Abstract
Purpose
to investigate the added value of qualitative and quantitative evaluation of diffusion weighted (DW) magnetic resonance (MR) imaging in response assessment after neoadjuvant chemo-radiotherapy (CRT) in patients with locally advanced rectal cancer (LARC).
Methods
31 patients with LARC (stage ≥ T3) were enrolled in the study. All patients underwent conventional MRI and DWI before starting therapy and after neoadjuvant CRT. All patients underwent surgery; pathologic staging represented the reference standard. For qualitative analysis, two radiologists retrospectively reviewed conventional MR images and the combined set of conventional and DW MR images and recorded their confidence level with respect to complete response (ypCR). For quantitative analysis, tumor’s apparent diffusion coefficient (ADC) values were measured at each examination. ADC pre-CRT, ADC post-CRT and Δ ADC post−ADC pre of the three groups of response (ypCR, partial response ypPR, stable disease ypSD) were compared. Receiver-operating characteristics (ROC) curve analysis was employed to investigate the discriminatory capability for ypCR, responders (ypCR, ypPR) and ypSD of each measure.
Results
addition of DWI to conventional T2-weighted sequences improved diagnostic performance of MRI in the evaluation of ypCR. A low tumor ADC value in the pre-CRT examination, a high ADC value in the post-CRT examination, a high Δ ADC post−ADC pre [>0.3 (×10−3 mm2/s)] were predictive of ypCR.
Conclusions
DW sequences improve MR capability to evaluate tumor response to CRT. Nevertheless, no functional MR technique alone seems accurate enough to safely select patients with ypCR.
Keywords: Chemoradiation, Magnetic resonance imaging, Diffusion-weighted imaging, Rectal cancer, Treatment response, Staging
1. Introduction
Rectal cancer is one of the most frequent neoplasias, with an incidence of 40 in 100,000 [1]. Over the last two decades its treatment has undergone many changes and innovation, thus more precise preoperative evaluation leaded to refined patient selection for appropriate treatment strategies. The tumor stage determines whether radiation and chemotherapy should be used in addition to surgery. In particular, T1-T2 N0 tumors are managed surgically, without neoadjuvant treatment whereas neoadjuvant chemo-radiotherapy (CRT) followed by total mesorectal excision (TME) surgery represents the standard treatment for locally advanced rectal carcinoma (≥T3; any T, N+) [2].
According to literature data 15–27% of the patients treated with CRT achieve a pathological complete response (ypCR), a partial response is seen in 54–75% and others show no response at all [3].
In view of these advances of CRT, alternative approaches to radical surgery have been proposed. Therefore, staging rectal cancer before and after CRT and assessing tumor response have become a very critical issue and imaging studies play a key role with relevant implications in patients’ management.
On one hand response assessment during CRT could possibly re-orientate non-responding patients to a different treatment modality (e.g. early surgery) or to treatment intensification (e.g. dose escalation or addition of targeted agents); on the other hand response assessment before surgery may enable physicians to offer patients who achieve a clinical complete response less extensive surgery, such as sphincter-saving local excision [4], or even a ‘wait-and-see’ policy [5], [6].
According to the European guidelines, magnetic resonance (MR) imaging is the most accurate technique for predicting tumor stage [5], [7]. Nevertheless, MRI, as a morphologic imaging modality, has inherent limitations in the differentiation of residual viable tumor from diffuse fibrotic change; therefore, conventional MRI sequences cannot be used to predict complete response to CRT [8], [9].
The reported overall accuracy of MRI in predicting the pathologic stage of nonirradiated rectal cancer is 71–91% (mean 85%) for T staging and 43–85% (75%) for N staging; on the other hand the reported overall accuracy of MRI in predicting the pathologic stage of irradiated rectal cancer is 47–54% (50%) for T staging and 64–68% (65%) for N staging [7], [10].
Since conventional MR sequences are insufficient for reliably assessing these critical issues there is considerable enthusiasm for employing functional imaging techniques such as diffusion-weighted (DW) MR imaging [11], which may depict microstructural and metabolic treatment-induced changes of the tumor before morphological changes become apparent. DWI allows to perform quantitative measures such as apparent diffusion coefficient (ADC); it may be used as a noninvasive imaging biomarker of tumor aggressiveness [12], [13] and to monitor and predict tumor response to CRT [14].
In recent years different imaging tools either volumetric (tumor volume reduction rate [15], magnetic resonance volumetry [16]) or functional [6] have been investigated as potential imaging-based biomarker of treatment response; in particular, the role of qualitative and quantitative DWI findings for prediction of tumor response to CRT has been evaluated.
Kim et al. [8] demonstrated that in patients with locally advanced rectal cancer, adding DW MR imaging to conventional MR imaging yields better diagnostic accuracy than use of conventional MR imaging alone in the evaluation of complete response to neoadjuvant CRT.
Other studies [6] have investigated the role of ADC measurements (potential markers of response being pre-CRT, post-CRT ADC measures and ΔADC) for prediction of treatment outcome and for early detection of tumor response in patients with locally advanced rectal cancer. Sometimes, however the obtained results are discordant and DWI seems not accurate enough to safely select patients for organ preservation.
The aims of our study were:
-
–
to investigate the added value of qualitative DW MRI evaluation in the response assessment after neoadjuvant CRT in patients with locally advanced rectal cancer;
-
–
to evaluate the diagnostic performance of rectal cancer’s ADC measurements, the quantitative parameter of diffusion, for the assessment of therapeutic response to CRT.
2. Materials and methods
2.1. Patients
This was a single institution retrospective cohort study. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and in accordance with recommendations of the local ethic committee. Informed consent was waived due to the retrospective study design.
Between October 2011 and May 2015, 47 patients with diagnosis of rectal cancer were considered for eligibility.
Inclusion criteria were as follows: histologically proven rectal carcinoma, staged on rectal MRI with DWI T3-4 and/or with positive regional lymph-node, neoadjuvant CRT, post-CRT rectal MRI with DWI, subsequent surgery.
Exclusion criteria were: previous CRT for primary rectal carcinoma or tumor in other organ (n = 1); contraindication to MR imaging examination (n = 2); delayed (more than 8 months after CRT), cancelled surgery or surgery performed in other institution (n = 2); insufficient quality of MR examination (e.g. owing to metal implants or movement artifacts) (n = 2); distant metastases (n = 4); lack of follow-up MR examination (n = 5).
All patients underwent a staging protocol before preoperative CRT, that included: digital rectal examination, complete blood tests, colonoscopy, contrast enhanced computed tomography (CT) of the chest and the abdomen, external pelvic phased-array MR examination and transrectal ultrasound.
2.2. Reference standard
Pretreatment stage (cT cN) was compared with pathologic stage (ypT ypN). Pathologic staging represented the reference standard and was based on the TNM staging system (VII ed.).
The response was graded as follows. No response to treatment was defined as stable disease (ypSD). A partial response (ypPR) to treatment was defined as downstaging, or reduction of at least one level in T or N staging between the baseline MR exam and histopathological staging. Pathological complete response (ypCR) was defined as the absence of any residual tumor cells detected in the operative specimen (ypT0 ypN0).
2.3. Neoadjuvant CRT treatment plan
CRT was performed by means of integration between an initial induction chemotherapy (ICT) and a subsequent concurrent radio-chemotherapy (CRCT), over a period of 9 weeks.
During the ICT phase, all patients received a FOLFOX 4 chemotherapy schedule for two cycles, with Oxaliplatin 85 mg/m2 in 3 h i.v. infusion (on day 1), 5-Fluoruracil 400 mg/m2 i.v. bolus, Folinic acid 200 mg/m2, 5-Fluoruracil 600 mg/m2 continuous intravenous infusion over 22 h (on day 1 and 2). The cycle was repeated after 14 days, by previous clinical and haematological examination.
A CT radiotherapy simulation was conducted during the ICT phase, in order to prepare the treatment plan.
The CRCT phase requires a continuous i.v. infusion of 5-Fluoruracil at 250 mg/m2 daily during all radiotherapy treatment period. Radiation therapy was conducted with patient in prone position, by means of a belly board position system, in order to reduce the dose to the small bowel.
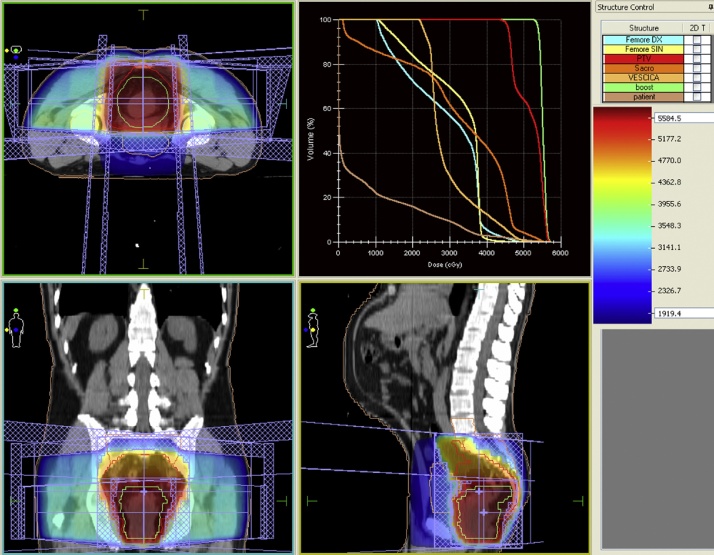
Radiotherapy was planned using a 3D-Conformal technique (Fig. 1), to ensure a coverage of the whole pelvis, including rectum, mesorectum, common iliac, internal and external iliac lymph nodes, obturator lymph nodes. This volume was defined as CTV 1 (Clinical Target Volume 1) and was irradiated to a dose of 45 Gy, with a conventional fractionation of 1,8 Gy/day. A second volume, called CTV2, was also defined to give a boost to the site of the primary tumor in the rectum, by means of a concomitant irradiation during the last six fractions, after an interval of 6 h from the first fraction. This volume received a dose of 9 Gy, with a fractionation of 1,5 Gy/day.
Fig. 1.
Radiotherapy plan. Radiotherapy dose distribution in axial, coronal and sagittal views. Planning was made through a 3D-conformal technique.
Using the above described concomitant-boost technique, a total dose of 54 Gy is given to the primary tumor. This dose is at least 10% higher than that conventionally used in the currently accepted protocols for neoadjuvant irradiation of rectal cancer.
2.4. Imaging technique
All patients were examined by MRI at two time points: about 1 week prior to CRT (pre-CRT MRI) and 7 weeks after the end of CRT (post-CRT MRI).
All pre-CRT and post-CRT MRI examinations were performed with a closed-configuration superconducting 1.5-T system (Signa HDxT; GE Healthcare, Milwaukee, Wis) with 57.2 mT/m gradient strength and 120 T/m/s slew rate, by using an eight-channel high-resolution torso coil with array spatial sensitivity technique (ASSET) parallel acquisition. Informed consent of MRI examination was obtained from all patients at the time of scanning after the nature and contraindications of the procedure were fully explained.
About three hours before the MR study, the patients performed a rectal cleansing with a water enema.
Table 1 summarizes our MR imaging protocol.
Table 1.
MRI protocol. Synoptic table summarizes the imaging parameters of MR sequences. Axial T2-weighted SSFSE sequence is used as second localiser. Sagittal T2-weighted FRFSE sequence is oriented parallel to the longitudinal axis of the rectum identified on the previous axial T2-weighted SSFSE sequence. Oblique coronal and oblique axial T2-weighted FRFSE sequences are oriented respectively perpendicular or parallel to the longitudinal axis of the rectal tumor.
| MRI protocol | Axial T2 W SSFSE | Sagittal T2 W FRFSE | Oblique coronal T2 W FRFSE | Oblique axial T2 W FRFSE | Axial DWI SE EPI |
|---|---|---|---|---|---|
| Repetition time/Echo time (m s) | 765/59 | 3560/100 | 3440/100 | 3440/100 | 5425/74.8 |
| Flip angle | 90° | 90° | 90° | 90° | 90° |
| Echo train length | – | 21 | 23 | 23 | – |
| Section thickness (mm) | 6 | 3 | 3 | 3 | 5 |
| Interslice gap (mm) | 0.6 | 0,3 | 0.3 | 0.3 | 1 |
| Bandwidht (kHz) | 31.25 | 25 | 31.25 | 31.25 | 250 |
| Field of view (cm) | 38 | 30 | 28 | 28 | 40 |
| Matrix | 320 × 288 | 288 × 224 | 288 × 224 | 288 × 224 | 160 × 160 |
| No. of averages | 0.54 | 3 | 4 | 4 | 8 |
| No. of images | 30 | 30 | 30 | 30 | 24 |
| Frequency direction | Right to left | Anterior to posterior | Right to left | Anterior to posterior | Right to left |
| Acquisition time | 24 s | 4 min 10 s | 4 min 41 s | 4 min 49 s | 2 min 59 s |
| b-value (sec/mm2) | – | – | – | – | 0−800 |
T2W = T2-weighted, SSFSE = single-shot fast spin-echo, FRFSE = fast relaxation fast spin-echo, DWI = diffusion-weighted imaging, SE = spin-echo, EPI = echoplanar imaging.
2.5. Image analysis
The evaluation of MR examinations was performed by two radiologists (a senior radiologist with 7 years of clinical experience in body MRI and a junior radiologist with 1 year of practice experience) who were blinded to information obtained at surgery and pathologic analysis.
Qualitative analysis was performed by the two radiologists in consensus. They reviewed the two image sets (the conventional MR image set and the combined set of conventional and DW MR images) in two different reading sessions over an 8-week period. To avoid any recall bias, the order of cases was changed in the second reading session.
First, the radiologists reviewed both the pre-CRT and post-CRT conventional MR images and recorded their confidence level with respect to the ypCR during the first reading session. CR was defined as an unidentified mass or wall thickening in the post-CRT MR examination. A partial response (PR) to treatment was defined as downstaging, or reduction of at least one level in T or N staging between the baseline pre-CRT MR exam and the post-CRT MR exam. No response to treatment (stable disease SD) was defined as stable disease between the two time points MR examinations.
At the second reading session, the reviewers recorded their confidence level with respect to the ypCR for the combined image set (the conventional MR image set, the pre- and post-CRT DW MR image set and the ADC map). CR was defined as nondepiction of high signal intensity in the corresponding tumor on DW MR images. The presence of residual high signal intensity on DW MR images (low signal intensity on the ADC map) in the corresponding tumor was considered a sign of a PR. SD was defined as stable disease between the two time points MR examinations. When the findings on DW MR images differed from those on conventional MR images, reviewers gave priority to the findings of the former ones.
In the quantitative assessment the measurements of the ADC were performed on both pre-CRT and post-CRT DW MR images by the two radiologists in consensus in a successive session, by using a workstation with diffusion analysis software (Advantage Windows version 4.6, General Electric Medical Systems, Milwaukee, WI, USA). To obtain the ADC measurements the radiologists placed at least three oval regions of interest (ROIs) (mean size 10 mm2; range 8–14 mm2) on the DW images (b = 0, b = 800 s/mm2) on the rectal wall in the area of brightest signal of the clearly visible tumor, resulting in a total of at least 3 subreadings for each lesion. The ROIs were delineated excluding cancer margins, distortion artifacts and macroscopically visible necrotic or cystic portions, and were automatically copied to the corresponding ADC map. T2-weighted images were observed to assist in correctly identifying the tumor. Following completion of therapy, if there was no residual tumor detectable on post-CRT images, the ROIs were traced on what was considered to be the normal residual rectal wall, as much as possible in the same area used in pre-CRT MR examination. Subsequently, the values of all subreadings of each time point examination were averaged and the mean ADC value was calculated for each lesion.
2.6. Statistical analysis
All data are presented as the mean ± standard deviation. The data were first tested for normality (Shapiro–Wilk’s test) and homoscedasticity (Levene’s test) and then compared by using one- or two-way analyses of variance (ANOVAs) followed by post hoc multiple comparisons by using Duncan’s test. In particular, the two-way ANOVA was performed by applying the mixed model for independent variables (ypCR, ypPR and ypSD groups) and repeated measures (ADC pre-CRT and ADC post-CRT examination). The one-way ANOVA was performed on difference between ADC post and ADC pre (Δ ADC post−ADC pre) of the three groups (ypCR, ypPR, ypSD).
Analyses were performed by using Statistica 7.0 for Windows and the significance level was established at p ≤ 0.05.
Diagnostic capabilities of the two image sets (the conventional MR image set and the combined set of conventional and DW MR images) for the diagnosis of CR compared with the reference standard were assessed by measuring accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Cases in which disease was understaged were considered as false negative, cases in which disease was overstaged were considered as false positive.
Receiver-operating characteristics (ROC) analysis with the area under the curve (AUC) was employed to investigate the discriminatory capability for ypCR, responders (ypCR, ypPR) and ypSD of each repeated measure (ADC pre-CRT, ADC post-CRT and Δ ADC post−ADC pre). For calculation of the sensitivity and specificity the optimal threshold was determined by giving equal weighting to sensitivity and specificity on the ROC curve [17]. Therefore, for each repeated measure we calculated AUC for the following independent variables:
-
–
ypCR versus ypPR and ypSD,
-
–
responders (ypCR, ypPR) versus ypSD,
-
–
ypSD versus responders (ypCR, ypPR).
We used 95% confidence intervals to express the statistical precision of the results. The above mentioned analysis was performed by using MedCalc software for Windows (MedCalc Software version 9.6.4.0, Mariakerke, Belgium).
3. Results
3.1. Patient demographics
31 patients met the above mentioned study criteria and were enrolled in the study (21 men and 10 women, mean age of 65 years with a range of 41–84 years).
The average interval between pre-CRT MR imaging for tumor staging and the start of the treatment was 8 days (range, 4–11 days). The average interval between the completion of CRT and post-CRT MR imaging for response evaluation was 51 days (range, 43–57 days). The average interval between post-CRT MR imaging and surgery was 9 days (range, 5–27 days).
Tumor location was as follows: anal canal, within 4.0 cm of the anal verge (n = 7); distal rectum, within 4.1–8.0 cm of the anal verge (n = 15); middle rectum, within 8.1–12.0 cm of the anal verge (n = 5); proximal rectum, within 12.1–16.0 cm of the anal verge (n = 4).
All patients underwent surgical excision: low/ultralow anterior resection (n = 26) and abdominoperineal resection (n = 5); 18/31 patients underwent also temporary diverting loop ileostomy.
At pathological evaluation tumor response was as follows:
-
–
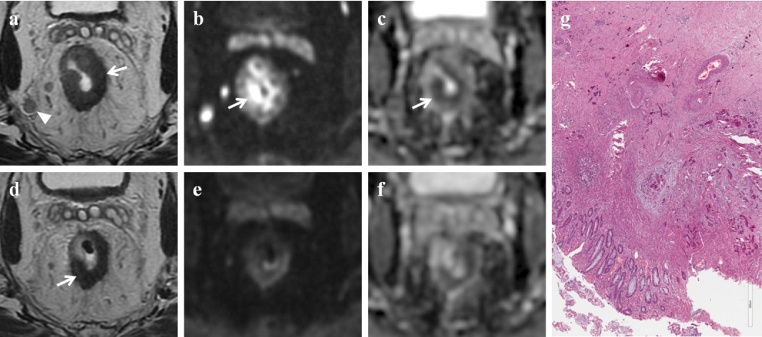
complete response (ypCR) 5/31 patients (16.1%) (Fig. 2);
-
–
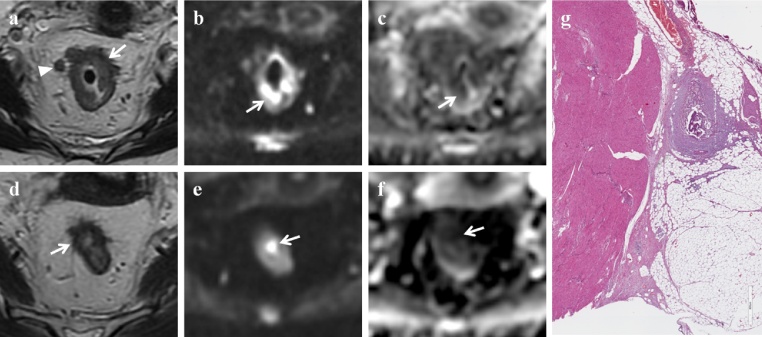
partial response (ypPR) 16/31 patients (51.6%) (Fig. 3);
-
–
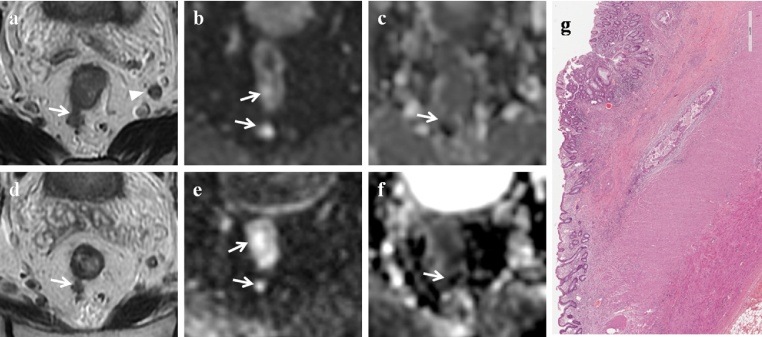
stable disease (ypSD) 10/31 patients (32.3%) (Fig. 4).
Fig. 2.
Complete response. MR images of a 70-year old man classified as complete responder on combined set of conventional and DW images and as partial responder on T2-weighted images. (a) Pre-CRT axial T2-weighted image shows neoplastic tissue in the middle rectum (white arrow) with nodal involvement (white arrowhead); the tumor has spread through the rectal wall into the perirectal fat for less than 5 mm (T3a N2 stage). (b) Pre-CRT DW (b = 800 s/mm2) image shows a focal high signal intensity area in the corresponding tumor (white arrow). (c) Pre-CRT ADC map at the same level shows reciprocal low signal intensity area due to the pathological tissue (white arrow). The mean ADC value was 0.752 × 10−3 s/mm2. (d) Post-CRT axial T2-weighted image shows a wall thickening of the rectum with low signal intensity (white arrow), not clearly depicted as fibrosis. (e) Post-CRT DW (b = 800 s/mm2) image shows no residual high signal intensity in the primary tumor bed. (f) Post-CRT ADC map at the same level: the mean ADC value was 1.076 × 10−3 s/mm2. On the basis of qualitative and quantitative DWI analysis the patient should be considered as complete responder. (g) Photomicrograph (H&E x800). Complete response (TRG: 4), absence of tumor remnants; fibrous reaction induced by the treatment and calcifications are evident.
Fig. 3.
Partial response. MR images of a 55-year old woman classified as partial responder both on combined set of conventional and DW images and on T2-weighted images. (a) Pre-CRT axial T2-weighted image shows wall thickening of the middle rectum (white arrow) with spread into the perirectal fat for less than 10 mm; there are also some lymph nodes (white arrowhead) into the mesorectal fat (T3b N2). (b) Pre-CRT DW (b = 800 s/mm2) image shows high signal intensity area in the corresponding tumor (white arrow). (c) Pre-CRT ADC map at the same level shows low signal intensity area due to the pathological tissue (white arrow). The mean ADC value was 0.804 × 10−3 s/mm2. (d) Post-CRT axial T2-weighted image shows tumor shrinkage and intermediate signal intensity tissue in the rectal wall, not clearly depicted as fibrosis. (e) Post-CRT DW (b = 800 s/mm2) image shows reduction of the high signal intensity area, which is now limited to a focal spot in the rectal wall (white arrow). (f) Post-CRT ADC map at the same level shows a focal low signal intensity area corresponding to the residual tumor (white arrow). The mean ADC value was 1.237 × 10−3 s/mm2. (g) Photomicrograph (H&E x800). Intermediate regression (TRG: 2 + 3), predominance of the fibrous reaction induced by the treatment and few tumor remnants.
Fig. 4.
Stable disease. MR images of a 77-year old man classified as stable disease both on combined set of conventional and DW images and on T2-weighted images. (a) Pre-CRT axial T2-weighted image shows neoplastic tissue in the middle rectum (white arrow) with nodal involvement (white arrowhead). The tumor has spread into the mesorectal fat for more than 10 mm (T3c N1 stage). (b) Pre-CRT DW (b = 800 s/mm2) image shows high signal intensity area due to neoplastic tissue (white arrows). (c) Pre-CRT ADC map at the same level shows low signal intensity area (white arrow). The mean ADC value was 0.937 × 10−3 s/mm2. (d) Post-CRT axial T2-weighted image shows a poor tumor reduction with persistence of neoplastic spread (white arrow) into the mesorectal fat (T3 stage). (e) Post-CRT DW (b = 800 s/mm2) image shows high signal intensity area in the corresponding tumor (white arrows). (f) Post-CRT ADC map at the same level shows low signal intensity area (white arrow). (g) Photomicrograph (H&E x600). Poor regression (TRG: 0 + 1), predominance of areas with tumor remnants surrounded by a poor fibrous reaction induced by the treatment.
3.2. Qualitative analysis
In the evaluation of CR, the diagnostic performance of the second reading session (combined set of conventional and DW MR images) was better than that of the first one (conventional MR image set) (Table 2). Additional DW MR image interpretation allowed to correct diagnostic errors made on the basis of conventional MR image interpretation alone (n = 3).
Table 2.
Statistical analysis. Diagnostic capabilities of the two image sets (the conventional MR image set and the combined set of conventional and DW MR images) for the diagnosis of complete response compared with the reference standard.
| T2 | T2 + DWI | |
|---|---|---|
| Accuracy% | 87.9 | 97 |
| Sensitivity% | 20 (15.1–55.1)a | 80 (44.9–151.1)a |
| Specificity% | 100 | 100 |
| PPV% | 100 | 100 |
| NPV% | 87.5 | 96.6 |
PPV: positive predictive value; NPV: negative predictive value; DWI: diffusion weighted imaging.
Confidence interval limits.
3.3. Quantitative analysis
Table 3 shows the overall ADC value of rectal cancer and the mean ADC value of ypCR, ypPR and ypSD groups at each time point.
Table 3.
Overall ADC value of rectal cancer and mean ADC value of ypCR, ypPR and ypSD groups at each time point (means ± standard deviation).
| Group | ADC pre-CRT (×10−3 mm2/s) | ADC post-CRT (×10−3 mm2/s) | Δ ADC post−ADC pre (×10−3 mm2/s) |
|---|---|---|---|
| overall ADC | 0.85 ± 0.09 | 1.13 ± 0.18 | 0.28 ± 0.21 |
| ypCR | 0.78 ± 0.05 | 1.28 ± 0.21 | 0.51 ± 0.18 |
| ypPR | 0.84 ± 0.07 | 1.16 ± 0.16 | 0.31 ± 0.19 |
| ypSD | 0.91 ± 0.12 | 1.01 ± 0.14 | 0.11 ± 0.09 |
ypCR: complete response; ypPR: partial response; ypSD: stable disease; CRT: chemo-radiotherapy.
The overall ADC value of rectal cancer in the pre-CRT examination was significantly lower than that in the post-CRT examination, as revealed by the examination effect of the two-way ANOVA (F1, 28 = 88.63; p < 0.00001) (ADC pre-CRT: 0.85 ± 0.09 × 10−3 mm2/s; ADC post-CRT: 1.13 ± 0.18 × 10−3 mm2/s).
In the ypCR and ypPR groups, the ADC value of rectal cancer in the pre-CRT examination was significantly lower than that in the post-CRT examination, whereas in the ypSD group the difference was not statistically significant, as revealed by post hoc comparisons on interaction of the two-way ANOVA (F2, 28 = 10.77; p = 0.0003) (post hoc comparisons: ypCR group, p = 0.00002; ypPR group, p = 0.00006; ypSD group, p = 0.07).
Moreover, as revealed by post hoc comparisons on interation of the two-way ANOVA (F2, 28 = 10.77; p = 0.0003), in the pre-CRT examination the ADC value of rectal cancer of ypCR group was significantly lower than that of ypSD group (p = 0.04); whereas no statistically significant difference was found between ADC value of rectal cancer of ypCR and ypPR groups (p = 0.2) and between ypPR and ypSD groups (p = 0.3). In the post-CRT examination the ADC values of rectal cancer were significantly different among the three groups of tumor response (ypCR vs. ypPR: p = 0.03; ypCR vs. ypSD: p = 0.0001; ypPR vs. ypSD: p = 0.01).
The Δ ADC post−ADC pre were significantly different among the three groups of tumor response, as revealed by post hoc comparisons of the one-way ANOVA (F2, 28 = 10.77; p = 0.0003) (post hoc comparisons: ypCR vs. ypPR: p = 0.02; ypCR vs. ypSD: p = 0.0001; ypPR vs. ypSD: p = 0.02).
The results of ROC curve analysis are shown in Table 4. The Δ ADC post−ADC pre showed the best diagnostic capability in identifying both ypCR and responders (ypCR, ypPR). In particular, when an ADC > 0.3 (×10−3 mm2/s) was used as the cut-off value for distinguishing between the ypCR and ypPR-ypSD groups [area under the ROC curve (AUC), 0.87], a sensitivity of 100% and a specificity of 70.37% were obtained. When an ADC > 0.2 (×10−3 mm2/s) was used as the cut-off value for distinguishing between the responders (ypCR, ypPR) and ypSD groups [area under the ROC curve (AUC), 0.873], a sensitivity of 72.73% and a specificity of 100% were obtained.
Table 4.
Statistical analysis. ROC curve analysis.
| ypCR vs ypPR and ypSD | responders (ypCR, ypPR) vs ypSD | ypSD vs responders (ypCR, ypPR) | ||
|---|---|---|---|---|
| ADC pre-CRT | OCV ADC (×10−3 mm2/s) | 0.8 | 0.9 | 0.9 |
| AUC | 0.793 | 0.718 | 0.718 | |
| Sensitivity% | 100 | 81.82 | 60 | |
| Specificity% | 66.67 | 60 | 81.82 | |
| ADC post-CRT | OCV ADC (×10−3 mm2/s) | 1.3 | 1.1 | 1.1 |
| AUC | 0.763 | 0.77 | 0.77 | |
| Sensitivity% | 60 | 77.27 | 70 | |
| Specificity% | 92.59 | 70 | 77.27 | |
| Δ ADC post−ADC pre | OCV ADC (×10−3 mm2/s) | 0.3 | 0.2 | 0.2 |
| AUC | 0.87 | 0.873 | 0.873 | |
| Sensitivity% | 100 | 72.73 | 100 | |
| Specificity% | 70.37 | 100 | 72.73 | |
ypCR: complete response; ypPR: partial response; ypSD: stable disease; CRT: chemo-radiotherapy; OCV ADC: optimal cut-off ADC value; AUC: area under the ROC curve.
4. Discussion
In this study we evaluated the diagnostic value of qualitative and quantitative DWI findings in the assessment of tumor response to neoadjuvant CRT in patients with locally advanced rectal cancer.
Our study demonstrated that:
-
–
the addition of DWI sequence’s qualitative assessment to conventional high-resolution T2-weighted sequences improves the diagnostic performance of MRI in the evaluation of ypCR (sensitivity 80%, specificity 100%);
-
–
in the ypCR group the ADC value of rectal cancer in the per-CRT examination was significantly lower than that in the post-CRT examination; in the pre-CRT examination the ADC value of ypCR group was significantly lower than that of ypSD group;
-
–
Δ ADC post−ADC pre (sensitivity 100%, specificity 70.37%) yields better diagnostic capability than ADC pre-CRT (sensitivity 100%, specificity 66.67%) and ADC post-CRT (sensitivity 60%, specificity 92.59%) in the evaluation of ypCR.
In our case series tumor response rate after CRT was 67.7%, in particular 16.1% of patients showed a complete tumor response and 51.6% a partial response. These data are substantially in line with those ones from scientific literature in which complete response rate ranges from 15% to 27% and partial response rate from 54% to 75% [3].
The first of our purposes was to investigate the added value of qualitative DW MRI evaluation in rectal cancer response assessment after neoadjuvant CRT.
Our results showed that in the evaluation of ypCR the diagnostic performance of the combined set of conventional and DW MR images was better than that of the conventional MR image set. Sensitivity improved from 20% to 80%, NPV from 87.5% to 96.6% and accuracy from 87.9% to 99.6%. In 3 cases the interpretation of additional DW MR images allowed us to correct diagnostic errors made on the basis of conventional MR image interpretation alone, differentiating viable tumor from fibrosis.
Our results are consistent with those of previous studies on rectal cancer [8], [18], [19], [20] in which adding DWI to conventional MR sequences was helpful for detecting viable tumor after neoadjuvant CRT. In a recently published systematic review Joye et al. [6] found that late qualitative DWI assessment can predict ypCR with a pooled specificity of 94% and an overall accuracy of 87%, thereby outperforming quantitative DWI measurements.
The second of our purposes was to evaluate the diagnostic performance of rectal cancer’s ADC measurements for the assessment of therapeutic response to CRT.
In our case series the overall ADC value of rectal cancer in the pre-CRT examination was significantly lower than that in the post-CRT examination. In the ypCR and ypPR groups, the ADC value in the pre-CRT examination was significantly lower than that in the post-CRT examination, whereas in the ypSD group the difference was not statistically significant.
Moreover, in the pre-CRT examination the ADC value of rectal cancer of ypCR group was significantly lower than that of ypSD group (p = 0.04); though no statistically significant difference was found between ADC value of rectal cancer of ypCR and ypPR groups (p = 0.2) and between ypPR and ypSD groups (p = 0.3).
In the post-CRT examination the ADC value of the ypCR group was significantly higher than that of ypPR and ypSD group. Similarly, the Δ ADC post−ADC pre were significantly different among the three groups of tumor response, being higher in the ypCR group.
Concerning the role of rectal cancer’s ADC value in predicting treatment outcomes our data largely confirm earlier reports. Dzik-Jurasz et al. found a negative correlation between the pretreatment tumor ADC value and the percentage shrinkage of the tumor after CRT in rectal cancer. DW MR imaging was effective for the pretreatment prediction of treatment outcome, since patients who responded to treatment had a lower ADC at presentation than those who did not respond [21]. Likewise, the association between high tumor ADC and poor response was consistent with the known relationship between necrosis and poor response to cancer treatment.
As for ROC curve analysis we found that among the three ADC measures (ADC pre-CRT, ADC post-CRT and Δ ADC post−ADC pre), the Δ ADC post−ADC pre showed the best diagnostic capability in identifying ypCR. In particular, when an ADC > 0.3 (×10−3 mm2/s) was used as the cut-off value for distinguishing between the ypCR and ypPR-ypSD groups [area under the ROC curve (AUC), 0.87], a sensitivity of 100% and a specificity of 70.37% were obtained.
Our results are very similar to those obtained by Kim et al. [22] that found the percentage of the ADC increase (cut-off value of 42%) to be an useful predictor for ypCR with a sensitivity of 100% and a specificity of 71%. Similarly, Lee et al. [23] reported that the percentage change in ADC was significantly correlated with pathological response.
Conversely in the study of Semedo et al. [4] Δ ADC post−ADC pre was not reliable in predicting ypCR (sensitivity of 54% and specificity of 64%).
Such a discrepancy between the results of the different studies can be explained by several factors; among these the definition of responder group (ypCR or downstaging) seems to be the most relevant. However, the ypCR, which was used in our as in other studies, is a more objective reference standard than tumor volume reduction rate. The variability depending on coil systems, scanners, magnetic field strength and MR imaging protocol (different b values), along with study population and design, different ADC measurement methods, interobserver variability and operator dependence on ROI positioning should also be considered.
In their systematic review, Joye et al. [6] collected the current evidence on the role of DWI in the prediction of ypCR before, during and after CRT for rectal cancer. They found the following data: a low pretreatment ADC, the change in ADC after 10–15 fractions of radiation therapy (ΔADCduring) and a high ADCpost value were significantly correlated with ypCR.
Consistent with this previous report, our results confirm the potential of pre-CRT examination ADC value to predict treatment response even before the start of therapy and the assumption that the change in ADC values (Δ ADC post−ADC pre) has the potential to provide a surrogate biomarker of treatment response in rectal cancer.
Nevertheless, as demonstrated by previous Authors [6], no technique alone is accurate enough to safely select patients with ypCR; indeed, in our study, although a specificity of 100%, qualitative DWI analysis showed a sensitivity of 80%, and quantitative ADC assessment, despite a sensitivity of 100%, yielded a specificity of 70%.
The present study has some limitations. The first limitation is due to its retrospective design which may predispose to selection bias. Second, we used 2 b-values (0 and 800 s/mm2) for ADC calculations and did not investigate the possible influence of other b-values. Third, our study lacks an early follow-up MR examination that could allow an earlier and accurate prediction of the pathological tumor response during CRT treatment, thus enabling to guide modifications of the treatment protocol [24].
5. Conclusions
In our series, use of qualitative DW MR imaging assessment in addition to conventional MR imaging yielded better diagnostic accuracy in the evaluation of ypCR to neoadjuvant CRT in patients with locally advanced rectal cancer.
A low tumor ADC value in the pre-CRT examination, a high ADC value in the post-CRT examination, a high Δ ADC post−ADC pre [>0.3 (×10−3 mm2/s)] were predictive of ypCR.
Nevertheless, although DW sequences improve MR capability to evaluate tumor response to CRT, no functional MR technique alone seems accurate enough to safely select patients with ypCR. Therefore, only a multidisciplinary approach based on the combination of morphological techniques (high-resolution T2-weighted sequences), different functional imaging modalities (qualitative and quantitative DWI), endoscopic findings, clinical data and laboratory parameters might allow to reach a sufficient diagnostic accuracy to select patients candidable for organ-sparing strategies.
The contribution of DW MRI in the study of rectal cancer needs further evaluation in a larger study cohort and prospective, multi-center trials are necessary to fully evaluate the impact of functional MRI on clinical decision making (surgery, conservative treatment or “wait and watch” approach).
Conflicts of interest
None.
All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence their work.
References
- 1.Maier A., Fuchsjäger M. Preoperative staging of rectal cancer. Eur. J. Radiol. 2003;47:89–97. doi: 10.1016/s0720-048x(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 2.Attenberger U.I., Pilz L.R., Morelli J.N., Hausmann D., Doyon F., Hofheinz R., Kienle P., Post S., Michaely H.J., Schoenberg S.O., Dinter D.J. Multi-parametric MRI of rectal cancer—do quantitative functional MR measurements correlate with radiologic and pathologic tumor stages? Eur. J. Radiol. 2014;83:1036–1043. doi: 10.1016/j.ejrad.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Maas M., Nelemans P.J., Valentini V. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 4.Semedo L.C., Lambregts D.M., Maas M. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260:734–743. doi: 10.1148/radiol.11102467. [DOI] [PubMed] [Google Scholar]
- 5.Kluza E., Rozeboom E.D., Maas M., Martens M., Lambregts D.M., Slenter J., Beets G.L., Beets-Tan R.G. T2 weighted signal intensity evolution may predict pathological complete response after treatment for rectal cancer. Eur. Radiol. 2013;23:253–261. doi: 10.1007/s00330-012-2578-z. [DOI] [PubMed] [Google Scholar]
- 6.Joye I., Deroose C.M., Vandecaveye V., Haustermans K. The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother. Oncol. 2014;113:158–165. doi: 10.1016/j.radonc.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Allen S.D., Padhani A.R., Dzik-Jurasz A.S., Glynne-Jones R. Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am. J. Roentgenol. 2007;188:442–451. doi: 10.2214/AJR.05.1967. [DOI] [PubMed] [Google Scholar]
- 8.Kim S.H., Lee J.M., Hong S.H., Kim G.H., Lee J.Y., Han J.K., Choi B.I. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253:116–125. doi: 10.1148/radiol.2532090027. [DOI] [PubMed] [Google Scholar]
- 9.Monguzzi L., Ippolito D., Bernasconi D.P., Trattenero C., Galimberti S., Sironi S. Locally advanced rectal cancer: value of ADC mapping in prediction of tumor response to radiochemotherapy. Eur. J. Radiol. 2013;82:234–240. doi: 10.1016/j.ejrad.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Kim D.J., Kim J.H., Lim J.S., Yu J.S., Chung J.J., Kim M.J., Kim K.W. Restaging of rectal cancer with MR imaging after concurrent chemotherapy and radiation therapy. Radiographics. 2010;30:503–516. doi: 10.1148/rg.302095046. [DOI] [PubMed] [Google Scholar]
- 11.Barbaro B., Vitale R., Leccisotti L., Vecchio F.M., Santoro L., Valentini V., Coco C., Pacelli F., Crucitti A., Persiani R., Bonomo L. Restaging locally advanced rectal cancer with MR imaging after chemoradiation therapy. Radiographics. 2010;30:699–716. doi: 10.1148/rg.303095085. [DOI] [PubMed] [Google Scholar]
- 12.Curvo-Semedo L., Lambregts D.M., Maas M., Beets G.L., Caseiro-Alves F., Beets-Tan R.G. Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J. Magn. Reson. Imaging. 2012;35:1365–1371. doi: 10.1002/jmri.23589. [DOI] [PubMed] [Google Scholar]
- 13.Zhi-hua Lu, Chun-hong Hu, Wei-xin Qian, Wen-hong Cao. Preoperative diffusion-weighted imaging value of rectal cancer: preoperative T staging and correlations with histological T stage. J. Clin. Imaging. 2015 doi: 10.1016/j.clinimag.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Koh D.M., Collins D.J. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am. J. Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 15.Neri E., Guidi E., Pancrazi F., Castagna M., Castelluccio E., Balestri R., Buccianti P., Masi L., Falcone A., Manfredi B., Faggioni L., Bartolozzi C. MRI tumor volume reduction rate vs tumor regression grade in the pre-operative re-staging of locally advanced rectal cancer after chemo-radiotherapy. Eur. J. Radiol. 2015;84:2438–2443. doi: 10.1016/j.ejrad.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Carbone S.F., Pirtoli L., Ricci V., Venezia D., Carfagno T., Lazzi S., Mourmouras V., Lorenzi B., Volterrani L. Assessment of response to chemoradiation therapy in rectal cancer using MR volumetry based on diffusion-weighted data sets: a preliminary report. Radiol. Med. 2012;117:1112–1124. doi: 10.1007/s11547-012-0829-3. [DOI] [PubMed] [Google Scholar]
- 17.Lambrecht M., Deroose C., Roels S., Vandecaveye V., Penninckx F., Sagaert X., van Cutsem E., de Keyzer F., Haustermans K. The use of FDG-PET/CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer. Acta Oncol. 2010;49:956–963. doi: 10.3109/0284186X.2010.498439. [DOI] [PubMed] [Google Scholar]
- 18.Sassen S., de Booij M., Sosef M. Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur. Radiol. 2013;23:3440–3449. doi: 10.1007/s00330-013-2956-1. [DOI] [PubMed] [Google Scholar]
- 19.Lambregts D.M., Vandecaveye V., Barbaro B. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann. Surg. Oncol. 2011;18:2224–2231. doi: 10.1245/s10434-011-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song I., Kim S.H., Lee S.J., Choi J.Y., Kim M.J., Rhim H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br. J. Radiol. 2012;85:577–586. doi: 10.1259/bjr/68424021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzik-Jurasz A., Domenig C., George M. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–308. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.H., Lee J.Y., Lee J.M., Han J.K., Choi B.I. Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur. Radiol. 2011;21:987–995. doi: 10.1007/s00330-010-1989-y. [DOI] [PubMed] [Google Scholar]
- 23.Lee E.M., Hong Y.S., Kim K.P. Phase II study of preoperative chemoradiation with S-1 plus oxaliplatin in patients with locally advanced rectal cancer. Cancer Sci. 2013;104:111–115. doi: 10.1111/cas.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musio D., De Felice F., Magnante A.L., Ciolina M., De Cecco C.N., Rengo M., Redler A., Laghi A., Raffetto N., Tombolini V. Diffusion-weighted magnetic resonance application in response prediction before, during, and after neoadjuvant radiochemotherapy in primary rectal cancer carcinoma. Biomed. Res. Int. 2013;2013:740195. doi: 10.1155/2013/740195. [DOI] [PMC free article] [PubMed] [Google Scholar]