Summary
Mechanistic target of rapamycin (mTOR) has been shown to play an important role in red blood cell physiology, with inhibition of mTOR signalling leading to alterations in erythropoiesis. To determine if mTOR inhibition would improve anaemia in sickle cell disease (SCD), mice with SCD were treated with the dual mTORC1/2 inhibitor, INK128. 1 week after daily oral drug treatment, erythrocyte count, haemoglobin, and haematocrit were all significantly increased while reticulocyte counts were reduced. These parameters remained stable during 3 weeks of treatment. Similar effects were observed following oral treatment with the mTORC1 inhibitor, sirolimus. Sirolimus treatment prolonged the lifespan of sickle cell erythrocytes in circulation, reduced spleen size, and reduced renal and hepatic iron accumulation in SCD mice. Following middle cerebral artery occlusion, stroke size was reduced in SCD mice treated with sirolimus. In conclusion, mTOR inhibition is protective against anaemia and organ damage in a murine model of SCD.
Keywords: mTOR, anaemia, sickle cell disease, sirolimus, stroke
Introduction
Sickle cell disease (SCD) is a relatively common inherited blood disorder affecting millions worldwide (GBD 2013 Mortality and Causes of Death Collaborators 2015) that occurs in approximately 1 out of every 500 African American births (https://www.nhlbi.nih.gov/news/spotlight/fact-sheet/sickle-cell-disease-research-care). When deoxygenated, the mutated haemoglobin tetramer polymerizes to form a network of fibrous polymers that cause erythrocytes to acquire a sickle shape (Savitt and Goldberg 1989). The rigid, sickled erythrocytes are subject to premature destruction resulting in haemolytic anaemia (Platt, et al 1991). Sickled erythrocytes also lead to microvascular occlusions, which lead to painful crises and end organ damage (Platt, et al 1991), including stroke(Platt, et al 1991). In addition to supportive treatment for acute complications, current therapies for SCD include blood transfusions (DeBaun, et al 2014) and treatment with hydroxycarbamide (also termed hydroxyurea; Lanzkron, et al 2008). Repeated blood transfusions have been shown to reduce the risk of recurrent stroke although iron overload with haemochromatosis is a long-term complication (Aliyu, et al 2006, Dos Santos, et al 2012). Prolonged treatment with hydroxycarbamide also appears to be beneficial in preventing some SCD complications (Lanzkron, et al 2008).
In addition to peripheral destruction of erythrocytes, impaired bone marrow erythropoiesis may contribute to anaemia and morbidity in SCD (Wu, et al 2005). Additional treatments are needed to further reduce the health burden of SCD patients. Given that the mechanistic target of rapamycin (mTOR) has been shown to affect erythropoiesis and to improve anaemia in some circumstances (Gan, et al 2008, Knight, et al 2014), the aim of this study was to test the effect of mTOR inhibition on anaemia and organ pathology in a murine model of SCD.
Methods
Mice
Mice carrying the homozygous sickle cell mutation (Hbbhβs/hβs) (Wu, et al 2006) along with strain-matched controls (Hbb+/+), as well as wild-type (WT) C57BL6/J male mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Eight-week-old mice were fed a standard laboratory rodent diet (#5001, TestDiet, Richmond, IN) in specific pathogen-free facilities. SCD and control experimental mice were generated by bone marrow transplantation (BMT) from Hbbhβs/hβs mice or Hbb+/+ donors to WT C57BL6/J male recipients. Non-bone marrow-transplanted SCD and control experimental mice were also used.
Bone marrow transplantation
BMT was performed as previously described (Luo, et al 2012). Briefly, donor mice for BMT were euthanized at 8-10 weeks of age and bone marrow was then flushed from femurs and tibias. Recipient mice were irradiated with 650 rads × 2 separated by a 3-h interval (total of 1300 rads). Each recipient mouse was administered a 200 μl bone marrow suspension in phosphate-buffered saline (PBS: 2 × 107cells/ml) via tail vein injection. Acid water (6 mM HCl, pH=2.5) was provided to animals beginning 4 days before BMT to 4 weeks following BMT. Recipient mice were housed in a specific pathogen-free animal facility.
Drug treatment
INK128 (Cayman Chemical, Ann Arbor, MI) was dissolved in dimethyl sulfoxide (DMSO) and then diluted with PBS to 0.1 mg/ml. Mice were gavaged daily with 1 mg/kg INK128. Sirolimus (Pfizer, New York, NY) was suspended in PBS at a concentration of 0.5 mg/ml. Mice were gavaged daily with 5 mg/kg sirolimus.
Haemoglobin electrophoresis
Haemoglobin electrophoresis was performed as previously described (Campbell, et al 2011). Briefly, 20 μl of whole blood, collected by retro-orbital bleeding, was used for analysis on a Bio-Rad Variant II Haemoglobin Testing System using an ion-exchange high performance liquid chromatography (HPLC) column (Bio-Rad, Hercules, CA). Separated haemoglobin fractions were detected by absorbance at 415 nm.
Cell counting
Complete blood counts were measured in the Unit for Laboratory Animal Medicine core at the University of Michigan using a Hemavet 950 haematology system (Drew Scientific, Miami Lakes, FL). Reticulocytes were manually counted after staining with New Methylene Blue “N” Stain (RICCA Chemical Company, Arlington, TX), according to manufacturer's instructions and expressed as a percentage of total erythrocytes.
Erythrocyte lifespan measurement
For analysis of erythrocyte lifespan, biotin labelling was used with intravenous injection of 50 mg/kg Sulfo-NHS-Biotin (Life Technologies, Grand Island, NY) to produce a pulse-label. Flow cytometry was then performed to determine decay of biotinylated cells and RBC lifespan at 4-day intervals.
Transfusion experiment
Sickle blood cells from sirolimus or PBS-treated SCD(BMT) donor mice were biotinylated with intravenous injection of 50 mg/kg Sulfo-NHS-Biotin (Life Technologies, Grand Island, NY). After terminal bleeding, the red blood cells were isolated by centrifugation at 1000 rpm for 10 min. WT(BMT) recipient mice orally treated with sirolimus (5 mg/kg in PBS, gavage) or PBS for one week were transfused with 300 μl erythrocytes at haematocrit of 50%. The percentage of biotinylated cells was monitored with flow cytometry in the following days. The percentage of biotinylated cells at 1 h after transfusion was set at 100% for each mouse.
Erythrocyte in vitro culture
Sickle red blood cells were isolated by centrifugation at 1000 rpm for 10 min. 2 ml of reticulocytes (2 × 106 /ml) were cultured for 48 h in 24-well plates with Iscove's modified Dulbecco's medium, 30% heat-inactivated fetal bovine serum, 1% deionized bovine albumin, 100 units/ml penicillin G, 100 μg/ml streptomycin, and 0.1 mM α-thioglycerol in the presence of sirolimus (10 μM) or vehicle control (Koury, et al 2005). CD71 expression level was measured with flow cytometry. Four wells were analysed for each group and the experiment was repeated three times.
Erythroid cell analysis in blood, bone marrow and spleen
Blood was collected from the retroorbital sinus and anticoagulated with 3.2% buffered sodium citrate. Erythroid cells were isolated by centrifugation at 1000 rpm for 10 min. Bone marrow extracted from femur was passed through a 25-gauge needle 30 times to obtain a single cell suspension. A section of spleen was cut and ground though a 100 μm cell strainer and passed through a 25-gauge needle 30 times to produce a single cell suspension. Cells were washed in PBS+2% fetal clalf serum (FCS), and then resuspended in PBS+2% FCS for flow cytometry analysis. In blood, CD71-positive erythrocytes were expressed as percentage of TER119 positive cells (erythrocytes). Erythroid cells at different developmental stages, including proerythroblasts (ProE, Ter119medCD71hi), early basophilic erythroblasts (Ery A , Ter119hiCD71hi), and late basophilic erythroblasts (EryB, Ter119hiCD71med) were detected and expressed as percentage of total bone marrow or spleen cells (Liu, et al 2006).
Flow cytometry
Fluorescein isothiocyanate (FITC)-conjugated Avidin (Life Technologies) was used for biotinylated cell detection. Biotin anti-mouse CD71 antibody (Biolegend, San Diego, CA), FITC-conjugated Avidin (Life Technologies) and allophycocyanin/cyanin 7 (APC/Cy7) anti-mouse TER-119/erythroid cells antibody (Life Technologies) were used to measure the maturation status of the cells. Cell counts of 2 × 106 in 200 μl PBS+2% FCS were incubated with fluorochrome-conjugated antibodies for 30 min followed by washing once in PBS+2% FCS. Flow cytometry was performed with a Gallios Flow Cytometer (Beckman Coulter, Indianapolis, IN).
Splenectomy
Mice were anesthetized with 2% isoflurane. A 1.5-2 cm laparotomy was made at the left lateral abdomen. The spleen was gently trapped and pushed free of adjacent tissues. A single knot of 6-0 nylon suture was used to tie off the splenic artery and another knot was used to tie off the efferent vein. Connective tissue was then cut away and the spleen was removed. The musculoperitoneal layer was closed with 6.0 nylon sutures and the skin was closed with interrupted 5-0 nylon sutures. Animals were placed on a heating pad until they were completely awake. 2 weeks following splenectomy, mice were bled for complete blood count measurement.
Tissue iron staining
For analysis of tissue iron, tissues were fixed in neutral-buffered formalin, and then dehydrated in 90% and then 70% ethanol, embedded in paraffin, and 6-μm thick sections cut. Iron deposition was determined with the Iron Stain Kit (Sigma-Aldrich, St Louis, MO). Three fields of each section were photographed and then percent area stained was calculated with automated computer software (ImageJ, National Institutes of Health, Bethesda, MD).
Stroke model
Stroke was induced by photochemical-mediated injury to the middle cerebral artery (MCA) similar to that previously described (Luo, et al 2014, Su, et al 2008) except that MCA exposure to the laser was performed through the parietal cranium after the left temporal muscle was transected and peeled back to expose the cranium. Rose Bengal (50 mg/kg) was injected via the tail vein while a 1.5-mW green light laser (540 nm) was applied to the middle cerebral artery for 30 min to induce thrombotic occlusion. The temporal muscle and skin were then sutured back in place. At 72 h later the brains were harvested, sectioned and stained with 2% 2, 3, 5-triphenyltetrazolium chloride (TTC) for infarct area measurement.
Statistics
Values are expressed as mean ± standard error of the mean. The statistical significance of differences between two groups was determined by the student 2-tailed t test. For multiple comparisons, results were analysed using two-way ANOVA, followed by Bonferroni post-test analysis or one-way ANOVA, followed by Dunnett post-test analysis if compared with the same group. P<0.05 was considered significant.
Ethics Statement
All procedures complied with the Principles of Laboratory and Animal Care established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals.
Results
Effect of mTOR inhibition on anaemia in SCD mice
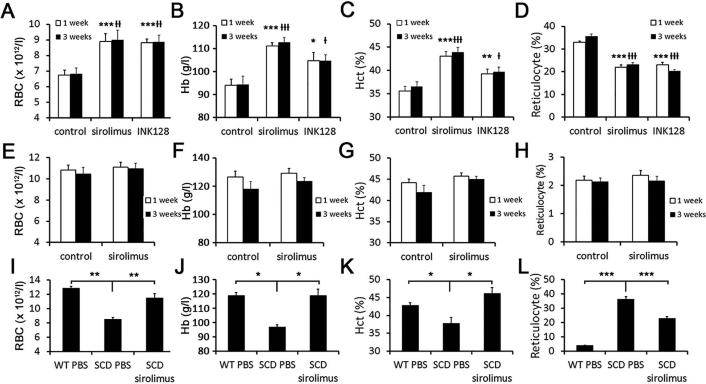
To test the effects of combined mTORC1 and mTORC2 inhibition in a murine model of SCD, we used the dual mTORC1/2 inhibitor, INK128, beginning 10 weeks after BMT. Following 1 week of daily oral gavage with either PBS or INK128 (1 mg/kg), mice treated with INK128 showed significantly higher erythrocyte counts, haemoglobin (Hb) and haematocrit (Hct), along with reduced reticulocyte counts (Figure 1A-D).
Figure 1. Complete blood count analysis.
(A - D) Erythrocyte counts (A), Hb (B), Hct (C) and reticulocyte percentage (D) of SCD(BMT) mice (n=10 per group; *p<0.05, **p<0.01, ***p<0.001 vs. control group after 1 week of treatment; +p<0.05, ++p<0.01, +++p<0.001 vs control group for 3 weeks treatment). (E - H) Erythrocyte counts (E), Hb (F), Hct (G) and reticulocyte percentage (H) of WT(BMT) mice (n=9 for PBS treatment and n=10 for sirolimus treatment). (I - L) Erythrocyte counts (I), Hb (J), Hct (K) and reticulocyte percentage (L) of non-BMT SCD mice treated with sirolimus or PBS (n=3 for WT mice treated with PBS, n=3 for SCD mice treated with PBS and n=6 for SCD mice treated with sirolimus; *p<0.05, **p<0.01, ***p<0.001 vs. SCD mice treated with PBS for 3 weeks). RBC red blood cells; Hb, haemoglobin; Hct, haematocrit; SCD, sickle cell disease; BMT, bone marrow transplantation; WT, wild-type; PBS, phosphate-buffered saline.
Treatment with the mTORC1 inhibitor, sirolimus, for 1 week with daily oral gavage (5 mg/kg), demonstrated similar beneficial effects on anaemic parameters compared to the PBS-treated group (Figure 1A-D). In contrast to SCD(BMT) mice, WT(BMT) mice treated with 1 week of sirolimus showed no significant difference in erythrocyte counts, Hb or Hct when compared to mice treated with PBS (Figure 1E-H). Following a more prolonged treatment regimen with INK128 or sirolimus (3 weeks), differences in anaemic parameters between drug-treated and PBS-treated SCD(BMT) mice persisted and remained similar to the 1-week treatment course (Figure 1A-D). No differences were observed in erythrocyte counts, Hb or Hct in WT(BMT) mice treated with 3 weeks of sirolimus compared to PBS (Figure 1E-H).
To exclude the possibility that mTOR inhibition was affecting late marrow-related events post-BMT that may only be relevant in the BMT model, we tested mTOR inhibition in non-transplanted SCD mice purchased from Jackson Laboratory (Stock Number:013071) along with strain-matched controls. Following 3 weeks of oral sirolimus treatment at 5 mg/kg/ day, erythrocyte counts, Hb and Hct increased to a similar extent as observed in the SCD(BMT) mice (Figure 1I-L), demonstrating that the effects of mTOR inhibition are not specific to BMT mice.
To determine whether the apparent beneficial effect of mTOR inhibition on anaemia was associated with a reduction in spleen weight, spleens were weighed from SCD(BMT) mice 3 weeks after treatment with sirolimus or INK128. Spleens from mice treated with either sirolimus or INK128 weighed less than spleens from PBS-treated mice while no differences in total body weight were observed between the groups (Table I).
Table I.
Body and spleen weight of SCD(BMT) mice treated with phosphate-buffered saline (PBS), sirolimus or INK128 for 3 weeks.
| PBS (n=10) | Sirolimus (n=10) | INK128 (n=10) | |
|---|---|---|---|
| Body weight (g) | 21.0±2.1 | 21.9±0.4 | 22.9±0.5 |
| Spleen (mg) | 562.5±31.4 | 486.1±16.0* | 407.8±26.3** |
P<0.05 vs. PBS
p<0.01 vs. PBS.
Effect of sirolimus treatment on fetal haemoglobin and erythrocyte lifespan in SCD mice
Sirolimus has been shown to increase fetal haemoglobin (HbF) in erythrocytes in vitro (Mischiati, et al 2004). To determine whether effects of mTOR inhibition on anaemia and erythrocyte turnover were due to increases in HbF, levels were measured in SCD(BMT) mice 3 weeks after treatment with sirolimus or PBS. The percentage of HbF was not different between sirolimus-treated and PBS-treated mice (1.92 ± 0.09% vs 1.89 ± 0.07%, respectively, p=0.76), indicating that effects on HbF are not the mechanism by which sirolimus improves anaemia in SCD mice.
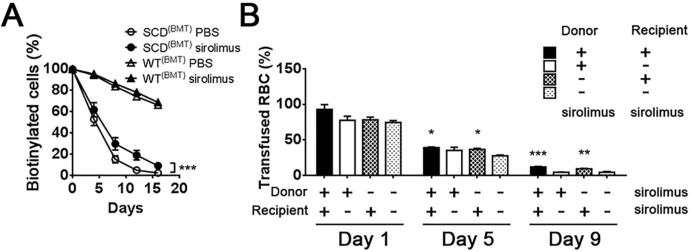
To determine whether the effect of mTOR inhibition on anaemia in SCD was associated with increased erythrocyte survival, biotin was injected in a pulse manner to label blood cells and the percentage of labelled cells was monitored with flow cytometry. Treatment with sirolimus or PBS in SCD(BMT) mice was initiated 1 week prior to biotin injection and continued throughout the monitoring phase. Erythrocyte lifespan was significantly prolonged in SCD(BMT) mice treated with sirolimus compared to mice treated with PBS. There was no effect of sirolimus treatment on erythrocyte lifespan in WT(BMT) mice compared to PBS treatment (Figure 2A).
Figure 2. Effect of sirolimus on sickle red blood cell.
(A) Red blood cell lifespan in SCD(BMT) mice (n=6 for PBS treatment and n=5 for sirolimus treatment) or WT(BMT) mice treated with sirolimus or PBS (n=6 for PBS treatment and n=5 for sirolimus treatment). (B) Survival of transfused sickle red blood cells (n=5 for each group with donor mice treated with sirolimus, and n=6 for each group with donor mice treated with PBS). *p<0.05, **p<0.01, ***p<0.001. SCD, sickle cell disease; BMT, bone marrow transplantation; WT, wild-type; PBS, phosphate-buffered saline.
To further clarify the target of sirolimus, sickle red blood cells from sirolimus or PBS-treated SCD(BMT) donor mice were labelled with biotin, isolated and then transfused into sirolimus or PBS-treated WT(BMT) recipient mice. The percentage of labelled cells was monitored with flow cytometry. In recipient mice treated with sirolimus, labelled sickle red blood cells survived longer, regardless of the donor source, compared to recipient mice treated with PBS (Figure 2B) suggesting that sirolimus targets the peripheral circulating erythroid cells.
Effect of sirolimus treatment on erythroid maturation in SCD mice
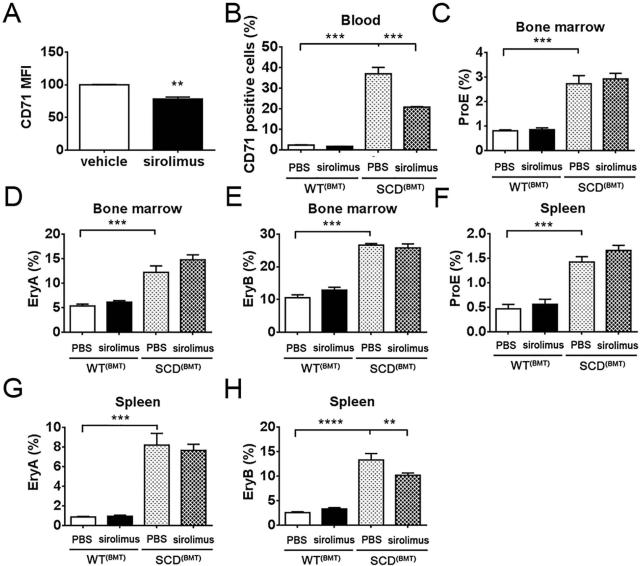
To further study the potential of sirolimus to promote maturation of peripheral sickle reticulocytes, erythrocytes from SCD(BMT) mice were isolated and cultured in vitro in the presence of sirolimus or vehicle control. After 48 h, CD71 expression was analysed with flow cytometry. Mean florescence intensity (MFI) was reduced on cells treated with sirolimus, indicating a more mature status (Figure 3A). Consistently, peripheral CD71+ cells were reduced in SCD(BMT) mice treated with sirolimus for 1 week compared to SCD(BMT) mice treated with PBS. (Figure 3B).
Figure 3. Effect of sirolimus on sickle red blood cell maturation.
(A) Mean fluorescence intensity of CD71 on sickle red blood cells cultured in vitro. (B) CD71 positive cell percentage in blood of SCD(BMT) mice treated with PBS (n=6) or sirolimus (n=5) and WT(BMT) mice treated with PBS (n=4) or sirolimus (n=3) for 1 week. (C - E) Percentage of proerythroblasts (C), early basophilic erythroblasts (D) and late basophilic erythroblasts (E) in bone marrow of SCD(BMT) mice treated with PBS (N=6) or sirolimus (n=5) and WT(BMT) mice treated with PBS (n=4) or sirolimus (n=3) for 1 week. (F - H) Percentage of ProE (F), EryA (G) and EryB (H) in spleen of SCD(BMT) mice treated with PBS (n=6) or sirolimus (n=5) and WT(BMT) mice treated with PBS (n=4) or sirolimus (n=3) for 1 week. *p<0.05, **p<0.01, ***p<0.001. MFI, mean fluorescence intensity; SCD, sickle cell disease; BMT, bone marrow transplantation; WT, wild-type; PBS, phosphate-buffered saline; ProE, proerythroblasts; EryA, early basophilic erythroblasts; EryB, late basophilic erythroblasts.
mTOR pathways are essential to cell growth and proliferation, and mTOR inhibition may therefore suppress bone marrow erythropoiesis (Aliyu, et al 2006), (Dos Santos, et al 2012). To study erythropoiesis in our SCD(BMT) mice, bone marrow cells were isolated and analysed for haematopoietic progenitor cells using flow cytometry following 1 week of treatment. The percentage of ProE, EryA and EryB was increased in bone marrow from SCD(BMT) mice compared to WT(BMT) mice, however, sirolimus treatment of SCD(BMT) mice had no effect on these cell populations (Figure 3C-E). Analysis of erythropoietic progenitor cells was also performed from spleens. The percentage of ProE, EryA and EryB of splenic cells was increased in SCD(BMT) mice compared with WT(BMT) mice while sirolimus treatment only reduced the ratio of EryB in SCD(BMT) mice (Figure 3F-H).
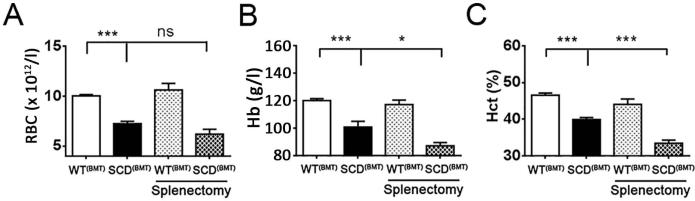
To further explore the role of the spleen in the pathology of sickle cell anaemia, splenectomy was performed in SCD(BMT) mice. Anaemia was not improved in SCD(BMT) mice 2 weeks after splenectomy (Figure 4), indicating the spleen is not a relevant target organ for sirolimus with regard to the improvement of anaemia in SCD.
Figure 4. Effect of splenectomy on sickle cell anaemia.
Erythrocyte counts (A), Hb (B), Hct (C) of SCD(BMT) mice with/without splenectomy (n=7 and n=10, respectively) or WT(BMT) mice with/without splenectomy (n=8 and n=10, respectively) 2 weeks after operation. *p<0.05, **p<0.01, ***p<0.001. RBC red blood cells; Hb, haemoglobin; Hct, haematocrit; SCD, sickle cell disease; BMT, bone marrow transplantation; WT, wild-type.
Effect of sirolimus treatment tissue iron deposition in SCD mice
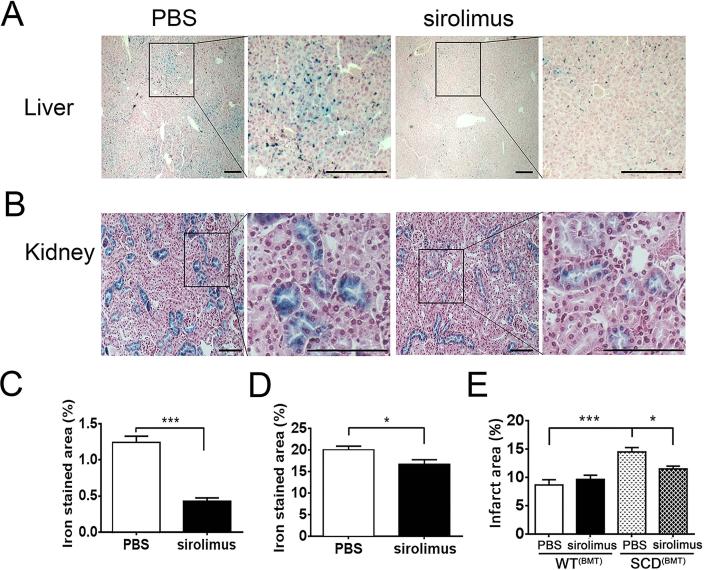
To determine whether sirolimus treatment affected tissue iron deposition, iron staining was performed on sections of liver and kidney from SCD(BMT) mice treated with PBS or sirolimus for 3 weeks. Iron deposition in WT(BMT) was not detectable (data not shown). Iron staining was reduced in livers (Figure 5A, C) and kidneys (Figure 5B, D) from mice treated with sirolimus compared to PBS. Iron staining in the kidneys was confined to the tubules within the renal cortex (Figure 5B).
Figure 5. Protective effect of sirolimus on SCD(BMT) tissue iron accumulation and stroke.
(A) Representative pictures of iron staining in liver (bar: 250 μm). (B) Representative pictures of iron staining in kidney (bar: 100 μm). (C) Quantification of iron stained area in liver (n=5 per group) (D) Quantification of iron stained area in kidney (n=5 per group). (E). Stroke size in brain of SCD(BMT) mice or WT(BMT) mice treated with sirolimus (n=9 per group) or PBS (n=8 per group). * p<0.05, ***p<0.001. SCD, sickle cell disease; BMT, bone marrow transplantation; WT, wild-type; PBS, phosphate-buffered saline.
Effect of sirolimus treatment on stroke volume in SCD mice
To further determine whether the apparent beneficial effects of sirolimus on anaemia in SCD might lead to other beneficial effects related to end organ damage, we induced stroke in mice treated with sirolimus or placebo. SCD(BMT) and WT(BMT) mice were treated with daily sirolimus or PBS for 1 week prior to stroke induction. The infarct area was increased in SCD(BMT) mice compared to WT(BMT) mice while stroke size was reduced in SCD(BMT) mice treated with sirolimus compared with PBS-treated SCD(BMT) mice (Figure 5E). No effect of sirolimus on stroke size was observed in WT(BMT) mice (Figure 5E).
Discussion
mTOR activity has been previously implicated in erythropoiesis (Gan, et al 2008, Knight, et al 2014). Both activation and inhibition of mTOR activity may contribute to anaemia (Gan, et al 2008, Knight, et al 2014). Ineffective erythropoiesis due to increased mTOR signalling has also been demonstrated in FOXO3 deficiency (Zhang, et al 2014). In β-thalassaemic mice, sirolimus treatment reversed the defect in erythropoiesis leading to enhanced erythroid cell maturation and less severe anaemia (Zhang, et al 2014). Other investigators have shown that chronic sirolimus treatment in rats leads to erythrocyte microcytosis without anaemia (Diekmann, et al 2012), while in humans, the effect of chronic sirolimus treatment on anaemia is controversial. For example, one study showed a higher incidence of anaemia in patients scheduled to undergo renal transplantation that were treated with sirolimus (Ekberg, et al 2010) while another study showed that anaemia correlates most with allograft dysfunction in renal transplant patients (Friend, et al 2007).
Sickle cell anaemia has been characterized as a disease in which ineffective erythropoiesis may contribute to disease severity (Wu, et al 2005). As ineffective erythropoiesis may be a therapeutic target in SCD, we hypothesized that inhibition of mTOR would be beneficial. To test this hypothesis we used a murine model of SCD because murine models of SCD are available that mimic many of the abnormalities observed in humans with SCD (Ryan, et al 1997), (Paszty, et al 1997), (Nagel 1998). BMT from a donor SCD mouse to WT recipients can be used to generate large numbers of age and sex-matched SCD mice on a relatively homogenous genetic background.
In this study, sirolimus treatment improved anaemia in SCD mice after just 1 week of treatment. Although sirolimus has been shown to upregulate HbF in erythrocytes in vitro (Mischiati, et al 2004), an effect of sirolimus on HbF after in vivo treatment was not observed in this study, indicating a distinct mechanism related to improved anaemia. Erythrocyte lifespan was mildly prolonged by sirolimus treatment indicating that the effect of sirolimus may prevent erythrocyte sickling. In a passive transfusion experiment, transfused sickled erythrocytes treated with sirolimus survived longer in circulation, indicating the target of mTOR inhibition may be the peripheral erythroid population; however, it is probable that many of these transfused erythrocytes were reticulocytes that remain sensitive to the maturation effects of sirolimus.
Splenomegaly secondary to chronic haemolysis is present in SCD mouse models and correlates with disease severity (Kean, et al 2003). The spleen may play an important role as a haematopoietic organ in mice, and the reductions in spleen size in SCD following mTOR inhibition observed in this study could be due to suppression of haematopoiesis. However, given that the reduction of spleen size was associated with improvements in anaemia, it is probably secondary to reduced erythrocyte sickling. There may also be other pathways involved in mTOR inhibition on spleen size as previous studies have shown that sirolimus leads to reduced spleen size in non-SCD human populations without compromising splenic function (Araujo, et al 2014). As splenectomy did not improve anaemia in SCD mice, the spleen is unlikely to be the relevant target organ for sirolimus with regards to the improvement of anaemia in SCD.
Although the high ratio of reticulocytes in SCD peripheral blood is considered a sign of stress haematopoiesis, a defect in reticulocyte terminal differentiation has also been reported (Chen, et al 2008). This defect may contribute to SCD because sickle reticulocytes are more susceptible to sickling with less capacity to resume a discoid shape compared to mature sickle erythrocytes (Onyike, et al 1995). The data showing that sirolimus promotes erythroid maturation both in vitro and in vivo indicate the mechanism may be due to correction of a maturation defect in circulating SCD erythrocytes. Mechanisms by which mTORC1 inhibition may affect reticulocyte maturation and/or prolong circulating half-life include the well-described induction of autophagy by mTORC1 inhibitors (Jung, et al 2010). Induction of reticulocyte autophagy could facilitate turnover and shrinkage of the plasma membranes (Mankelow, et al 2015, Ney 2011) as well as modify intracellular oxidative stress by affecting autophagy of reticulocyte mitochondria (Zhang, et al 2009).
Tissue iron deposition secondary to chronic haemolysis has also been shown to correlate with SCD severity in mice (Kean, et al 2003). Tissue iron may activate downstream oxidative stress pathways and constitute one of the mechanisms of organ damage in SCD (Thomas, et al 2009). This is especially true in SCD patients who receive chronic blood transfusions (Dos Santos, et al 2012). The reduced organ iron deposition suggests that sirolimus treatment may protect the vasculature and other organs in SCD in addition to improvements in anaemia. Reduced tubular iron deposition may be a consequence of decreased intravascular haemolysis and haemoglobinuria.
Stroke is one of the most devastating complications of SCD (Yawn, et al 2014). We have previously demonstrated that stroke volumes are larger in SCD mice following middle cerebral artery thrombotic occlusion (Luo, et al 2014) due to sickling involving the ischaemic microvasculature. Treatment with sirolimus reduced stroke area in this study, indicating that SCD outcomes may also be improved in this regard. The effects of sirolimus on stroke size are probably due to reduced sickling/vaso-occlusion in the ischaemic penumbra, which is consistent with the other effects we observed.
In conclusion, mTOR inhibition is associated with improvements in anaemia and reduced organ damage in a mouse mode of SCD. The mechanism appears to be related to maturation of circulating erythroid cells resulting in a longer lifespan. A clinical trial will be necessary to determine the relevance of these finding to humans. Further studies of downstream mTOR signalling pathways may also identify additional cellular targets that may prove beneficial in SCD.
Acknowledgments
This work was supported by the National Institutes of Health, National Heart, Lung. and Blood Institute (HL073150) (D.T.E.) and University of Michigan Medical School MCubed Funding (A.D.C., D.T.E.).
Footnotes
Author contributions
J.W., J.T., H.W, C.G., D.H. and A.C. performed experiments and analysed results. J.W. and D.T.E. designed the research and wrote the paper.
Conflict of interest disclosure
The authors declare no competing financial interests.
References
- Aliyu ZY, Tumblin AR, Kato GJ. Current therapy of sickle cell disease. Haematologica. 2006;91:7–10. [PMC free article] [PubMed] [Google Scholar]
- Araujo NC, Sampaio Goncalves de Lucena SB, da Silveira Rioja S. Effect of rapamycin on spleen size in longstanding renal transplant recipients. Transplant Proc. 2014;46:1319–1323. doi: 10.1016/j.transproceed.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Cui S, Shi L, Urbonya R, Mathias A, Bradley K, Bonsu KO, Douglas RR, Halford B, Schmidt L, Harro D, Giacherio D, Tanimoto K, Tanabe O, Engel JD. Forced TR2/TR4 expression in sickle cell disease mice confers enhanced fetal hemoglobin synthesis and alleviated disease phenotypes. Proc Natl Acad Sci U S A. 2011;108:18808–18813. doi: 10.1073/pnas.1104964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Wang Y, Telen MJ, Chi JT. The genomic analysis of erythrocyte microRNA expression in sickle cell diseases. PLoS One. 2008;3:e2360. doi: 10.1371/journal.pone.0002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Gordon M, McKinstry RC, Noetzel MJ, White DA, Sarnaik SA, Meier ER, Howard TH, Majumdar S, Inusa BP, Telfer PT, Kirby-Allen M, McCavit TL, Kamdem A, Airewele G, Woods GM, Berman B, Panepinto JA, Fuh BR, Kwiatkowski JL, King AA, Fixler JM, Rhodes MM, Thompson AA, Heiny ME, Redding-Lallinger RC, Kirkham FJ, Dixon N, Gonzalez CE, Kalinyak KA, Quinn CT, Strouse JJ, Miller JP, Lehmann H, Kraut MA, Ball WS, Jr., Hirtz D, Casella JF. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371:699–710. doi: 10.1056/NEJMoa1401731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann F, Rovira J, Diaz-Ricart M, Arellano EM, Vodenik B, Jou JM, Vives-Corrons JL, Escolar G, Campistol JM. mTOR inhibition and erythropoiesis: microcytosis or anaemia? Nephrol Dial Transplant. 2012;27:537–541. doi: 10.1093/ndt/gfr318. [DOI] [PubMed] [Google Scholar]
- Dos Santos TE, de Sousa GF, Barbosa MC, Goncalves RP. The role of iron overload on oxidative stress in sickle cell anemia. Biomark Med. 2012;6:813–819. doi: 10.2217/bmm.12.71. [DOI] [PubMed] [Google Scholar]
- Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, Ketteler M, Navratil P. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrol Dial Transplant. 2010;25:2004–2010. doi: 10.1093/ndt/gfp778. [DOI] [PubMed] [Google Scholar]
- Friend P, Russ G, Oberbauer R, Murgia MG, Tufveson G, Chapman J, Blancho G, Mota A, Grandaliano G, Campistol JM, Brault Y, Burke JT, Rapamune Maintenance Regimen Study, G. Incidence of anemia in sirolimus-treated renal transplant recipients: the importance of preserving renal function. Transpl Int. 2007;20:754–760. doi: 10.1111/j.1432-2277.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, Chin L, Williams DA, Kwiatkowski DJ, DePinho RA. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean LS, Manci EA, Perry J, Balkan C, Coley S, Holtzclaw D, Adams AB, Larsen CP, Hsu LL, Archer DR. Chimerism and cure: hematologic and pathologic correction of murine sickle cell disease. Blood. 2003;102:4582–4593. doi: 10.1182/blood-2003-03-0712. [DOI] [PubMed] [Google Scholar]
- Knight ZA, Schmidt SF, Birsoy K, Tan K, Friedman JM. A critical role for mTORC1 in erythropoiesis and anemia. Elife. 2014;3:e01913. doi: 10.7554/eLife.01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury MJ, Koury ST, Kopsombut P, Bondurant MC. In vitro maturation of nascent reticulocytes to erythrocytes. Blood. 2005;105:2168–2174. doi: 10.1182/blood-2004-02-0616. [DOI] [PubMed] [Google Scholar]
- Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, Witkop C, Bass EB, Segal JB. Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;148:939–955. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pop R, Sadegh C, Brugnara C, Haase VH, Socolovsky M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood. 2006;108:123–133. doi: 10.1182/blood-2005-11-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Campbell A, Wang H, Guo C, Bradley K, Wang J, Eitzman DT. P-selectin glycoprotein ligand-1 inhibition blocks increased leukocyte-endothelial interactions associated with sickle cell disease in mice. Blood. 2012;120:3862–3864. doi: 10.1182/blood-2012-07-444455. [DOI] [PubMed] [Google Scholar]
- Luo W, Su EJ, Wang J, Wang H, Guo C, Pawar A, Campbell AD, Lawrence DA, Eitzman DT. Increased stroke size following MCA occlusion in a mouse model of sickle cell disease. Blood. 2014;123:1965–1967. doi: 10.1182/blood-2014-01-549717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankelow TJ, Griffiths RE, Trompeter S, Flatt JF, Cogan NM, Massey EJ, Anstee DJ. Autophagic vesicles on mature human reticulocytes explain phosphatidylserine-positive red cells in sickle cell disease. Blood. 2015;126:1831–1834. doi: 10.1182/blood-2015-04-637702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischiati C, Sereni A, Lampronti I, Bianchi N, Borgatti M, Prus E, Fibach E, Gambari R. Rapamycin-mediated induction of gamma-globin mRNA accumulation in human erythroid cells. Br J Haematol. 2004;126:612–621. doi: 10.1111/j.1365-2141.2004.05083.x. [DOI] [PubMed] [Google Scholar]
- Nagel RL. A knockout of a transgenic mouse--animal models of sickle cell anemia. N Engl J Med. 1998;339:194–195. doi: 10.1056/NEJM199807163390310. [DOI] [PubMed] [Google Scholar]
- Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol. 2011;18:152–157. doi: 10.1097/MOH.0b013e328345213e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyike AE, Ohene-Frempong K, Horiuchi K. Sickling in vitro at venous and arterial oxygen tensions of reticulocytes from patients with sickle cell disease. Biochem Biophys Res Commun. 1995;211:504–510. doi: 10.1006/bbrc.1995.1842. [DOI] [PubMed] [Google Scholar]
- Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- Savitt TL, Goldberg MF. Herrick's 1910 case report of sickle cell anemia. The rest of the story. JAMA. 1989;261:266–271. [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Mackey MM, Diaz AA, Cox DP. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 2009;14:102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- Wu CJ, Krishnamurti L, Kutok JL, Biernacki M, Rogers S, Zhang W, Antin JH, Ritz J. Evidence for ineffective erythropoiesis in severe sickle cell disease. Blood. 2005;106:3639–3645. doi: 10.1182/blood-2005-04-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108:1183–1188. doi: 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE, Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- Zhang J, Randall MS, Loyd MR, Dorsey FC, Kundu M, Cleveland JL, Ney PA. Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood. 2009;114:157–164. doi: 10.1182/blood-2008-04-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Camprecios G, Rimmele P, Liang R, Yalcin S, Mungamuri SK, Barminko J, D'Escamard V, Baron MH, Brugnara C, Papatsenko D, Rivella S, Ghaffari S. FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis. Am J Hematol. 2014;89:954–963. doi: 10.1002/ajh.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]