281. doi: 10.1152/ajprenal.1987.253.2.F277. [DOI] [PubMed] [Google Scholar]
- 45.Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem. 2005;280:12781–12789. doi: 10.1074/jbc.M414118200. [DOI] [PubMed] [Google Scholar]
- 46.Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G776–G784. doi: 10.1152/ajpgi.00297.2001. [DOI] [PubMed] [Google Scholar]
- 47.Gekle M, Drumm K, Mildenberger S, Freudinger R, Gassner B, Silbernagl S. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J Physiol. 1999;520:709–721. doi: 10.1111/j.1469-7793.1999.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gekle M, Freudinger R, Mildenberger S. Inhibition of Na+-H+ exchanger-3 interferes with apical receptor-mediated endocytosis via vesicle fusion. J Physiol. 2001;531:619–629. doi: 10.1111/j.1469-7793.2001.0619h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gekle M, Volker K, Mildenberger S, Freudinger R, Shull GE, Wiemann M. NHE3 Na+/H+ exchanger supports proximal tubular protein reabsorption in vivo. Am J Physiol Renal Physiol. 2004;287:F469–F473. doi: 10.1152/ajprenal.00059.2004. [DOI] [PubMed] [Google Scholar]
- 50.Gill R, Nazir TM, Wali R, Sitrin M, Brasitus TA, Ramaswamy K, Dudeja PK. Regulation of rat ileal NHE3 by 1,25(OH)2-vitamin D3. Dig Dis Sci. 2002;47:1169–1174. doi: 10.1023/a:1015071014584. [DOI] [PubMed] [Google Scholar]
- 51.Girardi AC, Titan SM, Malnic G, Reboucas NA. Chronic effect of parathyroid hormone on NHE3 expression in rat renal proximal tubules. Kidney Int. 2000;58:1623–1631. doi: 10.1046/j.1523-1755.2000.00323.x. [DOI] [PubMed] [Google Scholar]
- 52.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca2+ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012;31:1999–2012. doi: 10.1038/emboj.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol. 2005;288:F530–F538. doi: 10.1152/ajprenal.00229.2004. [DOI] [PubMed] [Google Scholar]
- 54.Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol. 2003;284:F467–F473. doi: 10.1152/ajprenal.00352.2002. [DOI] [PubMed] [Google Scholar]
- 55.Grimellec CL, Poujeol P, Rouffignia C. 3H-inulin and electrolyte concentrations in Bowman’s capsule in rat kidney. Comparison with artificial ultrafiltration. Pflügers Arch. 1975;354:117–131. doi: 10.1007/BF00579943. [DOI] [PubMed] [Google Scholar]
- 56.Haas HG, Dambacher MA, Guncaga J, Lauffenbruger T. Renal effects of calcitonin and parathyroid extract in man. Studies in hypoparathyroidism. J Clin Invest. 1971;50:2689–2702. doi: 10.1172/JCI106770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol. 2010;2010:238080. doi: 10.1155/2010/238080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill JK, Brett CL, Chyou A, Kallay LM, Sakaguchi M, Rao R, Gillespie PG. Vestibular hair bundles control pH with (Na+, K+)/H+ exchangers NHE6 and NHE9. J Neurosci. 2006;26:9944–9955. doi: 10.1523/JNEUROSCI.2990-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hochberg Z, Moses AM, Richman RA. Parathyroid hormone infusion test in children and adolescents. Miner Electrolyte Metab. 1984;10:113–116. [PubMed] [Google Scholar]
- 60.Hou J, Goodenough DA. Claudin-16 and claudin-19 function in the thick ascending limb. Curr Opin Nephrol Hypertens. 2010;19:483–488. doi: 10.1097/MNH.0b013e32833b7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howlin KJ, Alpern RJ, Rector FC., Jr Amiloride inhibition of proximal tubular acidification. Am J Physiol Renal Fluid Electrolyte Physiol. 1985;248:F773–F778. doi: 10.1152/ajprenal.1985.248.6.F773. [DOI] [PubMed] [Google Scholar]
- 63.Hulter HN, Melby JC, Peterson JC, Cooke CR. Chronic continuous PTH infusion results in hypertension in normal subjects. J Clin Hypertens. 1986;2:360–370. [PubMed] [Google Scholar]
- 64.Jaumouille V, Krishnan D, Alexander RT. The calmodulin antagonist W-7 inhibits the epithelial Na+/H+ exchanger via modulating membrane surface potential. Channels (Austin) 2011;5:308–313. doi: 10.4161/chan.5.4.16548. [DOI] [PubMed] [Google Scholar]
- 65.Joseph C, Twombley K, Gattineni J, Zhang Q, Dwarakanath V, Baum M. Acid increases NHE8 surface expression and activity in NRK cells. Am J Physiol Renal Physiol. 2012;302:F495–F503. doi: 10.1152/ajprenal.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karlen J, Aperia A, Zetterstrom R. Renal excretion of calcium and phosphate in preterm and term infants. J Pediatr. 1985;106:814–819. doi: 10.1016/s0022-3476(85)80364-4. [DOI] [PubMed] [Google Scholar]
- 67.Kinsella JL, Aronson PS. Interaction of NH4+ and Li+ with the renal microvillus membrane Na+-H+ exchanger. Am J Physiol Cell Physiol. 1981;241:C220–C226. doi: 10.1152/ajpcell.1981.241.5.C220. [DOI] [PubMed] [Google Scholar]
- 68.Kinsella JL, Aronson PS. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol Renal Fluid Electrolyte Physiol. 1980;238:F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- 69.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 70.Kleeman CR, Bohannan J, Bernstein D, Ling S, Maxwell MH. Effect of variations in sodium intake on calcium excretion in normal humans. Proc Soc Exp Biol Med. 1964;115:29–32. [PubMed] [Google Scholar]
- 71.Kuntziger H, Amiel C, Roinel N, Morel F. Effects of parathyroidectomy and cyclic AMP on renal transport of phosphate, calcium, and magnesium. Am J Physiol. 1974;227:905–911. doi: 10.1152/ajplegacy.1974.227.4.905. [DOI] [PubMed] [Google Scholar]
- 72.Lassiter WE, Gottschalk CW, Mylle M. Micropuncture study of renal tubular reabsorption of calcium in normal rodents. Am J Physiol. 1963;204:771–775. [Google Scholar]
- 73.Ledoussal C, Lorenz JN, Nieman ML, Soleimani M, Schultheis PJ, Shull GE. Renal salt wasting in mice lacking NHE3 Na+/H+ exchanger but not in mice lacking NHE2. Am J Physiol Renal Physiol. 2001;281:F718–F727. doi: 10.1152/ajprenal.2001.281.4.F718. [DOI] [PubMed] [Google Scholar]
- 74.Leong PK, Yang LE, Lin HW, Holstein-Rathlou NH, McDonough AA. Acute hypotension induced by aortic clamp vs. PTH provokes distinct proximal tubule Na+ transporter redistribution patterns. Am J Physiol Regul Integr Comp Physiol. 2004;287:R878–R885. doi: 10.1152/ajpregu.00180.2004. [DOI] [PubMed] [Google Scholar]
- 75.Liu FY, Cogan MG. Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. J Clin Invest. 1989;84:83–91. doi: 10.1172/JCI114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu FY, Cogan MG. Axial heterogeneity in the rat proximal convoluted tubule. I. Bicarbonate, chloride, and water transport. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F816–F821. doi: 10.1152/ajprenal.1984.247.5.F816. [DOI] [PubMed] [Google Scholar]
- 77.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol Renal Physiol. 1999;277:F447–F453. doi: 10.1152/ajprenal.1999.277.3.F447. [DOI] [PubMed] [Google Scholar]
- 78.Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest. 2012;122:3355–3367. doi: 10.1172/JCI57407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marone CC, Wong NL, Sutton RA, Dirks JH. Effects of metabolic alkalosis on calcium excretion in the conscious dog. J Lab Clin Med. 1983;101:264–273. [PubMed] [Google Scholar]
- 80.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2010;298:R851–R861. doi: 10.1152/ajpregu.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyazaki E, Sakaguchi M, Wakabayashi S, Shigekawa M, Mihara K. NHE6 protein possesses a signal peptide destined for endoplasmic reticulum membrane and localizes in secretory organelles of the cell. J Biol Chem. 2001;276:49221–49227. doi: 10.1074/jbc.M106267200. [DOI] [PubMed] [Google Scholar]
- 82.Moe OW. Cohesion of epithelial ion homeostasis: implementing calcium transport with sodium transporters? Am J Physiol Renal Physiol. 2012;302:F941–F942. doi: 10.1152/ajprenal.00632.2011. [DOI] [PubMed] [Google Scholar]
- 83.Morel F, Roinel N, Le Grimellec C. Electron probe analysis of tubular fluid composition. Nephron. 1969;6:350–364. doi: 10.1159/000179738. [DOI] [PubMed] [Google Scholar]
- 84.Murayama Y, Morel F, Le Grimellec C. Phosphate, calcium and magnesium transfers in proximal tubules and loops of Henle, as measured by single nephron microperfusion experiments in the rat. Pflügers Arch. 1972;333:1–16. doi: 10.1007/BF00586037. [DOI] [PubMed] [Google Scholar]
- 85.Murer H, Hopfer U, Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976;154:597–604. [PMC free article] [PubMed] [Google Scholar]
- 86.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA. 2010;107:8011–8016. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagami GT. Ammonia production and secretion by the proximal tubule. Am J Kidney Dis. 1989;14:258–261. doi: 10.1016/s0272-6386(89)80198-2. [DOI] [PubMed] [Google Scholar]
- 88.Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest. 1988;81:159–164. doi: 10.1172/JCI113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 90.Ng RC, Rouse D, Suki WN. Calcium transport in the rabbit superficial proximal convoluted tubule. J Clin Invest. 1984;74:834–842. doi: 10.1172/JCI111500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noel J, Roux D, Pouyssegur J. Differential localization of Na+/H+ exchanger isoforms (NHE1 and NHE3) in polarized epithelial cell lines. J Cell Sci. 1996;109:929–939. doi: 10.1242/jcs.109.5.929. [DOI] [PubMed] [Google Scholar]
- 93.Noonan WT, Woo AL, Nieman ML, Prasad V, Schultheis PJ, Shull GE, Lorenz JN. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol Regul Integr Comp Physiol. 2005;288:R685–R691. doi: 10.1152/ajpregu.00209.2004. [DOI] [PubMed] [Google Scholar]
- 94.Numata M, Orlowski J. Molecular cloning and characterization of a novel (Na+,K+)/H+ exchanger localized to the trans-Golgi network. J Biol Chem. 2001;276:17387–17394. doi: 10.1074/jbc.M101319200. [DOI] [PubMed] [Google Scholar]
- 95.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 96.Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992;267:9331–9339. [PubMed] [Google Scholar]
- 97.Pan W, Borovac J, Spicer Z, Hoenderop JG, Bindels RJ, Shull GE, Doschak MR, Cordat E, Alexander RT. The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption. Am J Physiol Renal Physiol. 2012;302:F943–F956. doi: 10.1152/ajprenal.00504.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park K, Evans RL, Watson GE, Nehrke K, Richardson L, Bell SM, Schultheis PJ, Hand AR, Shull GE, Melvin JE. Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchanger-deficient mice. J Biol Chem. 2001;276:27042–27050. doi: 10.1074/jbc.M102901200. [DOI] [PubMed] [Google Scholar]
- 99.Pizzonia JH, Biemesderfer D, Abu-Alfa AK, Wu MS, Exner M, Isenring P, Igarashi P, Aronson PS. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am J Physiol Renal Physiol. 1998;275:F510–F517. doi: 10.1152/ajprenal.1998.275.4.F510. [DOI] [PubMed] [Google Scholar]
- 100.Preisig PA, Ives HE, Cragoe EJ, Jr, Alpern RJ, Rector FC., Jr Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest. 1987;80:970–978. doi: 10.1172/JCI113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1983;244:F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- 102.Rotin D, Grinstein S. Impaired cell volume regulation in Na+-H+ exchange-deficient mutants. Am J Physiol Cell Physiol. 1989;257:C1158–C1165. doi: 10.1152/ajpcell.1989.257.6.C1158. [DOI] [PubMed] [Google Scholar]
- 103.Rouse D, Ng RC, Suki WN. Calcium transport in the pars recta and thin descending limb of Henle of the rabbit, perfused in vitro. J Clin Invest. 1980;65:37–42. doi: 10.1172/JCI109657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rutherford PA, Pizzonia JH, Biemesderfer D, Abu-Alfa A, Reilly R, Aronson PS. Expression of Na+-H+ exchanger isoforms NHE1 and NHE3 in kidney and blood cells of rabbit and rat. Exp Nephrol. 1997;5:490–497. [PubMed] [Google Scholar]
- 105.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56:271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- 106.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci USA. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schor N, Ichikawa I, Brenner BM. Mechanisms of action of various hormones and vasoactive substances on glomerular ultrafiltration in the rat. Kidney Int. 1981;20:442–451. doi: 10.1038/ki.1981.160. [DOI] [PubMed] [Google Scholar]
- 108.Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 110.Sekine T, Cha SH, Hosoyamada M, Kanai Y, Watanabe N, Furuta Y, Fukuda K, Igarashi T, Endou H. Cloning, functional characterization, and localization of a rat renal Na+-dicarboxylate transporter. Am J Physiol Renal Physiol. 1998;275:F298–F305. doi: 10.1152/ajprenal.1998.275.2.F298. [DOI] [PubMed] [Google Scholar]
- 111.Seldin DW. Renal handling of calcium. Nephron. 1999;81(Suppl 1):2–7. doi: 10.1159/000046292. [DOI] [PubMed] [Google Scholar]
- 112.Suki WN. Calcium transport in the nephron. Am J Physiol Renal Fluid Electrolyte Physiol. 1979;237:F1–F6. doi: 10.1152/ajprenal.1979.237.1.F1. [DOI] [PubMed] [Google Scholar]
- 113.Suki WN, Rouse D, Ng RC, Kokko JP. Calcium transport in the thick ascending limb of Henle. Heterogeneity of function in the medullary and cortical segments. J Clin Invest. 1980;66:1004–1009. doi: 10.1172/JCI109928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suki WN, Schwettmann RS, Rector FC, Jr, Seldin DW. Effect of chronic mineralocorticoid administration on calcium excretion in the rat. Am J Physiol. 1968;215:71–74. doi: 10.1152/ajplegacy.1968.215.1.71. [DOI] [PubMed] [Google Scholar]
- 115.Sutton RA, Dirks JH. The renal excretion of calcium: a review of micropuncture data. Can J Physiol Pharmacol. 1975;53:979–988. doi: 10.1139/y75-136. [DOI] [PubMed] [Google Scholar]
- 116.Sutton RA, Wong NL, Dirks JH. Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int. 1979;15:520–533. doi: 10.1038/ki.1979.67. [DOI] [PubMed] [Google Scholar]
- 117.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 118.Twombley K, Gattineni J, Bobulescu IA, Dwarakanath V, Baum M. Effect of metabolic acidosis on neonatal proximal tubule acidification. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1360–R1368. doi: 10.1152/ajpregu.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wali RK, Baum CL, Bolt MJ, Brasitus TA, Sitrin MD. 1,25-dihydroxyvitamin D3 inhibits Na+-H+ exchange by stimulating membrane phosphoinositide turnover and increasing cytosolic calcium in CaCo-2 cells. Endocrinology. 1992;131:1125–1133. doi: 10.1210/endo.131.3.1324151. [DOI] [PubMed] [Google Scholar]
- 120.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol Renal Physiol. 1999;277:F298–F302. doi: 10.1152/ajprenal.1999.277.2.F298. [DOI] [PubMed] [Google Scholar]
- 121.Wang W, Kwon TH, Li C, Frokiaer J, Knepper MA, Nielsen S. Reduced expression of Na-K-2Cl cotransporter in medullary TAL in vitamin D-induced hypercalcemia in rats. Am J Physiol Renal Physiol. 2002;282:F34–F44. doi: 10.1152/ajprenal.0101.2001. [DOI] [PubMed] [Google Scholar]
- 122.Wang W, Li C, Kwon TH, Miller RT, Knepper MA, Frokiaer J, Nielsen S. Reduced expression of renal Na+ transporters in rats with PTH-induced hypercalcemia. Am J Physiol Renal Physiol. 2004;286:F534–F545. doi: 10.1152/ajprenal.00044.2003. [DOI] [PubMed] [Google Scholar]
- 123.Watanabe C, Kato Y, Ito S, Kubo Y, Sai Y, Tsuji A. Na+/H+ exchanger 3 affects transport property of H+/oligopeptide transporter 1. Drug Metab Pharmacokinet. 2005;20:443–451. doi: 10.2133/dmpk.20.443. [DOI] [PubMed] [Google Scholar]
- 124.Wittner M, Mandon B, Roinel N, de Rouffignac C, Di Stefano A. Hormonal stimulation of Ca2+ and Mg2+ transport in the cortical thick ascending limb of Henle’s loop of the mouse: evidence for a change in the paracellular pathway permeability. Pflügers Arch. 1993;423:387–396. doi: 10.1007/BF00374932. [DOI] [PubMed] [Google Scholar]
- 125.Worcester EM, Coe FL, Evan AP, Bergsland KJ, Parks JH, Willis LR, Clark DL, Gillen DL. Evidence for increased postprandial distal nephron calcium delivery in hypercalciuric stone-forming patients. Am J Physiol Renal Physiol. 2008;295:F1286–F1294. doi: 10.1152/ajprenal.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G335–G343. doi: 10.1152/ajpgi.00146.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol. 2004;287:F896–F906. doi: 10.1152/ajprenal.00160.2004. [DOI] [PubMed] [Google Scholar]
- 128.Yeh BI, Sun TJ, Lee JZ, Chen HH, Huang CL. Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J Biol Chem. 2003;278:51044–51052. doi: 10.1074/jbc.M306326200. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J, Bobulescu IA, Goyal S, Aronson PS, Baum MG, Moe OW. Characterization of Na+/H+ exchanger NHE8 in cultured renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F761–F766. doi: 10.1152/ajprenal.00117.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol Renal Physiol. 1999;276:F711–F719. doi: 10.1152/ajprenal.1999.276.5.F711. [DOI] [PubMed] [Google Scholar]