Summary
The human central nervous system follows a pattern of development typical of all mammals, but certain neurodevelopmental features are highly derived. Building the human CNS requires the precise orchestration and coordination of myriad molecular and cellular processes across a staggering array of cell types and over a long period of time. Dysregulation of these processes affects the structure and function of the CNS and can lead to neurological or psychiatric disorders. Recent technological advances and increased focus on human neurodevelopment have enabled a more comprehensive characterization of the human CNS and its development in both health and disease. The aim of this review is to highlight recent advancements in our understanding of the molecular and cellular landscapes of the developing human CNS, with focus on the cerebral neocortex, and the insights these findings provide into human neural evolution, function, and dysfunction.
Keywords: brain development, developmental milestones, species differences, evolution, genomics, regulatory elements, transcription factor, lateralization, neurodevelopmental disorders
Introduction
The human central nervous system (CNS) exhibits the organizing principles and developmental pattern typical of all mammals; it begins as a simple neural tube that breaks off from the embryonic ectoderm and gradually acquires mature organizational features through immensely complex and strictly regulated molecular and cellular processes. Studies of model organisms have provided fundamental insights into many human neurodevelopmental processes (Bae et al., 2015; Leone et al., 2008; Lui et al., 2011; Molyneaux et al., 2007; Nord et al., 2015; O’Leary et al., 2007; Rakic et al., 2009; Rash and Grove, 2006; Shibata et al., 2015; Taverna et al., 2014). However, despite commonalities in neurodevelopmental processes in mammals, there are compelling interspecies differences that yield clade and species-specific (or defining) features and, ultimately, differences in cognition and behavior. For example, the human brain as a whole, but most especially the association areas of the cerebral neocortex, develops more slowly than the brains of other mammals, and humans have a particularly long gestational time as well as childhood and adolescence (Figure 1) (Bogin, 1994; Gogtay et al., 2004; Petanjek et al., 2011; Stiles and Jernigan, 2010; Tau and Peterson, 2010; Yakovlev and Lecours, 1967). This prolonged developmental course and period of dependency allows, more so than in other species, environmental factors to shape the development of cognitive, emotional, and social capacities. In addition, the developing human CNS possesses certain divergent and highly derived features, such as expanded proliferative zones and diverse subtypes of neural stem and progenitor cells with enhanced proliferative capacities that facilitate brain expansion, especially of the neocortex (Bae et al., 2015; Bystron et al., 2006; Dehay et al., 2015; Gulden and Sestan, 2014; Howard et al., 2008; Lui et al., 2011; Taverna et al., 2014).
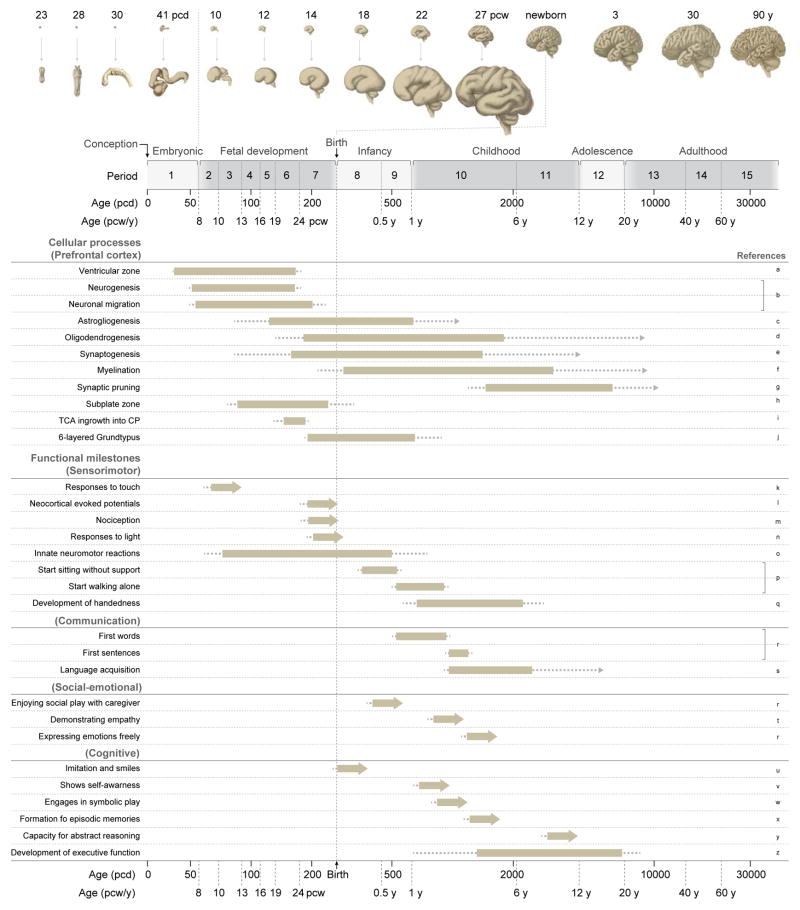
Figure 1. Timeline of Key Human Neurodevelopmental Processes and Functional Milestones.
The figure provides a summary of some key cellular processes in the developing prefrontal cortex and functional milestones. Illustrations in the top panel show the gross anatomical features of the developing and adult CNS, with prenatal brain features magnified. The second panel, which is duplicated at the bottom of the figure, provides a timeline of human development and the associated periods (designed by Kang et al., 2011), and age in postconceptional days (pcd), postconceptional weeks (pcw) and postnatal years (y). The schematic below details the approximate timing and sequence of key cellular processes and developmental milestones. Bars indicate the peak developmental period in which each feature is acquired; dotted lines indicate that feature acquisition occurs at these ages, though to a relatively minor degree; arrows indicate that the feature is present throughout life. Relevant references pertaining to each process or milestone are provided in the rightmost column: a, (Gould et al., 1990; Malik et al., 2013); b, (Bystron et al., 2006; Meyer, 2007; Workman et al., 2013); c, (Choi and Lapham, 1978; deAzevedo et al., 2003; Kang et al., 2011); d, (Kang et al., 2011; Yeung et al., 2014); e, (Huttenlocher, 1979; Kwan et al., 2012; Molliver et al., 1973; Petanjek et al., 2011); f, (Miller et al., 2012; Yakovlev and Lecours, 1967); g, (Huttenlocher, 1979; Petanjek et al., 2011); h, (Kostovic and Rakic, 1990), i, (Kwan et al., 2012; Kwan et al., 2008); j, (Aldama, 1930; Brodmann, 1909); k, (Humphrey and Hooker, 1959); l, (Eswaran et al., 2007); m, (Bellieni and Buonocore, 2012); n, (Polishuk et al., 1975); o, (Clowry, 2007; de Vries et al., 1985; Ianniruberto and Tajani, 1981; Johnson and Blasco, 1997; Van Dongen and Goudie, 1980); p, (W. H. O. Multicentre Growth Reference Study Group, 2006); q, (McManus et al., 1988; Ramsay, 1980); r, (Dosman et al., 2012; Gerber et al., 2010; Johnson and Newport, 1989); s, (Johnson and Newport, 1989); t, (Zahn-Waxler et al., 1992); u, (Meltzoff and Moore, 1977); v, (Amsterdam, 1972; Butterworth, 1990); w, (Harris, 2000); x, (Dumontheil, 2014); y, (Rajan et al., 2014); z, (Catts et al., 2013; Heaton et al., 1993).
Indicative of the biological challenge of precisely regulating diverse molecular and cellular processes over a protracted period of time and across myriad cell types and regions, the CNS exhibits regionally and temporally distinct patterns of vulnerability to various diseases and insults (Figure 2) (Kessler et al., 2007; Lee et al., 2014; Semple et al., 2013; Tebbenkamp et al., 2014). Thus, the emergence of the many neurodevelopmental processes that have enabled human brain complexity may have required a trade-off that rendered it particularly susceptible to certain disorders. Not surprisingly, it has become increasingly evident that studies involving commonly used experimental animals can neither fully model human neurodevelopment or disorders nor reliably predict if potential therapeutic compounds will work against human disease or have adverse effects. Moreover, the use of model organisms is limited by our evolutionary distance from these species. As such, null mutations of orthologous genes can result in vastly different phenotypes across species (Liao and Zhang, 2008). Therefore, a true understanding of how the human CNS is built and functions also requires direct analyses of human neural tissues and cells.
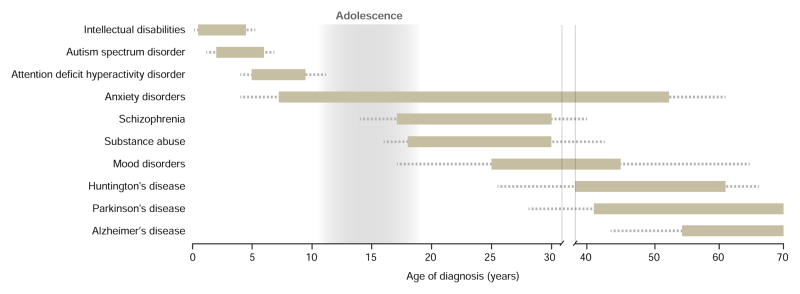
Figure 2. Psychiatric and Neurological disorders Have Discrete Ages of Onset.
The bars indicate the age range that each disorder commonly effects, with less frequent ages of diagnosis denoted as dotted lines. The light gray shading corresponds to adolescence. Note that the age of diagnosis is highly variable between brain disorders. Many psychiatric disorders emerge in adolescence and early adulthood. This variability is indicative of dysregulation of tightly controlled developmental processes and highlights the necessity of defining the spatiotemporal molecular and cellular processes in healthy and diseased human CNS. Based in part on data from (Kessler et al., 2007; Lee et al., 2014)
Unfortunately, the human CNS, in particular its development, is challenging to study for many reasons. However, the procurement of high-quality post-mortem developmental tissues, as well as recent advances in directed differentiation of induced human pluripotent stem (iPS) cells and other neural cell preparations (Brennand et al., 2011; Lancaster et al., 2013; Mariani et al., 2015; Pasca et al., 2015; Stein et al., 2014; van de Leemput et al., 2014; van den Ameele et al., 2014), coupled with the application of advanced histological, imaging, molecular, cellular, and genomic techniques, has provided new insights into dynamic cellular and molecular processes in human neurodevelopment and dysfunction in a wide array of neurological and psychiatric disorders (Brennand et al., 2011; Hu et al., 2014; Jeste and Geschwind, 2015; Lancaster et al., 2013; Mariani et al., 2015; Marin-Valencia et al., 2014; Stiles and Jernigan, 2010; Tau and Peterson, 2010; Tebbenkamp et al., 2014; Wen et al., 2014). Despite these advances, a more thorough understanding of the intricate processes underlying normal human CNS development is needed to answer many fundamental questions in biology and medicine and better recapitulate normal and abnormal human neurodevelopmental processes in model systems.
In this review, we will first briefly overview the basic principles of the organization and development of the human CNS, and highlight certain developmental features that differ from commonly studied mammals. Particular emphasis will be given to studies of the cerebral neocortex, also called the isocortex and neopallium, both because it has been the focus of many developmental and comparative studies and because of its importance in higher-order cognition, emotional regulation, and complex behaviors. We will next detail the current understanding of the transcriptional, epigenomic, and regulatory landscapes of the developing human neocortex and other regions of the CNS, noting the essential conserved features, as well as clade and species-specific differences, revealed by these studies. For a more comprehensive description of the state of knowledge on the evolution of the human CNS we recommend a number of recent reviews (Clowry et al., 2010; Dehay et al., 2015; Geschwind and Rakic, 2013; Lui et al., 2011; Marin-Padilla, 2014; Molnar and Pollen, 2014; Sherwood et al., 2012).
A Brief Overview of Human CNS Cellular Organization and Complexity
The human CNS is possibly the most complex biological tissue, comprising on average 86.1 billion neurons in the brain and spinal cord in males, along with a roughly equal number of glial cells (Herculano-Houzel, 2009; Herculano-Houzel et al., 2015). The neocortex alone contains approximately 16.34 billion neurons (Herculano-Houzel, 2009) and 164 trillion synapses, points of communication between neurons (Tang et al., 2001). In the entire adult CNS, there may be between several hundred trillion to well over a quadrillion synapses (see Box 1). Equally remarkable, the cerebral white matter of young adults contains approximately 149,000 to 176,000 kilometers of myelinated axons connecting these neurons (Marner et al., 2003). This immensely complex cellular organization comes at substantial metabolic cost, as the human brain uses 18% of the body’s oxygen at rest, but accounts for only 2.5% of a human’s total body weight (Kety and Schmidt, 1948).
Box 1. The Human CNS in Numbers. How Many Neurons and Synapses are there?
The human CNS contains approximately 86.1 billion neurons on average in 50 to 70 year-old males (Azevedo et al., 2009; Herculano-Houzel, 2015). There are approximately 16.34 billion neurons in the cerebral neocortex (Azevedo et al., 2009; Herculano-Houzel, 2009) and approximately 700 million additional neurons in the cerebral white matter when analyzed in young children (Sigaard et al., 2014). However, it should be noted that published estimates of the number of neocortical neurons varies by as much as a factor of 2 (between 14.7 to 32.0 billion neurons) (Pakkenberg and Gundersen, 1997; Pakkenberg et al., 2003). It was estimated that there are 164 trillion synapses in the adult human cerebral neocortex (Tang et al., 2001). The published estimates for the number of synapses an individual neocortical neuron receives also vary between approximately 7,200 (Pakkenberg et al., 2003; Tang et al., 2001) and 29,642 synapses (DeFelipe et al., 2002). The present authors did not find calculations for other human CNS regions. However, the average number of synapses per neuron certainly varies tremendously in other mammals and brain regions. In the rat brain, the number of synapses associated with a neuron ranges from an average of 2,186 for a calretinin-positive hippocampal interneuron (Gulyas et al., 1999), 31,700 for a hippocampal CA1 pyramidal neuron, to 175,000 for a cerebellar Purkinje neuron (Napper and Harvey, 1988). Thus, if we take the lower estimate for human neocortical neurons (i.e., 7,200) and assume that this establishes a lower boundary for a typical CNS neuron, there may be around 620 trillion synapses in the entire adult CNS. Remarkably, if we take the higher of these estimates for neocortical neurons (i.e., 29,642), then there may in fact be as many as 2.5 quadrillion synapses in the entire adult CNS.
Owing to its remarkable complexity, the human CNS takes over two decades to build via precisely regulated molecular and cellular processes governed both by a genetic blueprint and environmental factors. These processes are particularly dynamic during neurogenesis, when neurons are generated at staggering rates: approximately 3.86 million each hour during prenatal neocortical neurogenesis or 4.6 million each hour in the whole CNS over the course of developmental neurogenesis (Box 2). Moreover, synapses are formed at a rate of approximately 42.3 million synapses per minute in the late fetal and early postnatal neocortex (Box 2). Thus, it is not surprising that the relative energy and oxygen demands of the developing brain are markedly greater than that of the adult brain (Kennedy and Sokoloff, 1957). How the proper number of neuronal and glial cells is generated and how the myriads of distinct cell types are specified and assembled into complex neural circuitry during human development are among the greatest of scientific mysteries.
Box 2. The Human CNS in Numbers. What is the Rate of Neurogenesis and Synaptogenesis?
About 80% of the 16.34 billion neurons estimated to be present in the adult male cerebral cortex (Azevedo et al., 2009) are thought to be excitatory glutamatergic projection neurons (i.e., pyramidal and modified pyramidal neurons) (DeFelipe et al., 2002), which are produced from neural stem/progenitor cells within the VZ and SVZ of the developing cortical wall. The rest are GABAergic inhibitory interneurons, which recent studies have shown mostly originate from the ganglionic eminences of the ventral telencephalon (i.e., the subpallium) (Fertuzinhos et al., 2009; Hansen et al., 2013; Hansen et al., 2010; Ma et al., 2013). Thus, approximately 13 billion excitatory glutamatergic neurons must be generated in the VZ and SVZ of the neocortical wall within the approximately 141 days or 3,384 hours of neurogenesis in prenatal development (from 50–51 pcd [CS21 or the beginning of the 7th pcw] when immature neurons are first observed in the CP to 191 pcd [27 pcw], when neocortical excitatory neuron generation stops according to the translatingtime.org algorithm (Workman et al., 2013)). This suggests that approximately 3.86 million excitatory neurons are generated per hour during prenatal neocortical neurogenesis.
If we extend this analysis to the entire CNS, we would need to know when neurogenesis begins and ceases. CNS neurogenesis begins at approximately 32 pcd in the spinal cord and brain stem (Bayer and Altman, 2007). The vast majority of neurons in the human telencephalon are generated before birth and neocortical excitatory neuron generation ends around 27 pcw (Ernst et al., 2014; Larsen et al., 2006; Spalding et al., 2005). Substantial numbers of additional neocortical interneurons (Sanai et al., 2011), dentate gyrus neurons (Eriksson et al., 1998), and striatal interneurons (Ernst et al., 2014) are generated after this age, extending postnatally in some instances. On the other hand, 85% of the cerebellar granule cells are generated by the 11th postnatal month (Kiessling et al., 2014) and the external granule layer disappears by the end of the 18th postnatal month (Raaf and Kernohan, 1944). Thus, the bulk of CNS neurogenesis (i.e., approximately 86.4 billion neurons) occurs over approximately 781 days from 32 pcd to 813 pcd (i.e., approximately 234 prenatal pcd plus 547 postnatal days [birth to the 18th postnatal month]), which would mean that approximately 4.6 million neurons are generated per hour during the CNS developmental neurogenic period.
Neocortical synaptogenesis starts as early as 18 pcw (Kwan et al., 2012) and reaches its maximum synaptic density (the peak of formation) in the middle frontal gyrus (prefrontal cortex) after 15 months postpartum, which is considerably later than in the primary auditory and visual cortices (Huttenlocher and Dabholkar, 1997). The maximum dendritic spine density (a proxy for synapse) on pyramidal neurons of the dorsolateral prefrontal cortex occurs around the 7th postnatal year (Petanjek et al., 2011), after which there is a general decline in the density of spines, indicative of synapse elimination. Thus, the bulk of synapses found in the adult neocortex (i.e., approximately 164 trillion synapses in total according to Tang et al., 2001) are generated across approximately 2,695 days (140 prenatal and 2,555 postnatal days) at the rate of approximately 42.3 million synapses per minute or 700,000 synapses per second. However, the rate is likely higher as the density and the total number of synapses in the neocortex of a toddler is much higher than in the adult (Huttenlocher, 1979; Petanjek et al., 2011).
A Brief Overview of Human CNS Development
The CNS is amongst the earliest organ systems of the human body to begin its development prenatally and amongst the last to complete it postnatally; development continues into the mid-20s or perhaps even the 30s in certain regions and neural circuits (Gogtay et al., 2004; Petanjek et al., 2011; Yakovlev and Lecours, 1967). The human CNS undergoes remarkably rapid growth that exceeds the rate of any other organ systems from 4 postconceptional weeks (pcw) to the 3rd postnatal year (Gogtay et al., 2004; Petanjek et al., 2011; Yakovlev and Lecours, 1967). After this period, the rate of growth slows and processes such as synaptic maturation/pruning and myelination predominate. CNS development is also characterized by the emergence and disappearance of transient cellular compartments, cell types, and synaptic circuits (Bystron et al., 2008; Hoerder-Suabedissen and Molnar, 2015; Kostovic and Judas, 2006; Lui et al., 2011; Taverna et al., 2014). In humans, these cellular changes are arguably best understood in the cerebral neocortex, and we will use it as an example to highlight general features of neurodevelopment, some of which are illustrated in Figure 1.
General features of prenatal and postnatal neurodevelopment
During pregnancy, the developing CNS undergoes rapid and dramatic changes in its architecture. The average length of prenatal development (i.e., the time from the date of conception to birth) is 38 weeks and is typically divided into two primary periods, embryonic and fetal, or three equal trimesters. The embryonic period comprises the first 56 days or 8 weeks of pregnancy and can be divided into 23 Carnegie stages (CS), based solely on changes in morphologic features (O’Rahilly and Muller, 2006; Yamada et al., 2010). During this time span, the organ anlagen, including the primordium of the CNS, arise. The embryonic phase is also when the vast majority of congenital abnormalities occur (O’Rahilly and Muller, 2006). Moreover, due to its prolonged and dynamic development, the CNS is also sensitive to damaging effects of genetic (i.e. somatic mutations), epigenetic, and environmental insults for a longer period of time than other organs.
The anatomy of the prenatal human CNS has recently been systematically illustrated by several groups (Bayer and Altman, 2007; O’Rahilly and Muller, 2006; Yamada et al., 2010). Thus, we will only briefly summarize the sequence and timing of some key human prenatal neurodevelopmental events here. Human neurulation happens quite early, as is the case in all mammals, with the induction of the neural plate within the ectoderm immediately after the formation of the notochord. This is followed by the emergence of the neural groove, which is formed by neural folds that arise along each side of the midline of the neural plate on approximately the 23rd postconceptional day (pcd) and corresponding to CS 8 (O’Rahilly and Muller, 2006). Shortly thereafter, the neural folds fuse to form the neural tube, beginning in the center of neural plate and then proceeding both rostrally and caudally. The neuropores at the rostral and caudal ends of the neural tube close to separate the ventricular system from the amniotic fluid by 29 (CS 11) and 30 pcd (CS 12), respectively.
Due to its unequal growth, the neural tube is patterned along the rostro-caudal dimension of the CNS (i.e., neuraxis) into three major vesicles of the future brain: forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon). The spinal cord forms in the caudal region (O’Rahilly and Muller, 2006; Yamada et al., 2010). The three primary brain vesicles are subdivided and grow rapidly as prenatal development proceeds. In addition, the neural tube is patterned along the dorsal-ventral axis to establish defined compartments of neural progenitor cells that generate specific types of neural cells.
The fetal period is mainly characterized by the growth, differentiation, and subregionalization of organs (organogenesis). With respect to the CNS, there is a nearly 40-fold increase in the weight of the brain during fetal development (O’Rahilly and Muller, 2006). Among the most prominent morphological changes is the massive growth of the cerebral hemispheres, which first appear around 33 pcd or CS 14 (O’Rahilly and Muller, 2006), and the appearance of sulci and gyri on the cerebral surface, around the middle of prenatal development.
By birth, the gross anatomy of the CNS is reminiscent of its adult appearance and the repertoire of neurons found in the adult neocortex has been largely established (see Box 1). However, neurogenesis continues in several other regions (most prominently in the cerebellum, see Box 2), and glial cells are rapidly generated throughout infancy and early childhood. Early postnatal development is also characterized by massive outgrowth of dendrites and axons, followed by synaptogenesis and myelination, predominately in the forebrain and cerebellum. Thus, a newborn’s brain weighs just 26.2% (males) to 26.8% (females) of what it will in late adolescence, when the brain is at its heaviest (Dekaban, 1978). During the first 3 postnatal years, the brain continues to grow at a remarkable rate, attaining approximately 81.3% (females) to 87.6% (males) of its greatest weight (Dekaban, 1978). Though less dramatic than they are prenatally and in early postnatal development, substantial structural changes and molecular reorganization of neural circuits continue through late childhood and adolescence, paralleling the emergence of higher-order cognition and complex behavior.
Neuronal generation and migration
Neurogenesis in the human CNS begins shortly after the fusion of neural folds and proceeds at different rates across the neuraxis. The first neurons to appear are motor neurons in the ventral horn of the cervical spinal cord and neurons of certain cranial nerve nuclei in the brainstem at CS13 (28–32 pcd or 4 pcw) (Bayer and Altman, 2007; O’Rahilly and Muller, 2006). Neurogenesis continues throughout embryonic and fetal development (most prominently in the neocortex and cerebellum) and extends postnatally in certain regions (see Box 2). The wall of the neural tube initially comprises a pseudostratified layer of neuroepithelial cells called the ventricular zone (VZ), which lines the central cavity. These cells serve as the stem or progenitor cells for all neurons and macroglia (i.e., astrocytes and oligodendrocytes) of the CNS. Initially, each neuroepithelial cell division gives rise to two progenitor daughter cells. This process of symmetric division exponentially expands the number of progenitor cells.
Beginning at approximately 50–51 pcd (CS21 or the beginning of the 7th pcw), neocortical VZ cells begin to generate the earliest neurons of the emerging cortical plate (CP; future layers 2–6) (Bayer and Altman, 2007; Bystron et al., 2006; Meyer, 2007; O’Rahilly and Muller, 2006). At this time, another neurogenic proliferative compartment called the subventricular zone (SVZ) appears above the VZ and enlarges dramatically over the course of early and mid-fetal development (Bystron et al., 2008; Dehay et al., 2015; Fietz et al., 2010; Fietz et al., 2012; Florio et al., 2015; Hansen et al., 2010; Johnson et al., 2015; Meyer, 2007; Smart et al., 2002). Together these two zones give rise to all excitatory projection neurons (also known as pyramidal neurons) within the telencephalon and subsequently glial cells. Early on in embryonic neurogenesis, neuroepithelial progenitor cells of the VZ transition into another form of neural stem/progenitor cell, called radial glia (RG), which extend a very long process to the pial surface of the expanding neocortical wall (Bystron et al., 2006; Howard et al., 2008; Lui et al., 2011; Taverna et al., 2014). RG cell bodies largely reside in the VZ (apical or inner RG [iRG] cells) and SVZ (basal or outer RG [oRG] cells), where they divide symmetrically or asymmetrically, giving rise to a daughter RG cell and either an intermediate progenitor cell (IPC, also known as a transit amplifying cell) or nascent neuron (Bae et al., 2015; Bystron et al., 2008; Dehay et al., 2015; Lui et al., 2011; Taverna et al., 2014). Comparative studies of oRG cells in mouse, ferret, macaque, and human brain indicate that the expansion of this population correlates with brain size (Dehay et al., 2015; Fietz et al., 2010; Lui et al., 2011; Reillo and Borrell, 2012). In mammals with a large brain, the oRG cell population further subdivides the SVZ into inner and outer zones, the former populated mostly by iRG cells and IPCs and the latter composed mostly of oRG cells. While the oSVZ is negligible in rodents, its volume in macaque and human far exceeds that of the iSVZ (Smart et al., 2002). Therefore, the expansion of the neuronal cell population in the human brain appears in part due to a vast increase in the number of oRG cells that gives rise to highly proliferative IPCs.
Much of the expansion of human brain size and complexity is also a result of a relatively protracted time course of neurogenesis. For example, neocortical neurogenesis is predicted (see translatingtime.org; (Workman et al., 2013)) to last 11 days (11 to 22 pcd) in mouse, 67 days (45 to 112 pcd) in rhesus macaque, and 143 days (48 to 191 pcd) in human. These predictions appear quite accurate, as the first neurons forming the CP are detected within the wall of cerebral hemispheres in the incipient insula at approximately 50–51 pcd (Bayer and Altman, 2007; Bystron et al., 2006; Marin-Padilla, 2014; Meyer, 2007; O’Rahilly and Muller, 2006).
The subsequent migration of newly generated neurons from the SVZ/VZ and their postmitotic differentiation leads to the formation of new, often transient, structures and rapid expansion of the CNS. As is the case throughout the CNS, different subgroups of neocortical neurons have origins and migration modes that vary by subtype. Similar to rodents and other commonly studied mammals, human neocortical excitatory glutamatergic projection neurons (also known as pyramidal neurons) are generated from progenitor cells of the dorsal pallium and migrate radially into the CP (Fietz et al., 2010; Hansen et al., 2010). In contrast, human GABAergic interneurons are largely generated ventrally from the ganglionic eminences in the basal ganglia primordia and migrate tangentially into the CP (Fertuzinhos et al., 2009; Hansen et al., 2013; Hansen et al., 2010; Ma et al., 2013; Radonjic et al., 2014). Unlike in the rodent, most stem cells in the human ganglionic eminences do not appear to be located in the epithelium, but rather comprise a more distributed population suggestive of a mechanism for expansion of the interneuron progenitor pool (Hansen et al., 2013).
Before the start of neurogenesis a diverse group of early born “pioneer” neurons arrive by tangential migration from outside the neocortical primordium and settle immediately above the VZ to form the early marginal zone (MZ), also called the primordial plexiform layer* or preplate (Bystron et al., 2008; Marin-Padilla, 2014). These early-born “pioneer” neurons comprise a diverse group of cell types, including reelin-expressing Cajal-Retzius cells, which are important for establishing early synaptic circuits and laminar organization of the incipient CP “predecessor cells” and other cell types (Bystron et al., 2006; Marin-Padilla, 2014; Meyer, 2007; Zecevic, 1998).
Subsequently, newly born projection neurons migrate radially to form the CP below the pial surface by splitting the preplate into a superficial MZ (i.e., future layer 1) and a deep future subplate (SP; pre-SP) zone (Bystron et al., 2006; Marin-Padilla, 2014; Meyer, 2007; Molliver et al., 1973; Zecevic, 1998). The SP zone expands dramatically during pregnancy to become the largest compartment of the human fetal neocortical wall. It is enriched in extracellular matrix and filled with postmigratory glutamatergic projection neurons and GABAergic interneurons, migrating neurons, glial cells, and many ingrowing axon terminals forming transient synapses (Al-Jaberi et al., 2015; Hevner, 2007; Hoerder-Suabedissen and Molnar, 2015; Honig et al., 1996; Kostovic and Judas, 2006; Kostovic and Rakic, 1990; Kwan et al., 2008; Molliver et al., 1973; Wang et al., 2010). At its peak around 31 pcw, the human SP zone has around 3.6 billion cells (Samuelsen et al., 2003), which is an order of magnitude more than the total number of cells in the brain of mouse, rat, and many other mammals (Herculano-Houzel, 2009). While the SP zone undergoes gradual dissolution during late fetal and early postnatal development, many SP neurons survive and remain embedded in the adult white matter as so-called interstitial neurons (Hoerder-Suabedissen and Molnar, 2015; Honig et al., 1996; Kostovic and Rakic, 1990).
Newly born projection neurons migrate to their final laminar position in the emerging CP by attaching to the apical process of RG cells, following a precise inside-first, outside-last gradient (Bystron et al., 2008; Leone et al., 2008; Marin-Padilla, 2014; Meyer, 2007; Molyneaux et al., 2007; Shibata et al., 2015). Upon arriving at the CP, neurons are instructed to stop migrating and continue to differentiate. Most differentiation processes, such as the extension and elaboration of dendrites and the formation of synaptic connections, takes place only after neurons have assumed their final position in the CP; a process that likely lasts into early adulthood in humans (Huttenlocher, 1979; Koenderink and Uylings, 1995; Koenderink et al., 1994; Petanjek et al., 2008; Petanjek et al., 2011).
Glial cell genesis and differentiation
The generation of glial cells generally follows neurogenesis, peaking around birth, and is also a protracted postnatal process in humans (Jakovcevski et al., 2009; Miller et al., 2012; O’Rourke et al., 1992; Roessmann and Gambetti, 1986; Yeung et al., 2014). Astrocytes and oligodendrocyte precursor cells are derived from RG cells beginning in midgestation (Howard et al., 2008; Jakovcevski et al., 2009). Oligodendrocytes are robustly generated and migrate extensively through the first two postnatal years, while myelination continues postnatally in most brain regions, stretching well into the third decade in some areas such as fronto-parieto-temporal association cortex (Jakovcevski et al., 2009; Miller et al., 2012). Myelination is developmentally protracted in humans compared to chimpanzees in which myelination is largely completed in mid-adolescence (Miller et al., 2012). Given the inhibitory effects that myelin has on synaptogenesis and plasticity, this extended postnatal period of myelination expands the capacity for learning activities, memory, and complex sensory perception. Moreover, it indicates that gene regulatory processes and axon-glial interactions that govern myelination in humans may differ from those in mouse and other species, highlighting the importance for cross-species validation.
Less is known about human astrocyte development. The earliest astrocytes are generated from the direct transformation of RG cells followed by subsequent rounds of proliferation (Bystron et al., 2008; Choi and Lapham, 1978; deAzevedo et al., 2003; Howard et al., 2008). Astrocytes with mature-like morphological characteristics are observed as early as 15 pcw (Choi and Lapham, 1978; Howard et al., 2008). The astroglial population is likely largely non-proliferative and fully differentiated by the end of the first postnatal year (Kang et al., 2011; Sanai et al., 2011), which coincides with the peak in synaptic density (Huttenlocher, 1979) and is consistent with its previously reported role in synaptic formation and elimination (Clarke and Barres, 2013).
Laminar organization, regional patterning and lateralization of the human neocortex
In all mammals, including humans, at least two types of spatial information must be encoded in nascent excitatory projection (pyramidal) neurons in the CP: 1) their position in the radial direction, corresponding to their laminar position (Leone et al., 2008; Molyneaux et al., 2007; Shibata et al., 2015) and 2) their position in the tangential plane, corresponding to their particular cortical region/area identity, which work in mouse shows is governed by graded expression of transcription factors during early embryonic development, followed by extrinsic signaling from thalamic axonal inputs (Chou et al., 2013; O’Leary et al., 2007; Rakic et al., 2009; Rash and Grove, 2006). The physical separation of layers and areas is functionally determined and maintained through distinct compositions of neuronal cell-types and unique sets of afferent and efferent synaptic connections. The laminar identity of pyramidal neurons reflects their birth-order, with first-born neurons occupying the deepest layers and later-born neurons present in more superficial layers. The most superficial pyramidal neurons (layers 2 to 3), which exclusively make intratelencephalic connections, have been proposed to be over-represented in humans and contribute to certain human-specific cognitive and motor abilities (Marin-Padilla, 2014).
The formation of the human neocortical areal map is topographically matched, though slightly structurally and functionally asymmetric, between the left and right hemispheres (Amunts et al., 2003; Dehaene-Lambertz and Spelke, 2015; Sun and Walsh, 2006). This asymmetry plays a crucial role in functional lateralization of certain cognitive and motor functions (e.g. language and handedness). Neocortical structural asymmetry is first observed during the late mid-fetal period (Chi et al., 1977; Kasprian et al., 2011) and becomes more prominent during early postnatal development as functional asymmetries become more apparent (Amunts et al., 2003; Dehaene-Lambertz and Spelke, 2015; Sun and Walsh, 2006). Furthermore, the right hemisphere appears to mature faster than the left during late fetal and early postnatal development (Taylor, 1969; Thatcher et al., 1987).
Neural circuit assembly, maturation, and developmental plasticity
Dendritic and axonal outgrowth followed by the formation of synapses and myelination of axons are key cellular features associated with the functional maturation of the CNS. At midgestation, immature neocortical neurons have extended axons and begun to elaborate dendrites, initiating a protracted period of axon outgrowth, dendritic arborization, and synaptogenesis that extends into early childhood. These processes vary substantially between layers, areas, and subtypes of human neocortical neurons (Huttenlocher, 1979; Huttenlocher and Dabholkar, 1997; Koenderink and Uylings, 1995; Koenderink et al., 1994; Petanjek et al., 2011).
Synaptogenesis begins at the transition between embryonic and early fetal development and follows a specific spatio-temporal sequence. The very first synapses are found in the cervical spinal cord at CS17 (14 mm crown-rump length; estimated around 44 pcd or the beginning of the 6th pcw) (Okado et al., 1979) and precedes precocious movements and the first signs of reflex activity (Figure 1). Within the neocortical wall, the first synapses appear in the preplate between the 4th and 5th pcw (Zecevic, 1998), followed by their bilaminar distribution in the MZ above and in the SP below the emerging CP (Molliver et al., 1973; Zecevic, 1998). The SP zone represents a major site of early neocortical synaptogenesis and starting around 10 pcw there is a dramatic increase in synaptogenesis within this zone, which serves a transient “waiting” compartment for ingrowing cortical afferent axons (Hoerder-Suabedissen and Molnar, 2015; Honig et al., 1996; Kostovic and Rakic, 1990; Molliver et al., 1973). The basic features of the apical and basal dendrites of pyramidal neurons start to appear around 15 pcw, before the thalamocortical axons invade the CP (Mrzljak et al., 1988). The earliest synapses within the CP are observed around 18 pcw, terminating on prospective layer 5 projection (pyramidal) neurons (Kwan et al., 2012). Dendritic spines typically start to appear on immature neocortical pyramidal neurons and interneurons much later, between the 24th and 27th pcw (Mrzljak et al., 1988), which is after the massive ingrowth of thalamocortical axons into the CP starting around 21 pcw (Kostovic and Rakic, 1990; Molliver et al., 1973). This massive ingrowth of afferent axons coincides with intensive dendritic differentiation and synaptogenesis on dendrites of the prospective layer 3 and 5 pyramidal neurons; both of these processes rapidly increase during later perinatal phases (Mrzljak et al., 1988).
As noted above, many of these prenatal synapses and neural circuits are thought to be transient and the bulk of synaptogenesis in the neocortex and does not occur until later in prenatal development and early postnatal development (Huttenlocher, 1979). The ingrowth of thalamocortical axons and the intracortical burst of dendritic arborization and synaptogenesis that occurs in the late mid-fetal and early late fetal periods also coincide with both the beginning of the transformation of the CP into a six-layered ontogenetic lamination (Brodmann, 1909) and the appearance of evoked potentials (Figure 1). Rapid synaptogenesis (i.e., overproduction) continues during the first two postnatal years, peaking between 3 months and the beginning of the second year, depending on brain region (Huttenlocher, 1979). Subsequently, refinement of synaptic connections and dendritic pruning, mediated by the convergence of influences from intrinsic and extrinsic factors, seems a major task of the early postnatal brain, extending largely from the peak of synaptogenesis during the first few years (depending on the brain region) through the third decade in regions such as the prefrontal cortex (Huttenlocher and Dabholkar, 1997; Petanjek et al., 2011). This reorganization of synaptic circuits is thought to be essential for the functional specialization of neocortical areas and brain regions. There are two opposing scenarios that may describe spatio-temporal patterns of the reorganization and maturation of neocortical neural circuits in primates: (1) synaptogenesis proceeds concurrently in all areas (Rakic et al., 1986); (2) synaptogenesis proceed heterochronically across areas (Huttenlocher and Dabholkar, 1997), with the association areas pruning synapses and myelinating axons (Yakovlev and Lecours, 1967) later than many other areas and thus retaining longer juvenile characteristics.
Remarkably, the size of pyramidal neurons and length of dendrites increases dramatically during the first postnatal year, and continue, at a reduced rate, to around the 5th postnatal year (Koenderink and Uylings, 1995; Koenderink et al., 1994; Petanjek et al., 2008). Robust synaptic elimination and dendritic pruning are observed from early childhood through adolescence. Differential maturation of brain regions and neocortical areas helps explain many of the cognitive and behavioral changes seen in children, teens, and young adults. Not surprisingly, alterations in synaptogenesis, progression of myelination, and tractography over the same ages have been noted in certain neurological and psychiatric disorders (Lee et al., 2014; Stiles and Jernigan, 2010; Tau and Peterson, 2010), highlighting the importance of the appropriate regulation of these prolonged human neurodevelopmental processes.
These characterizations of human neurodevelopment represent a great effort to describe the cellular, physiological, and anatomical trajectories of the developing human brain, which extends deep into the 19th century. Only recently, however, have advancements in functional genomic techniques enabled comprehensive and unbiased characterizations of the molecular processes in the human postmortem developmental CNS tissues and neural cell culture systems. The remainder of this review will focus on our understanding of the spatio-temporal landscape of the RNA species (i.e., transcriptome), epigenetic features [i.e., “the epigenome”; see also an alternative view by Mark Ptashne (Ptashne, 2013)] and DNA regulatory elements (i.e., regulome) active in the developing human neocortex, and other brain structures, as well as insights into human developmental neurobiology provided by these studies.
The Transcriptional Landscape of the Developing Human CNS
The molecular and cellular processes discussed above are encoded in the immensely complex genome (see Box 3). Transcription is the first step in transferring genetic information into specific phenotypes and establishing unique molecular and, subsequently, cellular properties. The development of high-throughput microarray and sequencing technologies have greatly advanced our ability to explore the transcriptome in the developing human CNS. Some of the relevant resources on the developmental transcriptome of the human CNS include: www.humanbraintranscriptome.org, www.braincloud.jhmi.edu, and www.brainspan.org.
Box 3. Organization of the Human Nuclear Genome.
The diploid human nuclear genome of approximately 6.2 (in males) or 6.4 (in females) billion base pairs distributed across 46 chromosomes provides the blueprint for building the body and its functions. Current GENCODE estimates (/www.gencodegenes.org/stats/current.html; version 23) predict 60,498 genes. Of these, 19,881 are protein-coding genes, 25,813 are non-coding RNA (long and short) genes and the remaining are pseudogenes. A significant part of the remaining genome is believed to be cis-regulatory elements (CREs), the DNA sequences that regulate transcription. According to some estimates, the human nuclear genome harbors approximately 400,000 putative enhancers and 70,000 promoters (Encode Project Consortium et al., 2012). This highlights both the complexity of the human genome and the challenges in elucidating the molecular mechanisms underlying complex processes like neurodevelopment.
Global spatio-temporal dynamics of the human brain transcriptome
Recent genome-wide profiling studies of the developmental transcriptome of the human brain by several groups (Colantuoni et al., 2011; Ip et al., 2010; Jaffe et al., 2015; Johnson et al., 2009; Kang et al., 2011; Lambert et al., 2011; Mazin et al., 2013; Miller et al., 2014; Pletikos et al., 2014; Somel et al., 2009; Somel et al., 2011) have generated comprehensive datasets on coding and non-coding RNAs across multiple time points and brain regions. Moreover, these initial analyses have shed new light on the transcriptional architecture of brain regions and neocortical areas, neurodevelopmental processes, and the underlying biology of complex neurodevelopmental disorders. This work has revealed that the great majority of protein coding genes (at least 86% according to Kang et al., 2011) and an ever-expanding number of non-coding genes (see Box 3) are used at some point in the building of the human CNS. Of these, nine out of ten genes were differentially expressed or spliced across brain regions and/or time (Kang et al., 2011; Mazin et al., 2013). Such differences in the transcriptional architecture of brain regions and neocortical areas reflect their ontologies, different cellular make-ups, biological processes, and developmental timing (Figure 3A, B). Another common finding of these studies is that transcriptomes differ more prominently across time and space than they do between sexes, ethnicities or individuals, despite the underlying genetic differences. In particular, they have revealed remarkable dynamicity of gene expression during prenatal and early postnatal development (periods 1–9), accounting for approximately 2/3 of the variance in global expression, while the expression changes over several decades of adulthood (periods 13–15) accounted for a very small fraction of the variance (Figure 3A). When compared with dorsal pallial structures (amygdala, hippocampus and neocortex), the cerebellum is the most transcriptionally distinctive region in the developing brain, followed by thalamus and striatum (Figure 3B) (Kang et al., 2011; Numata et al., 2012). The transcriptional differences between putative areas of the developing neocortex were less robust than those between neocortex and other analyzed brain regions. A majority of these spatially enriched genes were also temporally regulated, and some were transiently enriched during a specific time window, including protracted epochs of postnatal development - the late childhood and adolescence. These findings reveal that regional transcriptomes are developmentally regulated and reflect cellular and functional differences.
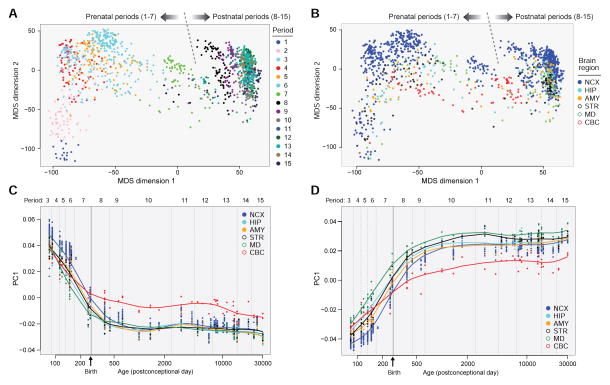
Figure 3. Global Spatio-Temporal Dynamics of the Human Brain Transcriptome.
(A) Multidimensional scaling (MDS) plot of global transcriptional differences across regions and time. The most pronounced differences (approximately two-thirds) occur during prenatal development (periods 1 to 7). By contrast, over four decades of adulthood (periods 13–15), less than 1% of genes are differentially expressed. Each dot represents a sample and is colored according to period as defined by Kang et al., 2011; the dotted line indicates birth.
(B) MDS plot in (A) colored by brain regions. The most prominent differences were between cerebellar cortex (CBC) and forebrain regions. Abbreviations: NCX: neocortex; HIP: hippocampus; AMY: amygdala; STR: striatum; MD: mediodorsal nucleus of thalamus.
(C) Weighted gene co-expression analysis identifies modules of co-expressed genes associated with distinct spatio-temporal expression patterns and biological processes in human brain development. Module M20 from Kang et al., 2011 shown here comprises genes that are downregulated simultaneously in different regions, with the exception of postnatal CBC, as the brain matures. M20 is enriched for genes encoding transcriptions factors involved in neurogenesis and pan-neuronal differentiation.
(D) Module M2 shown here comprises genes that are upregulated simultaneously, peaking first in childhood, in all regions, with the exception of postnatal CBC, as the brain matures. This module was enriched for genes associated with neuronal maturation processes like synaptic transmission, ion-transport, and calcium signaling. C and D were adapted with permission from Kang et al., 2011.
Transcriptional studies have also demonstrated correlations between gene expression dynamics and the morphological and functional development of brain regions, and consequently shed light on the timing of developmental processes and the onset of specific biological functions. For example, gene co-expression network analyses have revealed that the developmental brain transcriptome can be segregated into distinct modules or clusters of genes with highly correlated expression. The expression trajectories of the modules, which can be summarized using the module eigengene or an intramodular hub gene, often exhibit dynamic expression patterns across development, consistent with their roles in distinct biological processes (Johnson et al., 2009; Kang et al., 2011; Miller et al., 2014; Parikshak et al., 2013; Willsey et al., 2013). In Kang et al., 2011, the expression trajectories of the two largest modules comprising genes that are co-regulated across brain regions and time, indicate that many transcriptional processes are shared and coordinated across different regions as the brain develops (Figures 3C and D). In particular, the module enriched for genes associated with neuronal specification is most highly expressed embryonically and early fetally (Figure 3C), while the module enriched for genes associated with synaptic function and ion channels begins to rise late fetally and plateaus in early childhood (Figure 3D). In addition, many modules were very dynamic and distinct across spatial and temporal dimensions, and enriched for genes associated with distinct functions.
Recent studies of the developing postmortem human brains of individuals affected with autism spectrum disorder or fragile X syndrome have revealed distinct spatio-temporal patterns of transcriptional and post-transcriptional dysregulation (Kwan et al., 2012; Voineagu et al., 2011). Moreover, recent approaches have also successfully integrated the spatio-temporal dimensions of the human brain transcriptome with gene mutation discoveries to generate testable hypotheses about when and in which regions/cell types in the developing human brain the expression of disease-associated genes converge. In particular, these approaches have implicated the projection neurons in the prefrontal and primary motor-somatosensory cortex during mid-fetal development in autism spectrum disorder and the frontal cortex during fetal development in schizophrenia (Gulsuner et al., 2013; Kang et al., 2011; Lin et al., 2015; Parikshak et al., 2013; State and Sestan, 2012; Willsey et al., 2013). It is intriguing to speculate that selective dysfunction of spatially and temporally regulated gene expression may in part explain differences in the age of onset and affected neural circuits in neurological and psychiatric disorders (Figure 2). These observations further highlight the importance of deciphering the extent to which complex processes observed in the prolonged course of human development are recapitulated in model species.
Taken together, these recent transcriptomic analyses of human brain development across the spatial, temporal, and cellular dimensions have provided novel insights into normal neurodevelopmental processes and the interpretation of disease-associated mutations and underlying neuropathology.
Transcriptomic insights into human neural stem cell biology and evolution
In order to better understand the biology of and evolutionary changes in neural stem cell populations and the resultant expansion of certain progenitor compartments in brain evolution (e.g., oRG cell and the iSVZ) (see Figures 4A and B for schematic), recent studies have profiled differential gene expression in neocortical RG cells of humans and a few other species. In particular, by analyzing differential gene co-expression relationships between fetal human and mouse neocortial proliferative zones and cells, Lui and colleagues (Lui et al., 2014) revealed a number of genes that were enriched in either mouse or human RG cells (Figure 4C). By assessing genes expressed in human but not mouse RG cells, the study went on to determine that PDGFD encodes a platelet derived growth factor acting through the PDGFRB receptor, which is likewise enriched in human fetal RG cells, to regulate cell cycle progression and progenitor cell expansion in human but not mouse cortex.
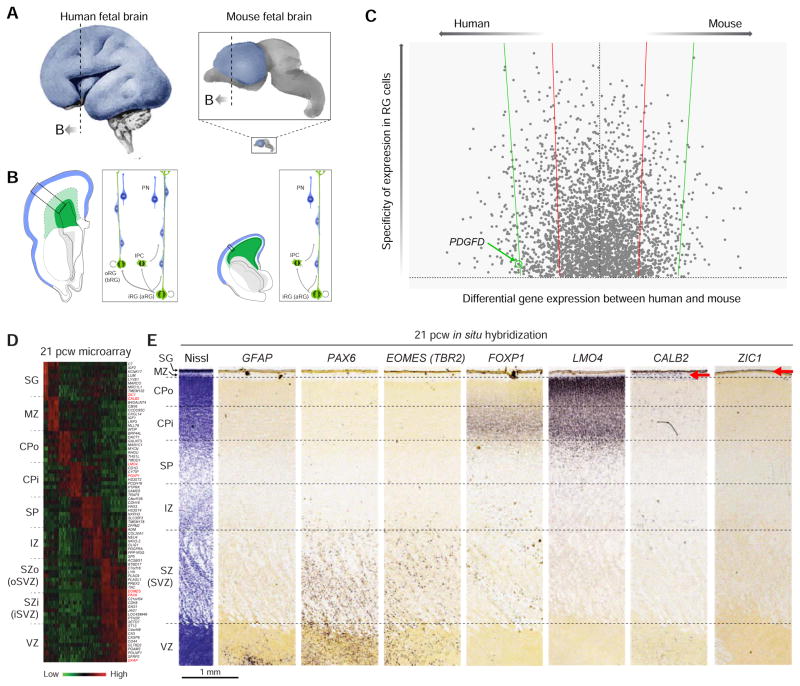
Figure 4. Divergent Organization and Transcritonal Profiles of Human and Mouse Neocortical Stem/Progentior Cells.
(A) Schematic representations of the mid-fetal human (left; 20 pcw) and mouse (right; 18.5 pcd) brain and neocortex (blue). The smaller image of the mouse brain indicates the approximate size difference between human and mouse brains and neocrtex (blue) at approximately equivalent age. The zoomed image of the mouse brain highlights differences in brain size and the position along the rostral caudal axis (dotted line) of the illustrations in (B). Adapted with permission from Gulden and Sestan, 2014.
(B) A schematic of the cellular composition of the human (left) and mouse (right) fetal forebrain wall detailing differences in neurogenic processes and stem/progenitor types between species. In mouse, apical or inner RG (aRG and iRG) cells (dark green) divide asymetrically to produce both a daughter intermediate progenitor cell (IPC) that subsequently divides symmetrically in the overlying SVZ (light green) and an excitatory projection neuron (PN) that migrates along RG fibers to the CP (blue). In human, the SVZ is greatly expanded and contains a large population of asymetrically dividing basal or outer RG (bRG and oRG) cells, which enables the production of greater numbers of neurons.
(C) A plot of the top 5,000 genes found to be enriched in RG cells by Lui and colleagues (Lui et al., 2014) are plotted. Ordinate values reflect the specificity of a gene within the neocortex to human RG cells while values along the abscissa represent expression differences between human and mouse RG. Red lines and green lines denote one- and two- standard deviations from the mean differential expression score. The vast majority of RG-enriched genes are similarly expressed in mouse and human. However, a considerable number of genes with high differential expression between species are observed, including a number of genes with high expression in human but not mouse RG cells. Among these genes, PDGFD was shown to increase the proliferative capacity of the neural progenitors. Adapted with permission from Figure 2d and Expanded Data Table 3 in Lui et al., 2014.
(D) A heatmap showing differential gene expression within discrete zones (layers) of the fetal neocortical wall at 21 pcw. These differences underlie the distinct cell types, cellular processes, and stages of maturation in each zone. Abbreviations: SG, subpial granular layer; MZ, marginal zone; CPo and CPi, outer and inner cortical plate; SP, subplate zone; IZ, intermediate zone; SZo and SZi, outer and inner subventricular zones; VZ, ventricular zone.
(E) A Nissl stain on the left delineates each fetal neocortical zone. Notable genes enriched in each are shown by in situ hybridization in the panels to the right, confirming findings of zone enriched expression identified by microarrays. The red arrows mark bands of enriched expression of calbindin2 (CALB2) and zic family member 1 (ZIC1) in the MZ and SG, respectively. Images in D and E were adapted with permission from Miller et al., 2014.
In a complementary approach, Florio and colleagues (Florio et al., 2015) used differential labeling of apical (iRG) and basal (oRG) RG cells to sort these two populations for RNA sequencing in mouse and human fetal neocortex. They found that global gene expression in mouse oRG cells was very similar to IPCs and immature neurons, while in human oRG cells it was highly similar to iRG cells. Of the genes that were preferentially expressed in human RG cells compared to iPCs and neurons, 56 lacked a mouse ortholog. Among these, the gene with the highest degree of iRG and oRG enrichment was the hominin-specific gene ARHGAP11B, which arose through a partial duplication of ARGHGAP11A after the human–chimpanzee split. Overexpression studies in mouse revealed that ARHGAP11B, but not ARHGAP11A, promoted basal progenitor cell proliferation and iRG delamination, ultimately leading to an increase in neuron numbers and ectopic cortical folding.
On the other hand, Johnson and colleagues (Johnson et al., 2015) employed a single cell sequencing approach to further profile the similarities and differences of the RG population in human, ferret, and mouse. They found that most “classical” RG markers were commonly expressed, confirming that oRG cells do indeed retain a definitive RG identity. oRG cells were enriched in targets of the neurogenin-family, which are proneural genes that are highly expressed in mouse iPCs but not mouse RG cells. In ferret, neurogenin2 (Neurog2) was found to be expressed in oRG cells, suggesting that this is a shared property of large gyrencephalic brains. Consistent with that interpretation, overexpression of human NEUROG2 in the developing ferret cortex promoted delamination of apical progenitors and an expansion of the oRG cell population with a concomitant increase in the rate of neurogenesis.
Together, these studies demonstrate that differential gene expression, including the emergence of hominin-specific genes, operated in evolution to promote increased diversity and complexity of neural stem/progenitor cells in species with large, and often gyrencephalic neocortices. One such example is the subdivision of SVZ in primates, and in other mammals with a large brain (Fietz et al., 2010; Hansen et al., 2010; Lui et al., 2011; Smart et al., 2002) to establish the expanded proliferative zone in the oSVZ that generates a greater number of neurons and glial cells. Moreover, oRG cells in the oSVZ exhibit distinct gene expression patterns compared to iRG cells, indicating that these cells are not merely “displaced” RG cells, but rather a related but distinct cell type with divergent properties.
Transcriptomic insights into laminar and regional patterning of neocortical neural circuits
Recently, in depth transcriptome analyses of the human prenatal neocortical wall have been carried out and have identified transcriptional signatures of different transient zones and the cell types within them, revealing both conserved patterns and species differences (Figures 4D and E) (Fietz et al., 2012; Ip et al., 2010; Johnson et al., 2009; Miller et al., 2014; Pletikos et al., 2014; Pollen et al., 2014). For instance, many of the genes previously implicated in the generation and specification of distinct subtypes of cortical projection neurons (Leone et al., 2008; Molyneaux et al., 2007; Shibata et al., 2015) and interneurons (Southwell et al., 2014) have well conserved expression gradients and domains among neural stem/progenitor cells in the VZ/SVZ or neurons in the SP and CP (Bayatti et al., 2008; Hansen et al., 2013; Hevner, 2007; Kwan et al., 2008; Ma et al., 2013; Radonjic et al., 2014), indicating that human neocortical patterning employs many of the same principles observed across mammalian species. This suggests a degree of homology. However, some of the same studies have also identified differences between humans, non-human primates and rodents in the expression of certain genes previously implicated in regional patterning, highlighting the role of species differences in the early patterning of the neocortex. For instance, species comparisons of the human and mouse SP zone transcriptomes have revealed many genes with enriched expression in the SP zone of both species, as well as genes that were enriched in human but not mouse (Hoerder-Suabedissen and Molnar, 2015; Miller et al., 2014). Given the essential role of the SP zone in establishing early neural circuits, it will be interesting to determine if human-enriched SP zone genes are important for establishing derived features of human early neocortical connectivity.
While the neocortex has a highly specialized laminar organization, its organization into functionally discrete regions and areas along the tangential dimension is arguably far more complex, especially in humans. Moreover, the molecular processes underlying the regional/areal parcellation of the neocortical CP are poorly understood, particularly in humans. Recent transcriptome studies have identified substantial regional/areal differences in the developing human neocortex (Johnson et al., 2009; Kang et al., 2011; Lambert et al., 2011; Miller et al., 2014; Pletikos et al., 2014). These works have revealed that each region/area has a unique temporally specified transcriptional profile, likely reflecting underlying biological processes involved in the patterning and differentiation of neural circuits in that region/area (Figures 5B and C). Interestingly, these regional/areal transcriptional profiles were largely bilaterally symmetrical across the two hemispheres at the population-level during fetal, early postnatal development and in adulthood (Johnson et al., 2009; Kang et al., 2011; Lambert et al., 2011; Miller et al., 2014; Pletikos et al., 2014). These findings suggest that transcriptional neocortical left-right asymmetry is either present at a level undetectable by using existing tissue-level transcriptomic methods and tissue samples or that other non-transcriptional mechanisms, such as neural activity, may play a critical role.
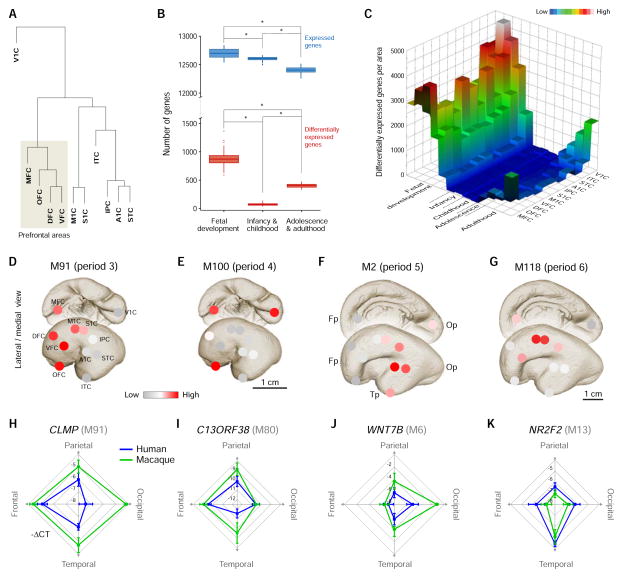
Figure 5. Transcriptional Differences Among Human Neocortical Areas are Temporally Regulated.
(A) Unsupervised hierarchical clustering of the 11 neocortical regions/areas profiled by Pletikos et al., 2014, based on the transcriptome of each area from the period of fetal development throughout adulthood, showing relative transcriptional differences. Abbreviations: OFC, orbital prefrontal cortex; DFC, dorsal prefrontal cortex; VFC, ventral prefrontal cortex; MFC, medial prefrontal cortex; M1C, primary motor cortex; S1C, primary somatosensory cortex; IPC, posterior inferior parietal cortex; A1C, primary auditory cortex; STC, superior temporal cortex; ITC, inferior temporal cortex; V1C, primary visual cortex.
(B) Boxplots of subsampling permutations show the number of expressed (blue) and differentially expressed (red) genes among neocortical areas across fetal development (periods 3–7), infancy (periods 8 and 9), childhood (periods 10 and 11), adolescence (period 12), and adulthood (periods 13–15). Note that the total number of genes expressed decreases over development. However, the number of differentially expressed genes observed over development exhibits a temporal hourglass pattern with the highest number in fetal development, a marked decline in infancy and childhood, and an increase in adolescence through adulthood.
(C) A 3D heatmap showing the number (post hoc Tukey test) of genes with differential expression between any two neocortical areas, demonstrating that the hourglass pattern of inter-areal differential expression persists in all neocortical areas, but is most prominent in MFC and V1C.
(D–G) Examples of gene co-expression modules (M) with a temporally regulated gradient-like expression pattern in the fetal neocortex (see circles with colored scale overlying each area). Modules M91, M100, M2, and M118 show frontal (D), medial fronto-occipital (E), posterior perisylvian (F) and middle perisylvian (G) enriched expression, respectively. Fp, frontal pole; Tp, temporal pole; Op, occipital pole.
(H–L) Radar charts showing shared and divergent expression gradients in human (blue) versus Rhesus macaque (green) fetal neocortex of specific intramodular hub genes (CLMP [M91], C13ORF38 [M80], WNT7B [M6], and NR2F2 [M13]). A–K were adapted with permission from Pletikos, et al. 2014.
Robust inter-areal transcriptional differences were particularly prominent during early and mid-fetal development and included specific transcriptional signatures associated with prospective prefrontal and perisylvian areas (Johnson et al., 2009; Kang et al., 2011; Miller et al., 2014; Pletikos et al., 2014), which are involved in some of the most distinctly human aspects of cognition and behavior. In addition, prefrontal/frontal-enriched graded expression along the anteroposterior axis of the CP and gradients with enrichment in temporal, occipital, occipito-temporal, perisylvian, and ventromedial areas were observed (Figures 5D–K). These strong early and mid-fetal inter-areal transcriptional differences and gradients diminish during late fetal periods and postnatal development, especially during infancy and childhood, and increase again after adolescence (Figures 5B and C) (Pletikos et al., 2014). We hypothesize that the greater inter-areal differences observed during early and mid-fetal development may in part reflect the topographically dynamic transcriptional programs that are necessary to establish area specific cortical and subcortical connectivity patterns. By contrast, in late fetal periods and early postnatal development, perhaps more general molecular programs for glial development, synaptic formation and elimination, and dendritic remodeling predominate. Differences in adolescence may reflect the varied maturational trajectories between certain neocortical areas (e.g., associative vs. primary areas), which may be important for late cognitive and sociobehavioral development.
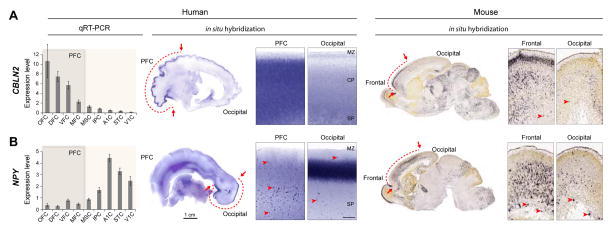
Interestingly, the fetal gene co-expression modules that showed prominent differential patterns among areas and gradient-like expression patterns (Figure 5D–G), particularly during fetal development, also displayed divergence between humans and rhesus macaques (Figures 5H–K) (Pletikos et al., 2014), suggesting a possible role for differences in neocortical topographic patterns of gene expression in the evolution of neural circuitry. Other recent studies have also reported genes and protein products with species-biased expression pattern in the developing human neocortex (Charrier et al., 2012; Florio et al., 2015; Han et al., 2013; Johnson et al., 2009; Johnson et al., 2015; Kang et al., 2011; Kwan et al., 2012; Lui et al., 2014; Miller et al., 2014; Pollard et al., 2006; Pollen et al., 2014). For instance, CBLN2, which is conserved in human and mouse, shows rostral enrichment. However, in situ hybridization data revealed species differences in laminar localization: in the fetal human frontal neocortex, CBLN2 is highly expressed throughout CP, while in mouse it is enriched selectively in the upper parts of CP (Figure 6A). Unlike CBLN2, NPY is expressed posteriorly in the occipital and parts of the temporal human CP compared to expression in anterior regions of mouse cortex (Figure 6B). Taken together, these findings indicate that, similar to other species the generation and differentiation of neocortical cell types and their assembly into functional neural circuits in humans is achieved through precise regulation of spatio-temporal gene expression involving conserved and divergent developmental programs.
Figure 6. Shared and Divergent Expression Patterns in the Fetal Human and Mouse Neocortex.
(A) CBLN2 is enriched throughout the CP of the mid-fetal human prefrontal cortex (PFC), whereas in mouse, at a comparable period of development, expression is enriched in the upper layers of frontal cortex.
(B) NPY has highly divergent expression along the rostral caudal axis in human versus mouse mid-fetal neocortex. In human, but not mouse, NPY is enriched in the mid-fetal occipito-temporal CP. The expression of NPY in sparsely distributed interneurons of the CP and SP zone is conserved. Human data were adapted with permission from Johnson et al., 2009. Mouse in situ hybridization images were obtained from the Allen Developing Mouse Brain Atlas (http://developingmouse.brain-map.org; (Thompson et al., 2014). Abbreviations are the same as in Figure 5.
The Regulatory and Epigenomic Landscapes of the Developing Human CNS
The spatio-temporal patterns of gene expression discussed above are achieved through complex regulatory processes involving several players that function in a combinatorial way (Nord et al., 2015; Shibata et al., 2015). But the tissue specificity, timing and level of a gene’s expression are greatly controlled by distal sequences like enhancers (sequences that increase expression), silencers (sequences that repress expression), and insulators (sequences that block interaction between enhancers and promoters), which along with core promoters are referred to as ‘cis-regulatory elements’ (CREs). CREs contain binding sites for multiple trans-acting regulatory proteins, primarily transcription factors. According to some estimates, the human genome harbors approximately 400,000 putative enhancers (Encode Project Consortium et al., 2012). Enhancer bound transcription factors along with mediator complex proteins loop DNA and bring enhancers and promoters in close proximity, leading to activation of gene expression. In addition to CREs and regulatory proteins, chromatin state or structure plays a critical role in the regulation of gene expression. Chromatin state is affected by epigenomic modifications like DNA methylation and post-translational modification of histone proteins and chromatin remodeling complexes. Here, we will give an overview of current understanding of transcriptional regulation in the developing human CNS.
Regulatory landscape of human neurodevelopment
Comparative genomic studies have shown that many of the genes encoding transcription factors and associated regulatory networks involved in the specification and differentiation of different subtypes of neurons and glia are highly conserved across mammals (Nord et al., 2015; Shibata et al., 2015). Moreover, studies indicate that nearly half of all pairwise regulatory interactions connecting mouse transcription factor genes have been maintained in orthologous human tissues, including brain (Ravasi et al., 2010; Stergachis et al., 2014). However, substantial turnover of CREs has been reported.
Evolutionary changes in CREs have been shown to directly underlie species differences, including in humans. For instance, in the case of Fezf2, a cortex specific enhancer, E4, is essential for corticospinal tract development mediated by SOX4 and SOX11 (Shim et al., 2012). The Sox binding sites in this enhancer emerged during tetrapod evolution and stabilized in mammals to establish a regulatory network that controls corticospinal tract specification and formation.
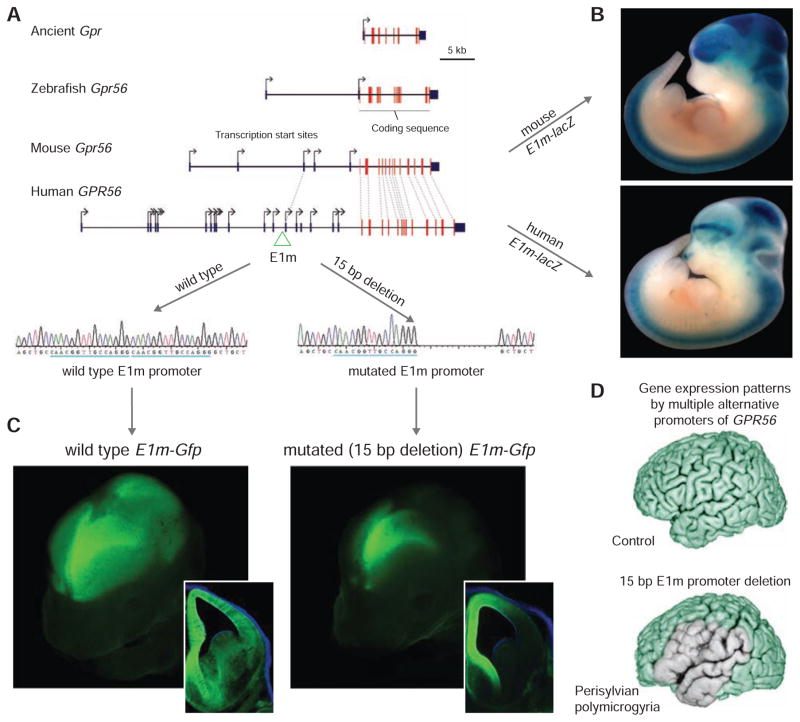
In addition to evolutionary changes in transcription factor binding sites, human-specific expansion, deletion, and de novo emergence of regulatory elements are also observed (Bae et al., 2014). For example, extensive variation in the cis-regulation of GPR56, a gene that promotes neural progenitor proliferation (Bae et al., 2014), is observed due to the expansion of the number of transcriptional start sites present in human GPR56 (17 start sites) as compared to its mouse ortholog Gpr56 (5 start sites; Figure 7A). The cis-element of one of these human alternative transcriptional start sites (E1m) can drive expression of a reporter gene in RG cells throughout the embryonic neocortex of a transgenic mouse (Figure 7B). Further experiments with sequences found in other mammals with a prominent sylvian fissure and gyrencephalic brain recapitulated this pattern of expression, suggesting that GPR56 was important for the evolutionary expansion of the perisylvian neocortex, a region involved in the processing of language and speech and other higher-order cognitive functions. Moreover, a 15 base pair deletion of the E1m cis-element caused perysylvian microgyria in humans and affected rostro-lateral GPR56 expression in mouse (Figures 7C and D). Taken together, these findings indicate that species differences in cis-regulatory elements are important for driving region and species-specific patterns of gene expression and contribute to the expansion of the neocortex.
Figure 7. Evolutionary Changes in a Cis-Regulatory Element Active in Neocortical Stem/Progenitor Cells.
(A) Schematic demonstrating expansion of transcriptional start sites over evolution in noncoding exon 1 of the Gpr56 gene (2 in zebra fish, 5 in mouse, and 17 in human), a gene that promotes proliferation of neural progenitor cells.
(B) A different expression pattern was observed after driving expression of lacZ (blue staining) with either the mouse or human variant of the promoter of one of the transcription start sites (E1m). The mouse element was able to recapitulate the full extent of mouse Gpr56 expression, but expression driven from the human element was restricted to a rostro-lateral band.
(C) Consistent with a role in driving rostra-lateral expression specifically, a 15 base pair deletion in the E1m element eliminated expression of GFP from the rostro-lateral forebrain in transgenic mice harboring an allele of Gfp driven by the E1m element.
(D) Deletion of this element was observed in patients with perisylvian polymicrogyria in accordance with a role in driving expression of GPR56 in the lateral neocortex (light green). In these patients, this mutation likely led to malformations of the perisylvian neocortex important for language among other functions. A–D were adapted with permission from (Bae et al., 2014).
Moving beyond the incremental advances provided by studies of individual CREs to a comprehensive understanding of gene regulation in the human brain requires genome-wide knowledge of the CREs that are active in human neurodevelopment. Comparative genomic methods have been applied by several groups (Lindblad-Toh et al., 2011; Margulies et al., 2007; Pennacchio et al., 2006; Pollard et al., 2006; Prabhakar et al., 2006; Siepel et al., 2005) to identify putative conserved CREs or regulatory RNAs, a disproportionate number of which are proposed to regulate human brain-expressed genes (Johnson et al., 2009; Pennacchio et al., 2006; Pollard et al., 2006). In addition to conserved sequences, many genomic regions that display accelerated sequence divergence in humans are predicted to be enhancers active during development, with a disproportionate number predicted to regulate human brain-expressed genes (Capra et al., 2013; Franchini and Pollard, 2015; Johnson et al., 2009; Lambert et al., 2011; Miller et al., 2014; Prabhakar et al., 2006).
In addition to genomic sequences, hallmark features of active CREs like chromatin accessibility, nucleosome depletion, or enrichment for specific post-translational histone modifications can also be assayed globally through techniques including DNAase hypersensitivity and chromatin immunoprecipitation followed by deep sequencing. Visel et al. (Visel et al., 2013) determined the genome-wide occupancy of the enhancer-associated proteins p300/CBP in human fetal neocortex to identify active enhancers. Enrichment of p300/CBP binding sites was observed near genes highly expressed in the fetal neocortex. By further characterizing a subset of highly conserved human enhancers that are active in the embryonic day 11.5 (e11.5) mouse telencephalon, Pattabiraman et al. (Pattabiraman et al., 2014) determined which progenitor domains generated cells that populated different subdivisions of the cerebral cortex and provided insights into the transcription networks that generate these subdivisions. Other groups have identified enhancers, on a genome-wide scale, in different brain regions and time points by mapping enrichment of histone 3 lysine 4 monomethylation (H3K4me1) and histone 3 lysine 27 acetylation (H3K27ac), characteristic marks of active enhancers (Reilly et al., 2015; Vermunt et al., 2014). Region specific enhancers along with co-regulated enhancers that form cell-type and context specific networks have also been identified. Comparative epigenetic profiling of promoters and enhancers present during human, rhesus macaque, and mouse corticogenesis has identified many regions that were gained in humans (Reilly et al., 2015). These regions were shown to be significantly enriched in modules of co-expressed genes that function in neuronal proliferation, migration, and cortical-map organization. Thus, the regulatory networks that govern gene expression in the developing human CNS are beginning to be elucidated; however, this information has yet to be fully placed into a functional context.
DNA methylation and histone modifications in human neurodevelopment
Epigenetic mechanisms, acting in concert with trans- and cis-components of the regulatory circuitry, play a critical role in regulating spatio-temporal expression patterns (Maze et al., 2014; Nord et al., 2015; Shibata et al., 2015). These mechanisms, including DNA methylation, histone modifications, and non-coding RNAs, can also be affected by various extrinsic factors, thus providing a molecular link between external cues and gene expression.
DNA methylation of cytosine, primarily at CpG nucleotides plays a key role in neural development and function and can be profiled at millions of CpGs in the human genome at single base resolution in an unbiased manner by applying sequencing technologies. Several studies have mapped global methylation patterns in human brain development using microarray platforms (Numata et al., 2012; Siegmund et al., 2007; Spiers et al., 2015). Global methylation in the human prefrontal cortex was observed to be age dependent, with fetal samples being the most distinct from early postnatal and adult samples (Numata et al., 2012), as DNA methylation levels changed rapidly during fetal development but slowed down after birth and with aging. Moreover, at loci that changed both in fetal and postnatal periods, demethylated states predominated during fetal periods with increasing methylation observed during the postnatal period.
DNA methylation patterns in the human prefrontal cortex during development were further studied by Lister et al. (Lister et al., 2013) by genome sequencing. Similar to array-based studies, they too observed widespread methylome reconfiguration during fetal to young adult development. Another key finding of their study is the accumulation of methylation in the non-CpG context (mCH) during early postnatal development (first two years postpartum) through adolescence, with a small decrease thereafter. Lister et al. noted that mCH accumulation during the early post-natal development period coincided with the primary phase of synaptogenesis. Further examination of the DNA methylation patterns in a cell type–specific manner found that mCH was more abundant than CpG methylation in adult neurons, but was at insignificant levels in glial cells. Accumulation of mCH and gene expression were negatively correlated; genes highly expressed in adult neurons lost both CpG and non-CpG methylation progressively during development.
Taken together these data indicate that dynamics in methylation of genomic DNA is an essential feature of gene regulation in human neurodevelopment. Moreover, it is intriguing to speculate that increased DNA methylation in infancy and childhood may be a mechanism underlying the reduction in interareal differences in gene expression and the increase in the expression of genes associated with synapse formation/regulation observed during these periods (Figures 3 and 6).
In addition to DNA methylation, histone modification is an essential mechanism for establishing cellular diversity and regulating the timing of developmental processes. Methylation (mono-, di- and tri-) and acetylation are the most extensively studied modifications, although many more have been observed (Tessarz and Kouzarides, 2014). Unfortunately, comprehensive ChIP-seq studies of histone modifications in human brain are limited due to the necessity for large amounts of tissue along with the variable quality of human post-mortem brain tissue. However, a few studies (Roadmap Epigenomics Consortium et al., 2015; Zhu et al., 2013) have generated chromatin state maps in a limited number of post-mortem human brains, which suggests that histone modification is indeed likely to be an important regulator of neuronal maturation in the human brain. Cheung et al. (Cheung et al., 2010) profiled histone 3 lysine 4 trimethylation (H3K4me3) across development in neuronal and non-neuronal cells of the human prefrontal cortex of 11 individuals ranging in age from 0.5 to 69 postnatal years. A key finding of their study was significant remodeling during postnatal development and aging of prefrontal neurons. Notably, H3K4me3 methylation of NEUROD1 and several members of the cadherin and semaphorin families was increased in infants compared to the oldest samples (greater than 60 years of age).
Non-coding RNAs in human neurodevelopment
Non-coding RNAs represent a large but poorly characterized component of the human transcriptome that play a critical role in transcriptional and post-transcriptional regulation to influence the overall transcriptional landscape and proteomic diversity (Morris and Mattick, 2014; O’Carroll and Schaefer, 2013)) (Box 3). They are broadly classified as short or long, based on their length. Short miRNAs bind their substrate mRNA and target it for degradation or inhibit its translation (O’Carroll and Schaefer, 2013). On the other hand, long non-coding RNAs (lncRNAs) and pseudogenes can act as regulators of transcription (Morris and Mattick, 2014), compete with endogenous RNAs, or act as microRNA sponges to post-transcriptionally regulate the levels and dynamics of protein-coding transcripts (Tay et al., 2014).
Recent studies have shown that a large number of all identified miRNAs and lncRNAs are expressed in the human and non-human primate brains and are spatially and temporally regulated (O’Carroll and Schaefer, 2013; Somel et al., 2010; Somel et al., 2011). For example, 31% of the miRNAs in the human prefrontal cortex were found to be affected by age and miRNA expression was negatively correlated with the expression of predicted target RNAs (Somel et al., 2010). miRNA mediated regulation was also implicated in the evolution of gene expression across the postnatal development of the prefrontal cortex and cerebellum in humans, chimpanzees, and macaques (Somel et al., 2011). Taken together, these data indicate that non-coding RNAs are an important regulatory mechanism in establishing the regional and temporal landscape of gene expression and contributed to human brain evolution.
Genome Mosaicism in Human Neurodevelopment
Recent advances in next generation sequencing and single cell genomics have enabled the detection of somatic genomic variations in specific cells and healthy and diseased human brains (Cai et al., 2015; Hu et al., 2014; Marin-Valencia et al., 2014). This work has broad ranging implications from uncovering new disease mechanisms to providing new insights into neurotypical development. For example, these studies have demonstrated that certain neurodevelopmental diseases are caused by de novo somatic mutations in neural progenitors that then expand clonally to produce aberrations in specific neural cell types and brain regions (Cai et al., 2015; Hu et al., 2014; Marin-Valencia et al., 2014).
Recent studies have also shown that somatic mutations and mobile DNA elements play a role in the generation of diversity and complexity in the developing human brain (Coufal et al., 2009). For example, mutations driven by L1 mediated retrotransposition, in which mobile elements relocate throughout the genome to inactivate genes and alter their expression profile, appear to occur in normal brain development. However, reports also indicate such mutations are rare in the human brain and the extent to which retrotransposition functionally contributes to aspects of normal human development remains to be determined (Cai et al., 2015).
Conclusions and Future Directions
The genomic revolution has left us in an unprecedented position, poised to decipher molecular and cellular process underlying neurodevelopment in humans and other not well studied mammals, such as non-human primates, more rapidly, precisely, and comprehensively than ever before. There is also an improved ability to detect genetic variations that confer risk for neurological and psychiatric disorders and an increased appreciation of the role of genomic mosaicism in development, evolution, and disease. However, a great deal of further work must be undertaken to know what makes us human: how our mind takes shape during development, why we suffer from certain diseases, how genetic and somatic variations cause diseases, and why certain therapeutic approaches developed using model systems do not work as effectively or lead to side effects in humans. Additional work is also needed to characterize the timing and sequence of cellular processes in human neurodevelopment, the sequence variants and gene expression differences observed in neurodevelopmental disorders, and the epigenetic and cis-regulatory elements that regulate the spatio-temporal dynamics of the transcriptome and proteomic diversity in both healthy and diseased individuals. Achieving these goals will require additional improvements in tissue procurement and processing to make tissue more accessible for advanced molecular and cellular techniques. Similar endeavors need to be applied for systematic comparisons with non-human primates, our closest relatives. Another major challenge before us is better understanding cellular heterogeneity and the properties of specific human cell types, which is now attainable through single-cell genomics and other techniques that allow for the isolation of discrete cell populations. Ultimately, advances in genomics and other disciplines must be leveraged to construct better experimental models of human neurodevelopment and disease.
Acknowledgments
We thank members of our laboratory for thoughtful discussions and comments on the manuscript. We apologize to all colleagues whose relevant studies were not cited because of space limitations, the broad scope of this review, and the emphasis on recent studies. Work in our laboratory was supported by the National Institutes of Health, the Foster-Davis Foundation (NARSAD), the Kavli Foundation, the James S. McDonnell Foundation, and the Simons Foundation.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Jaberi N, Lindsay S, Sarma S, Bayatti N, Clowry GJ. The early fetal development of human neocortical GABAergic interneurons. Cereb Cortex. 2015;25:631–645. doi: 10.1093/cercor/bht254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldama J. Cytoarchitektonik der Großhirnrinde eines 5 jährigen und eines 1 jährigen Kindes. Z Ges Neurol Psychiat. 1930;130:532–626. [Google Scholar]
- Amsterdam B. Mirror self-image reactions before age two. Dev Psychobiol. 1972;5:297–305. doi: 10.1002/dev.420050403. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Ditterich A, Zilles K. Broca’s region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Bae BI, Jayaraman D, Walsh CA. Genetic changes shaping the human brain. Dev Cell. 2015;32:423–434. doi: 10.1016/j.devcel.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae BI, Tietjen I, Atabay KD, Evrony GD, Johnson MB, Asare E, Wang PP, Murayama AY, Im K, Lisgo SN, et al. Evolutionarily dynamic alternative splicing of GPR56 regulates regional cerebral cortical patterning. Science. 2014;343:764–768. doi: 10.1126/science.1244392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayatti N, Sarma S, Shaw C, Eyre JA, Vouyiouklis DA, Lindsay S, Clowry GJ. Progressive loss of PAX6, TBR2, NEUROD and TBR1 mRNA gradients correlates with translocation of EMX2 to the cortical plate during human cortical development. Eur J Neurosci. 2008;28:1449–1456. doi: 10.1111/j.1460-9568.2008.06475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Atlas of Human Central Nervous System Development. 1–5. CRC Press LLC; 2007. [Google Scholar]
- Bellieni CV, Buonocore G. Is fetal pain a real evidence? J Matern Fetal Neonatal Med. 2012;25:1203–1208. doi: 10.3109/14767058.2011.632040. [DOI] [PubMed] [Google Scholar]
- Bogin B. Adolescence in evolutionary perspective. Acta Paediatr Suppl. 1994;406:29–36. doi: 10.1111/j.1651-2227.1994.tb13418.x. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Johann Ambrosius Barth Verlag; 1909. [Google Scholar]
- Butterworth G. Origins of self-perception in infancy. Chicago: University of Chicago Press; Harris, P. L; 1990. [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Bystron I, Rakic P, Molnar Z, Blakemore C. The first neurons of the human cerebral cortex. Nat Neurosci. 2006;9:880–886. doi: 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- Cai X, Evrony GD, Lehmann HS, Elhosary PC, Mehta BK, Poduri A, Walsh CA. Single-cell, genome-wide sequencing identifies clonal somatic copy-number variation in the human brain. Cell Rep. 2015;10:645. doi: 10.1016/j.celrep.2015.01.028. [DOI] [PubMed] [Google Scholar]
- Capra JA, Erwin GD, McKinsey G, Rubenstein JL, Pollard KS. Many human accelerated regions are developmental enhancers. Philos Trans R Soc Lond B Biol Sci. 2013;368:20130025. doi: 10.1098/rstb.2013.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, Fillman SG, Rothmond DA, Sinclair D, Tiwari Y, et al. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier C, Joshi K, Coutinho-Budd J, Kim JE, Lambert N, de Marchena J, Jin WL, Vanderhaeghen P, Ghosh A, Sassa T, et al. Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell. 2012;149:923–935. doi: 10.1016/j.cell.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung I, Shulha HP, Jiang Y, Matevossian A, Wang J, Weng Z, Akbarian S. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc Natl Acad Sci USA. 2010;107:8824–8829. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW. Radial glia in the human fetal cerebrum: a combined Golgi, immunofluorescent and electron microscopic study. Brain Res. 1978;148:295–311. doi: 10.1016/0006-8993(78)90721-7. [DOI] [PubMed] [Google Scholar]
- Chou SJ, Babot Z, Leingartner A, Studer M, Nakagawa Y, O’Leary DD. Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science. 2013;340:1239–1242. doi: 10.1126/science.1232806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowry G, Molnar Z, Rakic P. Renewed focus on the developing human neocortex. J Anat. 2010;217:276–288. doi: 10.1111/j.1469-7580.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowry GJ. The dependence of spinal cord development on corticospinal input and its significance in understanding and treating spastic cerebral palsy. Neurosci Biobehav Rev. 2007;31:1114–1124. doi: 10.1016/j.neubiorev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, Colantuoni EA, Elkahloun AG, Herman MM, Weinberger DR, et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478:519–523. doi: 10.1038/nature10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, Morell M, O’Shea KS, Moran JV, Gage FH. L1 retrotransposition in human neural progenitor cells. Nature. 2009;460:1127–1131. doi: 10.1038/nature08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries JI, Visser GH, Prechtl HF. The emergence of fetal behaviour. II Quantitative aspects. Early Hum Dev. 1985;12:99–120. doi: 10.1016/0378-3782(85)90174-4. [DOI] [PubMed] [Google Scholar]
- deAzevedo LC, Fallet C, Moura-Neto V, Daumas-Duport C, Hedin-Pereira C, Lent R. Cortical radial glial cells in human fetuses: depth-correlated transformation into astrocytes. J Neurobiol. 2003;55:288–298. doi: 10.1002/neu.10205. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J Neurocytol. 2002;31:299–316. doi: 10.1023/a:1024130211265. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Spelke ES. The Infancy of the Human Brain. Neuron. 2015;88:93–109. doi: 10.1016/j.neuron.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Dehay C, Kennedy H, Kosik KS. The Outer Subventricular Zone and Primate-Specific Cortical Complexification. Neuron. 2015;85:683–694. doi: 10.1016/j.neuron.2014.12.060. [DOI] [PubMed] [Google Scholar]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Dosman CF, Andrews D, Goulden KJ. Evidence-based milestone ages as a framework for developmental surveillance. Paediatr Child Health. 2012;17:561–568. doi: 10.1093/pch/17.10.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I. Development of abstract thinking during childhood and adolescence: the role of rostrolateral prefrontal cortex. Dev Cogn Neurosci. 2014;10:57–76. doi: 10.1016/j.dcn.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encode Project Consortium. Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Eswaran H, Haddad NI, Shihabuddin BS, Preissl H, Siegel ER, Murphy P, Lowery CL. Non-invasive detection and identification of brain activity patterns in the developing fetus. Clin Neurophysiol. 2007;118:1940–1946. doi: 10.1016/j.clinph.2007.05.072. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex. 2009;19:2196–2207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- Fietz SA, Lachmann R, Brandl H, Kircher M, Samusik N, Schroder R, Lakshmanaperumal N, Henry I, Vogt J, Riehn A, et al. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci USA. 2012;109:11836–11841. doi: 10.1073/pnas.1209647109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio M, Albert M, Taverna E, Namba T, Brandl H, Lewitus E, Haffner C, Sykes A, Wong FK, Peters J, et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science. 2015;347:1465–1470. doi: 10.1126/science.aaa1975. [DOI] [PubMed] [Google Scholar]
- Franchini LF, Pollard KS. Can a few non-coding mutations make a human brain? Bioessays. 2015;37:1054–1061. doi: 10.1002/bies.201500049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber RJ, Wilks T, Erdie-Lalena C. Developmental milestones: motor development. Pediatr Rev. 2010;31:267–276. doi: 10.1542/pir.31-7-267. quiz 277. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Howard S, Papadaki L. The development of ependyma in the human fetal brain: an immunohistological and electron microscopic study. Dev Brain Res. 1990;55:255–267. doi: 10.1016/0165-3806(90)90207-f. [DOI] [PubMed] [Google Scholar]
- Gulden FO, Sestan N. Neurobiology: building a bigger brain. Nature. 2014;515:206–207. doi: 10.1038/515206a. [DOI] [PubMed] [Google Scholar]
- Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, Rippey C, Shahin H, PS, et al. Consortium on the Genetics of, S., Group. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154:518–529. doi: 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Gennarino VA, Lee Y, Pang K, Hashimoto-Torii K, Choufani S, Raju CS, Oldham MC, Weksberg R, Rakic P, et al. Human-specific regulation of MeCP2 levels in fetal brains by microRNA miR-483-5p. Genes Dev. 2013;27:485–490. doi: 10.1101/gad.207456.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 2013;16:1576–1587. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Harris PL. The work of the imagination. Oxford; Malden, Mass: Blackwell Publishers; 2000. [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Kaas JH, de Oliveira-Souza R. Corticalization of motor control in humans is a consequence of brain scaling in primate evolution. J Comp Neurol. 2015 doi: 10.1002/cne.23792. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hevner RF. Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J Neuropathol Exp Neurol. 2007;66:101–109. doi: 10.1097/nen.0b013e3180301c06. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnar Z. Development, evolution and pathology of neocortical subplate neurons. Nat Rev Neurosci. 2015;16:133–146. doi: 10.1038/nrn3915. [DOI] [PubMed] [Google Scholar]
- Honig LS, Herrmann K, Shatz CJ. Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex. 1996;6:794–806. doi: 10.1093/cercor/6.6.794. [DOI] [PubMed] [Google Scholar]
- Howard BM, Zhicheng M, Filipovic R, Moore AR, Antic SD, Zecevic N. Radial glia cells in the developing human brain. Neuroscientist. 2008;14:459–473. doi: 10.1177/1073858407313512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WF, Chahrour MH, Walsh CA. The diverse genetic landscape of neurodevelopmental disorders. Annu Rev Genomics Hum Genet. 2014;15:195–213. doi: 10.1146/annurev-genom-090413-025600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T, Hooker D. Double simultaneous stimulation of human fetuses and the anatomical patterns underlying the reflexes elicited. J Comp Neurol. 1959;112:75–102. doi: 10.1002/cne.901120110. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ianniruberto A, Tajani E. Ultrasonographic study of fetal movements. Semin Perinatol. 1981;5:175–181. [PubMed] [Google Scholar]
- Ip BK, Wappler I, Peters H, Lindsay S, Clowry GJ, Bayatti N. Investigating gradients of gene expression involved in early human cortical development. J Anat. 2010;217:300–311. doi: 10.1111/j.1469-7580.2010.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AE, Shin J, Collado-Torres L, Leek JT, Tao R, Li C, Gao Y, Jia Y, Maher BJ, Hyde TM, et al. Developmental regulation of human cortex transcription and its clinical relevance at single base resolution. Nat Neurosci. 2015;18:154–161. doi: 10.1038/nn.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5. doi: 10.3389/neuro.05.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste SS, Geschwind DH. Developmental disorders. Curr Opin Neurol. 2015;28:89–90. doi: 10.1097/WCO.0000000000000188. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Blasco PA. Infant growth and development. Pediatr Rev. 1997;18:224–242. doi: 10.1542/pir.18-7-224. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cogn Psychol. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Wang PP, Atabay KD, Murphy EA, Doan RN, Hecht JL, Walsh CA. Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat Neurosci. 2015;18:637–646. doi: 10.1038/nn.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprian G, Langs G, Brugger PC, Bittner M, Weber M, Arantes M, Prayer D. The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cereb Cortex. 2011;21:1076–1083. doi: 10.1093/cercor/bhq179. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; normal values for cerebral blood flow and cerebral metabolic rate in childhood. J Clin Invest. 1957;36:1130–1137. doi: 10.1172/JCI103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psych. 2007;20:359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The Nitrous Oxide Method for the Quantitative Determination of Cerebral Blood Flow in Man: Theory, Procedure and Normal Values. J Clin Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling MC, Buttner A, Butti C, Muller-Starck J, Milz S, Hof PR, Frank HG, Schmitz C. Cerebellar granule cells are generated postnatally in humans. Brain Struct Funct. 2014;219:1271–1286. doi: 10.1007/s00429-013-0565-z. [DOI] [PubMed] [Google Scholar]
- Koenderink MJ, Uylings HB. Postnatal maturation of layer V pyramidal neurons in the human prefrontal cortex. A quantitative Golgi analysis. Brain Res. 1995;678:233–243. doi: 10.1016/0006-8993(95)00206-6. [DOI] [PubMed] [Google Scholar]
- Koenderink MJ, Uylings HB, Mrzljak L. Postnatal maturation of the layer III pyramidal neurons in the human prefrontal cortex: a quantitative Golgi analysis. Brain Res. 1994;653:173–182. doi: 10.1016/0006-8993(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M. Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol. 2006;48:388–393. doi: 10.1017/S0012162206000831. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Lam MM, Johnson MB, Dube U, Shim S, Rasin MR, Sousa AM, Fertuzinhos S, Chen JG, Arellano JI, et al. Species-dependent posttranscriptional regulation of NOS1 by FMRP in the developing cerebral cortex. Cell. 2012;149:899–911. doi: 10.1016/j.cell.2012.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Lam MM, Krsnik Z, Kawasawa YI, Lefebvre V, Sestan N. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci USA. 2008;105:16021–16026. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N, Lambot MA, Bilheu A, Albert V, Englert Y, Libert F, Noel JC, Sotiriou C, Holloway AK, Pollard KS, et al. Genes expressed in specific areas of the human fetal cerebral cortex display distinct patterns of evolution. PloS ONE. 2011;6:e17753. doi: 10.1371/journal.pone.0017753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CC, Bonde Larsen K, Bogdanovic N, Laursen H, Graem N, Samuelsen GB, Pakkenberg B. Total number of cells in the human newborn telencephalic wall. Neuroscience. 2006;139:999–1003. doi: 10.1016/j.neuroscience.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Sestan N, Weinberger DR, Casey BJ. Mental health. Adolescent mental health--opportunity and obligation. Science. 2014;346:547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao BY, Zhang J. Null mutations in human and mouse orthologs frequently result in different phenotypes. Proc Natl Acad Sci USA. 2008;105:6987–6992. doi: 10.1073/pnas.0800387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin GN, Corominas R, Lemmens I, Yang X, Tavernier J, Hill DE, Vidal M, Sebat J, Iakoucheva LM. Spatiotemporal 16p11.2 protein network implicates cortical late mid-fetal brain development and KCTD13-Cul3-RhoA pathway in psychiatric diseases. Neuron. 2015;85:742–754. doi: 10.1016/j.neuron.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad-Toh K, Garber M, Zuk O, Lin MF, Parker BJ, Washietl S, Kheradpour P, Ernst J, Jordan G, Mauceli E, et al. A high-resolution map of human evolutionary constraint using 29 mammals. Nature. 2011;478:476–482. doi: 10.1038/nature10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Nowakowski TJ, Pollen AA, Javaherian A, Kriegstein AR, Oldham MC. Radial glia require PDGFD-PDGFRbeta signalling in human but not mouse neocortex. Nature. 2014;515:264–268. doi: 10.1038/nature13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, et al. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16:1588–1597. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, Hu F, Zia MT, Hevner R, Zecevic N, Ballabh P. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33:411–423. doi: 10.1523/JNEUROSCI.4445-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies EH, Cooper GM, Asimenos G, Thomas DJ, Dewey CN, Siepel A, Birney E, Keefe D, Schwartz AS, Hou M, et al. Analyses of deep mammalian sequence alignments and constraint predictions for 1% of the human genome. Genome Res. 2007;17:760–774. doi: 10.1101/gr.6034307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell. 2015;162:375–390. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. The mammalian neocortex new pyramidal neuron: a new conception. Front Neuroanat. 2014;7:51. doi: 10.3389/fnana.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I, Guerrini R, Gleeson JG. Pathogenetic mechanisms of focal cortical dysplasia. Epilepsia. 2014;55:970–978. doi: 10.1111/epi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Maze I, Shen L, Zhang B, Garcia BA, Shao N, Mitchell A, Sun H, Akbarian S, Allis CD, Nestler EJ. Analytical tools and current challenges in the modern era of neuroepigenomics. Nat Neurosci. 2014;17:1476–1490. doi: 10.1038/nn.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazin P, Xiong J, Liu X, Yan Z, Zhang X, Li M, He L, Somel M, Yuan Y, Phoebe Chen YP, et al. Widespread splicing changes in human brain development and aging. Mol Syst Biol. 2013;9:633. doi: 10.1038/msb.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus IC, Sik G, Cole DR, Mellon AF, Wong J, Kloss J. The development of handedness in children. Br J Dev Psychol. 1988;6:257–273. [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:74–78. doi: 10.1126/science.897687. [DOI] [PubMed] [Google Scholar]
- Meyer G. Genetic control of neuronal migrations in human cortical development. Adv Anat Embryol Cell Biol. 2007;189:1–111. [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci USA. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Royall JJ, Aiona K, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver ME, Kostovic I, van der Loos H. The development of synapses in cerebral cortex of the human fetus. Brain Res. 1973;50:403–407. doi: 10.1016/0006-8993(73)90741-5. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Pollen A. How unique is the human neocortex? Development. 2014;141:11–16. doi: 10.1242/dev.101279. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271:355–386. doi: 10.1002/cne.902710306. [DOI] [PubMed] [Google Scholar]
- Napper RM, Harvey RJ. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J Comp Neurol. 1988;274:168–177. doi: 10.1002/cne.902740204. [DOI] [PubMed] [Google Scholar]
- Nord AS, Pattabiraman K, Visel A, Rubenstein JL. Genomic perspectives of transcriptional regulation in forebrain development. Neuron. 2015;85:27–47. doi: 10.1016/j.neuron.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata S, Ye T, Hyde TM, Guitart-Navarro X, Tao R, Wininger M, Colantuoni C, Weinberger DR, Kleinman JE, Lipska BK. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. 2012;90:260–272. doi: 10.1016/j.ajhg.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll D, Schaefer A. General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacology. 2013;38:39–54. doi: 10.1038/npp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Muller F. The Embryonic Human Brain: An Atlas of Developmental Stages. 3. New York: Wiley-Liss; 2006. [Google Scholar]
- O’Rourke NA, Dailey ME, Smith SJ, McConnell SK. Diverse migratory pathways in the developing cerebral cortex. Science. 1992;258:299–302. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- Okado N, Kakimi S, Kojima T. Synaptogenesis in the cervical cord of the human embryo: sequence of synapse formation in a spinal reflex pathway. J Comp Neurol. 1979;184:491–518. doi: 10.1002/cne.901840305. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, Horvath S, Geschwind DH. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O’Rourke NA, Nguyen KD, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman K, Golonzhka O, Lindtner S, Nord AS, Taher L, Hoch R, Silberberg SN, Zhang D, Chen B, Zeng H, et al. Transcriptional regulation of enhancers active in protodomains of the developing cerebral cortex. Neuron. 2014;82:989–1003. doi: 10.1016/j.neuron.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchio LA, Ahituv N, Moses AM, Prabhakar S, Nobrega MA, Shoukry M, Minovitsky S, Dubchak I, Holt A, Lewis KD, et al. In vivo enhancer analysis of human conserved non-coding sequences. Nature. 2006;444:499–502. doi: 10.1038/nature05295. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletikos M, Sousa AM, Sedmak G, Meyer KA, Zhu Y, Cheng F, Li M, Kawasawa YI, Sestan N. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron. 2014;81:321–332. doi: 10.1016/j.neuron.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishuk WZ, Laufer N, Sadovsky E. Fetal reaction to external light. Harefuah. 1975;89:395–396. [PubMed] [Google Scholar]
- Pollard KS, Salama SR, Lambert N, Lambot MA, Coppens S, Pedersen JS, Katzman S, King B, Onodera C, Siepel A, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–172. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]
- Pollen AA, Nowakowski TJ, Shuga J, Wang X, Leyrat AA, Lui JH, Li N, Szpankowski L, Fowler B, Chen P, et al. Low-coverage single-cell mRNA sequencing reveals cellular heterogeneity and activated signaling pathways in developing cerebral cortex. Nat Biotechnol. 2014;32:1053–1058. doi: 10.1038/nbt.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar S, Noonan JP, Paabo S, Rubin EM. Accelerated evolution of conserved noncoding sequences in humans. Science. 2006;314:786. doi: 10.1126/science.1130738. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Epigenetics: core misconcept. Proc Natl Acad Sci USA. 2013;110:7101–7103. doi: 10.1073/pnas.1305399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaf J, Kernohan JW. A study of the external granular layer in the cerebellum. The disappearance of the external granular layer and the growth of the molecular and internal granular layers in the cerebellum. Am J Anat. 1944;75:151–172. [Google Scholar]
- Radonjic NV, Ayoub AE, Memi F, Yu X, Maroof A, Jakovcevski I, Anderson SA, Rakic P, Zecevic N. Diversity of cortical interneurons in primates: the role of the dorsal proliferative niche. Cell Rep. 2014;9:2139–2151. doi: 10.1016/j.celrep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan V, Cuevas K, Bell MA. The Contribution of Executive Function to Source Memory Development in Early Childhood. J Cogn Dev. 2014;15:304–324. doi: 10.1080/15248372.2013.763809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Ramsay DS. Onset of unimanual handedness in infants. Infant Beh Dev. 1980;3:377–385. [Google Scholar]
- Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006;16:25–34. doi: 10.1016/j.conb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Ravasi T, Suzuki H, Cannistraci CV, Katayama S, Bajic VB, Tan K, Akalin A, Schmeier S, Kanamori-Katayama M, Bertin N, et al. An atlas of combinatorial transcriptional regulation in mouse and man. Cell. 2010;140:744–752. doi: 10.1016/j.cell.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reillo I, Borrell V. Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb Cortex. 2012;22:2039–2054. doi: 10.1093/cercor/bhr284. [DOI] [PubMed] [Google Scholar]
- Reilly SK, Yin J, Ayoub AE, Emera D, Leng J, Cotney J, Sarro R, Rakic P, Noonan JP. Evolutionary genomics. Evolutionary changes in promoter and enhancer activity during human corticogenesis. Science. 2015;347:1155–1159. doi: 10.1126/science.1260943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roadmap Epigenomics Consortium. Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessmann U, Gambetti P. Astrocytes in the developing human brain. An immunohistochemical study. Acta Neuropathol. 1986;70:308–313. doi: 10.1007/BF00686089. [DOI] [PubMed] [Google Scholar]
- Samuelsen GB, Larsen KB, Bogdanovic N, Laursen H, Graem N, Larsen JF, Pakkenberg B. The changing number of cells in the human fetal forebrain and its subdivisions: a stereological analysis. Cereb Cortex. 2003;13:115–122. doi: 10.1093/cercor/13.2.115. [DOI] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Bauernfeind AL, Bianchi S, Raghanti MA, Hof PR. Human brain evolution writ large and small. Prog Brain Res. 2012;195:237–254. doi: 10.1016/B978-0-444-53860-4.00011-8. [DOI] [PubMed] [Google Scholar]
- Shibata M, Gulden FO, Sestan N. From trans to cis: transcriptional regulatory networks in neocortical development. Trends Genet. 2015;31:77–87. doi: 10.1016/j.tig.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S, Kwan KY, Li M, Lefebvre V, Sestan N. Cis-regulatory control of corticospinal system development and evolution. Nature. 2012;486:74–79. doi: 10.1038/nature11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PloS ONE. 2007;2:e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, Clawson H, Spieth J, Hillier LW, Richards S, et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigaard RK, Kjaer M, Pakkenberg B. Development of the cell population in the brain white matter of young children. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu178. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somel M, Franz H, Yan Z, Lorenc A, Guo S, Giger T, Kelso J, Nickel B, Dannemann M, Bahn S, et al. Transcriptional neoteny in the human brain. Proc Natl Acad Sci USA. 2009;106:5743–5748. doi: 10.1073/pnas.0900544106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somel M, Guo S, Fu N, Yan Z, Hu HY, Xu Y, Yuan Y, Ning Z, Hu Y, Menzel C, et al. MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain. Genome Res. 2010;20:1207–1218. doi: 10.1101/gr.106849.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somel M, Liu X, Tang L, Yan Z, Hu H, Guo S, Jiang X, Zhang X, Xu G, Xie G, et al. MicroRNA-driven developmental remodeling in the brain distinguishes humans from other primates. PLoS Biol. 2011;9:e1001214. doi: 10.1371/journal.pbio.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Nicholas CR, Basbaum AI, Stryker MP, Kriegstein AR, Rubenstein JL, Alvarez-Buylla A. Interneurons from embryonic development to cell-based therapy. Science. 2014;344:1240622. doi: 10.1126/science.1240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Spiers H, Hannon E, Schalkwyk LC, Smith R, Wong CC, O’Donovan MC, Bray NJ, Mill J. Methylomic trajectories across human fetal brain development. Genome Res. 2015;25:338–352. doi: 10.1101/gr.180273.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State MW, Sestan N. Neuroscience. The emerging biology of autism spectrum disorders. Science. 2012;337:1301–1303. doi: 10.1126/science.1224989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, de la Torre-Ubieta L, Tian Y, Parikshak NN, Hernandez IA, Marchetto MC, Baker DK, Lu D, Hinman CR, Lowe JK, et al. A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron. 2014;83:69–86. doi: 10.1016/j.neuron.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergachis AB, Neph S, Sandstrom R, Haugen E, Reynolds AP, Zhang M, Byron R, Canfield T, Stelhing-Sun S, Lee K, et al. Conservation of trans-acting circuitry during mammalian regulatory evolution. Nature. 2014;515:365–370. doi: 10.1038/nature13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7:655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, De Groot DM, Gundersen HJ. Total regional and global number of synapses in the human brain neocortex. Synapse. 2001;41:258–273. doi: 10.1002/syn.1083. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E, Gotz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC. Differential rates of cerebral maturation between sexes and between hemispheres. Evidence from epilepsy. Lancet. 1969;2:140–142. doi: 10.1016/s0140-6736(69)92445-3. [DOI] [PubMed] [Google Scholar]
- Tebbenkamp AT, Willsey AJ, State MW, Sestan N. The developmental transcriptome of the human brain: implications for neurodevelopmental disorders. Curr Opin Neurol. 2014;27:149–156. doi: 10.1097/WCO.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 2014;15:703–708. doi: 10.1038/nrm3890. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Ng L, Menon V, Martinez S, Lee CK, Glattfelder K, Sunkin SM, Henry A, Lau C, Dang C, et al. A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron. 2014;83:309–323. doi: 10.1016/j.neuron.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Leemput J, Boles NC, Kiehl TR, Corneo B, Lederman P, Menon V, Lee C, Martinez RA, Levi BP, Thompson CL, et al. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68. doi: 10.1016/j.neuron.2014.05.013. [DOI] [PubMed] [Google Scholar]
- van den Ameele J, Tiberi L, Vanderhaeghen P, Espuny-Camacho I. Thinking out of the dish: what to learn about cortical development using pluripotent stem cells. Trends Neurosci. 2014;37:334–342. doi: 10.1016/j.tins.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Van Dongen LG, Goudie EG. Fetal movement patterns in the first trimester of pregnancy. Br J Obstet Gynaecol. 1980;87:191–193. doi: 10.1111/j.1471-0528.1980.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Vermunt MW, Reinink P, Korving J, de Bruijn E, Creyghton PM, Basak O, Geeven G, Toonen PW, Lansu N, Meunier C, et al. Large-scale identification of coregulated enhancer networks in the adult human brain. Cell Rep. 2014;9:767–779. doi: 10.1016/j.celrep.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Visel A, Taher L, Girgis H, May D, Golonzhka O, Hoch RV, McKinsey GL, Pattabiraman K, Silberberg SN, Blow MJ, et al. A high-resolution enhancer atlas of the developing telencephalon. Cell. 2013;152:895–908. doi: 10.1016/j.cell.2012.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W. H. O. Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Hoerder-Suabedissen A, Oeschger FM, Bayatti N, Ip BK, Lindsay S, Supramaniam V, Srinivasan L, Rutherford M, Mollgard K, et al. Subplate in the developing cortex of mouse and human. J Anat. 2010;217:368–380. doi: 10.1111/j.1469-7580.2010.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515:414–418. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, Reilly SK, Lin L, Fertuzinhos S, Miller JA, et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155:997–1007. doi: 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain (Regional development of the brain in early life) F.A. Davis Co; Philadelphia: 1967. [Google Scholar]
- Yamada S, Samtani RR, Lee ES, Lockett E, Uwabe C, Shiota K, Anderson SA, Lo CW. Developmental atlas of the early first trimester human embryo. Dev Dyn. 2010;239:1585–1595. doi: 10.1002/dvdy.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Radke-Yarrow M, Wagner E, Chapman M. Development of concern for others. Dev Psychol. 1992;28:126–136. [Google Scholar]
- Zecevic N. Synaptogenesis in layer I of the human cerebral cortex in the first half of gestation. Cereb Cortex. 1998;8:245–252. doi: 10.1093/cercor/8.3.245. [DOI] [PubMed] [Google Scholar]
- Zhu J, Adli M, Zou JY, Verstappen G, Coyne M, Zhang X, Durham T, Miri M, Deshpande V, De Jager PL, et al. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell. 2013;152:642–654. doi: 10.1016/j.cell.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]