Abstract
Background
Age 45 years is used as a cutoff in the staging of well-differentiated thyroid cancer (WDTC) as it represents the median age of most datasets. The aim of this study was to determine a statistically optimized age threshold using a large dataset of patients treated at a comprehensive cancer center.
Methods
Overall, 1807 patients with a median follow-up of 109 months were included in the study. Recursive partitioning was used to determine which American Joint Committee on Cancer (AJCC) variables were most predictive of disease-specific death, and whether a different cutoff for age would be found. From the resulting tree, a new age cutoff was picked and patients were restaged using this new cutoff.
Results
The 10-year disease-specific survival (DSS) by Union for International Cancer Control (AJCC/UICC) stage was 99.6, 100, 96, and 81 % for stages I–IV, respectively. Using recursive partitioning, the presence of distant metastasis was the most powerful predictor of DSS. For M0 patients, age was the next most powerful predictor, with a cutoff of 56 years. For M1 patients, a cutoff at 54 years was most predictive. Having reviewed the analysis, age 55 years was selected as a more robust age cutoff than 45 years. The 10-year DSS by new stage (using age 55 years as the cutoff) was 99.2, 98, 100, and 74 % for stages I–IV, respectively.
Conclusion
A change in age cutoff in the AJCC/UICC staging for WDTC to 55 years would improve the accuracy of the system and appropriately prevent low-risk patients being overstaged and overtreated.
Well-differentiated thyroid cancer (WDTC) is unusual amongst malignances since prognosis is closely linked with age at presentation. Young patients have an excellent prognosis and few will die of disease. However, older patients, particularly those with advanced local, regional, or distant disease, are at higher risk of disease-specific death.
The age cutoff at 45 years has been used by most of the major thyroid cancer staging systems for many years [grade, age, metastases, extent, size (GAMES), age, grade, extrathyroidal extension and size of tumor (AGES), National Thyroid Cancer Treatment Cooperative Study (NTCTCS) etc.]1-3 as it represents the median age of most large cohorts upon which such staging systems are based. Although some groups have used different age cutoffs,4,5 or considered age as a continuous variable,6,7 the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system for WDTC is unique in allocating disease stage dependent on age, with a cutoff at 45 years.8 The current system categorizes those under the age of 45 years with distant metastases as stage II, while considering all other patients under 45 years as stage I. Those aged 45 years or over are considered stage I or II when disease is limited to the thyroid, at least stage III in the presence of central neck (level VI) metastases, and stage IV when disease spreads to level VII, the lateral neck or beyond.
The AJCC/UICC staging system is not perfect but is easy to apply and has become internationally accepted. In time, this system may be replaced with a predictive tool that considers age as a continuous variable. However, this will not happen in the near future and therefore attempts should be made to maximize the accuracy and utility of the staging system in its current form.
Clinical experience suggests that a significant number of patients over the age of 45 years are at low risk of death and may not be appropriately considered to have advanced stage disease (stage III/IV).
The aim of this study was to determine a statistically optimized age threshold for application within the current AJCC/UICC model with a robust prognostic validity, using a large dataset of all WDTC patients treated at a comprehensive cancer center.
PATIENTS AND METHODS
Following approval by our Institutional Review Board, we analyzed patients with WDTC from an institutional database of 1810 consecutive patients who underwent primary surgery for differentiated thyroid cancer at Memorial Sloan Kettering Cancer Center (MSKCC) between 1986 and 2005. Of these patients, three had unresectable disease and were excluded, leaving 1807 patients for analysis. Data extracted included patient age, pT, pN and M status, and survival. Pathological details were determined from the original histopathology reports. Patients with evidence of distant metastasis at the time of presentation, based on imaging or histological features, or those in whom distant metastases were detected within 6 months of initial surgery, were considered M1. Details of death were determined from death certificates and hospital records where available. All patients who had evidence of active structural disease at the time of last follow-up and subsequently died during follow-up were considered to have died of disease. The median follow-up for the entire patient cohort was 109 months (range 52–291 months).
From the 1807 patients, 11 who were over the age of 45 years had missing data relating to T and N status, which prevented an AJCC/UICC stage being allocated; therefore, 1796 patients were included in the analysis by stage grouping.9 Eight patients had missing N status, and three patients had missing T and N status.
Recursive partitioning was used to determine which AJCC staging variables (T, N, M, and age) would be most predictive of risk for disease-specific death, and whether a different cutoff for age would be found. Disease-specific survival (DSS) was calculated from the date of surgery to the date of death due to disease or the last follow-up. Recursive partitioning is a nonparametric multivariate analysis that is used to create decision trees by breaking potential predictive variables into dichotomous variables. Patients are grouped (or partitioned) by similar outcomes, and decision points are created to direct patients to the optimal groupings. Often, this is reported in the form of a tree, which is built as a simple way to graphically explain the divisions, with a hazard rate calculated for each discreet group. Rates <1 refer to a low likelihood, while rates >1 refer to a higher likelihood of death from disease. For the purposes of recursive partitioning, of the 1807 patients in the cohort those with unknown pT status (n = 10) and pN status (n = 29) were excluded, as were those patients with no months of disease-specific follow-up (n = 152), leaving 1616 patients for this recursive partitioning. From the resulting tree, a new age cutoff was picked and patients were restaged using this new cutoff. All analysis was undertaken using R version 3.0.2 (cran.r-project.org), including the rpart.
RESULTS
Median age of the cohort was 46 years (range 4–94 years), and 72 % were female. The TNM and overall stage of the cohort are displayed in Table 1. Twenty-eight patients died of disease. A total of 1220 patients were stage I (62 %), 135 were stage II (8 %), 234 were stage III (13 %), and 207 were stage IV (17 %). The 10-year DSS by AJCC/UICC stage was 99.6, 100, 96, and 81 % for stages I–IV, respectively. The Kaplan–Meier plot of DSS by AJCC/UICC stage is shown in Fig. 1a.
TABLE 1.
TNM and AJCC/UICC stage for the cohort
| Variable | N (%) |
|---|---|
| Age, years | |
| <45 | 846 (47) |
| ≥45 | 961 (53) |
| pT | |
| T1 | 854 (47) |
| T2 | 332 (18) |
| T3 | 487 (27) |
| T4a | 119 (7) |
| T4b | 5 (0.3) |
| pN | |
| N0/Nx (N0) | 1205 (67) |
| N1a | 258 (14) |
| N1b | 315 (17) |
| M | |
| M0/Mx (M0) | 1755 (97) |
| M1 | 52 (3) |
| AJCC/UICC stage | |
| I | 1220 (68) |
| II | 135 (7) |
| III | 234 (13) |
| IV | 207 (11) |
AJCC American Joint Committee on Cancer, UICC Union for International Cancer Control
FIG. 1.

Disease-specific survival by AJCC/UICC stage. a Age cutoff of 45 years, b age cutoff of 55 years. AJCC American Joint Committee on Cancer, UICC Union for International Cancer Control
Recursive partitioning was performed to identify those factors most predictive of DSS, and the decision tree produced is shown in Fig. 2. The presence of distant metastasis at presentation was the most powerful predictor of DSS. For the M0 patients [who form the majority of the group (97 %)], age was the next most powerful predictor, with a cutoff of 56 years. For younger M0 patients, T status was most predictive, and for older M0 patients, N status was most predictive. For those patients with distant metastases at presentation (M1), an age cutoff at 54 years was most predictive. The number of patients within each group and the number of deaths of disease are shown in Table 2. A graphic representation of the model produced is shown in Fig. S1 (Electronic Supplementary Material).
FIG. 2.
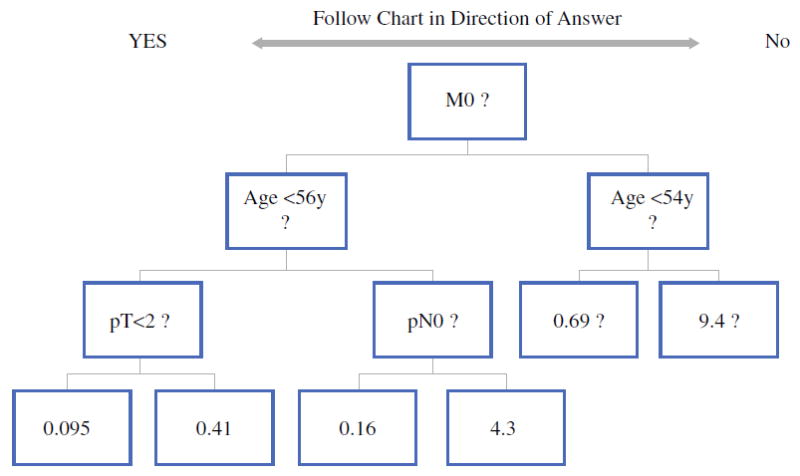
Recursive partitioning analysis of disease-specific survival. Patients are partitioned by outcome and the decision points created direct patients into optimal groupings. Each final division has a hazard rate shown
TABLE 2.
Recursive partitioning results by group and number of deaths of disease (DoDs)
| Group | N (%) | Number of DoDs |
|---|---|---|
| <56 years + M0 + T1 | 532 (33) | 0 |
| <56 years + M0 + T > 1 | 561 (35) | 4 |
| ≥56 years + M0 + N0 | 350 (22) | 0 |
| ≥56 years + M0 + N > 0 | 123 (8) | 12 |
| <54 years + M1 | 19 (1) | 0 |
| >54 years + M1 | 31 (2) | 12 |
Having reviewed the recursive partitioning analysis, 55 years was selected as the more robust age cutoff than 45 years. The 10-year DSS for the whole group stratified by age <45 versus >45 years was 100 versus 97.7 %. The 10-year DSS for the whole group stratified by age <55 versus >55 years was 99 versus 96.5 %.
The effect of this change in staging is shown in Table 3. All patients currently considered AJCC/UICC stage I remained stage I, while 66 of 135 patients (49 %) in stage II were reclassified as stage I. Ninety current stage III patients were reclassified as stage I (38 %), and 57 of 207 patients in stage IV were reclassified (28 %) to stage II (n = 6, 3 %) or stage I (n = 51, 25 %).
TABLE 3.
Changes in AJCC/UICC staging when age 45 years is substituted for age 55 years
| Age 55 I | Age 55 II | Age 55 III | Age 55 IV | |
|---|---|---|---|---|
| AJCC/UICC 45 I | 1220 | 0 | 0 | 0 |
| AJCC/UICC 45 II | 66 | 69 | 0 | 0 |
| AJCC/UICC 45 III | 90 | 0 | 144 | 0 |
| AJCC/UICC 45 IV | 51 | 6 | 0 | 150 |
AJCC American Joint Committee on Cancer, UICC Union for International Cancer Control
The 10-year DSS by new stage was 99.2, 98, 100, and 74 % for stages I–IV, respectively. The Kaplan–Meier graph of DSS by new stage is shown in Fig. 1b.
In total, no patients were upstaged and 213 patients (12 %) were downstaged. Of these 213 patients, 207 (97 %) migrated to stage I disease, and the remaining 6 patients (3 %) migrated to stage II disease. When DSS was analyzed in those 213 patients who were downstaged, there were 3 deaths. The 10-year DSS of this group, by new stage, was 96 % and 83 % for stage I and II, respectively. Two deaths were in patients with pT3 disease who moved from stage III to stage I disease. One of these was a papillary carcinoma tall cell variant (7.5 cm), and the other was a follicular carcinoma with areas of poor differentiation (5 cm). The remaining death was in a 52-year-old patient with T1 N1 M1 who had advanced nodal disease such that macroscopic clearance of disease in the neck was not achieved. In all patients, the mode of death was related to distant metastatic disease.
DISCUSSION
WDTC is a disease with an excellent prognosis10 and the vast majority of patients will not die of disease during prolonged follow-up. The AJCC/UICC model recognizes the prognostic importance of age on survival, with an age cutoff of 45 years.8 This number was selected as the median age of most major international datasets and has been adopted internationally. As with all cancers, the AJCC/UICC stratifies patients with WDTC stages I–IV, although, in contrast to other malignancies, the 10-year survival of even stage IV disease exceeds 80 % at 10 years.
The fact that <20 % of patients who are allocated a high disease stage (stage III or IV) will die of disease highlights the fact that many patients are overstaged using the current system. The impact of this overstaging on clinicians is to promote aggressive therapy for disease that is unlikely to be fatal. The impact on patients is to equate their disease with that of other patient groups with far more aggressive disease.
The current AJCC/UICC model, as applied to our cohort of 1807 patients, predicted a range of survivals between 100 and 81 % for stage I–IV. In total, 24 % of the cohort was classified as having disease of stage III or IV.
The aim of this study was to critically analyze age as a prognostic factor in WDTC within the structure of the AJCC/UICC staging system. A process of recursive partitioning was used to analyze factors prognostic of survival. The presence of distant metastases was found to be most predictive, followed by age. An age cutoff of 55 years was identified as most predictive of survival and was then applied using the current AJCC/UICC staging model.
The effects of this change were threefold. The spread of survival outcomes increased, with a 10-year survival of 99 % in stage I disease, falling to 74 % in stage IV disease. In addition, 12 % of patients were downstaged as a result of the change. Of the 213 patients affected by the change, there were only three deaths. In total, 8 % of our cohort migrated from an advanced stage (III/IV) to having stage I/ II disease. This constituted 33 % of those patients previously allocated a disease stage of III or IV. The vast majority of this group (96 %) migrated to having stage I disease, and had a 10-year DSS of 96 %. As patients with excellent prognosis migrate from stage IV group, those who remain have a more realistic prognosis, with a DSS of 74 % at 10 years (versus 81 % with the current age cutoff).
Our results are in keeping with those of other groups. Analysis of both national datasets and single-institution cohorts have confirmed that age 45 years may not be the most suitable cutoff for WDTC. Haymart used the surveillance, epidemiology, and end results (SEER) database to explore the relationship between age and outcome, confirming that patients over the age of 45 years have worse outcomes than younger patients.11 Using the SEER database, Oyer et al. confirmed that prognosis remains unaffected by age until 35 years.12 Bischoff et al. found, again within the SEER database, that survival deteriorated with age; however, no specific inflection point was seen at age 45 years, and survival for all patients under 65 years of age exceeded 90 %, raising the question of whether a cutoff at 45 years of age was appropriate.13 Jonklaas et al., through analysis of the National Thyroid Cancer Treatment Cooperative Study Group, confirmed the impact of gender under age 55 years, questioning whether an older cutoff than age 45 years would improve current staging systems.14 Ito et al. found that within a Japanese cohort of approximately 1000 patients, a change in survival was observed at 60 years.15 Mazurat et al. used multivariable hazard regression to show that DSS was not influenced by age until 55 years in a Canadian cohort of over 2000 patients with excellent follow-up.16
Our results again suggest that age 45 years is not the most statistically robust cutoff that can be used. The current staging system has developed from historical prognostic systems which used age 45 years as the median of the patient cohorts upon which they were based. It appears from the result of our work and others that the age cutoff should be raised. By raising the cutoff from 45 to 55 years of age in our cohort, the spread of outcomes experienced by stages I–IV is increased, while also appropriately downstaging a significant group of patients (12 %) who experienced excellent outcomes. If applied within the US, over 7000 patients would be affected annually.
This study has limitations due to the retrospective nature of its design. However, the need for large patient cohorts and long follow-up limit the feasibility of prospective studies in this field. Patients who were considered to have advanced-stage disease using the current AJCC/UICC staging system were likely to have been treated in a more aggressive manner, which could have affected the outcome for this group. The low number of disease-specific deaths also limits the conclusions we can draw. Although the use of national datasets, such as that provided by the SEER database, allow for large patient cohorts, they lack the level of accurate clinical data provided by single-institutional studies such as ours.17 In addition, the AJCC/UICC staging system is applied globally and in order to consider a change to such a system, data from patients across the world should be considered. Referral bias is a problem inherent to a single-institutional study such as this. Referral patterns to major US cancer centers may not be representative of international patient cohorts. To address these limitations, a multi-institutional, international dataset is being collected in order to validate the results shown by our analysis.
It is clear that no single cutoff point will be ideal as the concept that the biology of disease changes at a specific time point is inherently flawed. Other staging systems that consider not only disease burden but also response to therapy in a dynamic system have been suggested. However, the AJCC/UICC system is both simple to apply and widely accepted, and as such remains of international importance. Despite the fact that the use of age as a continuous variable may be more accurate than considering this as a categorical variable, the AJCC/UICC system remains the internationally accepted structure for staging. A transition away from the current model is likely over the coming years, but currently, within the constraints of the current staging system, a single age cutoff is required. Therefore, within this structure it is critical that the cutoff is set at the most appropriate point.
Although the current age cutoff of 45 years allows higher-risk patients to be correctly allocated to high-stage groups, it results in a significant number of low-risk patients being overstaged and overtreated. A change in the age cutoff in the AJCC/UICC staging for WDTC to 55 years would both improve the prognostic accuracy of the system and appropriately prevent low-risk patients from being overstaged and overtreated. Such a change should be considered in the next iteration of the AJCC/ UICC staging system.
Supplementary Material
Footnotes
DISCLOSURES Iain J. Nixon, Deborah Kuk, Volkert Wreesmann, Luc Morris, Frank L. Palmer, Ian Ganly, Snehal G. Patel, Bhuvanesh Singh, R. Michael Tuttle, Ashok R. Shaha, Mithat Gönen, and Jatin P. Shah have no disclosures to declare.
Electronic supplementary material The online version of this article (doi:10.1245/s10434-015-4762-2) contains supplementary material, which is available to authorized users.
References
- 1.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104(6):947–53. [PubMed] [Google Scholar]
- 2.Shah JP, Loree TR, Dharker D, Strong EW, Begg C, Vlamis V. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164(6):658–61. doi: 10.1016/s0002-9610(05)80729-9. [DOI] [PubMed] [Google Scholar]
- 3.Sherman SI, Brierley JD, Sperling M, et al. Prospective multi-center study of thyroiscarcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83(5):1012–21. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma. A study of the EORTC Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15(8):1033–41. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- 5.Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135(2):139–48. doi: 10.1016/s0039-6060(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 6.Randolph G. Surgery of the thyroid and parathyroid glands. 2. Philadelphia: Elsevier; 2013. [Google Scholar]
- 7.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1050–57. discussion 1057–1058. [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–74. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 9.Edge SB. AJCC cancer staging manual. 7. New York: Springer; 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 10.Nixon IJ, Ganly I, Patel SG, et al. Changing trends in well differentiated thyroid carcinoma over eight decades. Int J Surg. 2012;10(10):618–23. doi: 10.1016/j.ijsu.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Haymart MR. Understanding the relationship between age and thyroid cancer. Oncologist. 2009;14(3):216–21. doi: 10.1634/theoncologist.2008-0194. [DOI] [PubMed] [Google Scholar]
- 12.Oyer SL, Smith VA, Lentsch EJ. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg. 2012;147(2):221–26. doi: 10.1177/0194599812441587. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff LA, Curry J, Ahmed I, Pribitkin E, Miller JL. Is above age 45 appropriate for upstaging well-differentiated papillary thyroid cancer? Endocr Pract. 2013;19(6):995–97. doi: 10.4158/EP13029.OR. [DOI] [PubMed] [Google Scholar]
- 14.Urken ML, Mechanick JI, Sarlin J, Scherl S, Wenig BM. Pathologic reporting of lymph node metastases in differentiated thyroid cancer: a call to action for the College of American Pathologists. Endocr Pathol. 2014;25(3):214–8. doi: 10.1007/s12022-013-9282-7. [DOI] [PubMed] [Google Scholar]
- 15.Terris DJ, Snyder S, Carneiro-Pla D, et al. American thyroid association statement on outpatient thyroidectomy. Thyroid. 2013;23(10):1193–202. doi: 10.1089/thy.2013.0049. [DOI] [PubMed] [Google Scholar]
- 16.Urken ML, Milas M, Randolph GW, et al. Management of recurrent and persistent metastatic lymph nodes in well-differentiated thyroid cancer: a multifactorial decision-making guide for the Thyroid Cancer Care Collaborative. Head Neck. 2015;37(4):605–14. doi: 10.1002/hed.23615. [DOI] [PubMed] [Google Scholar]
- 17.Shah JP. Re: Extent of surgery affects papillary thyroid cancer. Ann Surg. 2008;247(6):1082–83. doi: 10.1097/SLA.0b013e3181758d93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


