Abstract
The anti-tumorigenic effects that type 1 interferons (IFN1) elicited in the in vitro studies prompted consideration of IFN1 as a potent candidate for clinical treatment. Though not all patients responded to IFN1, clinical trials have shown that patients with high risk melanoma, a highly refractory solid malignancy, benefit greatly from intermediate IFN1 treatment in regards to relapse-free and distant-metastasis-free survival. The mechanisms by which IFN1 treatment at early stages of disease suppress tumor recurrence or metastatic incidence are not fully understood. Intracellular IFN1 signaling is known to affect cell differentiation, proliferation, and apoptosis. Moreover, recent studies have revealed specific IFN1-regulated genes that may contribute to IFN1-mediated suppression of cancer progression and metastasis. In concert, expression of these different IFN1 stimulated genes may impede numerous mechanisms that mediate metastatic process. Though, IFN1 treatment is still utilized as part of standard care for metastatic melanoma (alone or in combination with other therapies), cancers find the ways to develop insensitivity to IFN1 treatment allowing for unconstrained disease progression. To determine how and when IFN1 treatment would be most efficacious during disease progression, we must understand how IFN1 signaling affects different metastasis steps. Here, we specifically focus on the anti-metastatic role of endogenous IFN1 and parameters that may help to use pharmaceutical IFN1 in the adjuvant treatment to prevent cancer recurrence and metastatic disease.
Keywords: Interferon, Cancer metastasis, Invasion, Colonization
1. Introduction
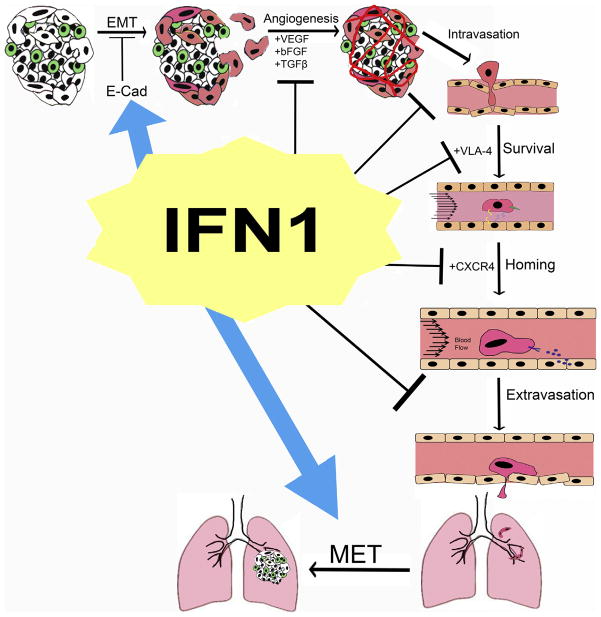
Cancer metastasis contributes to approximately 90% of cancer-related mortality [1,2]. Metastatic cancer cells (MCCs) possess molecular traits different than the tumor cells of the primary lesion, thus altering and often diminishing responses of these cells to therapeutic modalities designed to target primary tumor cells [2–4]. Severity of metastatic dissemination contributes to poor patient survival and quality of life [5,6]. The process of metastasis can be described as an “obstacle course” (Fig. 1) in which MCCs must overcome numerous and substantial hurdles (imposed by stromal cells in proximal and distal tissues) to enter and travel within lymph or blood vessels, survive the hostile environment within circulation, home to and arrive at metastasis-permissive tissues, and, finally, colonize a secondary site [4]. At the early stages of metastatic process, specific alterations in cellular adhesion molecules enable MCCs to interact with different cell types and extracellular matrix (ECM) molecules [7–9]. Furthermore, aberrant expression of cell adhesion molecules on MCCs alter intracellular signaling and cytoskeleton structure eliciting changes in cell morphology and increased cell migration/invasion [7,8].
Fig. 1.
Elements of metastatic processes and their regulators, whose activities are modulated by IFN1 are depicted.
Once MCCs utilize these changes to escape the confines of the primary tumor, they engage with endothelial cells and gain entrance into the circulatory system. MCCs that survive circulation then follow exact and complex homing signals to arrive at specific distal tissues, which are defined by expression of MCC-trapping molecules induced during the development of primary tumors. Cells that survive in the new metastatic site can remain dormant or rapidly proliferate to form metastatic lesions.
MCCs rely on intrinsic cellular and environmental factors to aid in the completion of this process [7,10,11]. Importance of understanding the mechanisms that contribute to suppressing MCCs from completing each step of the metastatic process cannot be overestimated. Advances in this area are likely to identify novel prognostic markers and molecular regulators as targets, which could potentially lead to therapies that extend cancer patients survival.
Anti-viral cytokines that belong to the superfamily of type 1 interferons (IFN1), are able to modulate cell growth, cell differentiation, and promote tumor immune surveillance [12,13]. These cellular functions are elicited when IFN1s bind to a cell surface receptor comprised of two subunits (IFNAR1 and IFNAR2). Interaction of IFN1 with the receptor leads to activation of receptor associated Janus kinases (JAKs) JAK1 and TYK2. Activated JAKs then phosphorylate IFNAR2 to recruit STAT1 and STAT2 Subsequent phosphorylation and dimerization of STAT1/2 and recruitment of IRF9 protein completes the assembly of the ISGF3 transcription factor that translocates to the nucleus, interacts with IFN-stimulated response elements and transactivates the expression of the interferon stimulated genes (ISGs) [14].
Cancer cells engineered to overexpress IFN1 exhibited an impaired metastatic potential [15,16]. Moreover, treatment with recombinant IFN1 also suppressed metastasis in mouse models [17,18]. Conversely, tumors grown in IFNAR1-null mice were shown to rapidly progress to development of metastases [19]. These studies demonstrated that IFN1 possess potent anti-metastatic properties. Furthermore, it was suggested that administration of pharmacologic IFN1 could be beneficial for patient treatment and resulted in FDA approval for the use of IFN1 in the treatment of hairy cell leukemia (1985), AIDS-related Kaposi’s sarcoma (1988), and melanoma (1995) as cancer chemotherapy agents [20]. Some of malignant melanoma patients receiving IFN1 treatment following surgery demonstrated improved relapse-free survival rate [21]. Although many patients failed to respond to IFN1 therapy and developed recurrence and metastasis, IFN1 continues to be the preferred adjuvant therapy in the treatment of melanoma patients at risk of developing metastasis [22]. Inability to suppress metastasis despite IFN1 adjuvant therapy in these patients suggests that IFN1 signaling in tumor or/and stromal cells may be impaired.
Understanding of specific mechanisms by which IFN1 can suppress metastatic process and how these mechanisms could be overcome by tumors represents a critical challenge for proper stratification of cancer patients to identify the cohorts that can benefit from the adjuvant therapy with IFN1. Furthermore, critical analysis of IFN1-affected pathways is required for identification of future promising therapeutic targets. This review aims to guide through understanding the role of IFN1 in maintaining normal cellular function and how loss of IFN1 signaling may contribute to an environment conducive to metastasis. The subsequent sections will describe conditions facilitating each step of metastasis and how IFN1 treatment may affect the tumor and/or stromal cells within that stage of the metastatic process.
2. The role of IFN1 in preventing tumor cell dissemination
The metastatic process begins when cancer cells alter their adhesive properties and begin shedding from the primary tumor mass [23,24]. Detachment from the primary tumor is often attributed to a process called epithelial-to-mesenchymal transition (EMT) in which cells lose epithelial markers in favor of expression of mesenchymal molecules that confer changes in cellular morphology and function [25]. A hallmark of EMT is downregulation of epithelial-cadherin (E-Cad) and upregulation of a mesenchymal cadherin, such as neuronal-cadherin (N-Cad) [24].
Cadherins are calcium-dependent cell adhesion molecules that form cell–cell contacts called adherens junctions (AJs) [23,24]. Classical cadherins, such as E-Cad, possess five extracellular domains, a single transmembrane domain, and two cytoplasmic domains. The five extracellular domains are required for homophilic cadherin binding [23,24]. For example, the extracellular domain of E-Cad can only interact with the extracellular domain of another E-Cad molecule [23]. The cytoplasmic domains bind the catenins p120 and β-catenin to affect intracellular signaling and reorganize the actin cytoskeleton [23]. This homophilic interaction at the AJs contribute to the establishment and maintenance of cell polarity and cellular identity within a tissue [23]. Loss of E-cad and upregulation of mesenchymal-associated cahderin results in loss of cellular polarity and mesenchymal-like cell properties [23,24]. Differential expression of cadherin between cells results in self-sorting and detachment of non-E-Cad expressing cells from the other cells in the tissue [24], which contributes to the diffusion of cancer cells into the stroma [23]. This invasion into the stroma occurs because “cadherin switching” alters intracellular signaling to affect cytoskeletal organization thus leading to loss of cell polarity, and increased migration and production of molecules that facilitate destruction of extracellular matrix (ECM) molecules [24]. Therefore, if maintaining E-Cad expression and function in cancer cells can suppress metastatic potential and IFN1 can upregulate E-Cad expression, expression of functional IFN1 receptor is required to elicit IFN1-mediated E-cad expression.
Though EMT-mediated cell detachment describes initiation of epithelial cancer metastasis, not all cancers derive from epithelial cells. Some cancer cells originate from specialized, non-epithelial cells, such as melanoma from melanocytes and glioma from glial cells. No matter the cell origin, cancer cells still undergo detachment and dissemination through aberrant E-Cad expression. Melanocytes, for example, express E-Cad to promote melanocyte-keratinocyte cellular adhesion which is important to the integrity of skin tissue architecture [24,26,27], while melanomas undergo upregulation of N-Cad to promote melanoma–melanoma and melanoma–fibroblast cell interaction [26,28].
It is important to note that numerous stimuli including oncogenic signaling, inflammation, tumor microenvironment stress etc. are capable of desensitizing cells and tissues to IFN1 by accelerating the ubiquitination and subsequent degradation of IFNAR1 chain of its receptor [29,30]. Furthermore, it has been shown that conditions associated with tumor growth stress that facilitates the loss of IFNAR1 [31–43], such as hypoxia [44] and exposure to VEGF [45], also contribute to EMT-mediated downregulation of E-Cad. It is possible that the IFNAR1 downregulation induced by the harsh conditions within the tumor microenvironment alters ISG expression to promote decreased E-Cad expression resulting in EMT and cancer cell dissemination. Studies have shown that in vitro treatment of cancer cells with IFN1 upregulates E-Cad protein expression [46,47]. This suggests that IFN1 treatment can maintain E-Cad expression to suppress EMT and prevent entrance into the metastatic process by affecting cadherin expression (Fig. 1). However, as melanocytes transform to melanoma cells, the cancerous cells acquire IFN1-insensitivity (likely through rapid IFNAR1 ubiquitination and subsequent protein degradation [29,30,42,43]). Once melanoma cells progress to IFNAR1 downregulation, E-Cad expression through IFN1 adjuvant therapy is no longer possible despite IFN1 production by the cancer or surrounding stromal cells. Therefore, IFN1 treatment would have to prevent the detached MCCs from entering the blood circulation.
3. The role of IFN1 in suppressing MCCs intravasation and extravasation
During cancer progression, cancer cells manipulate the malleability of the vascular endothelium to promote growth and metastasis [48,49]. The endothelial cells composing the various types of blood vessels throughout the body undergo changes to control nutrient/gas exchange into tissues by affecting cell–cell adhesion, regulate blood flow by changing vessel diameter, and modulate leukocyte trafficking via secretion of soluble proteins and expression of cell adhesion molecules [48]. Because cancer cells secreted factors, such as vascular endothelial growth factor (VEGF), blood vessels within the tumor tend to be leaky with irregular diameters, and have irregular blood flow [48]. These abnormalities promote intravasation of cancer cells into the tumor vasculature and extravasation from the systemic circulation.
Tumor cells injected intravenously into IFNAR1-null mice demonstrate accelerated metastasis, suggesting that IFNAR1 expression and subsequent IFN1 signaling in host (perhaps even the vascular endothelium) contribute to escape of tumor cells from circulation [19]. Treatment with IFN1 can suppress endothelial cell proliferation and migration, and affecting homophilic adhesion; all of which affect integrity of the vascular endothelium within the tumor and throughout the body [50–52]. Cancer cells and tumor infiltrating monocytes secrete a variety of factors, such as transforming growth factor-β1 (TGFβ1), basic fibroblast growth factor (bFGF), and VEGF to promote tumor growth, but also induce endothelial cell proliferation during angiogenesis [53]. Adenovirus IFN-β gene therapy was shown to induce expression of nitric oxide synthase and IFN-β resulting in reduction of TGFβ1 and bFGF [54], thus blocking endothelial cell proliferation. Though IFN-β is known for its apoptotic function, treatment of endothelial cells with IFN-β did not show cytotoxicity but decreased proliferation [50]. IFN-β or IFN-α treatment suppresses proliferation of endothelial cells with no loss of metabolic activity [55]. Together, these studies demonstrate how IFN-β suppresses angiogenesis via suppression of endothelial cell proliferation indirectly and directly (Fig. 1).
Proliferating cells lose the ability to form cell–cell and cell–substrate contacts. Inducing endothelial cell proliferation would therefore contribute to areas of vascular leakage, which may help with the migration of cancer cells in and out of the blood stream. Suppressing endothelial proliferation would thus suppress abnormal intratumoral angiogenesis and, accordingly, help to maintain the vascular integrity to inhibit invasion through the endothelium. However, IFN-β can also affect angiogenesis and vascular leakiness by altering vessel formation through suppression of endothelial cell migration. Cell migration also requires endothelial cell detachment from the rest of the vascular endothelium to create gaps that may contribute to intravasation and extravasation. IFN-β treatment of human umbilical vein endothelial cells (HUVEC) resulted in suppressed migration toward tumor conditioned media [55]. The IFN-β-mediated suppression of endothelial cell migration can be reversed using anti-IFN-β antibody. This suggests that IFN1 signaling in endothelial cells may play a role in intravasation and extravasation. Therefore, IFN-β treatment functions as an anti-angiogenic factor as well as a regulator of intratumoral and systemic blood vessel integrity (Fig. 1).
The vasculature of tumors consists of leaky blood vessels with slow blood flow that lowers efficacy of blood-borne therapeutics and facilitates entrance of tumor cells into circulation [56]. Intratumoral treatment using high dose IFN1 results normalizes blood vessel barrier function and blood flow [51,57]. Blood vessel barrier function is mediated by cell–cell adhesion. Endothelial cells can change their cell adhesive properties to create tight cell–cell junction needed for blood brain barriers or loose cell–cell interactions to allow for nutrient/gas exchange and mobilization of bone marrow derived cells. These functions are governed by tight junction proteins, such as occludin, and cell adhesion molecules, such as vascular endothelial cadherin (VE-Cad) and integrins. HUVEC cells treated with the type II interferon IFN-γ showed a dose dependent decreased expression of occludin [52] and VE-Cad [52,58]. Occludin is needed to maintain tight junction barriers to prevent metastasis. Loss of occludin results in reduced cell–cell adhesion to facilitate invasion. VE-Cadherin is a major component of endothelial cell–cell AJs. Loss of VE-Cad due to IFN-γ results in increased vascular permeability [52,58]. Treatment with IFN-β was able to rescue expression of both occludin and VE-Cad, thereby restoring barrier function [52]. IFN-β also induces expression of CD73 (also termed Ecto-5-prime nucleotidase) that converts adenosine monophosphate into adenosine and helps to reduce vascular permeability [59,60]. These effects of IFN1 in endothelial cells may help to increase the adenosine-mediated barrier function and, accordingly, limit the opportunities for tumor cells to egress from circulation.
Overall, these studies suggest that IFN1 functions to IFN-β decrease vascular leakage observed in the tumor microenvironment, and, in turn, likely to suppress intravasation of disseminated tumor cells into circulation. Moreover, promoting tight barrier cell–cell adhesion via IFN-β treatment may also suppress extravasation of circulating cancer cells within the distal metastatic site. Therefore, patients deficient in IFN1 signaling in the cancer cell may still benefit from systemic IFN1 treatment at this stage of the metastatic process to prevent disease progression. However, cancer cells and the cancer associated stroma surrounding the tumor vascular cells may also suppress IFN1 signaling in the endothelial cells to facilitate progression through the metastatic process. In this case, the integrity of IFN1 signaling in the systemic vascular endothelium will be of great importance in eliminating cancer cells that gain entrance to the circulation.
4. The role of IFN in suppression of MCCs survival in circulation
After invading the vascular endothelium, disseminated tumor cells must survive within the harsh environment of the circulatory system to avoid anoikis, the cell-contact deprivation-induced death. Cells, such as leukocytes, are able to survive the hemodynamic conditions within the vasculature by altering the expression of proteins that affect their binding to the endothelium and affect their cytoskeletons. The endothelial cells lining the inside of blood vessels express selectins, which are able to capture and tether circulating leukocytes. Specific cell–cell adhesive forces rely on the fluid shear forces to propel movement of the circulating leukocytes and affect their subsequent survival under these forces [61]. To combat cellular deformation caused by the fluid shear stress upon, circulating leukocytes can utilize surface projections and cytoplasmic processes to initiate active migration along the vessel walls [62]. IFN1 signaling along the vascular endothelium can further suppress metastasis by changing the hemodynamic conditions within blood vessels and altering expression of cell adhesion molecules that can promote cell survival/adhesion while in circulation.
The fluidic forces within blood vessels create superficial stresses near the vessel walls described as circumferential stress generated by pull pressure and shear stress due to blood flow. Normal shear stress conditions suppress leukocyte adhesion, and inhibit proliferation of endothelial cells and smooth muscle cells [63]. Cancer cells introduced into circulation undergo cell death an hour or two post injection and are subsequently cleared within ten minutes [64]. Changes in artery pressure, vascular resistance, and vascular stiffness can also affect shear stress [65]. Studies have shown that IFN1 treatment can affect different vascular systems to alter vessel diameter and blood flow [66–68], thus increasing shear stress. IFN-α treatment transiently increased pulmonary vascular resistance and pulmonary arterial pressure, which can result in increased blood flow [66,67], and, thus, shear stress. To decrease blood flow-associated shear stress, the vascular endothelium undergoes flow-mediated vasodilation [69]. However, IFN-α can also suppress flow-mediated vasodilation [68]. This suggests that systemic application of IFN-α can alter the hemodynamics of the patient to increase shear stress, thereby creating conditions more difficult for cancer cells to survive.
As previously mentioned, circulating tumor cells are able to withstand the shear stress by forming cell–cell contact with endothelial cells, thereby allowing changes in the cytoskeletal structure to promote migration, i.e. rolling adhesion in the direction of blood flow, and also extravasation. Survivor cancer cells able to withstand the circulation shear stress adapt by expressing the cell adhesion molecules similar to those found in circulating leukocytes, such as selectins and integrins, which also contribute to homing [70–72]. Selectins capture and tether allowing for the rolling of circulating cells along the vascular endothelium, while integrins rearrange the cytoskeleton to confer arrested cells on the endothelium survival under hydrodynamic shearing. Not unlike leukocytes, tumor cells express E-Selectin, L-Selectin, P-selectin, I-CAM1 and V-CAM1 to engage in rolling adhesion along the endothelium as well as the integrin VLA-4 to initiate arrest and survival of shear stress [61,73]. While IFN-α and IFN-β do not affect the expression of selectins, both affect expression of a variety of integrin molecules, including Very Late Antigen (VLA)-4, to suppress survival of cancer cells [74,75]. VLA-4 is an integrin β1 containing integrin complex that interacts with fibronectin [76]. Binding of fibronectin induces VLA-4 signaling that activates phosphoinositide 3-kinase (PI-3K)/AKT/BCL-2 signaling to promote cell resistance to anoikis, or anchorage-independent survival [77].
Survival of many cell types is anchorage dependent and requires environmental cues such as cell adhesion molecules and extracellular matrix proteins to activate intracellular survival mechanisms [76,78]. Cancer cells that fail to express cell adhesion molecules undergo anoikis. Expression of these pro-survival cell adhesion molecules is required but not sufficient to propel the MCC to the end of the metastatic process. The MCC also needs to acquire the ability to alter its cellular morphology to complete rolling adhesion and extravasation from circulation.
Alterations in the actin cytoskeleton and plasma membrane rigidity also play a role in the rolling adhesion within the vasculature to survive the shear stress. To elicit cellular migration, actin filaments are rapidly polymerized at cell protrusions called filopodia and lamellipodia, which comprises the Triton X-100 insoluble fraction [79]. IFN1-sensitive cells treated with IFN1 exhibit an increased recruitment of IFNAR1 to the Triton X-100 insoluble fraction, which was not observed in IFN1-insensitive cells [80,81]. These reports suggest that IFN1 and IFNAR interaction affects the actin-cytoskeleton organization (perhaps contributing to IFN1-mediated inhibition of cell proliferation and cell migration). Increasing the rigidity of the plasma membrane with reagents, such as the Acetyl-CoA carboxylase inhibitor Soraphen A, has been shown to diminish cancer cell migration and invasion [82]. In the case when cancer cells evolve to suppress IFN1 signaling, treatment with reagents to increase membrane rigidity may increase cancer cell death in circulation. However, if the patients’ blood contains MCCs, IFN1 signaling may already be suppressed in the MCCs and IFN1 treatment would be unable to regulate plasma membrane fluidity. In this case, the most beneficial treatments to design will modulate the homing capacity of MCCs by affecting chemokine receptor expression on MCCs or chemokine expression by stromal cells at the potential secondary site. Therefore, better understanding the role of IFN1 signaling in MCC homing is important for improving the efficacy of adjuvant therapies.
5. How IFN1 can suppress the homing of MCCs to the distant sites?
The ability of MCCs to home to specific tissues is an important step before colonization and completion of the metastatic process (Fig. 1). Many factors contribute to the arrival of MCCs to their specific destination, such as direction of blood from primary tumor, mechanical trapping of tumor cells in capillary beds within a distal tissue, cell surface adhesion molecules expressed on MCCs and chemokines expressed in the microenvironment within the secondary site [83,84]. The previous section has already discussed how IFN1 treatment can alter tumor vascularization to suppress metastatic potential; this section will focus on how IFN1 treatment affects the chemotaxis of MCCs able to survive the blood circulation to tissue specific sites. Studies have shown that the chemokines and chemokine receptors attributed to the homing of leukocytes to sites of injury or injection also participate in cancer metastasis, including CXCR4 [85,86] and CCR2 [86,87]).
The chemokine receptor CXCR4 was initially identified as a co-receptor for T-tropic HIV-1 and HIV-2 [84,88] and was later associated with promoting the growth, adhesion, and directional migration of immune cells in response to antigen-specific inflammation [84,85]. In normal tissue, most chemokine receptors, such as CXCR4, are quiescent. However, they are activated or upregulated in cancer cells in response to intrinsic genetic changes in the cancer cell or environmental stimuli. Cancer cells utilize aberrant CXCR4 expression and function to direct tissue specific metastasis [84,85]. CXCR4 is expressed in hematopoietic malignancies, such as CLL [88], and non-hematopoietic cancers, such as breast and melanoma [88], aiding in their survival and trafficking from the primary tumor to a secondary site, such as lung, bone, and brain [84,85,88,89].
Experiments using mouse orthotopic transplantation models have revealed that both blood flow directionality and cell surface protein expression contribute to successful homing [89–91]. The highly metastatic breast cancer cell line MDA-MB-231 is comprised of a highly heterogeneous population of cells, in which single-cell-derived progenies possess specific tissue tropisms [91]. Injection of MDA-MB-231 cells through the tail vein versus left ventricle deigned different metastatic patterns, with lung colonization favored over bone and brain [92]. In vivo selection of cells isolated from bone and lung metastatic lesions compared to the parental cell lines show preferential expression of genes unique to molecules found in their final destination [83,91,92]. This suggests that expression of certain genes mediate homing of MCCs to lung or bone. However, expression analysis found that both bone and lung tropic cells share CXCR4 expression [83,92]. Together, these reports suggest that while expression of CXCR4 by MCCs can aid is some tissue specificity; direction of blood flow contributes to MCC general directionality while other genetic markers provide further specificity for localization of metastatic lesions. Therefore, it is important to evaluate differential cell surface markers in different cancer types at different metastatic site to predict and target tissue tropism. Though CXCR4 upregulation in cancer cells and blood flow are important in the homing process, they are not sufficient to drive chemotaxis. The stromal cells at the secondary site contribute to the homing of MCCs by expressing the CXCR4 ligand CXCL12.
The chemokine CXCL12, also called stromal cell derived factor-1, is expressed by pericytes, endothelial cells, fibroblasts, and neurons [93]. Expression of CXCL12 by these stromal cells can be induced by pro-inflammatory factors, such as lipopolysaccharides, interleukin 1, and tumor necrosis factor α (TNF-α). CXCL12 contributes to vascularization during renal development and recruitment of bone marrow derived cells to sites of injury in the bone, heart, vascular endothelium, lung, and skin [93]. Upregulation of CXCL12 at injury sites creates a concentration gradient to attract CXCR4-expressing cells from the blood circulation to the site of injury [93]. The CXCL12 inducing agent TNFα, while not expressed in the plasma or serum of healthy individuals, is often detected in biopsies of cancer patients [94]. TNFα expression in epithelial cancer is frequently associated with poor prognosis [94], likely because the TNF-α expressed by cancer cells will initiate the CXCL12/CXCR4 signaling axis to promote MCCs homing to secondary metastatic site.
During chronic inflammation, such as multiple sclerosis [95] or hypertrophic scar formation [93], the CXCL12/CXCR4 signaling pathway recruiting CXCR4-expressing leukocytes can be suppressed with IFN1 treatment. Studies show that treatment with IFN1 is able to downregulate CXCR4 expression [93,95]. Without CXCR4 expression, cells fail to home to site of CXCL12 production. Therefore, it is possible that the IFN1 anti-metastatic effects are partially mediated by decreasing CXCR4 expression on MCCs to inhibit homing to secondary sites and prevent further metastatic progression. However, if patients have MCCs that have progressed to this point, IFN1 intervention may be too late to elicit a therapeutic response. In such cases, there is a pressing need to target other chemokine pathways associated with cancer cell homing to metastatic sites, such as CCL2 [96]. CCL2 is an IFN1-induced chemokine associated with the chemotaxis of CCR2+ prostate and breast cancer cells [86,97]. CCL2/CCR2 signaling upregulates integrin αvβ3 expression on the cell surface expression to promote MCC survival during the homing process [86]. If patients possess CCR2+ MCCs, systemic IFN1 therapy may have adverse effects. In this case, treatment with CCR2 antagonists would be most beneficial [98].
6. The role of IFN1 in suppression of metastatic colonization
In order for cancer cells to colonize a secondary site, the MCCs must relinquish mesenchymal phenotypes for more epithelial properties in a process called mesenchymal–epithelial transition (MET) [99]. Initiation of MET allows cancer cells to engage the non-cancer cells at the metastatic site and begin acclimation to the new microenvironment [99]. This allows for cancer cell proliferation. Part of the MET process is re-expression of epithelial markers, including E-Cad [99,100]. Re-expression of E-Cad reestablishes cell polarity and activates intracellular proliferation and survival signaling pathways, such as Raf-MEK-MAPK and PI3K [99]. Expression of E-Cad in metastatic lesions is associated with aggressive growth [101]. In the section of cancer cell dissemination process, I discussed how IFN1 treatment is able to restore E-Cad expression provided stable IFN1 signaling in the cancer cell. Downregulation of IFN1 signaling in MCCs would therefore be of great benefit, just as re-establishment of IFN1 signaling upon reaching the secondary site to re-introduce E-Cad would allow for metastatic colonization. It is here where IFN1 adjuvant therapy may, at least in theory, become detrimental to the treatment of cancer patients.
Though the cancer cells growing in the metastatic colony express E-Cad, they do not recover all their epithelial properties. And if they re-establish IFN1 signaling, retaining some mesenchymal properties may help evade IFN1-mediated cell apoptosis. Hematopoietic stem cells, for example, are activated from dormancy to induce cell proliferation upon IFN1 treatment [102]. It is, therefore, possible that IFN1 treatment in patients in which MCCs have formed micro-metastases would aggravate growth of metastatic tumors.
7. Conclusions
Clinical studies have found that IFN1 adjuvant therapy is most efficacious in patients at early stages of disease prior to establishment of distal metastasis [103]. Patients who already developed the metastatic disease are less responsive to treatment with IFN1 compared to those with non-metastatic tumors [104,105], which suggests cancer cell and/or the stromal cells relinquish their sensitivity to IFN1 to facilitate metastasis. Considering the effects IFN1 treatment has on cellular adhesion, the vascular endothelium, and chemotaxis, the observed IFN1-insensity in many patients may be in response to loss of IFN1 signaling in the early steps of the metastatic process.
Conversely, failure to elicit anti-tumorigenic effects in patients with metastatic disease may be due to re-establishment of IFN1 by cancer cells in the secondary site, which have managed to repurpose IFN1 signaling to aid in metastatic growth. Therefore, to achieve optimal response in cancer patients, IFN1 must be administered in early stages of disease while integrity of the IFN1 pathway (in both the cancer cell and stroma) is still present. Viable circulating MCCs collected from patient blood should be analyzed for cell surface markers that contribute to cell survival, such as VLA-4. If IFN1 treatment is not sufficient to create the harsh mechanical conditions to induce MCC death, anti-integrin therapies may be engaged rather than risk patients undergo IFN1 toxicity. Secondary metastatic lesions (proximal and/or distal) should be evaluated for MET. Application of IFN1 may prove more detrimental than therapeutic at this stage of disease.
Taken together, studies highlighted in this review demonstrate that IFN1 can be a valuable tool in combating development of metastatic disease by affecting both the cancer and stromal cells. However, because cancer cells are able to suppress IFN1 signaling intracellularly and in stromal cells, it would be critical to identify methods to either stabilize IFNAR1 expression or somehow else restore the IFN1 signaling in cancer cells prior to intravasation. Additional efforts may be also focused on maintaining the IFN1 signaling in the stroma to promote MCCs clearance from circulation or on suppressing IFN1 in colonizing MCCs to prevent MET and establishment of metastatic lesions. To further improve treatment of patients who are refractory to IFN1, strategies could include the identification and targeting of IFN-induced mediators that could help to restore and maintain E-Cad expression to prevent EMT and cancer cell dissemination or to regulate VE-Cad in endothelial cells and suppress cancer cells entrance and exit from circulation. In addition, suppressing VLA-4 to prevent cancer cell survival in circulation, and affecting CXCR4 expression to regulate cancer cell homing to the metastatic site may also improve the efficacy of adjuvant therapies.
Acknowledgments
All authors declare no conflict of interest with this study. Support for this work by the NIH/NCI PO1 CA165997 and RO1 CA092900 grants (to S.Y.F.) is greatly appreciated.
Abbreviations
- MCC
metastatic cancer cells
- IFN
interferon
- IFNAR1
type 1 interferon receptor subunit 1
- IFNAR2
type 1 interferon receptor subunit 2
- JAK
janus kinase
- ISG
interferon stimulated gene
- EMT
epithelial-to-mesenchymal transition
- MET
mesenchymal-to-epithelial transition
- E-Cad
epithelial cadherin
- N-Cad
neuronal cadherin
- AJ
adherens junction
- ECM
extracellular matrix
- VEGF
vascular endothelial growth factor
Contributor Information
Angélica Ortiz, Email: anortiz@vet.upenn.edu.
Serge Y. Fuchs, Email: syfuchs@vet.upenn.edu.
References
- 1.Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin–integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 2.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 3.Ganapathy V, Moghe PV, Roth CM. Targeting tumor metastases: drug delivery mechanisms and technologies. J Control Release. 2015 doi: 10.1016/j.jconrel.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 5.Huang J, Jatoi A. Morbidity and mortality in patients with cancer who become nonambulatory after spinal cord compression: a case series on end-of-life care. J Palliat Med. 2009;12:219–222. doi: 10.1089/jpm.2008.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venur VA, Ahluwalia MS. Prognostic scores for brain metastasis patients: use in clinical practice and trial design. Chin Clin Oncol. 2015;4:18. doi: 10.3978/j.issn.2304-3865.2015.06.01. [DOI] [PubMed] [Google Scholar]
- 7.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CF, Lira C, Chu K, Bilen MA, Lee YC, Ye X, et al. Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res. 2010;70:4580–4589. doi: 10.1158/0008-5472.CAN-09-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer RH, Vu M, Cheng YF, Ramos DM. Integrin expression in malignant melanoma. Cancer Metast Rev. 1991;10:49–59. doi: 10.1007/BF00046843. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi A, Hoffman LM, Welm AL, Lessnick SL, Beckerle MC. The EWS/ FLI oncogene drives changes in cellular morphology, adhesion, and migration in Ewing sarcoma. Genes Cancer. 2012;3:102–116. doi: 10.1177/1947601912457024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 1980;40:2281–2287. [PubMed] [Google Scholar]
- 12.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 14.Kalvakolanu DV, Borden EC. An overview of the interferon system: signal transduction and mechanisms of action. Cancer Invest. 1996;14:25–53. doi: 10.3109/07357909609018435. [DOI] [PubMed] [Google Scholar]
- 15.Kaido T, Bandu MT, Maury C, Ferrantini M, Belardelli F, Gresser I. IFN-alpha 1 gene transfection completely abolishes the tumorigenicity of murine B16 melanoma cells in allogeneic DBA/2 mice and decreases their tumorigenicity in syngeneic C57BL/6 mice. Int J Cancer. 1995;60:221–229. doi: 10.1002/ijc.2910600216. [DOI] [PubMed] [Google Scholar]
- 16.Dong Z, Greene G, Pettaway C, Dinney CP, Eue I, Lu W, et al. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-beta. Cancer Res. 1999;59:872–879. [PubMed] [Google Scholar]
- 17.Brunda MJ, Rosenbaum D, Stern L. Inhibition of experimentally-induced murine metastases by recombinant alpha interferon: correlation between the modulatory effect of interferon treatment on natural killer cell activity and inhibition of metastases. Int J Cancer. 1984;34:421–426. doi: 10.1002/ijc.2910340321. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura J, Mitsui K, Ishikawa T, Tanaka Y, Yamamoto R, Suhara Y, et al. Antitumor and antimetastatic activities of human recombinant interferon alpha A/D. Clin Exp Metast. 1985;3:295–304. doi: 10.1007/BF01585083. [DOI] [PubMed] [Google Scholar]
- 19.Rautela J, Baschuk N, Slaney CY, Jayatilleke KM, Xiao K, Bidwell BN, et al. Loss of host type-I IFN signaling accelerates metastasis and impairs NK-cell antitumor function in multiple models of breast cancer. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-15-0065. [DOI] [PubMed] [Google Scholar]
- 20.Wadler S, Schwartz EL. New advances in interferon therapy of cancer. Oncologist. 1997;2:254–267. [PubMed] [Google Scholar]
- 21.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U, et al. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 22.Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383:816–827. doi: 10.1016/S0140-6736(13)60802-8. [DOI] [PubMed] [Google Scholar]
- 23.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 25.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu MY, Wheelock MJ, Johnson KR, Herlyn M. Shifts in cadherin profiles between human normal melanocytes and melanomas. J Investig Dermatol Symp Proc. 1996;1:188–194. [PubMed] [Google Scholar]
- 27.Tang A, Eller MS, Hara M, Yaar M, Hirohashi S, Gilchrest BA. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci. 1994;107(Pt 4):983–992. doi: 10.1242/jcs.107.4.983. [DOI] [PubMed] [Google Scholar]
- 28.Haass NK, Herlyn M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Investig Dermatol Symp Proc. 2005;10:153–163. doi: 10.1111/j.1087-0024.2005.200407.x. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs SY. Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. J Interferon Cytokine Res. 2013;33:211–225. doi: 10.1089/jir.2012.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huangfu WC, Fuchs SY. Ubiquitination-dependent regulation of signaling receptors in cancer. Genes Cancer. 2010;1:725–734. doi: 10.1177/1947601910382901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharya S, Katlinski KV, Reichert M, Takano S, Brice A, Zhao B, et al. Triggering ubiquitination of IFNAR1 protects tissues from inflammatory injury. EMBO Mol Med. 2014;6:384–397. doi: 10.1002/emmm.201303236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbone CJ, Zheng H, Bhattacharya S, Lewis JR, Reiter AM, Henthorn P, et al. Protein tyrosine phosphatase 1B is a key regulator of IFNAR1 endocytosis and a target for antiviral therapies. Proc Natl Acad Sci USA. 2012;109:19226–19231. doi: 10.1073/pnas.1211491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharya S, HuangFu WC, Dong G, Qian J, Baker DP, Karar J, et al. Anti-tumorigenic effects of Type 1 interferon are subdued by integrated stress responses. Oncogene. 2013;32:4214–4221. doi: 10.1038/onc.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya S, Zheng H, Tzimas C, Carroll M, Baker DP, Fuchs SY. Bcr-abl signals to desensitize chronic myeloid leukemia cells to IFNalpha via accelerating the degradation of its receptor. Blood. 2011;118:4179–4187. doi: 10.1182/blood-2010-12-325373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattacharya S, Qian J, Tzimas C, Baker DP, Koumenis C, Diehl JA, et al. Role of p38 protein kinase in the ligand-independent ubiquitination and down-regulation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2011;286:22069–22076. doi: 10.1074/jbc.M111.238766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya S, HuangFu WC, Liu J, Veeranki S, Baker DP, Koumenis C, et al. Inducible priming phosphorylation promotes ligand-independent degradation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2010;285:2318–2325. doi: 10.1074/jbc.M109.071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Carvalho LP, Bhattacharya S, Carbone CJ, Kumar KG, Leu NA, et al. Mammalian casein kinase 1alpha and its leishmanial ortholog regulate stability of IFNAR1 and type I interferon signaling. Mol Cell Biol. 2009;29:6401–6412. doi: 10.1128/MCB.00478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng H, Qian J, Baker DP, Fuchs SY. Tyrosine phosphorylation of protein kinase D2 mediates ligand-inducible elimination of the Type 1 interferon receptor. J Biol Chem. 2011;286:35733–35741. doi: 10.1074/jbc.M111.263608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H, Qian J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Vascular endothelial growth factor-induced elimination of the type 1 interferon receptor is required for efficient angiogenesis. Blood. 2011;118:4003–4006. doi: 10.1182/blood-2011-06-359745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian J, Zheng H, Huangfu WC, Liu J, Carbone CJ, Leu NA, et al. Pathogen recognition receptor signaling accelerates phosphorylation-dependent degradation of IFNAR1. PLoS Pathog. 2011;7:e1002065. doi: 10.1371/journal.ppat.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng H, Qian J, Varghese B, Baker DP, Fuchs S. Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2. Mol Cell Biol. 2011;31:710–720. doi: 10.1128/MCB.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huangfu WC, Qian J, Liu C, Liu J, Lokshin AE, Baker DP, et al. Inflammatory signaling compromises cell responses to interferon alpha. Oncogene. 2012;31:161–172. doi: 10.1038/onc.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.HuangFu WC, Qian J, Liu C, Rui H, Fuchs SY. Melanoma cell-secreted soluble factor that stimulates ubiquitination and degradation of the interferon alpha receptor and attenuates its signaling. Pigment Cell Melanoma Res. 2010;23:838–840. doi: 10.1111/j.1755-148x.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Moreno O, Lecanda J, Green JE, Segura V, Catena R, Serrano D, et al. VEGF elicits epithelial–mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop. Exp Cell Res. 2010;316:554–567. doi: 10.1016/j.yexcr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 46.Masuda T, Saito H, Kaneko F, Atsukawa K, Morita M, Inagaki H, et al. Up-regulation of E-cadherin and I-catenin in human hepatocellular carcinoma cell lines by sodium butyrate and interferon-alpha. In Vitro Cell Dev Biol Anim. 2000;36:387–394. doi: 10.1290/1071-2690(2000)036<0387:uroeca>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Slaton JW, Karashima T, Perrotte P, Inoue K, Kim SJ, Izawa J, et al. Treatment with low-dose interferon-alpha restores the balance between matrix metalloproteinase-9 and E-cadherin expression in human transitional cell carcinoma of the bladder. Clin Cancer Res. 2001;7:2840–2853. [PubMed] [Google Scholar]
- 48.Dudley AC. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2:a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erdmann J, Vitale G, van Koetsveld PM, Croze E, Sprij-Mooij DM, Hofland LJ, et al. Effects of interferons alpha/beta on the proliferation of human micro- and macrovascular endothelial cells. J Interferon Cytokine Res. 2011;31:451–458. doi: 10.1089/jir.2009.0103. [DOI] [PubMed] [Google Scholar]
- 51.Dickson PV, Hamner JB, Streck CJ, Ng CY, McCarville MB, Calabrese C, et al. Continuous delivery of IFN-beta promotes sustained maturation of intratumoral vasculature. Mol Cancer Res. 2007;5:531–542. doi: 10.1158/1541-7786.MCR-06-0259. [DOI] [PubMed] [Google Scholar]
- 52.Minagar A, Long A, Ma T, Jackson TH, Kelley RE, Ostanin DV, et al. Interferon (IFN)-beta 1a and IFN-beta 1b block IFN-gamma-induced disintegration of endothelial junction integrity and barrier. Endothelium. 2003;10:299–307. doi: 10.1080/10623320390272299. [DOI] [PubMed] [Google Scholar]
- 53.Ciardiello F, Caputo R, Bianco R, Damiano V, Fontanini G, Cuccato S, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res. 2001;7:1459–1465. [PubMed] [Google Scholar]
- 54.Cao G, Su J, Lu W, Zhang F, Zhao G, Marteralli D, et al. Adenovirus-mediated interferon-beta gene therapy suppresses growth and metastasis of human prostate cancer in nude mice. Cancer Gene Ther. 2001;8:497–505. doi: 10.1038/sj.cgt.7700333. [DOI] [PubMed] [Google Scholar]
- 55.Albini A, Marchisone C, Del Grosso F, Benelli R, Masiello L, Tacchetti C, et al. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: a gene therapy approach. Am J Pathol. 2000;156:1381–1393. doi: 10.1016/S0002-9440(10)65007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu P, Zhang C, Chen J, Zhang R, Ren J, Huang Y, et al. Combinational therapy of interferon-alpha and chemotherapy normalizes tumor vasculature by regulating pericytes including the novel marker RGS5 in melanoma. J Immunother. 2011;34:320–326. doi: 10.1097/CJI.0b013e318213cd12. [DOI] [PubMed] [Google Scholar]
- 58.Herwig MC, Tsokos M, Hermanns MI, Kirkpatrick CJ, Muller AM. Vascular endothelial cadherin expression in lung specimens of patients with sepsis-induced acute respiratory distress syndrome and endothelial cell cultures. Pathobiology. 2013;80:245–251. doi: 10.1159/000347062. [DOI] [PubMed] [Google Scholar]
- 59.Kiss J, Yegutkin GG, Koskinen K, Savunen T, Jalkanen S, Salmi M. IFN-beta protects from vascular leakage via up-regulation of CD73. Eur J Immunol. 2007;37:3334–3338. doi: 10.1002/eji.200737793. [DOI] [PubMed] [Google Scholar]
- 60.Umapathy NS, Fan Z, Zemskov EA, Alieva IB, Black SM, Verin AD. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vascul Pharmacol. 2010;52:199–206. doi: 10.1016/j.vph.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong C, Lei XX. Biomechanics of cell rolling: shear flow, cell-surface adhesion, and cell deformability. J Biomech. 2000;33:35–43. doi: 10.1016/s0021-9290(99)00174-8. [DOI] [PubMed] [Google Scholar]
- 62.Coughlin MF, Sohn DD, Schmid-Schonbein GW. Recoil and stiffening by adherent leukocytes in response to fluid shear. Biophys J. 2008;94:1046–1051. doi: 10.1529/biophysj.107.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 2005;46:9–15. [PubMed] [Google Scholar]
- 64.Wei X, Sipkins DA, Pitsillides CM, Novak J, Georgakoudi I, Lin CP. Real-time detection of circulating apoptotic cells by in vivo flow cytometry. Mol Imag. 2005;4:415–416. doi: 10.2310/7290.2005.05148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Truong U, Fonseca B, Dunning J, Burgett S, Lanning C, Ivy DD, et al. Wall shear stress measured by phase contrast cardiovascular magnetic resonance in children and adolescents with pulmonary arterial hypertension. J Cardiovasc Magn Reson. 2013;15:81. doi: 10.1186/1532-429X-15-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanaoka M, Kubo K, Hayano T, Koizumi T, Kobayashi T. Interferon-alpha elevates pulmonary blood pressure in sheep–the role of thromboxane cascade. Eur J Pharmacol. 1999;370:145–151. doi: 10.1016/s0014-2999(99)00107-7. [DOI] [PubMed] [Google Scholar]
- 67.Nagaoka T, Sato E, Takahashi A, Yokohama S, Yoshida A. Retinal circulatory changes associated with interferon-induced retinopathy in patients with hepatitis C. Invest Ophthalmol Vis Sci. 2007;48:368–375. doi: 10.1167/iovs.06-0182. [DOI] [PubMed] [Google Scholar]
- 68.Takase B, Uehata A, Fujioka T, Kondo T, Nishioka T, Isojima K, et al. Endothelial dysfunction and decreased exercise tolerance in interferon-alpha therapy in chronic hepatitis C: relation between exercise hyperemia and endothelial function. Clin Cardiol. 2001;24:286–290. doi: 10.1002/clc.4960240406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okegawa T, Pong RC, Li Y, Hsieh JT. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 2004;51:445–457. [PubMed] [Google Scholar]
- 71.Kelly CP, O’Keane JC, Orellana J, Schroy PC, 3rd, Yang S, LaMont JT, et al. Human colon cancer cells express ICAM-1 in vivo and support LFA-1-dependent lymphocyte adhesion in vitro. Am J Physiol. 1992;263:G864–G870. doi: 10.1152/ajpgi.1992.263.6.G864. [DOI] [PubMed] [Google Scholar]
- 72.Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676731. doi: 10.1155/2012/676731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soilu-Hanninen M, Salmi A, Salonen R. Interferon-beta downregulates expression of VLA-4 antigen and antagonizes interferon-gamma-induced expression of HLA-DQ on human peripheral blood monocytes. J Neuroimmunol. 1995;60:99–106. doi: 10.1016/0165-5728(95)00059-b. [DOI] [PubMed] [Google Scholar]
- 74.Dhib-Jalbut S, Jiang H, Williams GJ. The effect of interferon beta-1b on lymphocyte-endothelial cell adhesion. J Neuroimmunol. 1996;71:215–222. doi: 10.1016/s0165-5728(96)00156-7. [DOI] [PubMed] [Google Scholar]
- 75.Jiang H, Williams GJ, Dhib-Jalbut S. The effect of interferon beta-1b on cytokine-induced adhesion molecule expression. Neurochem Int. 1997;30:449–453. doi: 10.1016/s0197-0186(96)00081-2. [DOI] [PubMed] [Google Scholar]
- 76.Zhan M, Zhao H, Han ZC. Signalling mechanisms of anoikis. Histol Histopathol. 2004;19:973–983. doi: 10.14670/HH-19.973. [DOI] [PubMed] [Google Scholar]
- 77.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 78.Guadamillas MC, Cerezo A, Del Pozo MA. Overcoming anoikis–pathways to anchorage-independent growth in cancer. J Cell Sci. 2011;124:3189–3197. doi: 10.1242/jcs.072165. [DOI] [PubMed] [Google Scholar]
- 79.Zucker MB, Masiello NC. The Triton X-100-insoluble residue (“cytoskeleton”) of aggregated platelets contains increased lipid phosphorus as well as 125I-labeled glycoproteins. Blood. 1983;61:676–683. [PubMed] [Google Scholar]
- 80.Pfeffer LM, Stebbing N, Donner DB. Cytoskeletal association of human alpha-interferon-receptor complexes in interferon-sensitive and -resistant lymphoblastoid cells. Proc Natl Acad Sci USA. 1987;84:3249–3253. doi: 10.1073/pnas.84.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfeffer LM, Landsberger FR. Interferon-alpha modulates the plasma membrane-cytoskeletal complex of human lymphoblastoid cells sensitive to the antiproliferative action of interferon-alpha. J Interferon Res. 1990;10:91–97. doi: 10.1089/jir.1990.10.91. [DOI] [PubMed] [Google Scholar]
- 82.Braig S, Schmidt BUS, Stroiber Katharina, Händel Chris, Möhn Till, Werz Oliver, Müller Rolf, Zahler Stefan, Koeberle Andreas, Käs Josef A, Vollmar Angelika M. Pharmacological targeting of membrane rigidity: implications on cancer cell migration and invasion. New J Phys. 2015;17:083007. [Google Scholar]
- 83.Horak CE, Steeg PS. Metastasis gets site specific. Cancer Cell. 2005;8:93–95. doi: 10.1016/j.ccr.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 84.Kim J, Mori T, Chen SL, Amersi FF, Martinez SR, Kuo C, et al. Chemokine receptor CXCR4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann Surg. 2006;244:113–120. doi: 10.1097/01.sla.0000217690.65909.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Boyle G, Swidenbank I, Marshall H, Barker CE, Armstrong J, White SA, et al. Inhibition of CXCR4-CXCL12 chemotaxis in melanoma by AMD11070. Br J Cancer. 2013;108:1634–1640. doi: 10.1038/bjc.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin TH, Liu HH, Tsai TH, Chen CC, Hsieh TF, Lee SS, et al. CCL2 increases alphavbeta3 integrin expression and subsequently promotes prostate cancer migration. Biochim Biophys Acta. 2013;1830:4917–4927. doi: 10.1016/j.bbagen.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 87.Lee PY, Li Y, Kumagai Y, Xu Y, Weinstein JS, Kellner ES, et al. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol. 2009;175:2023–2033. doi: 10.2353/ajpath.2009.090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 89.Cavallaro S. CXCR4/CXCL12 in non-small-cell lung cancer metastasis to the brain. Int J Mol Sci. 2013;14:1713–1727. doi: 10.3390/ijms14011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21:107–112. doi: 10.1016/j.semcancer.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 91.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 93.Ding J, Hori K, Zhang R, Marcoux Y, Honardoust D, Shankowsky HA, et al. Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS) Wound Repair Regen. 2011;19:568–578. doi: 10.1111/j.1524-475X.2011.00724.x. [DOI] [PubMed] [Google Scholar]
- 94.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metast Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 95.Wostradowski T, Gudi V, Pul R, Gingele S, Lindquist JA, Stangel M, et al. Effect of interferon-beta1b on CXCR4-dependent chemotaxis in T cells from multiple sclerosis patients. Clin Exp Immunol. 2015 doi: 10.1111/cei.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, et al. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, Cheng N. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. J Biol Chem. 2012;287:36593–36608. doi: 10.1074/jbc.M112.365999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roblek M, Calin M, Schlesinger M, Stan D, Zeisig R, Simionescu M, et al. Targeted delivery of CCR2 antagonist to activated pulmonary endothelium prevents metastasis. J Control Release. 2015;220:341–347. doi: 10.1016/j.jconrel.2015.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao D, Dai C, Peng S. Mechanism of the mesenchymal–epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 100.Chao YL, Shepard CR, Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Putzke AP, Ventura AP, Bailey AM, Akture C, Opoku-Ansah J, Celiktas M, et al. Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol. 2011;179:400–410. doi: 10.1016/j.ajpath.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 103.Eggermont AMM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383:816–827. doi: 10.1016/S0140-6736(13)60802-8. [DOI] [PubMed] [Google Scholar]
- 104.Repetto L, Giannessi PG, Campora E, Pronzato P, Vigani A, Naso C, et al. Tamoxifen and interferon-beta for the treatment of metastatic breast cancer. Breast Cancer Res Treat. 1996;39:235–238. doi: 10.1007/BF01806190. [DOI] [PubMed] [Google Scholar]
- 105.Lyrdal D, Stierner U, Lundstam S. Metastatic renal cell carcinoma treated with Peg-interferon alfa-2b. Acta Oncol. 2009;48:901–908. doi: 10.1080/02841860902795257. [DOI] [PubMed] [Google Scholar]