Abstract
The WASF3 gene is overexpressed in high-grade breast cancer and promotes invasion and metastasis but does not affect proliferation. The HER2/ERBB2/NEU gene is also frequently overexpressed in breast cancer and has been shown to promote invasion and metastasis in these tumors. Here we show that WASF3 in present in the HER2 immunocomplex and suppression of WASF3 function leads to suppression of invasion even in the presence of HER2 expression. Overexpression of both HER2 and WASF3 in non-metastatic MCF7 breast cancer cells promotes invasion and metastasis more significantly than either gene alone. HER2 forms homodimers as well as heterodimers with other HER family members and we now show that the ability of WASF3 to promote invasion is highly dependent on the HER2/HER3 heterodimer. The engagement of WASF3 with the HER2/HER3 complex facilitates its phospho-activation and transcriptional upregulation, which is facilitated by HER2/HER3 activation of JAK/STAT signaling. In breast cancer cells overexpressing HER2, therefore, WASF3 is specifically required to facilitate the invasion/metastasis response. Targeting WASF3, therefore, could be a potential therapeutic approach to suppress metastasis of HER2-overexpressing breast tumors.
Keywords: WASF3, HER2/HER3, JAK/STAT3, cancer, invasion, metastasis
INTRODUCTION
The majority of cancer deaths result from metastatic spread rather than the primary tumor. By identifying and targeting genes that control this phenotype, this aspect of cancer can potentially be controlled, and mortality reduced. Many genes have been associated with the metastasis process, either by promoting or suppressing the phenotype 1–3. In particular, overexpression of receptor tyrosine kinases have been associated with the metastatic phenotype 4,5. One of the most significant genetic changes in breast cancer is overexpression of the epidermal growth factor receptor 2 (HER2/ERBB2/NEU) gene, which is particularly associated with poor outcome in these patients and has been the major focus of targeted therapies using receptor antagonists 6.
HER2 has no known ligand and, even though homodimerization is preferred, its activation is achieved through heterodimerization with one of the other HER family members following ligand binding 7,8. Overexpression of the HER2 gene is not found in normal breast tissue or in benign breast lesions, and has been associated with the transition from carcinoma in situ to invasive breast cancer 9. Overexpression of HER2 is particularly found in subtypes of breast cancers that have a relatively high rate of metastasis 10,11. In support of a role for HER2 in cancer cell invasion, overexpression of HER2 in a variety of non-invasive cancer cell types leads to increased invasion potential, and knockdown of HER2 in invasive cancer cells suppresses phenotypes that are stimulated by this receptor, including metastasis 12,13. The HER2/HER3 heterodimer is considered the main oncogenic unit in HER2 positive breast cancer 14,15 and is activated by the NRG1 and NRG2 members of the neuregulin family 16,17. The ability of HER2/HER3 to promote metastasis is thought to be due to the activation of downstream genes such as matrix metalloproteinases (MMP), which are known to be involved in the metastasis process 18,19. Since HER2/HER3 signaling can also promote proliferation and survival, it is likely that the stimulation of these distinct downstream pathways is mediated by specific interacting partner proteins.
WASF3 is a member of the Wiskott-Aldridge family of proteins that are involved in actin polymerization, leading to changes in actin cytoskeletal dynamics that are responsible for cell movement and invasion 20,21. Studies in primary human breast cancers support a role for WASF3 in metastasis, since elevated WASF3 expression levels were part of the gene signature associated with the highly aggressive “claudin-low” subtype of tumors that includes the triple negative (ER−, PR−, HER2−) breast cancers (TNBC) 22. Knockdown of WASF3 in breast cancer cells leads to a suppression of invasion in vitro and metastasis in vivo, regardless of the genetic background of the cells, including their tyrosine kinase receptor expression pattern 21,23–32. The mechanism of action has been shown to involve gene regulation facilitated through suppression of the KISS1 tumor suppressor gene, which leads to increased expression of metastasis promoting genes such as ZEB1 and MMP9 as a result of NFƙB activation 23,28. WASF3 function can be regulated through a variety of mechanisms such as transcription suppression 24,26, reduced stabilization 25,30 and inhibition of activation 25, 29,31,32. Our previous study showed that stimulation of breast cancer cells with cytokines such as IL6 leads to JAK2 activation of STAT3, which directly promotes WASF3 transcription 26,29. HER2 also functions through the activation of the JAK-STAT pathway 16, suggesting that WASF3 may also mediate signal transduction from this receptor to promote metastasis. In this study, we demonstrate that HER2/HER3 facilitates WASF3 phospho-activation and promotes WASF3 transcription through JAK2 activation of STAT3 in response to NRG. Loss of WASF3 leads to attenuated epithelial-to-mesenchyme transition (EMT) and invasion induced by NRG. Thus, our results demonstrate that the ability of HER2 to promote cell invasion depends on WASF3 function and provides a mechanism where stimulation of this receptor specifically drives the invasion and metastasis phenotype.
RESULTS
NRG induces phospho-activation of WASF3 in HER2-positive breast cancer cells
Expression of WASF3 has been shown to be critical for the ability of breast cancer cells to be able to invade in vitro and metastasize in vivo 23–32. This invasion-promoting ability of WASF3 is dependent on its phosphorylation, which is induced by a number of growth factor receptors and cytokines 25,26,31. Since HER2 is one of the critical receptors that promotes invasion of breast cancer cells 14, we evaluated whether WASF3 is an essential conduit for this HER2 promotion of metastasis
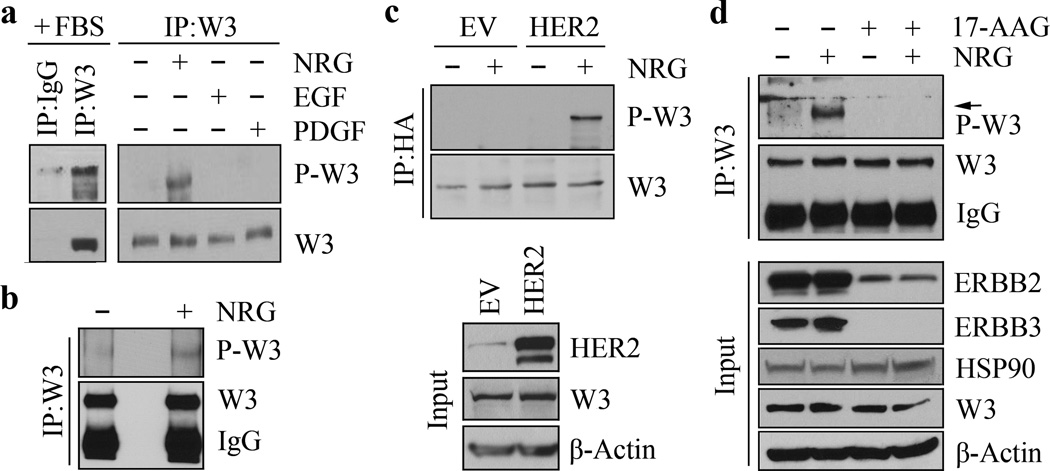
In HER2-positive breast cancer cells, HER2 can be selectively activated using ligands such as NRG. Short-term stimulation (10 min) of starved, HER2-positive SKBR3 breast cancer cells with 20 ng/ml NRG, for example, leads to enhanced WASF3 phosphorylation, compared with other growth factors such as EGF and PDGF which have no effect (Figure 1a). NRG treatment also promoted increased WASF3 phosphorylation levels in the BT474 HER2-positive breast cancer cell line (Figure 1b). The relative ability of breast cancer cells to invade and metastasize is related to their WASF3 levels and, as we have shown previously, low WASF3-expressing, low-invasive MCF7 cells, which also have low HER2 expression levels, can be induced to invade following exogenous expression of WASF3 in the presence of serum 28. To study the relationship between WASF3 and HER2 in these non-invasive cells, we overexpressed HER2 in WASF3 (HA tagged) overexpressing MCF7 cells. In the starved parental MCF7 cells expressing WASF3 alone, NRG did not induce its activation (Figure 1c). However, WASF3 was phospho-activated in the response to NRG in these cells when HER2 was also overexpressed (Figure 1c), demonstrating that WASF3 activation requires functional activation of HER2.
Figure 1. NRG induces WASF3 phospho-activation through HER2 signaling in breast cancer cells.
In the presence of serum, WASF3 (W3) is phospho-activated (a, left). Starved SKBR3 cells do not show WASF3 activation but when these cells are treated with NRG, phospho-activation of WASF3 is induced (a, right). Neither EGF nor PDGF can activate WASF3 in these cells (a, right). IP of WASF3 from NRG-treated BT474 breast cancer cells also shows activated WASF3 in the immunocomplex (b). Overexpression of HER2 in starved MCF7 cells expressing an HA-tagged WASF3 gene shows increased activation of WASF3 in response to NRG treatment compared with cells expressing the empty vector (EV) alone (c). Treatment of SKBR3 cells with 17-AAG leads to loss of WASF3 activation even in the presence of NRG through significantly suppressing HER2 and HER3 levels (d).
It is established that HSP90 inhibitors, such as 17-AAG, cause the proteasomal degradation of HSP90 client proteins, including a number of proteins involved in growth factor signaling 33,34. Of these clients, members of the HER family are particularly sensitive to 17-AAG treatment 34,35. When we treated HER2-postitive, SKBR3 cells with 5µM 17-AAG for 6 hours prior to NRG stimulation, neither HSP90 nor WASF3 levels were affected (Figure 1d), which confirms our previous observations 25. However, 17-AAG treatment resulted in suppression of NRG-induced WASF3 phosphorylation. This effect was accompanied by a significant reduction in HER2 levels and almost complete loss of HER3 (Figure 1d). These observations provide further support for the idea that phospho-activation of WASF3 is regulated by NRG-mediated HER2 signaling in HER2-positive breast cancer cells.
WASF3 is essential for NRG-induced epithelial–mesenchymal transition and invasion of HER2-positive breast cancer cells
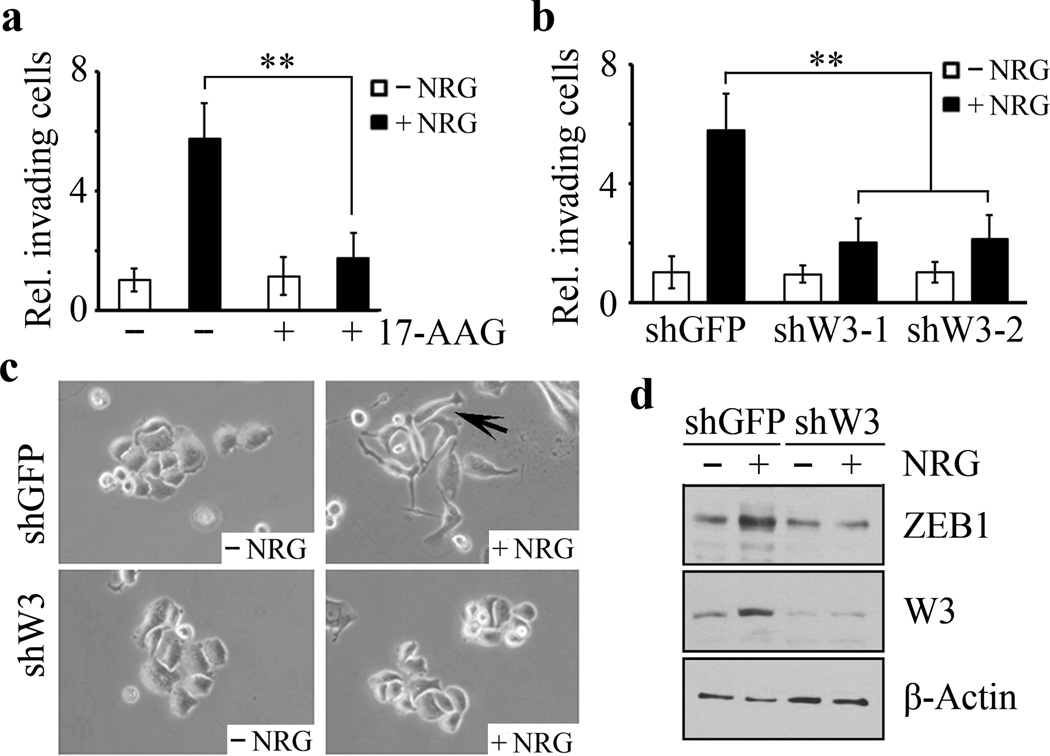
To investigate whether WASF3 plays an essential role in NRG-induced cell invasion, starved SKBR3 cells, which show limited invasion in the absence of NRG, experienced a significant increase in invasion when NRG was added (Figure 2a and Supplementary Figure S1). This response, however, is suppressed in SKBR3 cells treated with 17-AAG (Figure 2a and Supplementary Figure S1). This observation, together with the results shown in Figure 1d, suggest that tyrosine phosphorylation of WASF3 contributes, at least in part, to NRG-induced cell invasion. When WASF3 was knocked down in SKBR3 cells, invasion potential was much lower in response to NRG compared with control cells (Figure 2b and Supplementary Figure S1). Moreover, long-term treatment (4 days) of SKBR3 cells with NRG promotes EMT, whereas the WASF3 knockdown cells retained their typical epithelial cell phenotype (Figure 2c). We also found that, in the presence of NRG, the knockdown control cells showed a dramatic increase in levels of the ZEB1 mesenchymal marker compared with the WASF3 knockdown cells (Figure 2d). Collectively, these data indicate that the function of WASF3 is required for NRG-induced EMT and invasion of HER2-positive breast cancer cells.
Figure 2. WASF3 is essential for NRG-induced EMT and invasion in HER2-positive breast cancer cells.
Transwell invasion assays demonstrate that NRG-treated SKBR3 cells show increased invasion (a) which is suppressed following treatment with 17-AAG. When WASF3 is knocked down in these cells (shW3-1 and shW3-2), NRG treatment no longer leads to increased invasion (b) compared with knockdown control cells (shGFP). NRG facilitates transition of epithelial SKBR3 cells to a mesenchymal phenotype, but this change is suppressed when WASF3 is knocked down (c). NRG treatment leads to increased ZEB1 levels in cells expressing the control shRNA (shGFP) but not in WASF3 knockdown cells (shW3). ** p<0.01; Student’s t-test.
WASF3 is activated through binding to the HER2/HER3 heterodimer in the presence of NRG
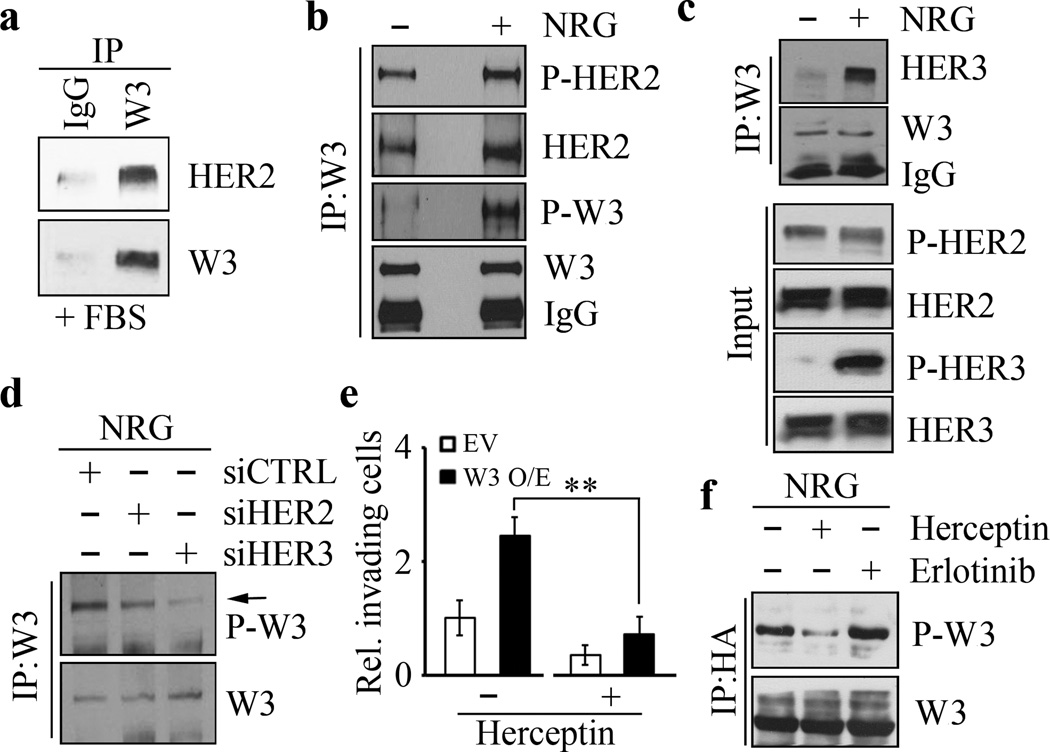
Confocal microscopy analysis shows that, in SKBR3 cells, HER2 is clearly located around the membrane perimeter and co-localizes with WASF3 (Supplementary Figure S2), suggesting WASF3 has been recruited to the cell membrane through an interaction with HER2. IP of WASF3 from SKBR3 cells demonstrated that HER2 was present in the WASF3 immunocomplex (Figure 3a). To exclude the influence of the various other growth factors in the serum, we starved SKBR3 cells overnight before NRG treatment. Interestingly, in starved cells, WASF3 activation is minimal, although it still binds to activated HER2 (Figure 3b). In the presence of NRG (10 minutes), however, increased HER2 levels were seen in the WASF3 immunocomplex with a concomitant increase in WASF3 activation levels (Figure 3b).
Figure 3. WASF3 is phosphorylated following NRG stimulation through binding to the HER2/HER3 heterodimer.
IP of WASF3 in the presence of serum leads to co-precipitation of HER2 (a) in SKBR3 cells. In the presence of NRG, there is only a modest increased in activated HER2 levels but the majority of the WASF3 protein in the immunocomplex is phospho-activated (b). IP of WASF3 in SKBR3 cells shows the presence of high levels of HER3 in the immunocomplex (c). Analysis of the NRG-treated SKBR3 cells shows only a modest increase in HER2 levels but a dramatic increase in activated HER3 (c). IP of WASF3 shows a decrease in WASF3 phosphorylation levels (d) either in HER2 knockdown (siHER2) or HER3 knockdown (siHER3) SKBR3 cells compared with the knockdown control cells (siCTRL). Treatment of SKBR3 cells overexpressing WASF3 with Herceptin shows a significant reduction in the invasion potential (e). Herceptin treatment of WASF3-overexpressing SKBR3 cells leads to a reduction in levels of activated WASF3, which is not seen in cells treated with the Erlotinib suppresser of EGFR signaling (f). ** p<0.01; Student’s t-test.
Since NRG-induced HER2 signaling depends on its dimerization with HER3 14, we investigated both the protein expression profile and phosphorylation status of HER2 and HER3 in response to NRG. Constitutively activated HER2 is seen in SKBR3 cells but NRG treatment significantly activates HER3 with only a slight increase in HER2 activation (Figure 3c). Following IP of WASF3 from these cells, we noted that, unlike HER2, HER3 was only present in the WASF3 immunocomplex following NRG treatment (Figure 3c), suggesting that HER2 dimerization with HER3 is essential for activation of WASF3. We then knocked down HER2 and HER3 using short interfering RNA (siRNA) (Supplementary Figure S3) and found that WASF3 cannot by phosphorylated in the absence of HER2 or HER3 (Figure 3d). Thus, WASF3 is apparently activated by the HER2/HER3 heterodimer in HER2-positive breast cancer cells.
Herceptin suppresses WASF3-dependent invasion of HER2-positive breast cancer cells
Herceptin treatment of HER2-positive metastatic breast cancer can suppress tumor development through direct binding with HER2, which prevents its function 36,37. When we treated SKBR3 cells with 1 µg/ml Herceptin for 48 hours, no change in HER2 protein levels was seen (Supplementary Figure S4). When SKBR3 cells are treated with Herceptin, however, NRG-induced invasion was suppressed (Figure 3e and Supplementary Figure S1). Overexpression of WASF3 in SKBR3 cells led to increased invasion following NRG treatment, but this increase can be suppressed by Herceptin treatment (Figure 3e and Supplementary Figure S1). IP analysis shows that WASF3 phosphorylation levels were significantly inhibited by Herceptin but not by Erlotinib (Figure 3f), excluding a role for EGFR in NRG-induced WASF3 activation. Taken together, our data demonstrate that HER2/HER3 signaling is specifically required for WASF3-directed invasion in HER2-positive breast cancer cells.
Upregulation of WASF3 following extended treatment of NRG in HER2-positive breast cancer cells
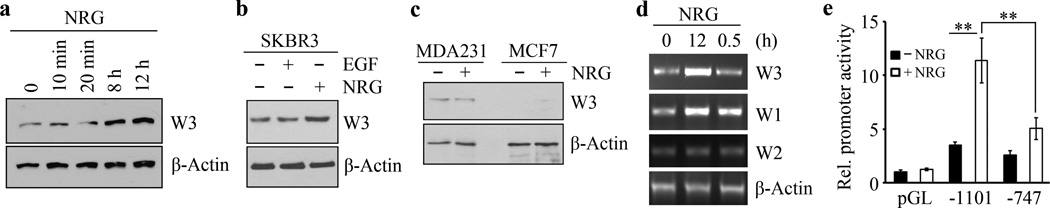
As shown in Figure 4a, short-term treatment (10 and 20 minutes) of SKBR3 cells had no effect on WASF3 protein levels but, when these cells were treated for longer periods (8 and 12 hours), a remarkable (~4-fold) increase in WASF3 protein levels was observed (Figure 4a and Supplementary Figure S5). To investigate whether long-term treatment with other growth factors such as EGF is involved in WASF3 upregulation, we treated starved SKBR3 cells with EGF for 12 hours but no significant increases in WASF3 levels were observed (Figure 4b), excluding a role for EGFR in WASF3 expression in these cells. There was also no increase in WASF3 levels in HER2-negative MDA-MB-231 or MCF7 cells treated with NRG for 12 hours (Figure 4c), which indicates that transcriptional regulation of WASF3 is involved in NRG-HER2 signaling.
Figure 4. The expression of WASF3 is upregulated following extended treatment of HER2-positive breast cancer cells with NRG.
Short term treatment (10–20 minutes) of SKBR3 cells with NRG does not lead to a significant increase in levels of WASF3, while longer term treatment (8–12 hours) leads to increased WASF3 levels (a). When starved SKBR3 cells are treated with NRG, there is an increase in WASF3 levels not seen in EGF treated cells (b). Treatment of MDA-MB-231 (MDA231) and MCF7 cells with NRG that do not express HER2 does not lead to any significant increase in WASF3 protein levels (c). RT-PCR analysis of expression levels of the WASF3 in SKBR3 cells shows a dramatic increase in WASF3 expression after 12 hours compared with 30 minutes, while WASF2 levels are not affected (d). NRG treatment also leads to a significant increase in WASF1 expression levels (d). When luciferase levels were studied (e) following NRG treatment of SKBR3 cells expressing high levels of the WASF3 promoter construct (−1101), high-level stimulation is seen compared with the WASF3 reporter construct (−747) which does not contain the STAT3 binding sites. ** p<0.01; Student’s t-test.
To investigate the effect of NRG on WASF3 mRNA levels we used RT-PCR to survey expression levels of the WASF family which consists of three members; WASF1, WASF2 and WASF3. As shown in Figure 4d, WASF2 expression is unaffected following NRG stimulation, but WASF1 shows increased expression levels similar to those seen for WASF3 when treated with NRG for 12 hours. We then used WASF3 promoter reporter constructs described previously 24,26 to investigate the response to NRG treatment. Treatment of cells carrying the promoter construct (+494/−1101 bp) that contained all three STAT3 DNA-binding sites (−894/−886 bp, −915/−906 bp and −926/−919 bp) with NRG significantly increased WASF3 promoter activation (Figure 4e). In contrast, a truncated promoter (+494/−747 bp), in which the STAT3 binding elements were deleted, showed a significantly suppressed response (Figure 4e).
NRG-induced upregulation of WASF3 is dependent on HER2/HER3-JAK2/STAT3 signal transduction
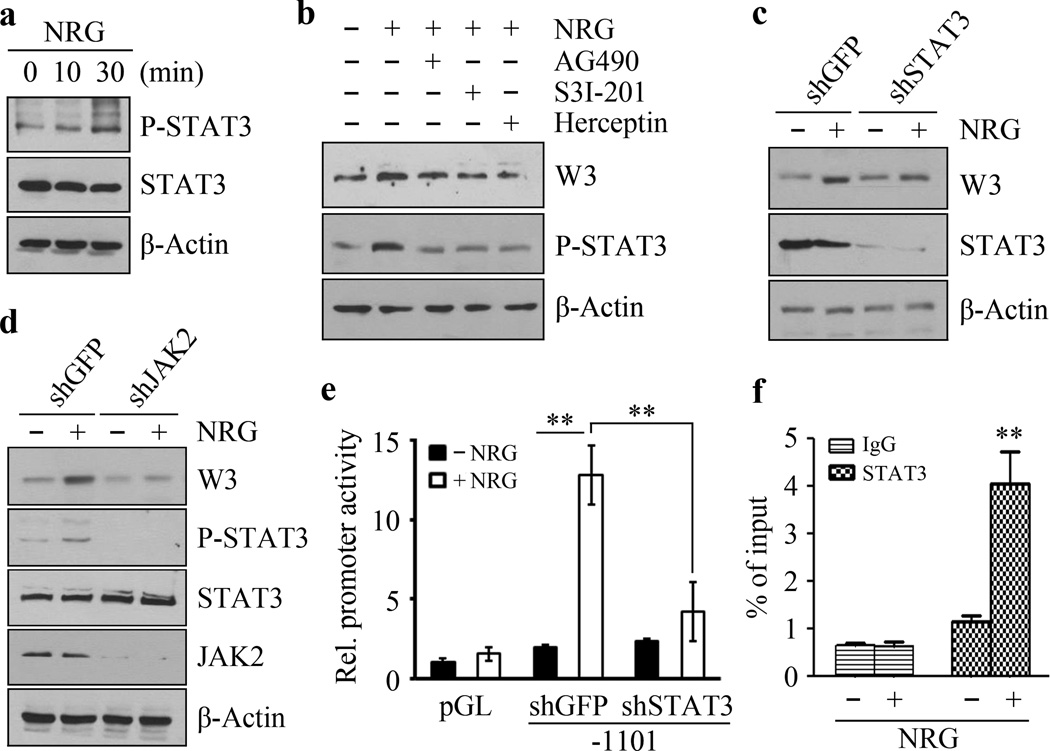
NRG activates the JAK-STAT signal transduction pathway through binding to its high-affinity receptor, the HER2/HER3 heterodimer 16. WASF3 expression levels are increased in response to NRG stimulation, which is presumably mediated through activation of STAT3 (see above), which we have shown is a direct regulator of WASF3 expression 26,29. To investigate the role of STAT3 signaling in the HER2/3 response further, we investigated its activation in the presence of NRG. As shown in Figure 5a, following treatment with NRG for 10 minutes, there is a slight increase in STAT3 phosphorylation at Tyrosine 705 (Y705) but, after 30 minutes, STAT3 was strongly phosphorylated at this site (Figure 5a). To determine the role of STAT3 in mediating signaling through the NRG-HER2/HER3-WASF3 axis, we pretreated SKBR3 cells with different inhibitors before NRG stimulation. Similar to the effect of blocking HER2 function by Herceptin, inhibition of either JAK2 activation with AG490, or STAT3 activation with S3I-201, NRG-treated SKBR3 cells showed suppression of phospho-STAT3 and no change in WASF3 levels (Figure 5b), indicating that JAK2/STAT3 signaling is required for WASF3 expression through NRG-HER2/HER3 signaling. When STAT3 was knocked down in these cells using shRNA, they were relatively insensitive to NRG induction of WASF3 (Figure 5c). The same phenotype was observed when JAK2 was knocked down (Figure 5d), which confirms that JAK2 activation of STAT3-induces WASF3 expression.
Figure 5. STAT3 is required for NRG-induced upregulation of WASF3.
When SKBR3 cells were treated with NRG for 30 minutes, there is a significant increase in STAT3 phosphorylation at Tyrosine 705 (Y705) (a). Only a slight increase in STAT3 phosphorylation was seen in the presence of NRG for 10 minutes (a). When these NRG-treated cells are treated with the AG490 JAK2 inhibitor, Y705 activation is suppressed (b). The same suppression is seen when the cells are treated with the either the S3I-201 STAT3 inhibitor, or the Herceptin recombinant, humanized anti-HER2 antibody (b). In STAT3 knockdown SKBR3 cells, NRG does not affect WASF3 expression levels (c). Similarly, knockdown of JAK2 also suppresses NRG-induced expression of WASF3 (d). In the luciferase reporter assay for WASF3, NRG treatment leads to a significant increase in activity, which is suppressed in STAT3 knockdown cells (e). ChIP-qPCR assays show increased levels of STAT3 at the WASF3 promoter-binding site in the presence of NRG (f). ** p<0.01; Student’s t-test.
We have shown previously that STAT3 regulates WASF3 transcription through direct binding to potential STAT3 binding sites in the promoter 26,29. When STAT3 was knocked down, NRG cannot induce luciferase activity in the +474/−1101 WASF3 promoter in these cells (Figure 5e). ChIP-qPCR assays confirmed increased levels of STAT3 at the WASF3 promoter binding sites in the presence of NRG in both HER2-positive SKBR3 (Figure 5f) and BT474 (Figure S6) cells. These data confirm the suggestion that upregulation of WASF3 following NRG treatment is mediated through HER2/HER3-JAK2/STAT3 signaling.
The critical role of HER2-WASF3 signaling in metastasis of breast cancer cells
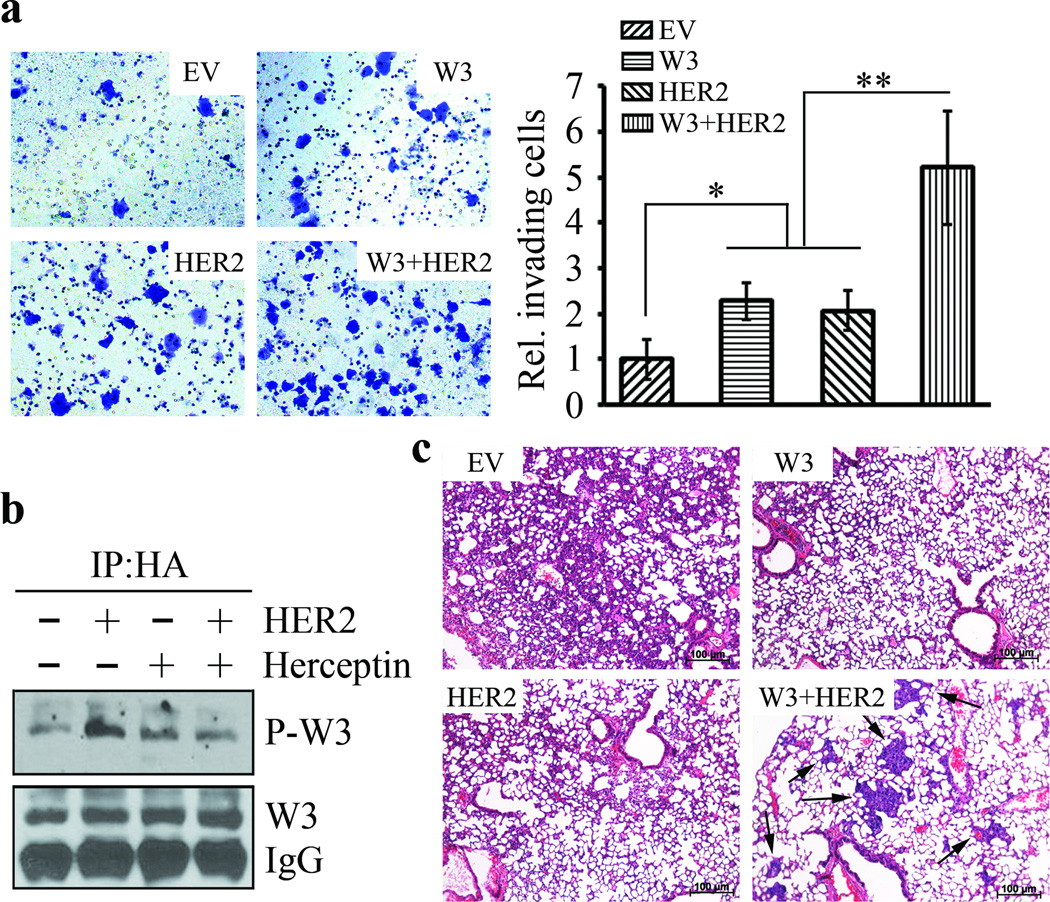
We have established that WASF3 regulates invasion and metastasis of breast cancer cells 23–32 and we next investigated whether HER2-WASF3 signaling is involved in cell invasion and metastasis in the presence of serum. HER2 negative MCF7 cells show low WASF3 expression levels. Although increased invasion levels could be induced when either WASF3 or HER2 were overexpressed individually in MCF7 cells, compared with cells carrying the empty vector (Figure 6a), co-expression of WASF3 and HER2 in these cells led to a much more significant increase in invasion levels. These observations correlate with enhanced WASF3 phospho-activation when HER2 was overexpressed in WASF3-overexpressing MCF7 cells and suppression of invasion in the presence of Herceptin (Figure 6b).
Figure 6. HER2-WASF3 signaling is critical for metastasis of breast cancer cells.
In vitro invasion assays show that invasion potential of MCF7 cells that overexpress either WASF3 or HER2 is slightly increased but a more significant increase in invasion levels was seen when both genes were overexpressed (a). When HER2 is expressed in WASF3-overexpressing MCF7 cells, enhanced WASF3 phospho-activation is seen, but this activation is suppressed following Herceptin treatment (b). In the experimental metastasis assay in SCID/NOD mice, expression of WASF3 and HER2 together leads to increased levels of metastasis to the lungs (arrows) which is not seen when either of these genes is expressed alone (c). * p<0.05, ** p<0.01; Student’s t-test.
To determine whether HER2-WASF3 signaling is essential for metastasis in vivo, we used the SCID/NOD mouse experimental metastasis assay described previously 38. Parental MCF7 cells form primary tumors in the mammary fat pad but do not metatasise in mice. When we injected 1.5×106 MCF7 cells that overexpressed either HER2 or WASF3 alone into the tail veins of five mice, analysis of the lungs after 12 weeks showed no surface nodules and histopathological analysis of the lungs showed no evidence of tumors either (Figure 6c). In contrast, mice injected with MCF7 cells expressing both WASF3 and HER2, showed small tumor foci throughout the lungs in two of the five mice after 12 weeks (Figure 6c). These observations further implicate HER2-WASF3 signaling in metastasis of breast cancer cells.
DISCUSSION
Growth factor receptor signaling is responsible for a wide variety of cellular responses involved in development, differentiation, proliferation, cell survival, invasion and metastasis. Since these functions can be cell type and developmental stage specific, the execution of the responses are likely dependent on the availability of cofactors that channel the signal through specific pathways. In terms of invasion and metastasis, we have shown that WASF3 is central to the execution of these phenotypes as a result of stimulation by growth factor receptors such as PDGFRA 31 and now HER2/HER3. When cells are cultured in serum, most receptor/ligand combinations can be activated but by starving cells, the role of specific receptor signaling pathways responsible specifically for invasion can be functionally isolated and, through ligand stimulation, the role of the individual interaction can be evaluated. In breast cancer cells such as SKBR3, we show here that WASF3 serves as the conduit from HER2/HER3 to signal invasion and metastasis in response to NRG ligand. Treatment with other ligands such as EGF and PDGF has no effect, showing the specificity for this response in these particular cells. Since MDA-MB-231 cells do not express HER2, NRG does not increase invasion potential but we previously showed that PDGF could specifically stimulate invasion in these cells 31. It is likely, therefore, that WASF3 may interact with many different receptors to facilitate invasion, in which case the specificity to signal invasion is provided by the dominant growth factor receptors expressed in the cells. The demonstration that the same downstream consequences of WASF3 signaling such as ZEB1 activation occurs regardless of the initiating receptor, supports a common downstream pathway regulated by WASF3 across cancer cell types. Interestingly, in our unpublished Mass Spectrometry analysis of the proteins present in the WASF3 immunocomplex in different cells lines such as MDA-MB-231 breast and PC3 prostate cancer cells, for example, we identified receptors such as PDGFRA, TGFBR1, IGF2R and EPHA10, all of which have been implicated in cancer metastasis. Indeed, recent studies demonstrate a potential relationship between WASF3 and TGFBR1 signaling in breast cancer metastasis 39. In other cell lines that predominantly overexpress EGFR or VEGFR, such as MDA-MB-231, suppression of WASF3 function also leads to loss of invasion 32, supporting the wider view that WASF3 is the nexus for transmitting the signal from growth factor stimulation to invasion and metastasis.
Stimulation of growth factor receptors will activate all responsive pathways, if the requisite cofactors are present in the cell. Cell proliferation signals, for example, are typically executed through the MEK-ERK axis and, as we contend, invasion is executed through WASF3, which accounts for the observation that, in WASF3 expressing cells, inactivation of WASF3 does not lead to suppression of proliferation and HER2/HER3 initiated MEK-ERK signaling is unaffected (Supplementary Figure S7). Invasion, however, is specifically inhibited. Therapeutic targeting of HER2, for example, will suppress cell proliferation but will also suppress invasion as we show here. Targeting the receptors that signal through WASF3, therefore, may provide one approach to suppress invasion and metastasis but needs to be customized to the receptor profile of each cancer. Targeting WASF3 directly, on the other hand, since it would specifically suppress invasion and metastasis signaling regardless of the receptor that is dominant in any given cancer, may potentially provide a broad ability to suppress invasion and metastasis throughout cancer types. Consistent with this suggestion, we note here that in order to promote invasion in non-invasive cells such as MCF7, it is necessary to express both WASF3 and a responsive receptor to achieve increased invasion and metastasis.
As suggested above, acquisition of invasion and metastatic potential by cancer cells requires a coincident and constitutive expression of the various cofactors that are required for these phenotypes. Loss of any of these contributors can suppress the phenotype. Previously we have shown that overexpression of WASF3 is dependent on STAT3 activation 26,29, which is responsible for its transcriptional upregulation. This may account for the delayed response in upregulation of WASF3 following NRG treatment, since the STAT3 must be activated and relocate to the nucleus to promote WASF3 expression. In turn, JAK2 activation is required not only to activate STAT3 and promote WASF3 synthesis but also to activate WASF3 at the membrane to initiate its function there. In this study, we have demonstrated that WASF3 engages the HER2/HER3 complex where it is presumably activated by JAK2, since knockdown of JAK2 leads to reduced WASF3 activation. Independently, JAK2 activates STAT3 which upregulates WASF3 transcription thereby promoting invasion and metastasis.
WASF3 is one of a three member family of highly homologous genes that are all implicated in cell movement and actin cytoskeleton reorganization 20. Of the three family members, however, WASF3 shows the most specific involvement in invasion and metastasis. From the discussion outlined above, this is possibly a result of its ability to engage different protein complexes and interact with different receptors thought adapter proteins. We have previously shown that WASF3 is involved with lamellipodia formation 31,38 whereas the other WASF family members appear to regulate other membrane structures 40. The WASF1 and WASF2 genes are not activated by IL6 promotion of STAT3 signaling 26 and our unpublished observations do not show STAT3 binding sites in their promoter regions. In addition, knockdown of WASF1 or WASF2 in breast and prostate cancer cells does not lead to suppression of invasion potential 41. Here we show that WASF2 expression is not affected by NRG activation of HER2/HER3, although WASF1 shows the same response as WASF3 in SKBR3 cells. There is some evidence for a role of WASF1 in invasion in ovarian cancer however 42. Clearly different responses are experienced by the different family members which, although all seem to regulate actin cytoskeleton reorganization, subtle differences in their molecular regulation dictate different functions, particularly in the regulation of the invasion and metastasis response, which may also be cell context dependent.
The MCF7 cell line does not invade in vitro or metastasize in vivo. In the present study we were able to induce metastasis in an experimental mouse model by overexpressing WASF3 and HER2. MCF7 cells, however, only express low levels of HER3 43, which might account for why only 2/5 mice developed lung metastases over the observation period, since the formation of HER2/HER3 dimers might be limited. The other possibility is that being a relatively poorly metastasizing cell line, it takes longer for the metastases to develop. We used the 12-week observation period based on our previous experience in the NOD/SCID strain 38 where lung metastases formed readily for other cell lines but for the MCF7 derivatives described here, it may require longer periods for metastases to fully develop in all animals. Nonetheless, the fact that we could demonstrate increased lung metastases in mice carrying MCF7 cells overexpressing HER2 and WASF3 supports the role of this signaling cascade in metastasis, although it may be necessary in the future to also overexpress HER3 to increase this potential further.
In summary, we show that WASF3 provides an intermediate to facilitate HER2/HER3 promotion of invasion and metastasis in breast cancer cells which operates through JAK2/STAT3 signaling. We also present evidence that WASF3 may act as an intermediate for other receptor tyrosine kinases, thereby providing a link between the ability of different receptor-ligand combinations to promote invasion and metastasis. In this case, the ability of different cells to respond to metastasis signals is determined by their expression profile for membrane bound receptors.
MATERIALS AND METHODS
Cell lines and standard assays
Breast cancer cell lines SKBR3, BT474, MCF7 and MDA-MD-231 were purchased from the American Type Culture Collection (ATCC, Rockville, MD) and maintained according to the supplier’s instructions. Transient transfections, luciferase reporter assays, Transwell invasion assay, immunoblotting, immunoprecipitation, immunofluorescence, lentiviral transduction and RT-PCR were carried out as described previously 23–26,28,38
Constructs, antibodies and reagents
The pLKO lentiviral vectors containing a shRNA against WASF3 were purchased from Open Biosystems (Huntsville, AL). Coding sequences of human HA tagged WASF3 was cloned into pCDH-CMV-MCS-EF1 lentivector (System Biosciences, Mountain View, CA) as described previously 28. The pGL-WASF3 promoter constructs (+494/−747, +494/−1101) were generated as described previously (24). pSIH1-puro-STAT3 shRNA was a gift from Dr. FA Sinicrope (Addgene plasmid #26596). The pCDNA3-HER2 overexpressing HER2 gene was kindly provided by Dr. Ren Neckers (Center for molecular modeling, National institutes of health, Bethesda, MD). siRNA duplexes targeting HER2 (siHER2) and HER3 (siHER3) and non-targeting siRNA (siCTRL) were synthesized by Dharmacon (Lafayette, CO). The following primary antibodies were used in our study: WASF3 (#2806), HER2 (#2242), P-HER2 (Y1221/1222, #2249), HER3 (#4754), P-HER3 (Y1289, #2842), STAT3 (#4904), P-STAT3 (Y705, #4113), ERK1/2 (#4695), P-ERK1/2 (T202/Y204, #9101) and JAK2 (#3230) (Cell Signaling Technology, MA), HA (#H9658), PY20 (#P4110) and β-Actin (#A5441) (Sigma, MO). Recombinant human Neuregulin-1/NRG1, EGF, PDGF were obtained from R&D systems (Minneapolis, MN). Herceptin (Trastuzumab) and Erlotinib were obtained from Dr. Hao Zhonglin (Georgia Regents University, Augusta, GA). The JAK inhibitor AG490, STAT3 inhibitor S3I-201 and HSP90 inhibitor 17-AAG were obtained from Selleckchem (Houston, TX).
Chromatin immunoprecipitation qPCR (ChIP-qPCR)
ChIP assays were performed using a ChIP assay kit (Millipore, Billerica, MA) as described previously (24,26,28). The primers (F: 5’-TTCCTCACATCTAGGGTGGA-3’; R: 5’-ACCAAGAGACCCTTGTATCA-3’) were used to amplify a proximal promoter region containing putative STAT3 binding sites in the WASF3 promoter. Each immunoprecipitated DNA sample was quantified using qPCR and all readings were normalized to the input. IgG was used as a negative control.
Experimental metastasis assays
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Georgia Regents University. Six-week-old female SCID/NOD mice were purchased from National Cancer Institute (Frederick, MD) and maintained in accordance with IACUC guidelines. 1.5×106 MCF7 cells carrying different exogenous genes were injected into eight-week-old female mice via the tail vein (five mice per group). The mice were euthanized on day 84 post-injection (12 weeks) and the lungs were removed from these mice for histological analyses as described previously 30,38.
Statistical analysis
Where indicated, the results were representative of at least three independent experiments performed in triplicate and were expressed as the mean±s.d. Different values among groups were compared using the Student’s t-test. For analysis of the in vivo metastasis data, statistical significance of the total number of tumors observed in different groups was assessed using a statistical test as described previously 30. For all statistical analyses, p<0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported in part by grant CA120510 from the National Institutes of Health. We would like to thank Yun Mei and Hao Zhang for the technical assistance.
Footnotes
Conflict of interest: The authors declare no conflicts of interest related to this work.
References
- 1.Sethi N, Kang Y. Unravelling the complexity of metastasis-molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11:735–748. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen DX, Massagué J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 4.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 5.Müller-Tidow C, Diederichs S, Bulk E, Pohle T, Steffen B, Schwäble J, et al. Identification of metastasis-associated receptor tyrosine kinases in non-small cell lung cancer. Cancer Res. 2005;65:1778–1782. doi: 10.1158/0008-5472.CAN-04-3388. [DOI] [PubMed] [Google Scholar]
- 6.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 8.Tsujioka H, Yotsumoto F, Shirota K, Horiuchi S, Yoshizato T, Kuroki M, et al. Emerging strategies for ErbB ligand-based targeted therapy for cancer. Anticancer Res. 2010;30:3107–3112. [PubMed] [Google Scholar]
- 9.Roses RE, Paulson EC, Sharma A, Schueller JE, Nisenbaum H, Weinstein S, et al. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1386–1389. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Vijver MJ, Peterse JL, Mooi WJ, Wisman P, Lomans J, Dalesio O, et al. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988;319:1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- 11.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberg JA, Wang Q, Katsumata M, Drebin J, Nagatomo I, Greene MI. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009;87:1–11. doi: 10.1016/j.yexmp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson E, Seachrist DD, DeLeon-Rodriguez CM, Lozada KL, Miedler J, Abdul-Karim FW, et al. HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42. J Biol Chem. 2010;285:29491–29501. doi: 10.1074/jbc.M110.136770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 15.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, et al. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res. 2012;72:2672–2682. doi: 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol. 2002;27:306–313. doi: 10.1165/rcmb.4850. [DOI] [PubMed] [Google Scholar]
- 17.Shankaran H, Wiley HS, Resat H. Modeling the effects of HER/ErbB1-3 coexpression on receptor dimerization and biological response. Biophys J. 2006;90:3993–4009. doi: 10.1529/biophysj.105.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosc DG, Goueli BS, Janknecht R. HER2/Neu-mediated activation of the ETS transcription factor ER81 and its target gene MMP-1. Oncogene. 2001;20:6215–6224. doi: 10.1038/sj.onc.1204820. [DOI] [PubMed] [Google Scholar]
- 19.Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10:7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- 20.Sossey-Alaoui K, Su G, Malaj E, Roe B, Cowell JK. WAVE3, an actin-polymerization gene, is truncated and inactivated as a result of a constitutional t(1;13)(q21;q12) chromosome translocation in a patient with ganglioneuroblastoma. Oncogene. 2002;21:5967–5974. doi: 10.1038/sj.onc.1205734. [DOI] [PubMed] [Google Scholar]
- 21.Sossey-Alaoui K. Surfing the big WAVE: Insights into the role of WAVE3 as a driving force in cancer progression and metastasis. Semin Cell Dev Biol. 2013;24:287–297. doi: 10.1016/j.semcdb.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng Y, Liu M, Cowell JK. Functional interrelationship between the WASF3 and KISS1 metastasis-associated genes in breast cancer cells. Int J Cancer. 2011;129:2825–2835. doi: 10.1002/ijc.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghoshal P, Teng Y, Lesoon L, Cowell JK. HIF1A induces expression of the WASF3 metastasis associated gene under hypoxic conditions. Int J Cancer. 2012;131:E905–E915. doi: 10.1002/ijc.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng Y, Ngoka L, Mei Y, Lesoon L, Cowell JK. HSP90 and HSP70 are essential for stabilization and activation of the WASF3 metastasis promoting protein. J Biol Chem. 2012;287:10051–10059. doi: 10.1074/jbc.M111.335000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng Y, Ghoshal P, Ngoka L, Mei Y, Cowell JK. Critical role of the WASF3 gene in JAK2/STAT3 regulation of cancer cell invasion. Carcinogenesis. 2013;4:1994–1999. doi: 10.1093/carcin/bgt167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng Y, Xie X, Walker S, White DT, Mumm JS, Cowell JK. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer. 2013;13:453. doi: 10.1186/1471-2407-13-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng Y, Mei Y, Hawthorn LA, Cowell JK. WASF3 regulates miR-200 inactivation by ZEB1 through suppression of KISS1 leading to increased invasiveness in breast cancer cells. Oncogene. 2014;33:203–211. doi: 10.1038/onc.2012.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng Y, Ross JL, Cowell JK. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAKSTAT. 2014;3:e28086. doi: 10.4161/jkst.28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng Y, Ren X, Li H, Shull A, Kim J, Cowell JK. Mitochondrial ATAD3A combines with GRP78 to regulate the WASF3 metastasis-promoting protein. Oncogene. 2015 doi: 10.1038/onc.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sossey-Alaoui K, Li X, Ranalli TA, Cowell JK. WAVE3-mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:21748–21755. doi: 10.1074/jbc.M500503200. [DOI] [PubMed] [Google Scholar]
- 32.Sossey-Alaoui K, Li X, Cowell JK. c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J Biol Chem. 2007;282:26257–26265. doi: 10.1074/jbc.M701484200. [DOI] [PubMed] [Google Scholar]
- 33.Atalay G, Cardoso F, Awada A, Piccart MJ. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann Oncol. 2003;14:1346–1363. doi: 10.1093/annonc/mdg365. [DOI] [PubMed] [Google Scholar]
- 34.Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:132–139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 35.Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez-Martín C, Lopez-Rios F, Aparicio J, Barriuso J, García-Carbonero R, Pazo R, et al. A critical review of HER2-positive gastric cancer evaluation and treatment: from trastuzumab, and beyond. Cancer Lett. 2014;351:30–40. doi: 10.1016/j.canlet.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Teng Y, Ren M, Cheney R, Sharma S, Cowell JK. Inactivation of the WASF3 gene in prostate cancer cells leads to suppression of tumorigenicity and metastases. Br J Cancer. 2010;103:1066–1075. doi: 10.1038/sj.bjc.6605850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor MA, Davuluri G, Parvani JG, Schiemann BJ, Wendt MK, Plow EF, et al. Upregulated WAVE3 expression is essential for TGF-β-mediated EMT and metastasis of triple-negative breast cancer cells. Breast Cancer Res Treat. 2013;142:341–353. doi: 10.1007/s10549-013-2753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 41.Teng Y, Bahassan A, Dong DY, Hanold LE, Ren X, Kennedy EJ, et al. Targeting the WASF3-CYFIP1 complex using stapled peptides suppresses cancer cell invasion. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-1680. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Zhou S, Tang L, Shen L, Xiao L, Duan Z, et al. WAVE1 gene silencing via RNA interference reduces ovarian cancer cell invasion, migration and proliferation. Gynecol Oncol. 2013;130:354–361. doi: 10.1016/j.ygyno.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Cai Z, Zhang G, Zhou Z, Bembas K, Drebin JA, Greene MI, et al. Differential binding patterns of monoclonal antibody 2C4 to the ErbB3-p185her2/neu and the EGFR-p185her2/neu complexes. Oncogene. 2008;27:3870–3874. doi: 10.1038/onc.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.