In this study, we found that patients with incurable cancer and their family caregivers experience high rates of depression and anxiety symptoms, with FC anxiety levels exceeding those of the patients. We identified patient and FC factors associated with FCs’ psychological distress, including patients’ coping strategies and prognostic understanding.
Keywords: palliative care, mood, advanced cancer, caregivers, depression, anxiety
Abstract
Background
Family caregivers (FCs) are critically important for patients with cancer, yet they may experience psychological distress related to caregiving demands. We sought to describe rates of depression and anxiety in FCs of patients with incurable cancer and identify factors associated with these symptoms to determine those at greatest risk for psychological distress.
Patients and methods
We performed a cross-sectional analysis of baseline data from a randomized trial of early palliative care. We assessed depression and anxiety using the Hospital Anxiety and Depression Scale in patients within 8 weeks of diagnosis of incurable lung or gastrointestinal cancer and their FCs. We also assessed patients' quality of life (Functional Assessment of Cancer Therapy-General), coping strategies (Brief COPE), and their report of the primary goal of their cancer treatment. We used linear regression with purposeful selection of covariates to identify factors associated with FC depression and anxiety symptoms.
Results
We enrolled 78.6% (n = 275) of potentially eligible FCs. The majority were female (69.1%) and married to the patient (66.2%). While the proportion of FCs and patients reporting depression did not differ (16.4% versus 21.5%, P = 0.13), FCs were more likely to report anxiety compared with patients (42.2% versus 28.4%, P < 0.001). Patients' use of acceptance coping was associated with lower FC depression (B = −0.42, P < 0.001), while emotional support coping was associated with higher FC depression (B = 0.69, P = 0.001) and lower FC anxiety (B = −0.70, P < 0.001). Patient report that their primary goal of their treatment was to ‘cure my cancer’ was associated with higher FC depression (B = 0.72, P = 0.03).
Conclusions
Patients with incurable cancer and their FCs report high levels of depression and anxiety symptoms. We demonstrated that patients' coping strategies and prognostic understanding were associated with FC depression and anxiety symptoms, underscoring the importance of targeting these risk factors when seeking to address the psychological distress experienced by FCs.
introduction
Family caregivers (FCs) are essential to the care of patients with cancer. Patients with cancer often require assistance from friends and family in addition to the care from their medical team [1]. Specifically, FCs help patients with transportation, finances, personal care, emotional support, and symptom management [2]. Many FCs take on this responsibility with little to no preparation or support, thus placing them at risk for psychological distress [3].
Despite the critically important care FCs provide to patients, FCs often experience negative physical and psychological consequences themselves related to caregiving demands [3–6]. Prior studies of FCs have shown that they report a substantial symptom burden, including fatigue and sleep disturbance [7]. Additionally, caregiving may result in psychological symptoms, such as depression and anxiety [8]. The burdens of caregiving may not only have detrimental effects on FCs' quality of life (QoL) [9], but also compromise the ability of FCs to provide the necessary assistance to their loved one [10]. Thus, FCs of patients with cancer are at-risk for experiencing emotional distress related to their caregiving responsibilities, and efforts to determine factors associated with this distress are needed in order to identify those at highest risk.
Although FCs may experience considerable psychological distress, research identifying salient factors associated with FC depression and anxiety are lacking. Characteristics related to both the FC and the patient likely play a role, including demographic factors such as their age and gender, but more specific risk factors for FC depression and anxiety are largely unknown [6, 9, 11]. Additionally, studies suggest that patients' prognostic understanding and coping strategies correlate with their psychological distress, yet the relationship between these patient-reported outcomes and FC psychological distress remains unclear [12–14]. To develop effective interventions targeting FC psychological distress, we need to better understand the factors associated with FC depression and anxiety.
In the present study, we sought to describe rates of depression and anxiety symptoms in FCs of patients with incurable cancer and identify factors associated with FC psychological distress. By studying the relationship between FC psychological distress and characteristics of both the patient and FC, we hope to identify those who may benefit from targeted interventions focused on addressing FCs' distress.
methods
We performed a cross-sectional analysis of baseline data from a randomized, controlled trial of early palliative care integrated with standard oncology care versus standard oncology care alone that enrolled patients newly diagnosed with incurable cancer and their FCs. For the current study, we analyzed data from both arms of the trial, but participants completed baseline measures before patient randomization and notification of study arm allocation. Study staff subsequently obtained clinical data from the medical record. The Dana Farber/Harvard Cancer Care Institutional Review Board approved the study protocol.
participants
All of the medical oncologists in the thoracic and gastrointestinal (GI) oncology clinics at Massachusetts General Hospital Cancer Center (Boston, MA) agreed to approach, recruit, and obtain consent from their patients. Study staff screened all patients presenting to the thoracic and GI oncology clinics and notified the treating clinicians via email regarding patient eligibility. For any patients not enrolled, study staff contacted the clinical staff in order to document reasons for study refusal.
Patient eligibility included: (i) incurable lung or non-colorectal GI cancer diagnosed within the previous 8 weeks; (ii) no prior therapy for metastatic disease; (iii) an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; (iv) age ≥18 years; (v) plan to receive care at the participating institution; and (vi) the ability to read and respond to questions in English or with minimal assistance from family or an interpreter. We excluded patients who were already receiving consultation from the palliative care service, needed immediate referral for palliative care or hospice, or had significant psychiatric or other comorbid illness that the treating clinicians felt prohibited informed consent or participation.
To identify patients with incurable disease, study staff reviewed clinical documentation in the electronic health record. If clinicians replied to the eligibility email that the patient was being treated with curative intent, we considered the patient ineligible for study participation. At the time of written informed consent, we asked study patients to identify their FC (e.g. relative or friend) upon whom they rely for help and who would likely accompany them to clinic visits. We invited this person to participate in the FC portion of the study. We did not exclude patients without an FC from participation in the trial, but for the current study, we limited our analyses to only the patients with a participating FC. FC eligibility included: (i) age ≥18 years; and (ii) the ability to read and respond to questions in English or with minimal assistance from family or an interpreter.
study measures
FCs self-reported their age, gender, race, ethnicity, religion, employment status, education level, presence of dependent children, their relationship status, whether they live with the patient, and their relationship to the patient. Patients completed a demographic questionnaire that included their race, ethnicity, religion, relationship status, income, and education level. We reviewed patients' electronic medical records to obtain information about their age, gender, cancer diagnosis and stage, ECOG performance status, and cancer therapy.
We measured FC and patient depression and anxiety symptoms using the Hospital Anxiety and Depression Scale (HADS), a 14-item questionnaire that contains two 7-item subscales assessing depression and anxiety symptoms during the past week [15]. Scores on each subscale range from 0 to 21, with scores higher than 7 denoting clinically significant depression or anxiety.
We assessed FC QoL using the Medical Outcomes Study 36-item Short Form Health Survey (SF-36), which provides a mental component summary and physical component summary (PCS) [16]. Higher scores for each component summary indicate better QoL. Additionally, we assessed patients' QoL using the Functional Assessment of Cancer Therapy-General (FACT-G) [17]. The FACT-G contains 28 items with subscales assessing well-being across four domains (physical, functional, emotional, and social) during the past week, with scores ranging from 0 to 112, and higher scores indicating better QoL.
We administered the Brief COPE to assess patients' use of different coping strategies. The Brief COPE is a 28-item questionnaire measuring 14 coping methods with two items for each method [18]. To minimize questionnaire burden for participants, we limited our assessment to the following seven coping strategies which we felt were most appropriate for our study population: active, denial, emotional support, behavioral disengagement, positive reframing, self-blame, and acceptance. Scores on each scale range from 2 to 8, with higher scores indicating greater use of that particular coping strategy.
To understand perceptions of prognosis, we used an item with established content validity, asking patients to choose the primary goal of their current cancer treatment from the following options: ‘to lessen suffering’, ‘to be able to keep hoping’, ‘to make sure I have done everything’, ‘to extend my life as long as possible’, ‘to cure my cancer’, ‘to help cancer research’, and ‘other’ [12]. Consistent with prior work, we considered the response ‘to cure my cancer’ to denote inaccurate prognostic understanding.
statistical analysis
We calculated descriptive statistics to analyze the frequencies, means, and standard deviations (SDs) of the study variables. We compared rates of clinically significant depression and anxiety symptoms (HADS subscale scores >7) between FCs and patients using McNemar's test. To identify potential factors associated with FC depression and anxiety (including both FC- and patient-related factors), we used multivariable linear regression modeling with purposeful selection of covariates [19]. We pre-specified the inclusion of age and gender regardless of their significance level into the models, given their known association with depression and anxiety [6, 11, 14]. We then tested the associations between FC-reported HADS-depression and both FC-related and patient-related factors, including sociodemographic, clinical, and self-reported factors. We incorporated covariates that were associated at a significance level <0.10 and any confounders (defined as any variable that changed the parameter estimate of another variable by >20% when removed from the model) into the final model. Similarly, we used multivariable linear regression modeling with purposeful selection of covariates to identify FC- and patient-related factors associated with FC-reported HADS-anxiety.
results
We enrolled 350 patients and 275 (78.6%) of their FCs from May 2011 to July 2015 in the randomized trial. Most of the FCs were white (93.1%), female (69.1%), and married to the patient (66.2%) (Table 1). Patients were primarily white (93.1%) and less than half were female (44.4%). Nearly one-third (32.7%, 82/251) of patients reported that the primary goal of their current cancer treatment was ‘to cure my cancer’.
Table 1.
Baseline characteristics of family caregivers and patients
| Characteristic | Family caregivers (n = 275) |
Patients (n = 275) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Age, years, mean (SD) | 57.4 (13.6) | 65.4 (10.7) | ||
| Female gender | 190 | 69.1 | 122 | 44.4 |
| Race | ||||
| White | 256 | 93.1 | 256 | 93.1 |
| Asian | 8 | 2.9 | 5 | 1.8 |
| African American | 6 | 2.2 | 6 | 2.2 |
| American Indian or Alaska Native | 1 | 0.4 | 4 | 1.5 |
| Other | 4 | 1.5 | 4 | 1.5 |
| Hispanic or Latino ethnic group | 8 | 3.1 | 7 | 2.6 |
| Relationship status | ||||
| Married | 222 | 80.7 | 206 | 74.9 |
| Single, never married | 30 | 10.9 | 21 | 7.6 |
| Divorced/separated | 8 | 2.9 | 25 | 9.1 |
| Widowed | 5 | 1.8 | 23 | 8.4 |
| Missing | 10 | 3.6 | 0 | 0.0 |
| Lives with patient | — | — | ||
| Yes | 208 | 75.6 | ||
| No | 57 | 20.7 | ||
| Missing | 10 | 3.6 | ||
| Relationship to patient | ||||
| Married | 182 | 66.2 | — | — |
| Child | 51 | 18.5 | — | — |
| Friend | 12 | 4.4 | — | — |
| Sibling | 12 | 4.4 | — | — |
| Parent | 6 | 2.2 | — | — |
| Non-cohabitating relationship | 2 | 0.7 | ||
| Other | 9 | 3.3 | — | — |
| Missing | 1 | 0.4 | — | — |
| Dependent children | 55 | 20.0 | 33 | 12.0 |
| Religion | ||||
| Catholic | 161 | 58.5 | 160 | 58.2 |
| Protestant | 45 | 16.4 | 50 | 18.2 |
| Jewish | 11 | 4.0 | 13 | 4.7 |
| Muslim | 2 | 0.7 | 1 | 0.4 |
| None | 27 | 9.8 | 31 | 11.3 |
| Other | 28 | 10.2 | 19 | 6.9 |
| Missing | 1 | 0.4 | 1 | 0.4 |
| Currently working | 153 | 55.6 | — | — |
| Education level | ||||
| ≤High school | 73 | 26.5 | 104 | 37.8 |
| >High school | 201 | 73.1 | 171 | 62.2 |
| Missing | 1 | 0.4 | 0 | 0.0 |
| Cancer type | ||||
| Lung | — | — | 149 | 54.2 |
| GI | — | — | 126 | 45.8 |
| ECOG performance status | ||||
| 0 | — | — | 71 | 25.8 |
| 1 | — | — | 177 | 64.4 |
| 2 | — | — | 27 | 9.8 |
SD, standard deviation; GI, gastrointestinal; ECOG, Eastern Cooperative Oncology Group.
The proportion of FCs and patients reporting depressive symptoms did not significantly differ (16.4% versus 21.5%, P = 0.13) (Figure 1). However, a greater proportion of FCs reported anxiety symptoms compared with patients (42.2% versus 28.4%, P < 0.001).
Figure 1.
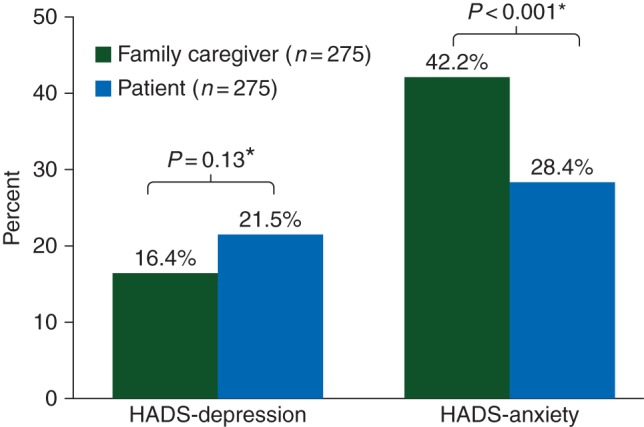
Family caregiver and patient depression and anxiety. *P values compare rates of clinically significant depression and anxiety symptoms (HADS subscale scores >7) between FCs and patients using McNemar's test. HADS, Hospital Anxiety and Depression Scale.
Using linear regression, we observed associations between FC depression and both FC- and patient-related factors (Table 2). Among the FC factors, greater anxiety (B = 0.50, SE = 0.04, P < 0.001) was significantly associated with worse FC depression. Among the patient factors, greater depression (B = 0.10, SE = 0.04, P = 0.03), worse social well-being (B = −0.13, SE = 0.05, P = 0.01), reporting that the primary goal of their current cancer treatment was ‘to cure my cancer’ (B = 0.72, SE = 0.33, P = 0.03), greater use of emotional support (B = 0.69, SE = 0.21, P = 0.001), and less use of acceptance coping (B = −0.42, SE = 0.11, P < 0.001) were associated with greater FC depression.
Table 2.
Multivariable model of characteristics associated with family caregiver depression
| Covariates | Unstandardized |
Standardized |
|||
|---|---|---|---|---|---|
| B | SE | 95% CI | β | P value | |
| Family caregiver | |||||
| Age | −0.01 | 0.01 | −0.03 to 0.02 | −0.03 | 0.55 |
| Female | 0.29 | 0.35 | −0.39 to 0.98 | 0.04 | 0.40 |
| Working | −0.21 | 0.36 | −0.92 to 0.51 | −0.03 | 0.57 |
| HADS-anxiety | 0.50 | 0.04 | 0.43 to 0.57 | 0.63 | <0.001 |
| Patient | |||||
| HADS-depression | 0.10 | 0.04 | 0.01 to 0.18 | 0.11 | 0.03 |
| FACT-G social well-being | −0.13 | 0.05 | −0.23 to −0.03 | −0.13 | 0.01 |
| Reports primary treatment goal: ‘to cure my cancer’ | 0.72 | 0.33 | 0.06 to 1.38 | 0.10 | 0.03 |
| Positive reframing coping | 0.15 | 0.09 | −0.04 to 0.33 | 0.08 | 0.12 |
| Emotional support coping | 0.69 | 0.21 | 0.28 to 1.10 | 0.17 | 0.001 |
| Acceptance coping | −0.42 | 0.11 | −0.63 to −0.20 | −0.19 | <0.001 |
R2 = 0.570, Adjusted R2 = 0.551, F(10 230) = 30.493, P < 0.001.
SE, standard error; HADS, Hospital Anxiety and Depression Scale; Functional Assessment of Cancer Therapy-General (FACT-G).
We also found associations between FC anxiety and multiple FC and patient factors (Table 3). Among the FC factors, younger age (B = −0.04, SE = 0.02, P = 0.047), female gender (B = 0.83, SE = 0.40, P = 0.04), being married to the patient (B = 1.66, SE = 0.47, P < 0.001), Catholic religion (B = 1.09, SE = 0.37, P = 0.004), higher SF-36 PCS (B = 0.05, SE = 0.02, P = 0.01), and greater HADS-depression (B = 0.80, SE = 0.05, P < 0.001) were significantly associated with worse FC anxiety. Among the patient factors, less use of emotional support coping (B = −0.70, SE = 0.20, P < 0.001) was associated with greater FC anxiety.
Table 3.
Multivariable model of characteristics associated with family caregiver anxiety
| Covariates | Unstandardized |
Standardized |
|||
|---|---|---|---|---|---|
| B | SE | 95% CI | β | P value | |
| Family caregiver | |||||
| Age | −0.04 | 0.02 | −0.07 to −0.001 | −0.11 | 0.047 |
| Female | 0.83 | 0.40 | 0.04 to 1.62 | 0.09 | 0.04 |
| Married to patient | 1.66 | 0.47 | 0.74 to 2.60 | 0.18 | <0.001 |
| Catholic religion | 1.09 | 0.37 | 0.36 to 1.82 | 0.12 | 0.004 |
| SF-36 PCS | 0.05 | 0.02 | 0.01 to 0.10 | 0.11 | 0.01 |
| HADS-depression | 0.80 | 0.05 | 0.70 to 0.90 | 0.64 | <0.001 |
| Patient | |||||
| Lives with dependent children | −0.88 | 0.62 | −2.11 to 0.34 | −0.07 | 0.16 |
| Emotional support coping | −0.70 | 0.20 | −1.10 to −0.31 | −0.15 | <0.001 |
R2 = 0.566, Adjusted R2 = 0.552, F(8256) = 41.668, P < 0.001.
SE, standard error; SF-36, Medical Outcomes Study Short Form-36 Health Survey; PCS, physical component summary; HADS, Hospital Anxiety and Depression Scale.
discussion
We found high levels of depression and anxiety in patients with incurable cancer and their FCs. Notably, the rate of anxiety in FCs exceeded that of the patients. Additionally, we are the first to demonstrate how patients' coping strategies relate to FCs' psychological distress. Importantly, we also discovered a novel association between patient prognostic understanding and FC depression. Collectively, our findings highlight the substantial emotional morbidity experienced by FCs of patients with incurable cancer and help identify those at greatest risk for psychological distress who may benefit from targeted palliative care and psychosocial interventions.
Nearly half of the FCs in our study reported high rates of anxiety symptoms, a significantly larger proportion than the patients. Patients in our study were newly diagnosed with incurable cancer, which may help explain why FCs reported such high anxiety rates. Soon after diagnosis, family and friends are thrust into their new caregiver role with little preparation [3, 20]. This lack of preparation may result in low confidence in their caregiving ability and anxiety related to the uncertain future demands of the FC role [20, 21]. Additionally, patients with incurable cancer often experience high symptom burden which may place added strain on the FC [7, 21, 22]. To better understand the nature of FC anxiety, investigators should explore the relationships among FC psychological distress, caregiving self-efficacy, and the patients' symptom burden [23]. FCs represent a vulnerable population, at particularly high risk for psychological distress. Thus, interventions targeting FCs' anxiety earlier in the patients' disease course may prevent ongoing FC burden and enhance their ability to provide assistance to the patient.
To our knowledge, this is the first study to show that patients' coping strategies are associated with FC depression and anxiety. Notably, emotional support coping by the patient was associated with higher FC depression, yet less anxiety. The items assessing emotional support coping ask patients to report if they are getting emotional support or comfort and understanding from someone [18]. FCs in our study likely represent those to whom the patient is referring when responding to these questions. Thus, FC provision of emotional support to the patient may help relieve FC anxiety and worry, but also engender a sense of sadness related to their loved one's life-limiting illness. Provision of emotional support is an important component of the FC role, and to fully understand this relationship between patients' use of emotional support coping and FC depression and anxiety will require further investigation. We also demonstrated that patients' use of acceptance coping was significantly associated with lower depression in FCs. While this finding supports the adaptive nature of this coping strategy, it also suggests that patients' use of adaptive coping strategies, such as those that are more positive or constructive, may improve both patient and FC outcomes [24]. By understanding the relationship between patients' use of certain coping strategies and FC psychological distress, we can begin to design interventions that encourage patients to utilize more adaptive coping mechanisms.
Interestingly, we found a novel relationship between patients' prognostic understanding and FC depression. Specifically, patient report that their primary goal of their current cancer treatment was ‘to cure my cancer’ was significantly associated with worse FC depression. This finding is hypothesis-generating and suggests that overly optimistic patient perceptions of prognosis may contribute to FC depression or vice versa. Additionally, nearly one-third of patients chose this treatment goal, thus indicating high levels of inaccurate prognostic understanding in this sample of patients with incurable cancer. Research suggests that both patients and their FCs desire honest prognostic disclosure, and our findings further support the importance of discussions to ensure accurate prognostic understanding among patients with incurable cancer [25, 26]. Until now, no studies had investigated how cancer patients' prognostic understanding influences FC depression and further work is undeniably needed to better understand the mechanisms underlying how patients' prognostic understanding relates to FCs' psychological distress.
Several limitations of our study warrant discussion. First, we carried out this study at an academic cancer center with a patient and FC sample lacking racial and ethnic diversity. Secondly, we did not collect information about patient or FC use of additional services, such as social work, financial, or mental health services, which may affect FC distress. However, we collected these data early in the patients' disease course, thus participants were unlikely to have received these services. In addition, our analyses do not account for patient and FC mental health history. Thirdly, as this is a cross-sectional analysis, we cannot confirm the directionality of the associations we found. Fourthly, to understand patients' perceptions of their prognosis, we asked them to choose the primary goal of their current cancer treatment, rather than other potentially related constructs such as an estimate of their life expectancy. Further, patients in this sample were only recently diagnosed with incurable cancer and patients' perceptions of their prognosis may change over time. Additionally, we did not collect information about other strategies that this patient and FC population may use to cope with illness (e.g. religious coping or substance use). Finally, this study lacks information regarding the change in FC psychological distress over time and how the factors associated with this distress vary throughout patients' cancer course. Further investigation is needed to assess the longitudinal trajectory of FCs' psychological distress, as their needs and those of the patient likely change as the disease progresses.
In summary, we demonstrated that patients with incurable cancer and their FCs experience high rates of depression and anxiety symptoms, with FC anxiety levels exceeding those of the patients. Importantly, factors associated with FC psychological distress include characteristics related to both the FC and the patient, highlighting the need to design interventions that target both of these groups. Additionally, patients' coping strategies and prognostic understanding are associated with FCs' psychological distress. Notably, these are potentially modifiable factors for clinicians to consider when addressing patient and FC depression and anxiety. Future efforts to address the substantial psychological distress experienced by FCs of patients with incurable cancer and meet the needs of this vulnerable population are warranted, ideally accounting for both patient and FC factors.
funding
This work was supported by the National Institute of Nursing Research R01 (NR012735 to JST); and the National Cancer Institute K24 (CA181253 to JST).
disclosure
The authors have declared no conflicts of interest.
references
- 1.Siegel K, Raveis VH, Houts P, Mor V. Caregiver burden and unmet patient needs. Cancer 1991; 68: 1131–1140. [DOI] [PubMed] [Google Scholar]
- 2.Given BA, Given CW, Kozachik S. Family support in advanced cancer. CA Cancer J Clin 2001; 51: 213–231. [DOI] [PubMed] [Google Scholar]
- 3.van Ryn M, Sanders S, Kahn K et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology 2011; 20: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron JI, Franche RL, Cheung AM, Stewart DE. Lifestyle interference and emotional distress in family caregivers of advanced cancer patients. Cancer 2002; 94: 521–527. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, Shaffer KM, Carver CS, Cannady RS. Quality of life of family caregivers 8 years after a relative's cancer diagnosis: follow-up of the National Quality of Life Survey for Caregivers. Psychooncology 2016; 25: 266–274. [DOI] [PubMed] [Google Scholar]
- 6.Park B, Kim SY, Shin JY et al. Prevalence and predictors of anxiety and depression among family caregivers of cancer patients: a nationwide survey of patient-family caregiver dyads in Korea. Support Care Cancer 2013; 21: 2799–2807. [DOI] [PubMed] [Google Scholar]
- 7.Palos GR, Mendoza TR, Liao KP et al. Caregiver symptom burden: the risk of caring for an underserved patient with advanced cancer. Cancer 2011; 117: 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grov EK, Dahl AA, Moum T, Fossa SD. Anxiety, depression, and quality of life in caregivers of patients with cancer in late palliative phase. Ann Oncol 2005; 16: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 9.Wadhwa D, Burman D, Swami N et al. Quality of life and mental health in caregivers of outpatients with advanced cancer. Psychooncology 2013; 22: 403–410. [DOI] [PubMed] [Google Scholar]
- 10.Northouse LL, Mood D, Kershaw T et al. Quality of life of women with recurrent breast cancer and their family members. J Clin Oncol 2002; 20: 4050–4064. [DOI] [PubMed] [Google Scholar]
- 11.Drabe N, Klaghofer R, Weidt S et al. Mutual associations between patients’ and partners’ depression and quality of life with respect to relationship quality, physical complaints, and sense of coherence in couples coping with cancer. Psychooncology 2015; 24: 442–450. [DOI] [PubMed] [Google Scholar]
- 12.El-Jawahri A, Traeger L, Park ER et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer 2014; 120: 278–285. [DOI] [PubMed] [Google Scholar]
- 13.Tarakeshwar N, Vanderwerker LC, Paulk E et al. Religious coping is associated with the quality of life of patients with advanced cancer. J Palliat Med 2006; 9: 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nipp RD, El-Jawahri A, Fishbein JN et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer 2016; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 17.Cella DF, Tulsky DS, Gray G et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993; 11: 570–579. [DOI] [PubMed] [Google Scholar]
- 18.Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med 1997; 4: 92–100. [DOI] [PubMed] [Google Scholar]
- 19.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujinami R, Sun V, Zachariah F et al. Family caregivers’ distress levels related to quality of life, burden, and preparedness. Psychooncology 2015; 24: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher KL, Stewart BJ, Archbold PG. Mutuality and preparedness moderate the effects of caregiving demand on cancer family caregiver outcomes. Nurs Res 2007; 56: 425–433. [DOI] [PubMed] [Google Scholar]
- 22.Teunissen SC, Wesker W, Kruitwagen C et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage 2007; 34: 94–104. [DOI] [PubMed] [Google Scholar]
- 23.Hendrix CC, Bailey DE Jr, Steinhauser KE et al. Effects of enhanced caregiver training program on cancer caregiver's self-efficacy, preparedness, and psychological well-being. Support Care Cancer 2016; 24: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou ES, Penedo FJ, Bustillo NE et al. Longitudinal effects of social support and adaptive coping on the emotional well-being of survivors of localized prostate cancer. J Support Oncol 2010; 8: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang ST, Liu TW, Tsai CM et al. Patient awareness of prognosis, patient-family caregiver congruence on the preferred place of death, and caregiving burden of families contribute to the quality of life for terminally ill cancer patients in Taiwan. Psychooncology 2008; 17: 1202–1209. [DOI] [PubMed] [Google Scholar]
- 26.Clayton JM, Butow PN, Tattersall MH. The needs of terminally ill cancer patients versus those of caregivers for information regarding prognosis and end-of-life issues. Cancer 2005; 103: 1957–1964. [DOI] [PubMed] [Google Scholar]


