Abstract
Background and Purpose
The opioid antagonist nalmefene (selincro®) was approved for alcohol‐related disorders by the European Medicines Agency in 2013. However, there have been no studies regarding the effectiveness of nalmefene when alcohol is used in combination with cocaine.
Experimental Approach
Using operant alcohol self‐administration in Wistar rats and qRT‐PCR, we evaluated (i) the dose–response curve for s.c. and p.o. nalmefene; (ii) the effects of nalmefene with increasing concentrations of alcohol; (iii) the efficacy of nalmefene on cocaine‐potentiated alcohol responding; and (iv) the gene expression profiles of histone deacetylases (Hdac1–11) in peripheral blood in vivo and in the prefrontal cortex, heart, liver and kidney post mortem.
Key Results
S.c. (0.01, 0.05, 0.1 mg·kg−1) and p.o. (10, 20, 40 mg·kg−1) nalmefene dose‐dependently reduced alcohol‐reinforced responding by up to 50.3%. This effect of nalmefene was not dependent on alcohol concentration (10, 15, 20%). Cocaine potentiated alcohol responding by approximately 40% and nalmefene (0.05 mg·kg−1) reversed this effect of cocaine. Alcohol increased Hdac gene expression in blood and nalmefene prevented the increases in Hdacs 3, 8, 5, 7, 9, 6 and 10. In the other tissues, alcohol and nalmefene either did not alter the gene expression of Hdacs, as in the prefrontal cortex, or a tissue‐Hdac‐specific effect was observed.
Conclusions and Implications
Nalmefene might be effective as a treatment for alcohol‐dependent patients who also use cocaine. Also, the expression of Hdacs in peripheral blood might be useful as a biomarker of alcohol use and drug response.
Abbreviations
- Hdac
histone deacetylase
- qRT‐PCR
quantitative RT‐PCR
Tables of Links
| TARGETS | ||||
|---|---|---|---|---|
| GPCRs a | Enzymes b | |||
| μ receptor | HDACs | Hdac3 | Hdac6 | Hdac9 |
| δ receptor | Hdac1 | Hdac4 | Hdac7 | Hdac10 |
| κ receptor | Hdac2 | Hdac5 | Hdac8 | Hdac11 |
| LIGANDS |
|---|
| Cocaine |
| Naltrexone |
| Nalmefene |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a, 2015b).
Introduction
Nalmefene, formerly known as nalmetrene, is an opioid antagonist that is structurally similar to the μ‐opioid receptor antagonist naltrexone. Nalmefene is obtained by the replacement of the ketone group at the six‐position of naltrexone with a methylene group (Hahn and Fishman, 1975). This subtle structural change seems to explain the increased affinity of nalmefene for the μ‐opioid receptor and its longer half‐life compared with naltrexone (Weinstein et al., 1971). Animal preclinical studies demonstrated that nalmefene is effective in reducing alcohol consumption and that it could be used as an alternative pharmacotherapy to naltrexone (June et al., 1998). In 2013, the results from several clinical trials demonstrated that nalmefene could be used to reduce alcohol consumption in patients with alcohol dependence (van den Brink et al., 2013; Gual et al., 2013; Mann et al., 2013). As a result, selincro® (nalmefene hydrochloride dehydrate), marketed by the Danish pharmaceutical company Lundbeck, was approved for alcohol‐related disorders by the European Medicines Agency (EMA) in 2013.
In 2015, the first clinical experiment in which nalmefene was used for the treatment of cocaine‐related behaviours (i.e. cocaine craving) was published (Grosshans et al., 2015). These authors found that nalmefene caused an abatement of craving for cocaine and prevented relapse of cocaine consumption. This is an interesting point because it has been estimated that for patients with an alcohol disorder the likelihood of having other addictive disorders is seven times greater than in the rest of the population (Regier et al., 1990). In fact, 50–90% of cocaine‐dependent subjects are also likely to be dependent on alcohol as well (Lacoste et al., 2010), and there are studies demonstrating that the concurrent abuse of alcohol and cocaine increases the incidence of neurological and cardiac emergencies (Farooq et al., 2009). Also, the co‐consumption of alcohol and cocaine is common among recreational drug users. The concurrent use of alcohol and cocaine increases between 47 and 58% during the weekend compared with week days (Rodríguez‐Álvarez et al., 2015). Therefore, one of the aims of the present study was to examine the effects of the opioid antagonist nalmefene on alcohol seeking and identify its effects on alcohol‐cocaine interactions.
Several authors have suggested that alcohol and cocaine are linked to the differential expression profiling of chromatin modification enzymes (Botia et al., 2012; Kennedy and Harvey, 2015) and that the expression of histone deacetylase genes (Hdac1–11) in peripheral blood might be used as a biomarker for the pathophysiology of psychiatric disorders (Hobara et al., 2010). That means that the diagnosis, prognosis and pharmacological responses of alcoholic individuals could potentially benefit from using the gene expression profile of Hdacs as a biomarker. In this context, we have recently demonstrated that an increase in Hdac gene expression within the peripheral blood is associated with chronic alcohol consumption and that pharmacological treatments can prevent alcohol‐induced changes in genes involved in epigenetic mechanisms, such as DNA methyltransferases (Echeverry‐Alzate et al., 2014; López‐Moreno et al., 2015). For these reasons, the second aim of this study was to investigate whether a gene expression profile of Hdacs could be linked to the behavioural results obtained.
Methods
Group sizes, randomization and blinding
The exact group size for each experimental group has been provided for every group/condition within the figure legends. All the values correspond to independent values, not replicates, including values from the alcohol and benzoylecgonine analysis and quantitative RT‐PCR (qRT‐PCR) experiments. The minimum group size was n = 7 for the nalmefene group in the later experiments (Figures 6, 7, 8). Each animal was randomly assigned to an experimental group, and the nalmefene treatment order was fully counterbalanced. The researchers treating the animals (by injection or p.o.) were aware of the pharmacological treatments of each group. Nevertheless, the responses of the animals, to acquire alcohol, were automatically registered by the software, and the experimenters had no access to the administration panel. In addition, the statistical analysis was performed by different researchers.
Figure 6.
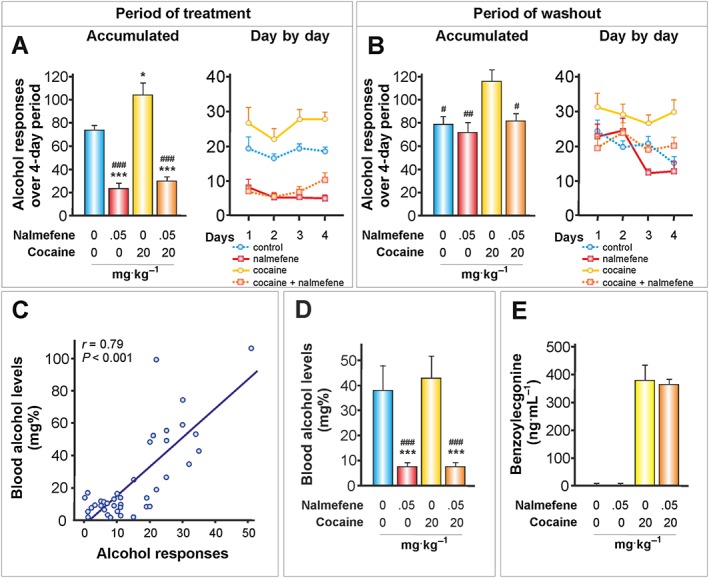
The effects of nalmefene on cocaine‐alcohol interactions (I): before alcohol self‐administration. Cocaine (20 mg·kg−1, i.p.) was injected daily 18 h before the operant alcohol self‐administration session, and nalmefene was administered s.c. 20 min before the alcohol session. (A, B) Data represent the mean ± SEM of the accumulated or day by day alcohol responses to 20% v·v−1 alcohol (n = 11, 7, 11, 11 per group). * P < 0.05, *** P < 0.001 compared with the vehicle group; # P < 0.05, ## P < 0.01, ### P < 0.001 compared with the cocaine group. (C) Scatter plot of alcohol responses during the 30‐min alcohol session and blood alcohol levels determined immediately after the test session. Alcohol responses were significantly correlated with the blood alcohol levels (n = 11, 7, 11, 11 per group). (D) Nalmefene reduced the blood alcohol levels according to the reduction in the number of alcohol responses independently of cocaine treatment. *** P < 0.001 compared with the vehicle group; ### P < 0.001 compared with the cocaine group (n = 11, 7, 11, 11 per group). (E) Benzoylecgonine (ng·mL−1) was examined 18.5 h after cocaine administration and immediately after alcohol self‐administration (n = 10–10 per group). Nalmefene did not alter the metabolism of cocaine.
Figure 7.
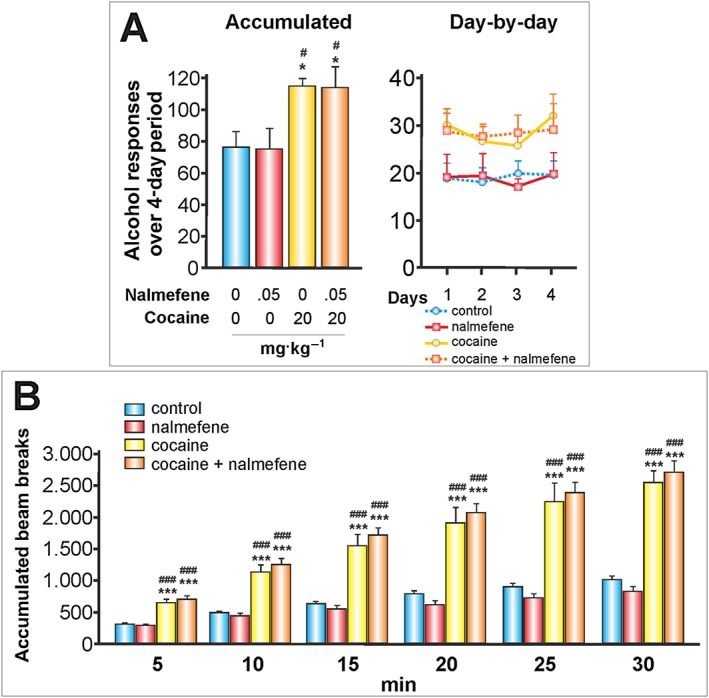
The effects of nalmefene on cocaine‐alcohol interactions (and II): before cocaine administration. Nalmefene was injected s.c. 20 min before the administration of cocaine (20 mgKg, i.p.). Nalmefene administered 18 h before alcohol self‐administration was not effective in reducing alcohol responses or in preventing cocaine‐induced increases in alcohol responses. Nalmefene was not effective in preventing cocaine‐induced locomotor sensitization. (A) Data represent the mean ± SEM of the accumulated or day by day operant alcohol responses to 20% v·v−1 alcohol (n = 11, 7, 11, 11 per group). * P < 0.05 compared with the vehicle group; # P < 0.05 compared with the nalmefene group. (B) Motor activity, mean ± SEM of the accumulated beam breaks for the 30 min after cocaine administration (n = 11, 7, 11, 11 per group). *** P < 0.001 compared with the vehicle group; ### P < 0.001 compared with the nalmefene group.
Figure 8.
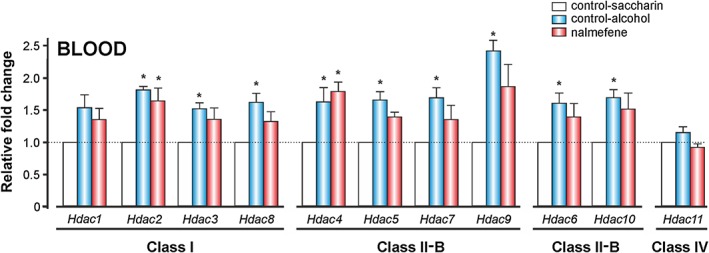
The effects of alcohol and nalmefene on Hdac gene expression in peripheral blood in vivo. The animals were treated with alcohol and nalmefene as described in Figure 6. Blood samples from the rat tail vein were collected in vivo immediately after alcohol self‐administration. Data represent the mean ± SEM (n = 9, 8, 7 per group) of the relative fold change obtained using the 2ΔCt method. * P ≤ 0.004 compared with the control‐saccharin group.
Normalization and statistical comparison
No data from the behavioural experiments were normalized. For the qRT‐PCR experiments, the 18S ribosomal RNA gene was used as an internal control for normalization according to the method described by Schmittgen and Livak (2008) in Nature Protocols and mentioned in the text (see below, Further Methods subheading).
For the statistical comparisons, only independent values have been used, and data are represented as the mean ± SEM. All the statistical analysis performed have been fully described (it has been noted when independent or repeated measures were used), and all the results and most relevant parameters from the ANOVA tests are shown in Table 1. The threshold for statistical significance was defined previously (P < 0.05) and maintained throughout all the manuscript, and when relevant, a Bonferroni correction was applied (i.e. in the gene expression study).
Table 1.
Results of the ANOVAs
| Figure 2 | A | C | ||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| nalmefene | 23.13 | 3, 79 | <0.0001 | nalmefene | 1.09 | 3, 82 | 0.36 | |
| days | 1.91 | 3, 237 | 0.13 | days | 6.66 | 1, 82 | <0.05 | |
| interaction | 1.58 | 9, 237 | 0.12 | interaction | 0.96 | 3, 82 | 0.41 | |
| B | D | |||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| nalmefene | 21.58 | 3, 81 | <0.0001 | nalmefene | 1.39 | 3, 81 | 0.25 | |
| days | 1.98 | 3, 243 | 0.12 | days | 6.02 | 1, 81 | <0.05 | |
| interaction | 1.26 | 9, 243 | 0.26 | interaction | 0.67 | 3, 81 | 0.57 | |
| Figure 3 | A | C | ||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| nalmefene | 23.52 | 3, 80 | <0.0001 | nalmefene | 0.98 | 3, 83 | 0.41 | |
| days | 1.48 | 3, 240 | 0.22 | days | 4.36 | 1, 83 | <0.05 | |
| interaction | 3.44 | 9, 240 | <0.005 | interaction | 1.44 | 3, 83 | 0.24 | |
| B | D | |||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| nalmefene | 17.98 | 3, 83 | <0.0001 | nalmefene | 0.81 | 3, 84 | 0.49 | |
| days | 1.69 | 3, 249 | 0.17 | days | 4.49 | 1, 84 | <0.05 | |
| interaction | 3.37 | 9, 249 | <0.005 | interaction | 1.00 | 3, 84 | 0.39 | |
| Figure 4 | A | B | ||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| nalmefene | 0.48 | 3, 36 | 0.69 | nalmefene | 2.41 | 3, 36 | 0.08 | |
| intervals | 287.30 | 5, 180 | <0.0001 | |||||
| interaction | 0.70 | 15, 180 | 0.78 | |||||
| C | D | |||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| nalmefene | 0.65 | 3, 37 | 0.58 | nalmefene | 0.59 | 3, 38 | 0.63 | |
| intervals | 261.37 | 5, 185 | <0.0001 | |||||
| interaction | 0.82 | 15, 185 | 0.65 | |||||
| Figure 5 | A | C | ||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| nalmefene | 33.51 | 2, 37 | <0.0001 | nalmefene | 0.11 | 2, 37 | 0.9 | |
| EtOH conc. | 132.87 | 2, 74 | <0.0001 | days | 2.45 | 3, 111 | 0.07 | |
| interaction | 3.77 | 4, 74 | <0.01 | interaction | 2.81 | 6, 111 | <0.05 | |
| B | D | |||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| nalmefene | 34.71 | 2, 37 | <0.0001 | nalmefene | 0.07 | 2, 37 | 0.94 | |
| EtOH conc. | 2.97 | 2, 74 | 0.06 | days | 3.57 | 3, 111 | <0.05 | |
| interaction | 5.35 | 4, 74 | <0.005 | interaction | 2.48 | 6, 111 | <0.05 | |
| Figure 6 | A | B | ||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| treatment | 31.44 | 3, 36 | <0.0001 | treatment | 5.92 | 3, 36 | <0.005 | |
| days | 3.26 | 3, 108 | <0.05 | days | 5.93 | 3, 108 | <0.005 | |
| interaction | 0.88 | 9, 108 | 0.54 | interaction | 2.13 | 9, 108 | <0.05 | |
| D | E | |||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| treatment | 8.05 | 3, 39 | <0.0001 | treatment | 0.06 | 1, 18 | 0.81 | |
| Figure 7 | A | B | ||||||
| Factor | F value | D.F. | Sig. | Factor | F value | D.F. | Sig. | |
| treatment | 5.23 | 3, 36 | <0.005 | treatment | 24.36 | 3, 36 | <0.0001 | |
| days | 1.75 | 3, 108 | 0.16 | intervals | 158.6 | 5, 180 | <0,0001 | |
| interaction | 0.70 | 9, 108 | 0.71 | interaction | 14.42 | 15, 180 | <0.0001 | |
| Figure 8 | Blood | |||||||
| Factor | F value | D.F. | Sig. | |||||
| Hdac | 4.42 | 10, 264 | <0.0001 | |||||
| treatment | 64.31 | 2, 264 | <0.0001 | |||||
| interaction | 1.51 | 20, 264 | 1.51 |
Validity of animal species, model selection, ethical statement and animal details
We used male Wistar rats in these studies is an abundance of information about the physiology, genetics, behaviour and cognitive functioning of these animals. Furthermore, we used the operant alcohol self‐administration model, which is known to be reliable, have a high face validity and predictive validity for humans (Koob et al., 2003). Fifty‐six male Wistar rats (Harlan, Barcelona, Spain) were used. Forty had access to alcohol, and 16 only had access to saccharin in the operant self‐administration procedures. Rats were purchased when they were 7 weeks old, and they weighed 375–475 g at the start of the pharmacological treatments. All research was conducted in strict adherence to the European Directive 2010/63/EU and Royal decree 53/2013 (BOE, 2013) on the protection of animals used for scientific purposes. The Ethics Committee of the Faculty of Psychology of the Complutense University of Madrid approved the study. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Experimental procedures, housing and husbandry and interpretation (3Rs)
The experimental procedures are detailed below, in the experimental design section. Since their arrival, animals were housed in groups of four per cage (transparent polycarbonate, 1.815 cm2 − Eco‐Pure Premium bedding from Datesand, Manchester, UK) in a specific pathogen‐free and temperature‐ and humidity‐controlled environment (21 ± 1°C), on a 12‐h reverse light/dark cycle (lights off at 08:00 h). Experimental sessions were performed during the dark phase. Food and water were available ad libitum except as specified below. The method of killing was by decapitation in order to obtain tissues from brain, heart, liver and kidney. All efforts were made to minimize animal suffering and to reduce the number of animals used. For instance, in order to reduce the number of Wistar rats, the same animals were used throughout the four studies described here (see Figure 1).
Figure 1.
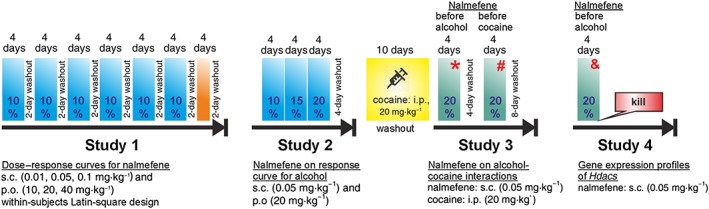
Experimental timeline. The animals were introduced daily to the operant alcohol self‐administration chambers without exception. The washout periods refer always to nalmefene. There was not a washout period for cocaine. The orange bar indicates when motor activity and strength/motor coordination tests were performed. * Denotes blood collection in vivo for the analysis of blood alcohol levels and levels of benzoylecgonine; # denotes evaluation of motor cocaine‐induced locomotor sensitization; & denotes blood collection in vivo for the analysis of Hdac gene expression.
Further methods
Experimental design
-
1
Study 1. Dose–response curves for s.c. and p.o. nalmefene
The aim of this study was to investigate the effects of nalmefene on alcohol self‐administration. For this purpose, we used an animal model with a high predictive validity for humans: operant alcohol self‐administration in rats (Koob et al., 2003). It has been reported that s.c. nalmefene is between 3.200‐ and 6.400‐fold more potent than p.o. nalmefene (June et al., 1998). Therefore, we performed two dose–response curves for nalmefene. In the first, nalmefene was administered s.c. (0.01, 0.05, 0.1 mg·kg−1) 20 min before the test, and in the second, it was administered p.o. (10, 20, 40 mg·kg−1) 90 min before. Each dose of nalmefene was administered over four consecutive days using a within‐subjects Latin‐square design with a 2‐day washout period between each dose. After it was observed that nalmefene significantly reduced the number of alcohol responses, we evaluated the motor activity and motor strength/coordination to rule out any impairment induced by nalmefene.
-
1
Study 2. The effects of nalmefene on a dose–response curve for alcohol
After ruling out any motor/coordination impairment, we examined the effectiveness of nalmefene on increasing doses of alcohol. This premise is supported by the fact that the alcohol content in alcoholic beverages is linked to the risk of becoming a heavy drinker, to the severity of alcohol dependence and to the adherence to treatments for alcoholism (Jensen et al., 2002; Baltieri et al., 2009). For this study, we chose the medium s.c. and p.o. doses of nalmefene (0.05 and 20 mg·kg−1, respectively), because they were the lowest effective doses for reducing alcohol responses and the g of alcohol Kg‐1 of body weight. The alcohol content (10, 15 and 20%) was increased every four consecutive days and was followed by a 4‐day washout period of nalmefene.
-
1
Study 3. The effects of nalmefene on alcohol‐cocaine interactions
Based on the results of study 2, in which 0.5 mg·kg−1 of nalmefene s.c. was found to be effective at reducing alcohol responses with all alcohol concentrations tested, we decided to use this dose of nalmefene for studying alcohol‐cocaine interactions. For this, we used the methodology previously employed by our group (Echeverry‐Alzate et al., 2012, 2014). It is noted that cocaine (20 mg·kg−1, i.p.) was injected 6 h after the self‐administration of alcohol. This means that the animals return to the operant chamber 18 h after the cocaine injection. Using this protocol, cocaine‐induced place‐conditioned motor sensitization and motor hyperactivity in the operant ethanol chambers would be avoided (Antoniou et al., 1998; Stromberg and Mackler, 2005). After a 10‐day period of daily injections of cocaine during which an increase in alcohol responses was observed, the animals were treated for four consecutive days with nalmefene before alcohol self‐administration. Blood samples were collected in vivo immediately after alcohol self‐administration on the fourth day to determine blood alcohol levels as well as the levels of benzoylecgonine, one of the two primary metabolites of cocaine.
To gain a deeper insight into the interaction between nalmefene and cocaine, following a new 4‐day washout period of nalmefene, the animals were treated with s.c. nalmefene 20 min before they were injected with cocaine. The goal was to differentiate between the effects of nalmefene before alcohol self‐administration and before the cocaine challenge.
-
1
Study 4. Gene expression profiles of Hdacs
After a final 8‐day washout period for nalmefene, we studied the gene expression profiles of Hdacs in peripheral blood in vivo. The interest in studying Hdac gene expression lies in its novelty as a mechanism that underlies excessive alcohol consumption (Pandey et al., 2008; Warnault et al., 2013) and its potential role as an alcohol biomarker (López‐Moreno et al., 2015). This study focused on this second aspect. For this, the same experimental conditions and results described in Figure 6 were replicated for four more days; during which time, nalmefene was again administered before alcohol self‐administration. Blood samples from the rat tail were collected in vivo immediately after the alcohol self‐administration session. Then, as a complementary study, they were collected post mortem to determine the gene expression profiles of Hdacs in the prefrontal cortex, heart, liver and kidney. The prefrontal cortex was chosen rather than other brain regions because of its major role in goal‐directed behaviours, its contribution to the development and maintenance of alcoholism (Lu and Richardson, 2014; Pfarr et al., 2015), and because nalmefene has a very high occupancy of μ‐opioid receptors in this brain area (Ingman et al., 2005). The animals were killed immediately after tail blood collection, and the appropriate tissues were collected.
Drugs and general procedures for pharmacological treatments
Nalmefene (17‐cyclopropylmethyl‐4, 5α‐epoxy‐6‐methylenemorphinan‐3,14‐diol) was provided as a hydrochloride dehydrate salt by the pharmaceutical company Lundbeck (Copenhagen, Denmark) as a generous gift. The doses of nalmefene were calculated based on the salt and administered either by p.o. gavage at a volume of 3 mL·kg−1 or s.c. at a volume of 1 mL·kg−1, in the scapular region. Nalmefene‐control animals were treated with water (p.o.) or saline (s.c.). According to its peak plasma concentration, nalmefene was injected s.c. or administered p.o., 20 and 90 min, respectively, before alcohol self‐administration. Because the absorption of nalmefene is affected by the presence of food in the stomach, the animals were deprived of food 12 h before the p.o. treatment with nalmefene or water (the control group) (EMA). The doses of nalmefene were chosen according to previous studies in Wistar rats in which the effects of nalmefene on alcohol self‐administration were investigated (June et al ., 1998; Walker and Koob, 2008).
Cocaine hydrochloride (Sigma‐Aldrich, S.L., Madrid, Spain) was dissolved in physiological saline and injected i.p. at a volume of 1 mL·kg−1. Cocaine‐control animals were injected with saline. The cocaine dose is expressed as the salt and was selected according to previous results (Echeverry‐Alzate et al., 2012, 2014). Alcohol solutions were prepared every 4 days from 96% alcohol (Alcoholes Aroca, S.L., Madrid, Spain).
Operant self‐administration and motor/coordination experiments
Apparatus and procedure
The operant alcohol sessions were conducted in eight modular chambers enclosed in sound‐attenuating cubicles (Med Associates Inc., St. Albans, VT, USA). The exhaust fans were inactivated because the fans increased the rate of alcohol evaporation. The chambers were equipped with two retractable levers located 7 cm above a grid floor on either side of a drinking reservoir positioned in the centre of the front panel of the chamber and 4 cm above the grid floor. The levers were counterbalanced to respond as the active lever (delivering 0.1 mL) or as the inactive lever. Auditory or visual cues were not present at any time. Training was conducted as follows: the rats were placed on a restricted water intake schedule for 12 h ranging from 2 to 4 days to facilitate the training in lever pressing. For the rest of the experiments, the animals had access to food and water ad libitum except as specified for the p.o. treatment with nalmefene. During the first 4 days of training, animals received a 1% w·v−1 saccharin solution (Sigma‐Aldrich, S.L., Madrid, Spain) in the dipper. Thereafter, the following sequence was used on a fixed‐ratio 1 schedule of reinforcement: 0.2% saccharin for three sessions, 0.2% saccharin and 0.2% alcohol for three sessions, 0.16% saccharin and 2% alcohol for three sessions, 0.12% saccharin and 4% alcohol for three sessions, 0.08% saccharin and 6% alcohol for three sessions, 0.04% saccharin and 8% alcohol for three sessions, 0.02% saccharin and 10% alcohol for three sessions and 10% alcohol for the rest of the sessions. The treatment with nalmefene started 19 days after 10% alcohol. Then, after the second study, 20% alcohol remained until the animals were killed. All the operant self‐administration sessions lasted 30 min under a fixed‐ratio 1 schedule 7 days a week for the entire study. Sixteen animals that had access only to saccharin (0.005%) and did not receive any pharmacological treatment during the study were used as the control group for the genetic expression experiments (the calibrator group – i.e. the non‐alcohol‐treated group).
The locomotor activity of the rats was assessed during 30 min using six custom‐made 40 × 35 × 35 cm rectangular boxes, and the boxes were equipped with eight photocells arranged in two lines (4 and 8 cm above the floor) that detected the locomotor activity as beam breaks. Strength and motor coordination were evaluated by the rod suspension test. The rat was held by the base of the tail and suspended over a horizontal rod (2 mm in diameter) until it grasped the rod with both forepaws. The body of the rat was then slowly lowered below the rod, and the animal had to support its body weight. The rod was suspended at a height of 60 cm above a soft blanket. The time from release of the suspended rat until it let go of the rod was the latency in seconds (maximum time allowed was 60 s). There were two trials the day before test (habituation), with an inter‐trial interval of 4 min, and one trial in the test day, after treatment with nalmefene or vehicle (Goettl et al ., 2001; Ingram et al ., 1994; Thullier et al ., 1996).
Alcohol and benzoylecgonine analysis
To determine blood alcohol concentrations and benzoylecgonine, 250 μL of blood was collected from the rat tail vein into a capillary tube (Microvette CB 300 K2E) that contained EDTA dipotassium salt. Each rat was placed on a towel on a table and held gently in place. The end of the tail was held, fixed between two fingers, onto the table. Using a scalpel, a diagonal incision, 2‐mm‐long, was made at 15 mm from the end of the tail. The collection of the blood took approximately 90 s, maximum. The whole blood was centrifuged for 15 min at 1500× g using a refrigerated centrifuge, and the plasma was stored at −80°C until use. The alcohol concentration was measured using the EnzyChrom ethanol assay kit following the protocol recommended by the manufacturer (Bioassay Systems, Hayward, CA, USA). All measurements were performed in duplicate. Benzoylecgonine, a main metabolite of cocaine, was measured using the Cocaine Metabolite Direct Elisa Benzoylecygonine Assay Kit, following the manufacturer's instructions (Bio‐Quant, Heidelberg, Germany).
Real time quantitative PCR experiments
Real time quantitative PCR was performed using a LightCycler 480‐II machine (Roche, Barcelona, Spain) with SYBR Green real time qPCR master mix (Applied Biosystems, Warrington, UK) and specific primers at 300 nm concentrations (see Supporting Information Table [Link]). The melting curves analysis showed only a single clear peak, and the sizes of the PCR products were confirmed by agarose gel electrophoresis. A 10‐fold dilution series of the template was used to amplify each gene to validate the efficiency of each assay and to confirm that the amplification efficiencies of the target and reference genes were comparable (indicated by a near‐zero slope value for both the target and reference genes). The 18S ribosomal RNA gene was used as an internal control for normalization. The saccharin‐vehicle group (non‐alcohol‐treated group) was used as a calibrator, and the 2–ΔΔCT method was used to analyse the expression data (Schmittgen and Livak, 2008).
Blood samples from the rat tail were collected in vivo using capillary tubes (Microvette CB 300 K2E) immediately after the alcohol self‐administration session as described above. Total RNA was isolated from whole blood using Trizol LS Reagent (Life Technologies, Carlsbad, USA). Then, the animals were killed by decapitation. Prefrontal cortex, heart, liver and kidney were collected and immediately dissected on ice and were quickly frozen on dry ice at −80°C. Total RNA was isolated using TriPure Isolation Reagent (Roche) and was stored at −80°C. One microgramme of total RNA was reverse transcribed into cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche).
Statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Four types of ANOVA were used according to the nature of the variables. Data from Figures 2, 3, 4A, 4C, 6A, 6B and 7 were analysed using a two‐way mixed ANOVA (within‐subjects: either days or intervals; between‐groups: treatment). Data from Figures 4B, 4D, 6D, 6E were analysed using a one‐way ANOVA. Data from Figure 5 were analysed using a three‐way mixed ANOVA (within‐subjects: days and ethanol concentrations; between‐groups: treatment). The data from Figure 8 and Supporting Information Fig. [Link] were analysed using a two‐way ANOVA for each tissue (between‐groups: Hdac × treatment). Bonferroni correction was applied for each tissue (P = 0.05/11 = 0.004), and thus, only P values ≤0.004 were considered significant. These analyses were performed after controlling for assumptions (e.g. Levene's test to assess variance homogeneity among groups), and the anomalous values detected through the spss box plot analysis (IBM, Chicago, IL, USA) were discarded. Except for Figure 8 and Supporting Information Fig. [Link], after confirming the significance of the primary findings using ANOVA, a significance level of P< 0.05 was applied to all remaining statistical analyses. The spss statistical software package (version 20.0) for Windows was used for all statistical analyses. All ANOVA results are detailed in Table 1.
Figure 2.
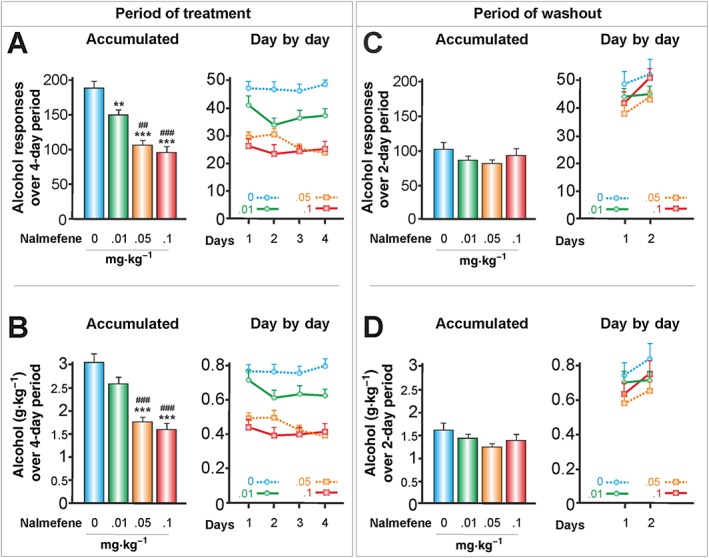
The effects of s.c. nalmefene on responses to alcohol and alcohol consumed in g kg‐1 body weight (30‐min session). Nalmefene was administered for four consecutive days with a washout period of 2 days between doses (Latin square design). According to its peak plasma concentration, nalmefene was injected s.c. 20 min before the operant alcohol self‐administration session (control group, n = 16; nalmefene groups, n = 24). (A, C) Data represent the mean ± SEM of the accumulated or day by day alcohol responses to 10% v·v−1 alcohol, and (B, D) represent their corresponding g alcohol consumed kg‐1 body weight. ** P < 0.01, *** P < 0.001 compared with the vehicle group; ## P < 0.01, ### P < 0.001 compared with the lowest dose of nalmefene. There were no significant differences throughout the withdrawal of nalmefene (washout period).
Figure 3.
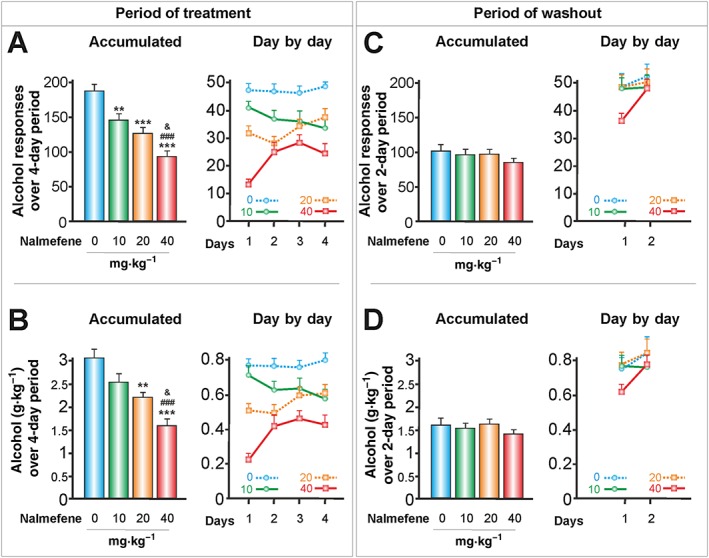
The effects of p.o. nalmefene on responses to alcohol and alcohol consumed in g kg‐1 body weight (30 min session). According to its peak plasma concentration, nalmefene was injected p.o. 90 min before the operant alcohol self‐administration session (control group, n = 16; nalmefene groups, n = 24). (A, C) Data represent the mean ± SEM of the accumulated or day by day alcohol responses to 10% v·v−1 alcohol, and (B, D) represent their corresponding g alcohol consumed kg‐1 body weight. ** P < 0.01, *** P < 0.001 compared with the vehicle group; ### P < 0.001 compared with the lowest dose of nalmefene; & P < 0.05 compared with the intermediate dose of nalmefene. There were no significant differences throughout the washout period. See Figure 1 legend for methodological details.
Figure 4.
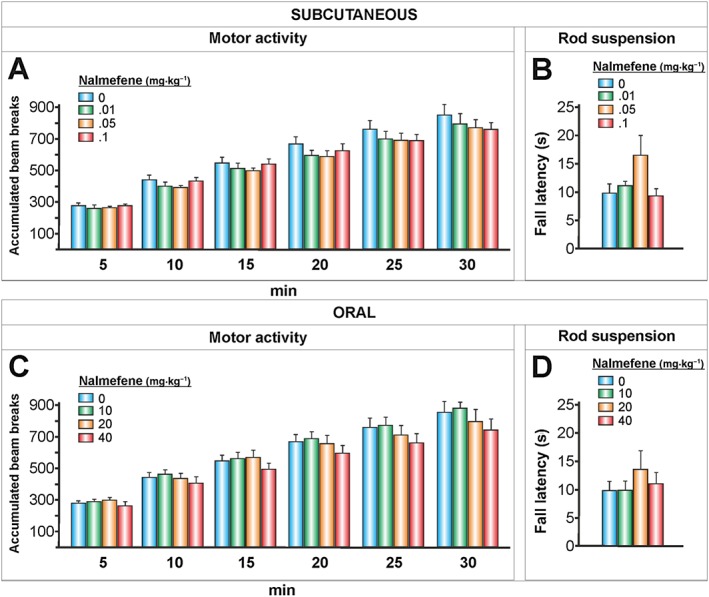
The effects of nalmefene on motor activity and strength/motor coordination. Nalmefene was injected s.c. or administered p.o. (20 and 90 min, respectively, according to its peak plasma concentration) before each test. Motor activity (A, C), mean ± SEM of the accumulated beam breaks for the 30 min following nalmefene administration (A, n = 10 per group; C, n = 11, 10, 10, 10 per group). Rod suspension (B, D), mean ± SEM of the latency to fall in seconds (B, n = 10 per group; D, n = 11, 11, 10, 10 per group) There were no significant differences in motor activity or strength/motor coordination.
Figure 5.
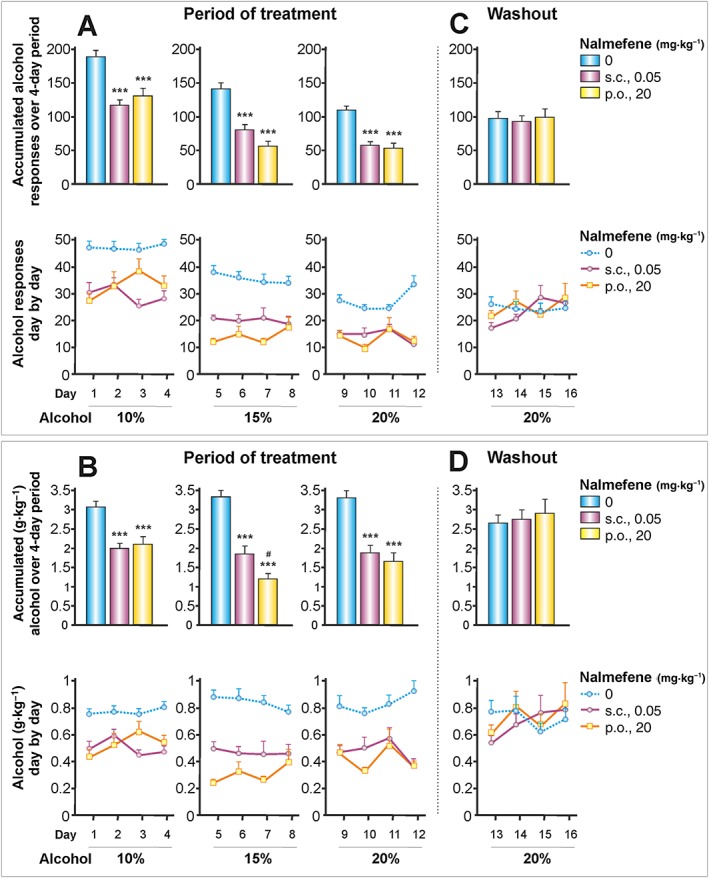
The effects of nalmefene on increased concentrations of alcohol. Nalmefene was injected s.c. or administered p.o. as previously described. The concentration of alcohol was increased every 4 days up to a maximum of 20% and was followed by a washout period of 4 days. (A–D) Data represent the mean ± SEM (n = 12, 14, 14 per group). Note that while the number of alcohol responses decreased according to the increase in alcohol content, the g alcohol consumed kg‐1 body weight remained stable. *** P < 0.001 compared with the vehicle group; # P < 0.05, compared with the s.c. dose of nalmefene. There were no significant differences in the washout period.
Results
-
1
Study 1. Dose–response curves for s.c. and p.o. nalmefene
S.c. and p.o. nalmefene reduced dose‐dependent levels of alcohol responses (Figures 2A and 3A) and the consumption of alcohol, as measured in g kg‐1 body weight (Figures 2B and 3B). With respect to accumulated alcohol responses over a 4‐day period, the highest doses of s.c. and p.o. nalmefene led to reductions of 49.5 and 50.3%, respectively, in alcohol responses. The lowest doses of s.c. and p.o. nalmefene caused a significant reduction in alcohol responses, but they did not reduce the consumption of alcohol in g kg‐1 body weight. This finding suggests that the values of alcohol responses may be more sensitive to the effects of nalmefene under our experimental conditions.
There were only some slight differences between alcohol responses each day. While the highest dose of s.c. nalmefene revealed rather a flat slope (Figure 2A), the highest dose of p.o. nalmefene had a greater effect on the first day of treatment (Figure 3A). This effect was regarded as a significant interaction (see Table 1) and is consistent with findings from clinical trials with alcohol‐dependent patients (Sinclair et al., 2014).
Throughout the washout periods for nalmefene, there were no significant differences between nalmefene doses, either in the accumulated alcohol responses (Figures 2C and 3C) or in the in g alcohol consumed kg‐1 body weight (Figures 2D and 3D). Only an interaction between nalmefene and days was observed (see Table 1).
These results led us to question whether the observed reduction in alcohol responses induced by nalmefene could be associated with a motor impairment. Therefore, we conducted an additional experiment to examine the spontaneous locomotor activity and the strength/motor coordination of the animals after the s.c. and p.o. nalmefene treatments (Figure 4). We did not find any significant effects of nalmefene on animal motor activity or coordination, although the s.c. dose of 0.05 mg·kg−1 of nalmefene exhibited a tendency (P = 0.08) to increase the animals' strength/coordination (Figure 4B). See Table 1 for ANOVA results. Compared with the control group, at the end of the treatments, the p.o. doses of nalmefene of 20 and 40 mg·kg−1 caused a significant reduction in the body weight of rats of 1.87 and 1.75 g respectively.
-
1
Study 2. The effects of nalmefene on a dose–response curve for alcohol
Figures 5A and 5B depict two essential effects. Firstly, nalmefene continued to be effective in reducing alcohol consumption independently of the concentration of alcohol consumed (10, 15 and 20% v·v−1). Secondly, as long as the concentration of alcohol continued to increase, the number of alcohol responses decreased (Figure 5A). However, it is important to note that Figure 5B shows that the animals maintained their intake in terms of g alcohol kg‐1 body weight, thus suggesting a set‐point for the psychoactive effects of alcohol.
Overall, it is concluded that both s.c. and p.o. doses of nalmefene were equally effective in reducing alcohol responses and g alcohol kg‐1 body weight. The only exception was the combination of p.o. nalmefene and 15% alcohol concentration (Figure 5B, upper‐middle panel), where the dose of p.o. nalmefene caused a greater reduction in g alcohol consumed kg‐1 body weight than did the s.c. dose.
After the treatment was interrupted (washout period for nalmefene), the levels of alcohol consumption were restored to those of the control group. For detailed ANOVA results, see Table 1. Note that a reduced version of the three‐way mixed ANOVA is provided due to space limitations. At the end of this experiment, it was observed that the p.o. dose of nalmefene of 20 mg·kg−1 reduced the weight of the animals by 2.5% compared with the control group.
-
1
Study 3. The effects of nalmefene on alcohol‐cocaine interactions
As presented in Figure 6A, nalmefene was effective at preventing the increase in alcohol responses induced by cocaine. While cocaine led to a 40.5% increase in alcohol responses, nalmefene caused a reduction of 68.9% in the nalmefene group and 71.1% in the cocaine + nalmefene group. However, and unexpectedly, when the treatment with nalmefene was interrupted, the cocaine + nalmefene group did not return immediately to the same levels of alcohol responses as the cocaine group (Figure 6B, washout period).
As anticipated, a strong positive correlation (r (38) = 0.79, P < 0.001) was found between the blood alcohol levels and the number of alcohol responses (Figure 6C). Mean blood alcohol levels were between 38 and 43 mg% in the control groups and between 7.5 and 7.6 mg% in the nalmefene groups (Figure 6D). Nalmefene did not alter the metabolism of cocaine, given as a chronic treatment at a dose of 20 mg·kg−1, as no change in benzoylecgonine levels was detected (Figure 6E).
To gain a deeper insight into the interaction between nalmefene and cocaine, two additional experiments were performed. Firstly, we administered nalmefene before the cocaine treatment (i.e. 18 h prior to the alcohol session), which is in contrast to the previous tests wherein nalmefene was given prior to alcohol self‐administration. Under these conditions, nalmefene was not effective at reducing alcohol responses or at preventing an increase in these responses induced by cocaine (Figure 7A). Furthermore, nalmefene had no effect on cocaine‐induced locomotor sensitization (Figure 7B). Cocaine significantly increased the locomotor activity of the animals, between 150 and 166%. Thus, the 18% reduction induced by nalmefene was not significant. For detailed ANOVA results, see Table 1.
-
1
Study 4. Gene expression profiles of Hdacs
The gene expression profile of Hdacs1–11 from peripheral blood in vivo is presented in Figure 8. Alcohol self‐administration caused a general increase in the expression of all Hdacs, although Hdac1, 3 and 11 did not support the Bonferroni's correction for multiple comparisons. The significant increase in Hdac gene expression was between 62 and 142%. However, nalmefene prevented these increases, except with respect to Hdac2 and 4.
The results regarding the other tissues analysed post mortem were more heterogeneous (Supporting Information Fig. S1). Specifically, the gene expression of histone deacetylases was not affected by any treatment in the prefrontal cortex; alcohol caused a reduction in the expression of Hdac9 in the heart which was prevented by nalmefene and nalmefene caused a reduction in Hdac2 in the liver. Despite that nalmefene increased Hdac1, 4 and 9, in the kidney, only Hdac7 survived the Bonferroni's correction (P ≤ 0.004).
Discussion
Using a reliable animal model of alcohol self‐administration, the results of the four studies performed herein have replicated previous findings and provided new answers regarding the effectiveness of nalmefene in reducing alcohol use. The first study confirmed that s.c. and p.o. nalmefene reduced dose‐dependent responses to alcohol and did not induce significant impairment of motor/coordination. In the second study, we demonstrated that nalmefene decreased alcohol consumption independently of alcohol content. Thereafter, we found that nalmefene reversed cocaine‐induced increases in alcohol consumption. In the final study, we demonstrated that nalmefene reduced the increased Hdac3, 8, 5, 7, 9, 6, 10 gene expression in peripheral blood in vivo induced by alcohol self‐administration. Accordingly, the discussion is divided into four brief sections corresponding to the studies above.
The results of the first study are consistent with previous works using operant alcohol self‐administration in Wistar rats or using alcohol‐preferring rats (June et al., 1998; June et al., 2004; Walker and Koob, 2008; Nealey et al., 2011). Until now, there is no evidence that nalmefene activates other receptors than the opioid receptors, mainly μ and δ (Soyka, 2014). Also, nalmefene acts as a partial agonist at κ receptors, which when activated leads to an elevation of serum prolactin, and a reduction of dopamine in brain regions implicated in alcohol addiction (Bart et al., 2005). In line with this, it has been demonstrated that the effects of nalmefene on alcohol‐motivated behaviours are explained by the blockade of opioid receptors within the nucleus accumbens and ventral tegmental area (June et al., 2004, Nealey et al., 2011). Because nalmefene acts as a full agonist at the μ‐opioid receptor, it has been suggested that genetic variants in this receptor might cause different responses to opioid treatments. However, this is still debatable because the results are heterogeneous (e.g. Arias et al., 2008; Bilbao et al., 2015). Also, it should be noted that there are some studies that show that nalmefene reduces the intake of natural rewards such as saccharin and a highly preferred food (June et al., 2004; Cottone et al., 2008). Interestingly, s.c. injections of nalmefene are thought to optimize its bioavailability. According to June et al. (1998), nalmefene was between 3.200 and 6.400‐fold more potent when administered s.c. rather than p.o. Here, both s.c. 0.01 mg·kg−1 and p.o. 10 mg·kg−1 doses of nalmefene were effective at reducing responses to alcohol, thus indicating that under our experimental conditions, s.c. nalmefene injection was 1.000‐fold more potent than was p.o. nalmefene. To the best of our knowledge, there have been no publications in which clinical trials have investigated the effect of injected nalmefene on alcohol addiction. Our results indicate that a pilot trial of injectable nalmefene for treating alcohol dependence should be considered, as occurred with naltrexone, which eventually resulted in the approval of Vivitrol® by the FDA (Lee et al., 2012; Sullivan et al., 2013).
Longitudinal studies have shown that the risk of becoming a heavy drinker or of developing an alcohol‐use disorder is associated with the alcoholic beverage preference. While the highest risk is associated with low and high alcohol contents, that is, beers and spirits/liquors, the lowest risk was associated with moderate alcohol content, such as wines (Jensen et al., 2002; Grønbaek et al., 2004; Flensborg‐Madsen et al., 2008; Siegel et al., 2011). In our second study, the animals had access to different concentrations of alcohol. The increase in the concentration of alcohol was accompanied by a decrease in the number of responses to alcohol. Control animals managed to maintain the total amount of of alcohol consumed in g kg‐1 body weight. This result revealed that the animals adjusted their responses to alcohol according to a specific psychoactive dose of alcohol. Nevertheless, s.c. and p.o. nalmefene reduced the responses to alcohol as well as the total amount of alcohol consumed in g kg‐1 body weight independently of the alcohol content. Therefore, it is assumed that, to some extent, nalmefene reduces the psychoactive alcohol set‐point. Alcohol content is also considered a significant factor as previous findings have shown that spirit drinkers and beer drinkers differ in their adherence to pharmacological treatments. For instance, beer‐preferring drinkers exhibited a higher degree of adherence to topiramate and naltrexone than did spirit‐preferring drinkers (Baltieri et al., 2009). Moreover, it is relevant to note that in our experiments, 96% alcohol diluted in water was used. Future preclinical studies using nalmefene should consider increasing the ecological validity of the alcohol drinking solutions, for example, using commercial beers, wines and spirits.
Previously, we reported that repeated cocaine injections induced an increase in the response to 10% alcohol (Echeverry‐Alzate et al., 2012, 2014). Herein, we extended this finding by showing that cocaine also increased the responses to a 20% alcohol solution. However, more interesting was the fact that nalmefene fully reversed the cocaine‐induced increase in the response to alcohol. Surprisingly, while in studies 1 and 2, the effects of nalmefene on alcohol responses disappeared within the first 2 days after nalmefene withdrawal, herein, the effects of nalmefene did not disappear until the sixth day (data not shown). This might suggest that nalmefene would be more effective in reducing alcohol consumption in groups with higher levels of alcohol use. As expected, blood alcohol levels were directly linked to the number of alcohol responses, and these values are similar to previous findings using Wistar rats and operant alcohol self‐administration (Gilpin et al., 2009). Benzoylecgonine, one of the two primary metabolites in cocaine (Schindler and Goldberg, 2012), did not show significant differences between the groups after 18 h of cocaine administration, indicating that nalmefene did not directly affect cocaine metabolization. Accordingly, we questioned whether treating the animals with nalmefene before the cocaine injection instead of treating the animals prior to introducing them to the operant alcohol chambers could prevent the cocaine‐induced increase in responses to alcohol. This experiment revealed that nalmefene was not effective in preventing the effects of cocaine on alcohol self‐administration nor did the nalmefene affect cocaine‐induced locomotor sensitization. In addition, we observed that nalmefene was not effective in reducing responses to alcohol when nalmefene was administered 18 h prior to alcohol exposure. This finding is consistent with nalmefene's posology, which indicates that one tablet of nalmefene should be taken 1 to 2 h prior to the anticipated time of drinking (EMA). Finally, it is noted that the mechanism by which repeated cocaine administration increases the responses to alcohol remains unknown. It has been demonstrated that nalmefene (40 mg·day−1) suppresses the craving for alcohol and alcohol‐induced stimulation without interacting with cocaine (Drobes et al., 2004). Under our experimental conditions, among other explanations, we cannot determine whether cocaine increases the craving for alcohol, or whether it increases the threshold for the rewarding effects of alcohol leading the animal to increase its consumption, or whether this increase is caused by cocaine abstinence. Other possible explanations might be that cocaine produces anxiogenic actions, and the animals increase their alcohol self‐administration because alcohol has anxiolytic effects (Hendler et al., 2013; Ettenberg et al., 2015); or as cocaine has anorectic properties, the increase in alcohol responses would be driven by a caloric deficit (Barson et al., 2011; Soares et al., 2010). Therefore, further studies are needed to investigate the psychobiological mechanism that accounts for this finding.
HDACs have been implicated in many diseases (cancer, cardiovascular and psychiatric diseases; for review, see Abend and Kehat, 2015; Sun. et al., 2013), and many efforts are currently being deployed to investigate their role as possible biomarkers. For instance, several PET radiotracers have been developed for visualizing HDAC activity in Alzheimer's disease (Couto and Millis, 2015) although gene expression from peripheral blood is one of the most promising biomarkers (Mizuarai et al., 2010). Furthermore, pharmacological treatments are able to alter the activity of HDACs. For instance, valproic acid, which is used to treat epilepsy and bipolar disorder, is an inhibitor of HDACs (Al Ameri et al., 2014). In the final study, we aimed to evaluate the gene expression profiles of Hdacs in peripheral blood in vivo. This is not a new concept. For example, some authors have demonstrated that the expression of Hdacs (1–11) in peripheral blood was associated with the pathophysiology of mood disorders (Hobara et al., 2010). Herein, we have developed this idea further by examining alcohol self‐administration because recent studies have provided evidence that HDACs are involved in alcohol‐related behaviours (Warnault et al., 2013; Pandey et al., 2015). In this study, it is noted that we did not investigate the functionality of HDACs or the relationship between mRNA and protein levels. Rather, we investigated whether mRNA levels of HDACs could be used as a biomarker of alcohol use and drug response. Recently, we have demonstrated that the gene expression profiles of Hdacs in peripheral blood in vivo after alcohol intake or intoxication are similar in humans and animals. In both cases, alcohol increased the expression of most Hdacs (López‐Moreno et al., 2015). Nevertheless, it is important to highlight that while the first exposure to alcohol reduces Hdacs gene expression in several tissues (Kirpich et al., 2012; Botia et al., 2012; Pandey et al., 2015), repeated alcohol exposure causes this reduction to disappear or results in an increase in gene expression (López‐Moreno et al., 2015). Not only have we replicated some of these results but we have also demonstrated that nalmefene reduces the increases in Hdac3, 8, 5, 7, 9, 6, 10 gene expression induced by alcohol in peripheral blood in vivo. Taking into consideration the gene expression profiles from the prefrontal cortex, heart, liver and kidney, it seems that, on the one hand, peripheral blood would not be the most reliable sample regarding the changes in Hdac gene expression that occur in those tissues after alcohol consumption. Actually, blood showed a general increase in Hdac gene expression, whereas the other tissues showed a more tissue‐Hdac‐specific effect. On the other hand, peripheral blood would be among one of the best samples to use as a biomarker of alcohol use and drug response.
Conclusion
In conclusion, our results corroborate previous findings and provide further evidence regarding the efficacy of nalmefene on alcohol‐related behaviours, which also includes the co‐administration of cocaine. Furthermore, we have demonstrated that Hdac gene expression in peripheral blood in vivo has potential as a putative biomarker for alcohol use and nalmefene treatment.
Author contributions
J.C.‐C, A.G. and J.A.L.‐M. made the conception and design of the study and drafted the manuscript. J.C.‐C., V.E.‐A., K.M.B. and E.G. acquired, analysed and interpreted the data. R.N., F.R.d.F., R.M. and A.G. interpreted the data and made critical revisions for the manuscript.
Conflict of interest
Nalmefene was provided by the pharmaceutical company Lundbeck (Copenhagen, Denmark) as a gift, but no financial support from Lundbeck was received for these studies. A.G. has received consultancy fees and research grants from Lundbeck outside the submitted work.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 The effects of alcohol and nalmefene in Hdac gene expression in the prefrontal cortex, heart, liver and kidney. The animals were treated with alcohol and nalmefene as described in Figure 6. Data represent the mean ± SEM (n = 9, 8, 7 per group) of the relative fold change obtained using the 2ΔCt method. *P ≤ 0.004 compared with the control‐saccharin group; #P ≤ 0.004 compared with the control‐alcohol group.
Table S1 Details of the rat primers used for quantitative real time PCR of each gene.
Supporting info item
Supporting info item
Acknowledgements
This work was supported by The European Foundation for Alcohol Research (to J.A.L.M., F.R.d.F., R.M. and R.N.), the Fondo de Investigación Sanitaria (Red de Trastornos Adictivos, FEDER, RD12/0028/0015 to J.A.L.M., RD12/0028/001 to F.R.d.F., RD12/0028/023 to R.M., RD12/0028/0014 to R.N.) and Ministerio de Ciencia e Innovación (SAF2011‐26818 to J.A.L.M.).
Calleja‐Conde, J. , Echeverry‐Alzate, V. , Giné, E. , Bühler, K. ‐M. , Nadal, R. , Maldonado, R. , Rodríguez de Fonseca, F. , Gual, A. , and López‐Moreno, J. A. (2016) Nalmefene is effective at reducing alcohol seeking, treating alcohol‐cocaine interactions and reducing alcohol‐induced histone deacetylases gene expression in blood. British Journal of Pharmacology, 173: 2490–2505. doi: 10.1111/bph.13526.
References
- Abend A, Kehat I (2015). Histone deacetylases as therapeutic targets–from cancer to cardiac disease. Pharmacol Ther 147: 55–62. [DOI] [PubMed] [Google Scholar]
- Al Ameri M, Al Mansouri S, Al Maamari A, Bahi A (2014). The histone deacetylase (HDAC) inhibitor valproic acid reduces ethanol consumption and ethanol‐conditioned place preference in rats. Brain Res 1583: 122–131. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E, Papadopoulou‐Daifoti Z, Hyphantis T, Marselos M (1998). D‐amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev 23: 189–196. [DOI] [PubMed] [Google Scholar]
- Arias AJ, Armeli S, Gelernter J, Covault J, Kallio A, Karhuvaara S et al. (2008). Effects of opioid receptor gene variation on targeted nalmefene treatment in heavy drinkers. Alcohol Clin Exp Res 32: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltieri DA, Daró FR, Ribeiro PL, De Andrade AG (2009). The role of alcoholic beverage preference in the severity of alcohol dependence and adherence to the treatment. Alcohol 43: 185–195. [DOI] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF (2011). Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiol Behav 104 (1): 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ (2005). Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology 30: 2254–2262. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH et al. (2015). A pharmacogenetic determinant of mu‐opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice. Biol Psychiatry 77: 850–858. [DOI] [PubMed] [Google Scholar]
- Botia B, Legastelois R, Alaux‐Cantin S, Naassila M (2012). Expression of ethanol‐induced behavioral sensitization is associated with alteration of chromatin remodeling in mice. PLoS One 7: e47527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP (2008). Opioid‐dependent anticipatory negative contrast and binge‐like eating in rats with limited access to highly preferred food. Neuropsychopharmacology 33: 524–535. [DOI] [PubMed] [Google Scholar]
- Couto PJ, Millis RM (2015). PET imaging of epigenetic influences on Alzheimer's disease. Int J Alzheimers Dis 2015: 575078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K (2004). Effects of naltrexone and nalmefene on subjective response to alcohol among non‐treatment‐seeking alcoholics and social drinkers. Alcohol Clin Exp Res 28: 1362–1370. [DOI] [PubMed] [Google Scholar]
- Echeverry‐Alzate V, Tuda‐Arízcun M, Bühler KM, Santos Á, Giné E, Olmos P et al. (2012). Cocaine reverses the naltrexone‐induced reduction in operant ethanol self‐administration: the effects on immediate‐early gene expression in the rat prefrontal cortex. Neuropharmacology 63: 927–935. [DOI] [PubMed] [Google Scholar]
- Echeverry‐Alzate V, Giné E, Bühler KM, Calleja‐Conde J, Olmos P, Gorriti MA et al. (2014). Effects of topiramate on ethanol‐cocaine interactions and DNA methyltransferase gene expression in the rat prefrontal cortex. Br J Pharmacol 171: 3023–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (European Medicines Agency) (2013) EPAR summary for the public. Selincro [Online] Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐Summary_for_the_public/human/002583/WC500140303.pdf (accessed 8/3/2015).
- Ettenberg A, Fomenko V, Kaganovsky K, Shelton K, Wenzel JM (2015). On the positive and negative affective responses to cocaine and their relation to drug self‐administration in rats. Psychopharmacology (Berl) 232: 2363–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq MU, Bhatt A, Patel M (2009). Neurotoxic and cardiotoxic effects of cocaine and ethanol. J Med Toxicol 5: 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flensborg‐Madsen T, Knop J, Mortensen EL, Becker U, Makhija N, Sher L et al. (2008). Beverage preference and risk of alcohol‐use disorders: a Danish prospective cohort study. J Stud Alcohol Drugs 69: 371–377. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN (2009). Operant behavior and alcohol levels in blood and brain of alcohol‐dependent rats. Alcohol Clin Exp Res 33: 2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettl VM, Wemlinger TA, Colvin AE, Neff NH, Hadjiconstantinou M (2001). Motoric behavior in aged rats treated with GM1. Brain Res 906: 92–100. [DOI] [PubMed] [Google Scholar]
- Grønbaek M, Jensen MK, Johansen D, Sørensen TI, Becker U (2004). Intake of beer, wine and spirits and risk of heavy drinking and alcoholic cirrhosis. Biol Res 37: 195–200. [DOI] [PubMed] [Google Scholar]
- Grosshans M, Mutschler J, Kiefer F (2015). Treatment of cocaine craving with as‐needed nalmefene, a partial κ opioid receptor agonist: first clinical experience. Int Clin Psychopharmacol 30: 237–238. [DOI] [PubMed] [Google Scholar]
- Gual A, He Y, Torup L, van den Brink W, Mann K, ESENSE 2 Study Group (2013). A randomised, double‐blind, placebo‐controlled, efficacy study of nalmefene, as‐needed use, in patients with alcohol dependence. Eur Neuropsychopharmacol 23: 1432–1442. [DOI] [PubMed] [Google Scholar]
- Hahn EF, Fishman J (1975). Narcotic antagonists. V. Stereochemistry of reactions at C‐6 in 14‐hydroxynoroxymorphone derivatives. J Org Chem 40: 31–34. [DOI] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW (2013). Stimulant and sedative effects of alcohol In: Sommer WH, Spanagel R. (eds). Behavioral neurobiology of alcohol addiction. Springer‐Verlag: Berlin Heidelberg, pp. 489–511. [DOI] [PubMed] [Google Scholar]
- Hobara T, Uchida S, Otsuki K, Matsubara T, Funato H, Matsuo K et al. (2010). Altered gene expression of histone deacetylases in mood disorder patients. J Psychiatr Res 44: 263–270. [DOI] [PubMed] [Google Scholar]
- Ingman K, Hagelberg N, Aalto S, Någren K, Juhakoski A, Karhuvaara S et al. (2005). Prolonged central mu‐opioid receptor occupancy after single and repeated nalmefene dosing. Neuropsychopharmacology 30: 2245–2253. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Joseph JA, Spangler EL, Roberts D, Hengemihle J, Fanelli RJ (1994). Chronic nimodipine treatment in aged rats: analysis of motor and cognitive effects and muscarinic‐induced striatal dopamine release. Neurobiol Aging 15: 55–61. [DOI] [PubMed] [Google Scholar]
- Jensen MK, Andersen AT, Sørensen TI, Becker U, Thorsen T, Grønbaek M (2002). Alcoholic beverage preference and risk of becoming a heavy drinker. Epidemiology 13: 127–132. [DOI] [PubMed] [Google Scholar]
- June HL, Grey C, Warren‐Reese C, Durr LF, Ricks‐Cord A, Johnson A et al. (1998). The opioid receptor antagonist nalmefene reduces responding maintained by ethanol presentation: preclinical studies in ethanol‐preferring and outbred Wistar rats. Alcohol Clin Exp Res 22: 2174–2185. [PubMed] [Google Scholar]
- June HL, Cummings R, Eiler WJ II, Foster KL, McKay PF, Seyoum R et al. (2004). Central opioid receptors differentially regulate the nalmefene‐induced suppression of ethanol‐ and saccharin‐reinforced behaviors in alcohol‐preferring (P) rats. Neuropsychopharmacology 29: 285–299. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Harvey E (2015). Histone deacetylases as potential targets for cocaine addiction. CNS Neurol Disord Drug Targets 14: 764–772. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich I, Ghare S, Zhang J, Gobejishvili L, Kharebava G, Barve SJ et al. (2012). Binge alcohol‐induced microvesicular liver steatosis and injury are associated with down‐regulation of hepatic Hdac 1, 7, 9, 10, 11 and up‐regulation of Hdac 3. Alcohol Clin Exp Res 36: 1578–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Kieffer BL, Heyser CJ, Katner SN, Ciccocioppo R et al. (2003). Animal models of motivation for drinking in rodents with a focus on opioid receptor neuropharmacology. Recent Dev Alcohol 16: 263–281. [DOI] [PubMed] [Google Scholar]
- Lacoste J, Pedrera‐Melgire M, Charles‐Nicolas A, Ballon N (2010). Cocaine and alcohol: a risky association. Presse Med 39: 291–302. [DOI] [PubMed] [Google Scholar]
- Lee JD, Grossman E, Huben L, Manseau M, McNeely J, Rotrosen J et al. (2012). Extended‐release naltrexone plus medical management alcohol treatment in primary care: findings at 15 months. J Subst Abuse Treat 43: 458–462. [DOI] [PubMed] [Google Scholar]
- López‐Moreno JA, Marcos M, Calleja‐Conde J, Echeverry‐Alzate V, Bühler KM, Costa‐Alba P et al. (2015). Histone deacetylase gene expression following binge alcohol consumption in rats and humans. Alcohol Clin Exp Res 39: 1939–1950. [DOI] [PubMed] [Google Scholar]
- Lu YL, Richardson HN (2014). Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience 277: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Bladström A, Torup L, Gual A, van den Brink W (2013). Extending the treatment options in alcohol dependence: a randomized controlled study of as‐needed nalmefene. Biol Psychiatry 73: 706–713. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuarai S, Irie H, Kotani H (2010). Gene expression‐based pharmacodynamic biomarkers: the beginning of a new era in biomarker‐driven anti‐tumor drug development. Curr Mol Med 10: 596–607. [DOI] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM (2011). κ‐opioid receptors are implicated in the increased potency of intra‐accumbens nalmefene in ethanol‐dependent rats. Neuropharmacology 61: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A (2008). Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci 28: 3729–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H (2015). Potential role of adolescent alcohol exposure‐induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis pii: S0969‐9961(15)00091–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schönig K et al. (2015). Losing control: excessive alcohol seeking after selective inactivation of cue‐responsive neurons in the infralimbic cortex. J Neurosci 35: 10750–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL et al. (1990). Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 264: 2511–2518. [PubMed] [Google Scholar]
- Rodríguez‐Álvarez T, Racamonde I, González‐Mariño I, Borsotti A, Rodil R, Rodríguez I et al. (2015). Alcohol and cocaine co‐consumption in two European cities assessed by wastewater analysis. Sci Total Environ 536: 91–98. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Goldberg SR (2012). Accelerating cocaine metabolism as an approach to the treatment of cocaine abuse and toxicity. Future Med Chem 4: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ (2008). Analyzing real‐time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Bladström A, Torup L, Gual A Chick J (2014). Fast onset of action with as‐needed use nalmefene in the treatment of alcohol dependence. 37th Annual RSA Scientific Meeting, Bellevue, Washington, USA, 21–25 June 2014
- Siegel MB, Naimi TS, Cremeens JL, Nelson DE (2011). Alcoholic beverage preferences and associated drinking patterns and risk behaviors among high school youth. Am J Prev Med 40: 419–426. [DOI] [PubMed] [Google Scholar]
- Soares B, Lima Reisser AA, Farrell M, Silva de Lima M (2010). WITHDRAWN: dopamine agonists for cocaine dependence. Cochrane Database Syst Rev 17: CD003352. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl. Acids Res. 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M (2014). Nalmefene for the treatment of alcohol dependence: a current update. Int J Neuropsychopharmacol 17: 675–684. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA (2005). The effect of cocaine on the expression of motor activity and conditioned place preference in high and low alcohol‐preferring Wistar rats. Pharmacol Biochem Behav 82: 314–319. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Bisaga A, Mariani JJ, Glass A, Levin FR, Comer SD et al. (2013). Naltrexone treatment for opioid dependence: does its effectiveness depend on testing the blockade? Drug Alcohol Depend 133: 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Kennedy PJ, Nestler EJ (2013). Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology 38: 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thullier F, Lalonde R, Mahler P, Joyal CC, Lestienne F (1996). Dorsal striatal lesions in rats. 1: effects on exploration and motor coordination. Arch Physiol Biochem 104: 300–306. [DOI] [PubMed] [Google Scholar]
- van den Brink W, Aubin HJ, Bladström A, Torup L, Gual A, Mann K (2013). Efficacy of as‐needed nalmefene in alcohol‐dependent patients with at least a high drinking risk level: results from a subgroup analysis of two randomized controlled 6‐month studies. Alcohol Alcohol 48: 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF (2008). Pharmacological evidence for a motivational role of kappa‐opioid systems in ethanol dependence. Neuropsychopharmacology 33: 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine A, Barak S, Ron D (2013). Chromatin remodeling–a novel strategy to control excessive alcohol drinking. Transl Psychiatry 3: e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SH, Pfeffer M, Schor JM, Indindoli L, Mintz M (1971). Metabolites of naloxone in human urine. J Pharm Sci 60: 1567–1568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The effects of alcohol and nalmefene in Hdac gene expression in the prefrontal cortex, heart, liver and kidney. The animals were treated with alcohol and nalmefene as described in Figure 6. Data represent the mean ± SEM (n = 9, 8, 7 per group) of the relative fold change obtained using the 2ΔCt method. *P ≤ 0.004 compared with the control‐saccharin group; #P ≤ 0.004 compared with the control‐alcohol group.
Table S1 Details of the rat primers used for quantitative real time PCR of each gene.
Supporting info item
Supporting info item


