Abstract
Background and Purpose
A decrease in fractional anisotropy (FA) of the ipsilesional corticospinal tract (CST) distal to stroke lesions in the subacute (e.g., 30 days) and chronic phase has been correlated with poor motor outcomes, but it is unclear whether FA values obtained within the acute stroke phase (here defined as 80 hours after onset) can predict later outcome.
Methods
Fifty-eight patients underwent an assessment of motor impairment in the acute phase and at 3 months using the Upper Extremity Fugl-Meyer (UE-FM) assessment. FA values, obtained within 80 hrs after stroke onset, were determined in two regions of interest: cerebral peduncle (CP) and a stretch of the CST caudal to each stroke lesion (Nearest-5-Slice – N5S)).
Results
The FA laterality index (FA-LI) for the CP-ROI was a poor predictor of 3-months outcome (R2 =0.044, p=0.137), while the slope over the FA-LIs of the N5S showed a relatively weak but significant prediction (R2=0.11, p=0.022) with the affected side having lower FA values. Initial UE-FM (R2=0.69, p<0.001) and the weighted CST lesion load (wCST-LL) (R2=0.71, p<0.001) were strong predictors of 3-months outcome. In multivariate analyses controlling for initial UE-FM, wCST-LL, and Days-of-Therapy, neither the FA-LI of the CP nor the slope over the FA-LI of the N5S significantly contributed to the prediction of 86% of the variance in the UE-FM at 3 months.
Conclusions
FA reductions of the CST can be detected near the ischemic lesion in the acute stroke phase, but offer minimal predictive value to motor outcomes at 3 months.
Keywords: fractional anisotropy, diffusion tensor imaging, lesion mapping, acute stroke, MRI, outcomes
Introduction
Motor impairment is a common consequence after ischemic stroke, leading to major disability and poor quality of life.1 Recovery after a stroke is quite variable and remains challenging to predict, although recent studies have related recovery to the effect that a lesion has on the motor system.2–4 The corticospinal tract (CST) is the primary descending motor pathway connecting cortical motor regions with neurons in the spinal cord. Injury from ischemic stroke leads to anterograde degeneration of axons and myelin sheaths of affected tracts, commonly known as Wallerian degeneration (WD).5–7 Studies have shown that measures of the integrity of the CST in the chronic stroke phase closely correlate with motor outcome after stroke.4, 8–15 Furthermore, evidence of CST atrophy and other signal changes thought to be indicative of WD on conventional MRI16 have been correlated with poor motor outcome in chronic stroke patients.17, 18 It seems that these changes develop at later stages and might be too subtle to be quantified in the acute stroke phase and therefore might not be useful as a prognostic tool for clinicians, patients, and caregivers.19 One prospective study found a decrease in the apparent diffusion coefficient (ADC) of the ipsilesional CST within 12 hours of stroke in patients with initial severe motor impairment20, but predictions of outcome were not very strong. Another imaging marker, fractional anisotropy, derived from Diffusion Tensor Imaging (DTI), quantifies the organization (i.e. degree of alignment) and integrity of white matter tracts in vivo using information about the predominant direction and degree of water diffusion. Variation of FA in normal tissue is expected and is related to the orientation of axonal membranes and myelin sheaths.21, 22 Studies of subacute and chronic stroke patients have demonstrated a decrease in FA of the CST distal to the infarct thought to be the result of Wallerian degeneration, but previous studies did not find any FA changes in the CST distal to the stroke lesion at either 12 hours or 3 days after a stroke. 19, 23–29.
Other variables determined in the acute stroke phase, such as the initial motor impairment,10, 30 lesion size and location quantified as the CST lesion load,2, 31 and corticospinal tract integrity 32, 33 determined at later stages have already been shown to correlate with post-stroke motor outcome.34 We found that the weighted CST lesion load – a combined measure of the acute stroke lesion overlapped with a canonical corticospinal tract – predicted post-stroke motor outcomes at 3 months better than clinical measures of motor impairment, particularly for patients with severe initial motor impairment.2
However, it remains unclear whether fractional anisotropy, a direct DTI-derived measure of the affected motor tract, measured in the acute phase of stroke, contributes to motor outcome predictions at 3 months when new and innovative ways of assessing signal changes are applied.
Thus, this study aimed to examine FA differences (as compared to a matched control group and as compared to the unaffected hemisphere) in the approximate location of the CST (using a canonical tract incorporated into each patient’s routine clinical MRIs done within the acute stroke phase) and to examine how those differences can predict motor outcomes at 3 months either alone or in combination with other variables (e.g., weighted CST-Lesion Load).
Methods
Subjects
This is a retrospective analysis of a prospectively collected cohort of patients with varying degrees of initial motor impairment following first-time ischemic hemispheric stroke. The study was approved by the local institutional review board. Inclusion criteria for this study were as follows: (1) First-time, acute, hemispheric ischemic stroke; (2) age >18 years old; (3) MRI with diffusion-weighted imaging (DWI), diffusion-tensor imaging (DTI), and FLAIR sequences obtained within 80 hours after stroke onset; (4) at least mild upper extremity motor impairment, defined as Upper Extremity Fugl-Meyer31 (UE-FM) score <60 measured between 2 and 6 days after stroke onset; and (5) completed follow-up assessment approximately 3 months after their stroke (mean follow-up was 90 days ± 18 days (SD)). Patients were excluded if they met any of the following: (1) primary intraparenchymal hemorrhage or subarachnoid/subdural/epidural hemorrhage; (2) bihemispheric strokes; (3) stroke lesion affecting the brainstem; (4) history of prior stroke demonstrated on CT or MRI, or the medical record; (5) documented history of dementia, medically uncontrolled depression, or any non-stroke neurological disorder causing motor impairment; (6) clinical evidence for a recurrent stroke before the follow-up visit.
Twelve healthy right-handed control subjects served as an age-matched control group (9 males; mean age: 56.5 ± 14.8 years). They were scanned using a 3T GE MRI scanner with image parameters identical to those described in a previous publication31.
MRI Protocol
All patient images used for analysis were standard-of-care, clinical MRI scans (on a 1.5 Tesla GE MRI scanner) obtained within 80 hours (mean 25.7 hrs (±14)) after stroke onset. Diffusion tensor images were obtained using single-shot spin-echo EPI sequence with 2.5×2.5×5mm3 voxel resolution, with 24 slices in total, and 30 non-collinear directions with a b-value of 1000s/mm2. Reconstructed FA maps were used for analyses. FLAIR image sequence had resolution of 1.3×0.8×5.0mm3, slice thickness 5mm, and a total of 24 slices.
Image Processing
All MRI sequences for patients and control subjects were normalized to the same standardized space using SPM5 (Wellcome Department of Neurology, London, UK) implemented in MATLAB (The Mathworks, Inc., Natick, MA). Appropriate SPM5 templates with isotropic voxels (2×2×2mm) were used for each set of images (for details of the normalization process, see detailed description in Feng et al., 20152).
Lesion maps were manually drawn for each patient on the normalized DWI using MRIcro (http://www.mccauslandcenter.sc.edu/mricro/mricro/index.html) by investigators who were blind to the motor impairment of the patients.
Construction of the Canonical CST and weighted CST Lesion Load was done as previously described (Feng et al., 2015). For the selection of ROIs described below, a threshold was applied to these tracts such that each contained only those voxels shared by at least 8 of the 12 individual tracts (see Zhu et al., 201031 for more details). Lesion maps were overlaid onto the CST mask to calculate a weighted lesion load value for each patient (for details see Feng et al., 2015, Zhu et al., 20102,31)
Image Analysis
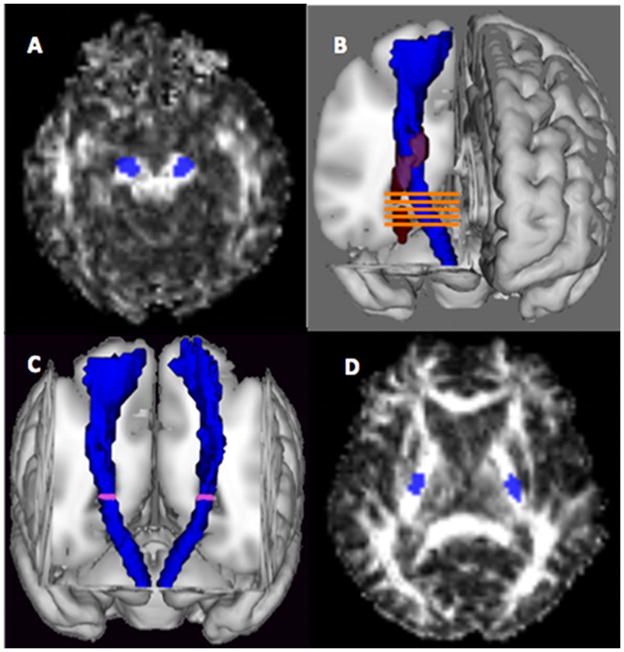
The canonical CSTs were overlaid on the spatially normalized images for each patient and each control subject to define two regions of interest (ROIs) bilaterally (see Figure 1): The cerebral peduncle-ROI and the “Nearest-5-Slices” ROI. The CP-ROI was used because the posterior limb of the internal capsule (another region used in the literature) was involved in the lesion in several patients and because the course of the CST in the CP can be easily identified.
Figure 1. Region of interest definition.
(A) Cerebral peduncle ROI. (B) Nearest-5-Slices ROI. Lesion is shown in red and canonical CST is shown in blue). (C) The canonical CST (shown in blue) with the first slice of the Nearest-5-Slices marked (D) The mean lowest slice of overlap for the patient group, shown here, was found to lie within the posterior limb of the internal capsule.
The “Nearest-5-Slices” ROI (N5S) was defined as follows. The lesion map for each patient was overlaid with the canonical CST and the lowest axial slice where the lesion overlapped with the CST was determined. Two axial slices below this overlapping slice (i.e., leaving at least a one slice buffer between the lesion and the “Nearest-5-Slices ROI”), the entire cross-sectional area of the CST on this slice and the subsequent four slices were used to define a N5S ROI on both hemispheres. To explore whether a trend could be seen moving along the CST distal to the lesion, a calculation of the FA LI for the nearest slice ROI and the next four descending slices was constructed for each patient. A slope across the LI of the 5 slices was calculated. We refer to this variable as the slope of the Nearest-5-Slices (S-N5S) FA laterality index. We first calculated a FA laterality index for each of the nearest 5 slices, then we regressed the FA LI across the 5 slices, and finally extracted the slope value from the regression equation.
Average FA, ADC, and FLAIR values were calculated for all CP-ROIs. To compare the stroke-affected hemisphere (A) with the unaffected hemisphere (U), a laterality index was calculated for each CP-ROI/sequence comparison using the following formula, shown here for FA: (FAA-FAU)/(FAA+FAU). Resulting values fall between −1 and 1, with positive LIs indicating a higher FA on the affected hemisphere and negative LIs indicating a higher FA on the unaffected hemisphere.
Since it was not known whether a normal control group would show a hemispheric laterality in the measures of interest, we calculated a LI for each CP-ROI/sequence comparison for each control subject, using the following formula: (FAL-FAR)/(FAL+FAR). Positive LIs indicate a higher FA on the left (L) and negative LIs indicate a higher FA on the right (R). To create a suitable “Nearest-5-Slices ROI” in the control group, a distribution of the slices was derived for each patient. The mean axial slice ± 1 standard deviation of this distribution was determined for the patient group; the mean axial slice was located at the level of the internal capsule (z=8.2, MNI) and a total of 17 slices (8 slices superior and 8 slices inferior to this mean axial slice) were used to define a comparable “nearest-5-slices ROI” in the healthy control group. A LI was calculated for each of these 17 slices and the LIs were averaged across these 17 slices for each control subject. For S-N5S values, each slice FA LI was corrected for laterality differences before calculating overall slope for each subject over the 5 slices (described in detail in the “Statistical Analysis” section below).
Statistical Analysis
All values are reported as mean ± SD. Fifty-eight patients met criteria for inclusion; three subjects were identified as extreme outliers in the wCST Lesion Load values in the regression diagnostics and excluded. To test for significant hemispheric asymmetry in FA and FLAIR signal intensity in control subjects, a one sample t-test was used to compare left-vs.-right LI with an expected mean LI=0 for both the CP-ROI and Nearest-5-Slices ROI. For all sequence type/ROI combinations in which a significant left vs. right difference was found in controls, each patient’s affected-vs.-unaffected LI was adjusted by mean left vs. right LI determined in controls to correct for this “normal” hemispheric asymmetry. In these cases, the LI of a patient with a left hemisphere lesion was determined by subtracting the mean LI of the normal control group from the patient’s LI. For patients with right hemisphere lesions, the LI ratio was determined as described for left hemisphere lesion patients, but then multiplied by −1 so that the affected FA was uniformly on the same side for all patients. For all sequence type/ROI combinations in which no significant side-to-side difference was found in the control group, each patient’s uncorrected affected vs. unaffected hemisphere LI was used.
Kruskal-Wallis one-way analysis of variance was used to test for an effect of imaging days post-stroke on imaging variables.
Univariate regression analysis was used to test the predictive value of several variables with regard to 3-month UE-FM score. Multiple regression analysis was subsequently done to test whether the imaging variable (FA-LI) could improve upon the predictive value of the initial UE-FM after controlling for effects of other variables.
Results
Demographic and clinical characteristics are shown in Table 1. Mean lesion volume was 39.0(±53.4) cc and mean weighted-CST-Lesion Load was 4.02(±2.94) cc. MR imaging occurred 25.7 (±14.0) hours post-stroke. Regression analysis of MR imaging time after stroke onset showed no effect of imaging day post-stroke for any FA LI sequence/ROI combination (data not shown, all p>0.05). Four FA images were of poor quality, and four FA images could not be normalized due to imaging artifacts, leaving 50 of 58 (86%) FA images available for analysis. Two ADC images were of poor quality and an additional seven ADC images could not be normalized due to imaging artifacts, leaving 51 of 58 (88%) ADC images available for analysis. Seven FLAIR images could not be satisfactorily normalized due to distortions, leaving 51 of 58 (88%) FLAIR images available for analysis.
Table 1.
Patients’ Demographical and Clinical Data Characteristics
| All Patients | |
|---|---|
| # of patients | 58 |
| Age (Yrs) | 61.3 ± 14.2 |
| Gender (%, Female) | 34 |
| Lesion side (%, Right) | 64 |
| Lesion Volume (in cc) | 39.01 ± 53.44 |
| Weighted CST-LL (in cc) | 4.02 ± 2.94 |
| Handedness (%, Right) | 97 |
| NIHSS, baseline | 10.5 ± 7.4 |
| NIHSS, 3-months | 4.3 ± 5.0 |
| UE-FM, baseline | 25.7 ± 19.2 |
| UE-FM, 3-months | 42.5 ± 23.5 |
| Imaging hours post-stroke | 26.4 ± 14.0 |
| t-PA/reperfusion therapy (%) | 39 |
| Hypertension (%) | 73 |
| Hyperlipidemia (%) | 63 |
| Diabetes (%) | 44 |
| Coronary artery disease (%) | 15 |
| Atrial Fibrillation (%) | 19 |
| Smoking (%) | 37 |
Laterality Indices in Controls and Patients
In controls, there was a subtle, but significant hemispheric asymmetry in FA for both the CP and nearest slice equivalent, with LIs indicating slightly higher FA values on the right for both ROIs. All controls were right-handed, thus, the higher FA values indicated that more alignment was seen in the non-dominant CST. There was no significant asymmetry in ADC or FLAIR for either ROI (see also Table 2).
Table 2.
Laterality Index Analysis in Controls and Patients
| Controlsa | Patientsb | |
|---|---|---|
| CP | ||
| FA | −.028 ± .019* | −.016 ± .047τ |
| ADC | −.028 ± .048 | .019 ± .128 |
| FLAIR | .004 ± .013 | −.018 ± .044 |
| Slope of LIs of Nearest 5-Slices | ||
| FA | −.031 ± .010 * | .011 ± .021* τ |
| ADC | .001 ± .014 | −.004 ± .017 |
| FLAIR | .007 ± .012 | .001 ± .012 |
Laterality Index calculated using (FAL-FAR)/(FAL+FAR)
Laterality Index calculated using (FAA-FAU)/(FAA+FAU)
Significantly different from zero with p<0.05
Adjusted for significant L vs. R asymmetry in control group
In patients, there was a significant difference in mean FA LI for the Nearest-5 slices slope indicating lower FA of the ipsilesional CST (see also Figure 2). There was no significant difference between groups for any other sequence/ROI combination (all p>0.05).
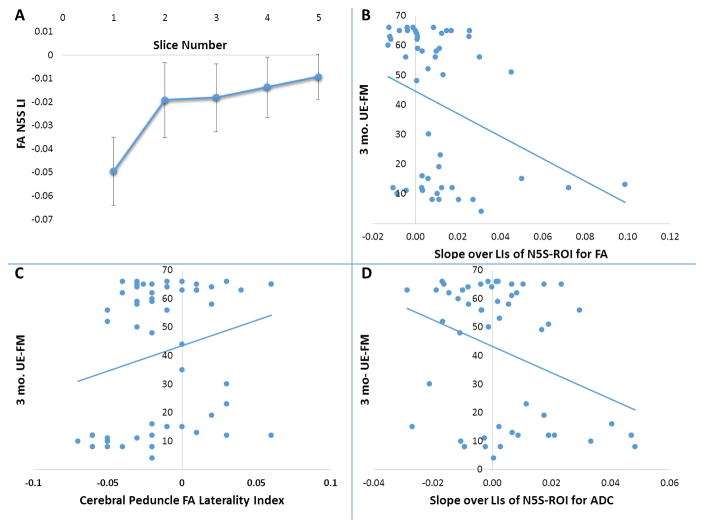
Figure 2. FA laterality Indices and 3-months UE-FM Outcome.
FA laterality indices are shown for each slice of the N5S ROI (Fig. 2A). Slice 1 is the slice closes to the lesion. The 3-month UE-FM scores are plotted against the Slope of the LIs of the Nearest-5-Slices for the FA (Fig. 2B), as well as against the FA laterality index of the CP-ROI (Fig. 2C), and against the Slope of the LIs of the Nearest-5-Slices for the ADC (Fig. 2D). Negative FA or ADC laterality indices indicate lower values on the lesional hemisphere compared to the unaffected hemisphere.
Mean difference of FA laterality between the nearest slice (slice 1) and the furthest slice (slice 5) were significantly different when assessed with a paired t-test (p<0.001) (Figure 2A).
Motor Outcome Prediction
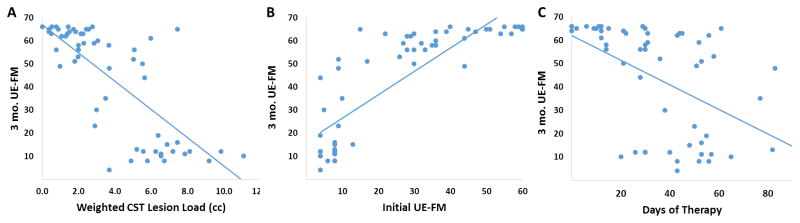
Table 3, Figure 2B–D, and Figure 3A–C show the results of the regression analyses. The Slope of the FA laterality index for the Nearest-5-Slices (N5S) ROI (S-N5S) showed a weak, significant trend (Fig. 2B) as a predictor of 3-month UE-FM score in univariate analysis (R2=0.105, p=0.02) while the FA LI for the Cerebral Peduncle (CP) ROI did not show any trend (R2=0.044, p=0.14; Fig. 2C). The slope of the ADC laterality index for the N5S ROI was also weakly predictive of 3- months outcome (R2=0.113, p=0.02; Fig 2D). No other variable was predictive of 3-month outcome (p>0.05, data not shown). However, the FA and ADC variables contributed only modestly and the regressions seemed highly susceptible to outliers. Initial UE-FM (R2=0.69, p<0.001) was a much better predictor than any FA or ADC variable in univariate analysis, comparable to the wCST lesion load prediction (R2=0.71, p<0.001; Fig. 3A+B)). Days of therapy also significantly predicted motor outcome (R2=0.249, p<0.001; Fig. 3C), although much less than initial UE-FM and wCST-LL.
Table 3.
Regression analyses for predicting 3-months UE-FM score
| Variable, Univariate | N | R | p-value |
|---|---|---|---|
| Initial UE-FM | 58 | 0.687 | <0.001 |
| S-N5S FA LI | 50 | 0.105 | 0.022 |
| FA LI CP | 50 | 0.044 | 0.137 |
| DoT | 56 | 0.247 | <0.001 |
| CST Lesion Load | 55 | 0.710 | <0.001 |
| S-N5S ADC LI | 51 | 0.113 | 0.02 |
|
|
|||
| Variables, Multivariate | |||
|
|
|||
| ^ DoT, CST_LL, UE-FM, + S-N5S FA LI Initial | 0.859 | <0.001*, 0.249** | |
| ^ DoT, CST_LL, UE-FM, + S-N5S ADC LI Initial | 0.849 | <0.001*, 0.334** | |
both models were significant in predicting 3 months outcome controlling for DoT, CST-LL and initial UE-FM.
partial regression p-value (which was non-significant); this p-value assesses if there is unique or significant contribution in the model for outcome prediction for the slope of the LIs of the N5S for FA or ADC after controlling for the effect of all other variables in the multivariate model.
DoT (Days of therapy), wCST_LL, and initial UE-FM were controlled for in the multivariate regression analyses.
Figure 3.
wCST-LL (A), initial UE-FM (B), and Days-of-Therapy (C) regressed against 3-months UE-FM Outcome.
In multivariate analyses, neither the slopes over the LIs of the N5S for the FA nor for the ADC significantly improved the R2 of our overall model including initial UE-FM, days of therapy, and wCST-LL for predicting 3-month UE-FM (FA: overall R2 = 0.859, p<0.001; Slope over LIs of N5S for FA partial p=0.25; ADC: overall R2 = 0.849, p<0.001; Slope over LIs of N5S for ADC partial p=0.33, see table 3).
Discussion
We demonstrated that subtle changes in asymmetry of FA values derived from a canonical tract of the CST incorporated into a patient’s brain imaging were detectable (close to the lesion) early after ischemic stroke, but not in the cerebral peduncle. In particular, the most pronounced FA asymmetry was detected in the slice that was closest to the ischemic lesion. The slope of the FA laterality indices of the Nearest-5-Slices and the slope of the ADC laterality indices of the Nearest-5-Slices were weak, significant predictors of 3-month UE-FM score, but neither measure significantly improved upon the predictive value of initial UE-FM for 3-month motor outcome in a multivariate analysis. We did not find significant changes in FLAIR signal in any of the ROIs.
There is evidence in the literature that FA values are lower in the affected CST in the chronic stage after stroke due to the beginning of Wallerian degeneration of the tract.23–27 There is a correlation between the degree of FA asymmetry and the motor deficit at a chronic time point.25, 27 It has not been established how early this process begins in humans and whether imaging markers can detect subtle effects of Wallerian degeneration in the acute stroke phase. Pathologic evidence suggests that the process of tract degeneration begins quite early, with axonal degeneration and myelin degradation demonstrated as early as 2 days after stroke in an experimental animal model5. In contrast, studies in humans did not find such evidence for Wallerian Degeneration so early. A study of 9 patients by Thomalla et al.28 was the first one to show a decrease in FA in the affected descending CST at the level of the cerebral peduncle at 9 days after stroke onset, but patients were scanned on average 9 days after stroke. A prospective study by Puig et al.19 found absolute and relative FA decreases at 30 days after stroke, but not at 12 hours or 3 days after stroke. Both of these studies, however, examined FA changes at the level of the brainstem only, relatively far away from the ischemic lesion. The lack of an effect in the cerebral peduncle ROI in our study is in agreement with these earlier publications.
We were able to detect subtle changes in FA earlier than previous studies on routine clinical MRIs done on average 26 (+/−14) hours after an ischemic stroke by placing ROIs closer to the lesion, using our Nearest-5-Slices (N5S) ROI, and calculating the slope over these 5 laterality indices of the FA and the ADC. Without pathological correlation, it is not possible to state with certainty that the changes detected in this study represent early Wallerian degeneration. The lack of significant differences in hemispheric asymmetry of FLAIR signal for our Slope of Nearest-5-Slices does, however, suggest that our findings are not simply due to perilesional edema or an increase in cellularity secondary to inflammation within the tract and could potentially reflect early disintegration of white matter fibers (either myelin sheath disintegration or axon collapse).
The degree of hemispheric asymmetry in the Slope of the Nearest-5-Slices FA laterality index was not a good predictor of 3-month motor outcome. Statistically, the additional predictive value offered by any FA value is null and other predictors such as the initial UE-FM and the CST lesion load are so strong that regional FA could not contribute anything to their predictive value.
Our study has several limitations. First, we used high-resolution DTI for ROI definitions and FA comparisons in control subjects, but our clinical MRIs had lower-resolution. This is unlikely to introduce bias to our results, since the main FA data was derived from the patient group. Second, we chose to use only laterality indices, rather than absolute FA values, for the analyses to avoid inappropriate comparisons between the different imaging techniques. Third, we included only patients with their first ischemic hemispheric stroke, which may limit somewhat the generalizability of our findings to a broader group of acute stroke patients.
Acknowledgments
Sources of Funding:
This study was supported by grants from the NIH (1RO1 DC008796, 3R01DC008796-02S1, R01 DC009823-01), the Mary Crown and William Ellis Fund, the Richard and Rosalyn Slifka Fund, and the Tom and Suzanne McManmon Fund. Christopher Doughty was supported by the Doris Duke Charitable Foundation. Wuwei Feng is supported by the American Heart Association (14SDG1829003), the South Carolina Clinical & Translational Research Institute through NIH (UL1 RR029882 and UL1 TR000062), and the NIH (P20GM109040).
Footnotes
Disclosures: None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, et al. Corticospinal tract lesion load - a potential imaging biomarker for stroke motor outcomes. Annals of neurology. 2015;78:860–870. doi: 10.1002/ana.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Annals of neurology. 2015;77:132–145. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Annals of neurology. 2015;78:848–859. doi: 10.1002/ana.24472. [DOI] [PubMed] [Google Scholar]
- 5.Iizuka H, Sakatani K, Young W. Corticofugal axonal degeneration in rats after middle cerebral artery occlusion. Stroke; a journal of cerebral circulation. 1989;20:1396–1402. doi: 10.1161/01.str.20.10.1396. [DOI] [PubMed] [Google Scholar]
- 6.Matsusue E, Sugihara S, Fujii S, Kinoshita T, Ohama E, Ogawa T. Wallerian degeneration of the corticospinal tracts: Postmortem mr-pathologic correlations. Acta Radiol. 2007;48:690–694. doi: 10.1080/02841850701342112. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn MJ, Johnson KA, Davis KR. Wallerian degeneration: Evaluation with mr imaging. Radiology. 1988;168:199–202. doi: 10.1148/radiology.168.1.3380957. [DOI] [PubMed] [Google Scholar]
- 8.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: Early prediction of functional outcome after stroke: The epos cohort study. Stroke; a journal of cerebral circulation. 2010;41:745–750. doi: 10.1161/STROKEAHA.109.572065. [DOI] [PubMed] [Google Scholar]
- 9.Smania N, Paolucci S, Tinazzi M, Borghero A, Manganotti P, Fiaschi A, et al. Active finger extension: A simple movement predicting recovery of arm function in patients with acute stroke. Stroke; a journal of cerebral circulation. 2007;38:1088–1090. doi: 10.1161/01.STR.0000258077.88064.a3. [DOI] [PubMed] [Google Scholar]
- 10.Zarahn E, Alon L, Ryan SL, Lazar RM, Vry MS, Weiller C, et al. Prediction of motor recovery using initial impairment and fmri 48 h poststroke. Cereb Cortex. 2011;21:2712–2721. doi: 10.1093/cercor/bhr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arac N, Sagduyu A, Binai S, Ertekin C. Prognostic value of transcranial magnetic stimulation in acute stroke. Stroke; a journal of cerebral circulation. 1994;25:2183–2186. doi: 10.1161/01.str.25.11.2183. [DOI] [PubMed] [Google Scholar]
- 12.Catano A, Houa M, Caroyer JM, Ducarne H, Noel P. Magnetic transcranial stimulation in acute stroke: Early excitation threshold and functional prognosis. Electroencephalogr Clin Neurophysiol. 1996;101:233–239. doi: 10.1016/0924-980x(96)95656-8. [DOI] [PubMed] [Google Scholar]
- 13.Dachy B, Biltiau E, Bouillot E, Dan B, Deltenre P. Facilitation of motor evoked potentials in ischemic stroke patients: Prognostic value and neurophysiologic correlations. Clin Neurophysiol. 2003;114:2370–2375. doi: 10.1016/s1388-2457(03)00252-9. [DOI] [PubMed] [Google Scholar]
- 14.Escudero JV, Sancho J, Bautista D, Escudero M, Lopez-Trigo J. Prognostic value of motor evoked potential obtained by transcranial magnetic brain stimulation in motor function recovery in patients with acute ischemic stroke. Stroke; a journal of cerebral circulation. 1998;29:1854–1859. doi: 10.1161/01.str.29.9.1854. [DOI] [PubMed] [Google Scholar]
- 15.Heald A, Bates D, Cartlidge NE, French JM, Miller S. Longitudinal study of central motor conduction time following stroke. 2. Central motor conduction measured within 72 h after stroke as a predictor of functional outcome at 12 months. Brain. 1993;116(Pt 6):1371–1385. doi: 10.1093/brain/116.6.1371. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn MJ, Mikulis DJ, Ayoub DM, Kosofsky BE, Davis KR, Taveras JM. Wallerian degeneration after cerebral infarction: Evaluation with sequential mr imaging. Radiology. 1989;172:179–182. doi: 10.1148/radiology.172.1.2740501. [DOI] [PubMed] [Google Scholar]
- 17.Sawlani V, Gupta RK, Singh MK, Kohli A. Mri demonstration of wallerian degeneration in various intracranial lesions and its clinical implications. J Neurol Sci. 1997;146:103–108. doi: 10.1016/s0022-510x(96)00299-7. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H, Tashiro K. Brunnstrom stages and wallerian degenerations: A study using mri. Tohoku J Exp Med. 1992;166:471–473. doi: 10.1620/tjem.166.471. [DOI] [PubMed] [Google Scholar]
- 19.Puig J, Pedraza S, Blasco G, Daunis IEJ, Prats A, Prados F, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR. American journal of neuroradiology. 2010;31:1324–1330. doi: 10.3174/ajnr.A2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeVetten G, Coutts SB, Hill MD, Goyal M, Eesa M, O’Brien B, et al. Acute corticospinal tract wallerian degeneration is associated with stroke outcome. Stroke; a journal of cerebral circulation. 2010;41:751–756. doi: 10.1161/STROKEAHA.109.573287. [DOI] [PubMed] [Google Scholar]
- 21.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 22.Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, et al. Water diffusion changes in wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 23.Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 25.Moller M, Frandsen J, Andersen G, Gjedde A, Vestergaard-Poulsen P, Ostergaard L. Dynamic changes in corticospinal tracts after stroke detected by fibretracking. J Neurol Neurosurg Psychiatry. 2007;78:587–592. doi: 10.1136/jnnp.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radlinska B, Ghinani S, Leppert IR, Minuk J, Pike GB, Thiel A. Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology. 2010;75:1048–1054. doi: 10.1212/WNL.0b013e3181f39aa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe T, Honda Y, Fujii Y, Koyama M, Matsuzawa H, Tanaka R. Three-dimensional anisotropy contrast magnetic resonance axonography to predict the prognosis for motor function in patients suffering from stroke. J Neurosurg. 2001;94:955–960. doi: 10.3171/jns.2001.94.6.0955. [DOI] [PubMed] [Google Scholar]
- 28.Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early wallerian degeneration of the pyramidal tract after ischemic stroke. NeuroImage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 29.Thomalla G, Glauche V, Weiller C, Rother J. Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2005;76:266–268. doi: 10.1136/jnnp.2004.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabilitation and neural repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 31.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke; a journal of cerebral circulation. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain : a journal of neurology. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 34.Stinear C. Prediction of recovery of motor function after stroke. Lancet neurology. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]