Abstract
Background
The impact of decision aids on prostate cancer screening outcomes has been inconsistent.
Purpose
We assessed whether pre-existing attitudes moderated the impact of decision aids on screening.
Methods
Men aged 45–70 (56.2% Caucasian; 39.9% African-American) were randomly assigned to a Print decision aid (N=630), a Web decision aid (N=631), or Usual Care (N=632). Telephone interviews assessed Pro/Con screening attitudes and screening behaviors at baseline, one-month, and 13-months post-randomization.
Results
Logistic regression analyses revealed significant arm by attitude interactions: higher baseline Cons scores predicted lower screening in the Print (OR=0.60 (95%CI: 0.40, 0.92)) and Web (OR=0.61 (95%CI: 0.40, 0.91)) arms, but not in usual care (OR=1.34 (95% CI: 0.90, 2.00)).
Conclusions
The decision aids amplified the impact of men’s baseline attitudes about limitations of screening: compared to the usual care arm, men in both decision aid arms were less likely to be screened when they perceived more limitations of screening.
Keywords: prostate cancer, patient decision aids, prostate cancer screening, decision making
Prostate cancer is the second leading cause of male cancer death [1]. Although prostate cancer screening is widespread, there is no conclusive evidence that it reduces prostate cancer mortality [2]. In fact, there are concerns that prostate cancer screening may lead to over diagnosis, resulting in unnecessary treatment and associated morbidity [3]. The mixed evidence about the benefits of screening and concerns about harm led the U.S. Preventive Services Task Force to recommend against routinely screening all men for prostate cancer. Instead, the Task Force promotes informed and shared decision making based on each individual’s values and preferences [4]. Unfortunately, many men are unaware of the controversy surrounding screening [5,6]. One way to promote informed decision making about screening is by offering decision aids. Decision aids are tools that provide information about a condition and the possible benefits, harms, and uncertainties about potential options. Several randomized controlled trials have evaluated decision aids for prostate cancer screening, typically among primary care patients. Findings have been mixed regarding the impact of decision aids on men’s screening rates, with some studies finding reduced screening [7], no change [8,9], or increased screening [10].
To further examine the impact of decision aids on screening rates, we conducted a randomized controlled trial (NCT00196807) comparing two decision aids (one print-based and one web-based) against usual care (no intervention) on decision making and screening outcomes [11]. These decision aids were developed with the goal of improving prostate cancer knowledge and clarifying values in order to promote informed decision making [12]. The randomized controlled trial showed that decision aid use resulted in increased knowledge and satisfaction with the decision, as well as reduced decisional conflict, relative to usual care [11]. However, the decision aids did not affect screening rates at 13-months post-randomization, relative to the usual care arm [11]. Finally, there were no differences between the two decision aids on screening behavior, knowledge, or decisional satisfaction at 13-months post-randomization. In a process evaluation of the decision aids, we found that use was greater in the print arm compared to the web arm [13]. These results suggest that when it is not possible to provide this age cohort with their preferred decision aid medium, print materials may be more highly used than web-based materials.
As the decision aids did not yield differences in screening rates relative to usual care, we have conducted follow-up analyses exploring whether baseline factors may have moderated the impact of the decision aids. A growing body of literature suggests that there are a variety of factors that influence participation in cancer control behaviors, including demographic, situational, and psychosocial factors [14–17]. Specifically, research has found that attitudes may reduce patients’ intentions to engage in cancer-related behaviors, such as information seeking [18] and treatment decision making [19]. As a result, the Ottawa Decision Support Framework suggests that patient attitudes should be considered in the development and implementation of decision aids [20]. Thus, we examined whether pre-existing attitudes towards the benefits and limitations of screening moderated the impact of the decision aids on screening outcomes.
Methods
Participants
As detailed in the previous report of the randomized controlled trial [11], participants were men aged 45–70 years old with no prior history of prostate cancer, the ability to provide informed consent, English speaking, living independently (e.g., nursing home occupants were excluded), and who had an outpatient visit in the last 24 months at one of the three primary care accrual sites (Georgetown University Hospital, Washington Hospital Center, or MedStar Physician Partners).
Materials and Procedures
Eligible men were mailed invitation letters between October 2007 and January 2010. Research staff subsequently contacted men to describe the study, confirm eligibility, obtain verbal consent, and conduct the 20–25 minute baseline interview with those enrolled. As displayed in the CONSORT figure (Figure 1), following the baseline assessment, we randomized participants to one of the three study arms: usual care (n=632), print decision aid (n=630), or web decision aid (n=631). After randomization, we mailed written consent forms along with a booklet (print arm) or log in instructions and a website URL (web arm; http://prostatedecision.georgetown.edu). Men completed the follow-up telephone interviews at one-month and 13-months post-baseline. All procedures were approved by the Georgetown University Oncology Institutional Review Board.
Figure 1.
CONSORT Diagram
At the baseline assessment, we assessed demographic information (age, marital status, education, race/ethnicity, income, having a regular doctor, health insurance status, and employment status), clinical information (comorbidities, personal and family history of prostate cancer and other cancers), and screening history (whether men had discussed prostate cancer screening with their doctor, and prostate cancer lifetime screening history). To assess baseline attitudes, men rated the personal importance of eight screening benefits (Pros; e.g. “How important is finding the cancer early?”) and seven risks or limitations (Cons; e.g. “How important is the possibility that you may have a biopsy that turns out to be unnecessary?”). These items were adapted from a scale designed to assess the importance of the pros and cons of breast cancer screening [21]. All 15 items were rated on a scale of 1 to 3 (“not at all important” to “very important”). We calculated average scores for Pros and Cons separately. Because the distributions of these scales were significantly skewed (Cons Skew z= −8, p<.01; Pros Skew z= −25.7, p<.01), we used a median split to categorize scores as high or low for both Pros and Cons. At the 13-month interview, men reported whether they had received a prostate-specific antigen test and/or a digital rectal exam during the one-year study period since randomization.
Data Analysis
We used logistic regression to evaluate whether randomization group and baseline attitudes predicted self-reported screening behavior at 13-months and if baseline attitudes moderated the effect of the randomization group on screening. The regression models included terms for group, attitudes (Pros and Cons were analyzed separately), and the interaction term between group and attitudes, controlling for age, race, education, marital status, family history of prostate cancer, and ever screened for prostate cancer (which were all individually significant predictors of screening in bivariate analyses, p <.05). Missing data were minimal (<1%) for all variables assessed at baseline.
Results
Descriptive Information
Baseline demographic, clinical, and prostate cancer screening variables are presented in Table 1. Almost 40% of participants were African-American, 23.8% had a high school education or less, and 59.3% were screened in the year before enrollment. With regard to attitudes towards the Pros and Cons of screening, participants reported an average importance rating of 2.15 (SD=0.56) for the Cons of screening and 2.75 (SD=0.25) for the Pros. There were no significant differences between the Print, Web, and UC arms on any of the demographic, clinical, prostate cancer screening, or Pro/Con attitude variables.
Table 1.
Baseline demographic and clinical characteristics (N = 1879#)
| Print (n = 628) | Usual Care (n = 626) | Web (n = 625) | All (N = 1879) | P Value1 | |
|---|---|---|---|---|---|
| Age (Mean (SD)) | 56.7 (6.8) | 56.9 (6.8) | 57.0 (6.8) | 56.9 (6.8) | .699 |
| Race2 | |||||
| White | 351 (55.9) | 352 (56.2) | 352 (56.4) | 1055 (56.2) | .973 |
| African-American | 250 (39.8) | 250 (39.9) | 250 (40.1) | 750 (39.9) | |
| Other | 27 (4.3) | 24 (3.8) | 22 (3.5) | 73 (3.9) | |
| Education2 | |||||
| High school or less | 146 (23.4) | 155 (24.9) | 144 (23.2) | 445 (23.8) | .694 |
| Some college or college degree | 268 (42.9) | 249 (40.0) | 249 (40.0) | 766 (41.0) | |
| Graduate work or degree | 210 (33.7) | 219 (35.2) | 229 (36.8) | 658 (35.2) | |
| Marital status2 | |||||
| Married | 416 (66.3) | 432 (69.1) | 430 (69.0) | 1278 (68.2) | .490 |
| Employment status | |||||
| Not Employed | 72 (11.5) | 79 (12.6) | 79 (12.6) | 230 (12.2) | .599 |
| Employed full-time/part-time | 438 (69.7) | 447 (71.4) | 428 (68.5) | 1313 (69.9) | |
| Retired | 118 (18.8) | 100 (16.0) | 118 (18.9) | 336 (17.9) | |
| Income | |||||
| ≤75k | 211 (38.9) | 228 (40.6) | 213 (38.9) | 652 (39.5) | .479 |
| 75k–150k | 189 (34.9) | 189 (33.6) | 171 (31.2) | 549 (33.2) | |
| >150k | 142 (26.2) | 145 (25.8) | 164 (29.9) | 451 (27.3) | |
| Refused | 86 | 64 | 77 | 227 | |
| Health insurance | |||||
| Yes | 618 (98.4) | 619 (98.9) | 611 (97.8) | 1848 (98.4) | .295 |
| Comorbidities2 | |||||
| 0 | 220 (35.3) ‡ | 194 (31.0) | 213 (34.1)‡ | 627 (33.5) | .460 |
| 1 | 202 (32.4) | 212 (34.2) | 193 (30.9) | 609 (32.5) | |
| ≥2 | 202 (32.4) | 218 (34.8) | 218 (34.9) | 638 (34.0) | |
| Family history of PCa | |||||
| Yes | 120 (20.2) | 142 (24.0) | 153 (25.3) | 415 (23.2) | .090 |
| Don’t Know/Missing | 33 | 34 | 21 | 88 | |
| Ever screened3 | |||||
| Yes | 534 (85.4) | 527 (84.9) | 552 (88.5) | 1613 (86.3) | .140 |
| Pros2 (Mean (SD)) | 2.8 (0.24) | 2.8 (0.25) | 2.7 (0.26) | 2.7 (0.25) | .384 |
| Cons2 (Mean (SD)) | 2.2 (0.55) | 2.2 (0.55) | 2.1 (0.57) | 2.1 (0.56) | .234 |
N = 14 participants were diagnosed with prostate cancer during the course of the study and were thus removed from all analyses, making the final N = 1879.
Group differences assessed using ANOVAs and chi square tests.
Variable is missing < 1% of data.
Men screened in past year due to symptoms were removed
% do not add up to 100 due to rounding
PCa = Prostate Cancer
Overall, 84.1% of participants completed the 13-month assessment, and 58.3% of men reported having been screened for prostate cancer since the baseline assessment [11].
Baseline Attitudes as Moderator of the Impact of the Decision Aids on Screening
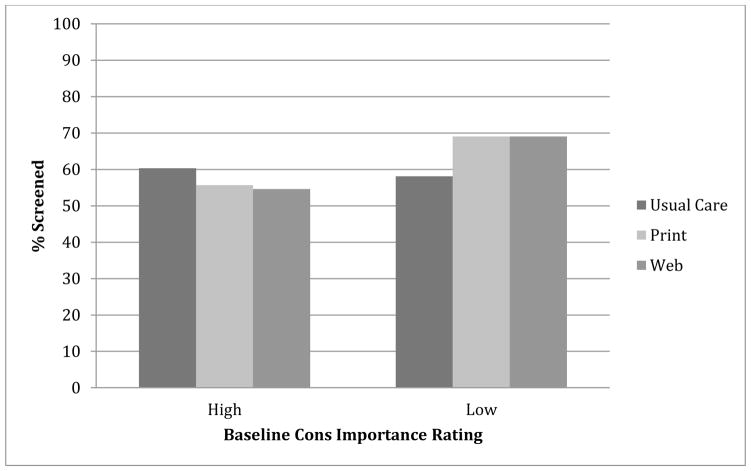
As displayed in Table 2 and Figure 2, there was a statistically significant interaction between randomization group and baseline Cons ratings that predicted screening outcomes. Follow-up examination of the interaction revealed that among men who were randomized to the Print- or Web-based decision aids, those with high baseline Cons ratings were significantly less likely to get screened than those with low baseline Cons ratings. However, for men in the usual care arm, there was no significant effect of baseline attitudes on screening decisions. Among the men who rated baseline Cons as having low importance, men in the Print and Web arms were significantly more likely to be screened than those in the usual care arm. Among men who rated baseline Cons as having high importance, there was a marginally significant effect, indicating that the web arm was less likely to undergo screening than the usual care arm (p< .10). In per-protocol models that included only those men who reported that they used the decision aid to which they were assigned, the interactions of baseline Cons and group remained significant and the association increased slightly, as expected.
Table 2.
Results from logistic regression models predicting screening at 13 months
| Demographic and attitude variables (N=1460) | OR | 95% CI | P value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Cons Model | Marital Status (ref=not married) | 1.243 | 0.971 | 1.590 | 0.084 | |
| Education (ref=high school or less) | 0.111 | |||||
| some college/degree | 1.226 | 0.902 | 1.666 | 0.193 | ||
| graduate work/degree | 1.428 | 1.023 | 1.993 | 0.036 | ||
| Race (ref=white) | 1.034 | 0.803 | 1.330 | 0.797 | ||
| Age (per 1 year) | 1.057 | 1.039 | 1.076 | <0.001 | ||
| Family History of PCa (ref=no) | 1.229 | 0.938 | 1.610 | 0.135 | ||
| Ever screened for PCa (ref=no) | 3.173 | 2.179 | 4.620 | <0.001 | ||
| Cons (ref=low) | 1.133 | 0.856 | 1.501 | 0.383 | ||
| Treatment group (ref=UC) | 0.022 | |||||
| 1.606 | 1.118 | 2.307 | 0.010 | |||
| Web | 1.414 | 0.998 | 2.003 | 0.051 | ||
| Cons by Group Interaction | 0.006 | |||||
| Print by Cons | 0.542 | 0.341 | 0.863 | 0.010 | ||
| Web by Cons | 0.543 | 0.339 | 0.868 | 0.011 | ||
|
| ||||||
| Stratified Effects | ||||||
|
| ||||||
| Effect of Attitudes (Low as reference) by Treatment Group | UC | 1.343 | 0.901 | 2.002 | 0.148 | |
| 0.604 | 0.397 | 0.920 | 0.019 | |||
| Web | 0.607 | 0.403 | 0.913 | 0.016 | ||
|
| ||||||
| Effect of Treatment (UC as reference) Group by Attitude Levels | Low | 1.790 | 1.207 | 2.653 | 0.004 | |
| Web | 1.543 | 1.059 | 2.248 | 0.024 | ||
|
| ||||||
| High | 0.811 | 0.553 | 1.188 | 0.282 | ||
| Web | 0.711 | 0.480 | 1.055 | 0.090 | ||
|
| ||||||
| Pros Model | Marital Status (ref=not married) | 1.269 | 0.992 | 1.622 | 0.057 | |
| Education (ref=high school or less) | 0.061 | |||||
| some college/degree | 1.271 | 0.939 | 1.719 | 0.121 | ||
| graduate work/degree | 1.486 | 1.070 | 2.063 | 0.018 | ||
| Race (ref=white) | 1.003 | 0.780 | 1.289 | 0.983 | ||
| Age (per 1 year) | 1.057 | 1.039 | 1.075 | <0.001 | ||
| Family History of PCa (ref=no) | 1.237 | 0.945 | 1.620 | 0.121 | ||
| Ever screened for PCa (ref=no) | 3.204 | 2.203 | 4.659 | <0.001 | ||
| Pros (ref=low) | 1.109 | 0.753 | 1.632 | 0.602 | ||
| Treatment group (ref=UC) | 0.255 | |||||
| 1.349 | 0.946 | 1.924 | 0.098 | |||
| Web | 1.158 | 0.817 | 1.642 | 0.409 | ||
| Pros by Group Interaction | 0.500 | |||||
| Print by Pros | 0.721 | 0.416 | 1.250 | 0.244 | ||
| Web by Pros | 0.818 | 0.471 | 1.419 | 0.475 | ||
Note. UC= Usual Care; PCa = Prostate Cancer;
Figure 2.
Moderation Effect of Baseline Cons Ratings by Study Arm on Screening Outcomes at 13-Months
The same modeling approach was performed using baseline ratings of the importance of the Pros of screening. There was no significant interaction between Pros ratings and group on screening behavior, and no significant impact of Pros on screening. Similarly, per-protocol analyses indicated no significant impact of the interaction of Pros and group on screening.
Discussion
Many professional groups, such as the American Cancer Society, now recommend that prostate cancer screening should be treated as a joint decision between patients and their doctors, taking into account each individual’s values. Unfortunately, primary care doctors have little time to counsel men on their decisions regarding screening. Decision aids are one way of promoting informed decision making without increasing the burden on physicians. As the impact on actual screening outcomes has been mixed, we examined whether pre-existing attitudes toward prostate cancer screening influenced the impact of decision aids. Specifically, we analyzed whether patients’ baseline attitudes regarding the benefits and risks of screening moderated the impact of the decision aids on screening outcomes.
Results suggested that both decision aids amplified existing attitudes about the risks and limitations of screening: among men who rated the limitations of screening as being less important to them, those who were randomized to a decision aid were significantly more likely to be screened than those who received usual care. In contrast, among patients who reported that the potential limitations of screening were more important to them, there was only a marginally significant screening difference between those assigned to use the web decision aid and those assigned to usual care. Men’s ratings of the importance of the potential benefits of screening did not predict screening behavior or moderate the effect of randomization arm on screening. Thus, among men who were less concerned about the limitations of screening, the decision aids resulted in increased screening rates, while the screening rates of men who were more concerned about the limitations of screening were not significantly impacted by the decision aids.
Several limitations should be considered in interpreting these results. First, the Pros and Cons scales were developed for the current study and have not been previously validated. Of particular note, the Pros scale had a restricted range, with very few men reporting that the benefits of prostate cancer screening were of low importance to them. This restriction may have limited our ability to examine the impact of men’s attitudes about the benefits of screening on screening rates. Second, the current study relied on self-reported prostate cancer screening, which can bias screening results and should be interpreted with caution [22,23]. Although we had also collected screening outcomes from medical records, these data were incomplete due to unreturned medical record consent forms and missing records. Importantly, self-reported screening outcomes were concordant with findings from the medical record data [11]. Further, there does not appear to be a systematic difference between self-report and medical record data, as both methods have been used in studies reporting increased screening, decreased screening, and no change.
Regarding study strengths, this study is one of the largest and most racially and socioeconomically diverse trials conducted on this topic. The trial followed men for a full year in order to evaluate the long-term impact of the decision aids on screening behaviors. Importantly, this study is among the first to highlight the impact of baseline attitudes about screening. Our findings suggest that decision aid developers should consider assessing baseline attitudes about the behavior under study, and build in ways of addressing pre-existing beliefs in a direct fashion. This may be particularly important to address when there are widely held pre-existing attitudes that are not supported by the data, such as prostate cancer screening.
Overall, these findings suggest that men’s pre-existing values and attitudes about the limitations and risks of prostate cancer screening were a critical factor in prostate cancer screening decision making. As a primary goal of informed decision making models is the clarification of patients’ goals and values [20], it is important that decision aids and medical professionals help men to understand what is important to them. Future studies should examine the impact of pre-existing beliefs on the interpretation of information presented in decision aids, the extent to which decision aids may serve to amplify vs. challenge those pre-existing beliefs, and their ultimate impact on behavioral outcomes.
Acknowledgments
Funding/Support: This work was supported by grants from the National Cancer Institute (R01CA119168-01) and Department of Defense (PCO51100) to Dr. Taylor. In addition, the project was supported by the Lombardi Comprehensive Cancer Center (LCCC) Biostatistics and Bioinformatics Shared Resource and an LCCC Cancer Center Support Grant
We are grateful to the participants for contributing their time; to Janet Ohene-Frempong, MA, our plain language consultant, who contributed to the editing of the intervention materials; to the interviewers who conducted telephone assessments: Sara Edmond, Caroline Dorfman, Elisabeth Kassan, David Dawson, William Tuong, Elizabeth Parker, Sofiya Penek, Samantha Barry, Lisa Haisfield, and to Susan Marx for administrative support.
Footnotes
Conflict of Interest and Ethical Standards: Authors Starosta, Luta, Tomko, Schwartz, and Taylor declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Accessed June 23, 2014]. Available at http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. [Google Scholar]
- 2.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 3.Justman S. Uninformed consent: mass screening for prostate cancer. Bioethics. 2012;26:143–148. doi: 10.1111/j.1467-8519.2010.01826.x. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 5.Diefenbach M, Turner G, Carpenter KM, et al. Cancer and patient-physician communication. J Health Commun. 2009;14(Suppl 1):57–65. doi: 10.1080/10810730902814079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gigerenzer G, Mata J, Frank R. Public knowledge of benefits of breast and prostate cancer screening in Europe. J Natl Cancer Inst. 2009;101:1216–1220. doi: 10.1093/jnci/djp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheridan SL, Golin C, Bunton A, et al. Shared decision making for prostate cancer screening: the results of a combined analysis of two practice-based randomized controlled trials. BMC Med Inform Decis Mak. 2012;12:130. doi: 10.1186/1472-6947-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepore SJ, Wolf RL, Basch CE, et al. Informed decision making about prostate cancer testing in predominantly immigrant black men: a randomized controlled trial. Ann Behav Med. 2012;44:320–330. doi: 10.1007/s12160-012-9392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams RM, Davis KM, Luta G, et al. Fostering informed decisions: a randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer. Patient Educ Couns. 2013;91:329–336. doi: 10.1016/j.pec.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamatiou K, Skolarikos A, Heretis I, et al. Does educational printed material manage to change compliance with prostate cancer screening? World J Urol. 2008;26:365–373. doi: 10.1007/s00345-008-0258-z. [DOI] [PubMed] [Google Scholar]
- 11.Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med. 2013;173:1704–1712. doi: 10.1001/jamainternmed.2013.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfman CS, Williams RM, Kassan EC, et al. The development of a web- and a print-based decision aid for prostate cancer screening. BMC Med Inform Decis Mak. 2010;10:12. doi: 10.1186/1472-6947-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomko C, Davis KM, Luta G, Krist AH, Woolf SH, Taylor KL. A comparison of web-based versus print-based decision aids for prostate cancer screening: participants' evaluation and utilization. J Gen Intern Med. 2014 Sep 3; doi: 10.1007/s11606-014-2994-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consedine NS, Morgenstern AH, Kudadjie-Gyamfi E, Magai C, Neugut AI. Prostate cancer screening behavior in men from seven ethnic groups: the fear factor. Cancer Epidemiol Biomarkers Prev. 2006;15:228–237. doi: 10.1158/1055-9965.EPI-05-0019. [DOI] [PubMed] [Google Scholar]
- 15.Gorin MA, Soloway CT, Eldefrawy A, Soloway MS. Factors that influence patient enrollment in active surveillance for low-risk prostate cancer. Urology. 2011;77:588–591. doi: 10.1016/j.urology.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 16.McDowell ME, Occhipinti S, Chambers SK. Classifying the reasons men consider to be important in prostate-specific antigen (PSA) testing decisions: evaluating risks, lay beliefs, and informed decisions. Ann Behav Med. 2013;46:322–335. doi: 10.1007/s12160-013-9508-4. [DOI] [PubMed] [Google Scholar]
- 17.Silberbogen AK, Busby AK, Ulloa EW. Impact of psychological distress on prostate cancer screening in U.S. military veterans. Am J Mens Health. 2013;8:399–408. doi: 10.1177/1557988313516357. [DOI] [PubMed] [Google Scholar]
- 18.Ross L, Kohler CL, Grimley DM, Green BL, Anderson-Lewis C. Toward a model of prostate cancer information seeking: identifying salient behavioral and normative beliefs among African American men. Health Educ Behav. 2007;34:422–440. doi: 10.1177/1090198106290751. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Dailey RK, Eggly S, Neale AV, Schwartz KL. Men's perspectives on selecting their prostate cancer treatment. J Natl Med Assoc. 2011;103:468–478. doi: 10.1016/s0027-9684(15)30359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor AM, Bennett CL, Stacey D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009:CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Rakowski W, Andersen MR, Stoddard AM, et al. Confirmatory analysis of opinions regarding the pros and cons of mammography. Health Psychol. 1997;16:433–441. doi: 10.1037//0278-6133.16.5.433. [DOI] [PubMed] [Google Scholar]
- 22.Chan JM, Jou RM, Carroll PR. The relative impact and future burden of prostate cancer in the United States. J Urol. 2004;172:S13–S16. [PubMed] [Google Scholar]
- 23.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]