Abstract
Background
Staphylococcus aureus is one of the earliest bacterial pathogens to colonize the lungs of children with cystic fibrosis and is an important contributor to pulmonary exacerbations. The adaptive host response to S. aureus in cystic fibrosis remains inadequately defined and has important implications for pathogenesis and potential interventions. The objectives of this study were to determine the functional antibody response to select staphylococcal exotoxins (LukAB, alpha-hemolysin, and PVL) in children with cystic fibrosis and to evaluate the relationship of this response with pulmonary exacerbations.
Methods
Fifty children with cystic fibrosis were enrolled and followed prospectively for 12 months. Clinical characteristics and serologic profiles were assessed at routine visits and during pulmonary exacerbations, and functional antibody assessments were performed to measure neutralization of LukAB-mediated cytotoxicity.
Results
For each antigen, geometric mean titers were significantly higher if S. aureus was detected at the time of exacerbation. For LukAB, GMTs were significantly higher at exacerbation follow-up compared to titers during the exacerbation, consistent with expression during human disease, and the humoral response capably neutralized LukAB-mediated cytotoxicity. Moreover, the presence of a positive S. aureus culture during a pulmonary exacerbation was associated with a 31-fold higher odds of having a LukA titer ≥1:160, suggesting potential diagnostic capability of this assay.
Conclusions
The leukotoxin LukAB is expressed by S. aureus and recognized by the human adaptive immune response in the setting of pulmonary infection in cystic fibrosis. Anti-LukAB antibodies were not only predictive of positive staphylococcal culture during exacerbation, but also functional in the neutralization of this toxin.
Keywords: Staphylococcus aureus, Cystic Fibrosis, Exotoxins, LukAB, Serology
INTRODUCTION
Cystic Fibrosis (CF) is the most common fatal genetic disease among Caucasians in the United States, affecting ~30,000 people in the US and approximately 70,000 children and adults worldwide. Pulmonary disease is the leading contributor to the morbidity and mortality observed in CF and is associated with substantial physical, psychological, and financial cost 1. The progressive decline in pulmonary function observed in CF is thought to be the result of two primary processes. First, chronic infection and inflammation lead to airway remodeling and ultimately destruction of normal airway architecture. Second, acute insults, typically due to bacterial overgrowth in the lungs (bronchopneumonia/pulmonary exacerbations), lead to more rapid decline in respiratory status 2. Despite our knowledge of the devastating ramifications of pulmonary exacerbations in patients with CF, there are neither standardized definitions for pulmonary exacerbations nor data to guide treatment strategies for these acute episodes 3.
Staphylococcus aureus is one of the earliest bacterial pathogens to colonize the lungs of children with cystic fibrosis and has been demonstrated to be an important contributor to pulmonary exacerbations. S. aureus can be found in as many as 60-70% of patients with CF, and is a primary or co-pathogen in many pulmonary exacerbations4. The problem of S. aureus is compounded by the dramatic increase in community-associated methicillin-resistant S. aureus (CA-MRSA) disease, and currently circulating clones of MRSA appear to have increased virulence and pulmonary-tropism 5,6. As of 2012, the prevalence of MRSA in patients with CF in the United States had increased to 26.5%, from 2% in 2001 7. Patients with MRSA-related CF exacerbations experience a two-fold greater decline in forced expiratory volumes (FEV1) compared to those unaffected by MRSA 8. Moreover, in a longitudinal study of ~20,000 CF patients (ages 6-45 years), MRSA was detected in nearly 6,000 patients and was independently associated with an increased risk of death9.
The severity of disease attributed to S. aureus is highly variable among children with CF. This variability may be related to differences in the virulence of the infecting strain, or to the genetic background of the host (e.g., nature of the CFTR gene mutation, modifier genes or other epigenetic factors). S. aureus virulence relies, in part, on the production of a group of cytolytic toxins10 including alpha-hemolysin and the bicomponent leukotoxins, notably Panton-Valentine leukocidin (PVL) and the recently described LukAB11,12. Recent work from our group demonstrated that invasive staphylococcal infection elicits a functional and potent humoral response to LukAB13. Data regarding the adaptive response to S. aureus in children with CF are limited, and the antibody response to key staphylococcal antigens, particularly those with vaccine or therapeutic potential, remains largely unknown14-16.
The primary aim of this study was to define the seroprevalence of antistaphylococcal antibodies targeting key staphylococcal exotoxins (LukAB, alpha-hemolysin, and PVL) in children with CF. We further sought to evaluate the relationship between anti-staphylococcal antibodies, pulmonary exacerbation and disease severity in patients with CF. We observed that a high-titer antibody response to LukAB was strongly correlated with S. aureus-positive respiratory cultures, and that this antibody response successfully neutralizes LukAB-mediated cytotoxicity.
METHODS
Study Design and Patient Enrollment
This was a prospective cohort study of pediatric patients (between 1 and 18 years of age) cared for at the Pediatric Cystic Fibrosis Center of the Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center (VUMC). Potential study participants were identified at either an outpatient visit in the CF Center or during an acute hospitalization for a pulmonary exacerbation at VUMC. Informed consent was obtained for each study participant, assent was obtained for children age 7 and above, and participants were screened for the following exclusion criteria: primary or secondary immune compromise (including long-term oral or parenteral corticosteroids), history of malignancy, and receipt of intravenous immunoglobulin (IVIG) or blood products in the past 12 months. Subjects on long-term antimicrobial therapy (e.g. inhaled antibiotics) were eligible for the study. This study was approved by the Vanderbilt University Medical Center Institutional Review Board.
Enrolled subjects were followed for 12 months (July 2012 – June 2013). Serum samples were obtained at the time of enrollment, 6 and 12 months following enrollment, at the time of an acute pulmonary exacerbation, and 1-3 months following acute pulmonary exacerbation. Samples were then classified into three groups: disease free baseline (enrollment, 6 month, 12 month), acute pulmonary exacerbation, and pulmonary exacerbation follow-up. Pulmonary exacerbations were diagnosed by the clinical care team and were defined as an acute change in clinical symptoms necessitating antimicrobial therapy, as previously described3,17. Exacerbations were diagnosed due to some or all of the following: increased cough/sputum production, decrease in FEV1 (>10%), weight loss/decline in BMI%, new or increased symptoms on auscultatory exam, hemoptysis, or increased baseline oxygen requirement. These exacerbations were further classified as mild, moderate or severe. Mild exacerbations were defined as exacerbations managed with outpatient oral antibiotic therapy. Moderate exacerbations included patients hospitalized for intensive airway clearance therapy as well as intravenous antimicrobial therapy. Severe exacerbations were defined as those during which the patient required either new or increased supplemental oxygen above baseline, or those that required admission to the Intensive Care Unit. Sera were obtained by centrifugation of unheparinized whole blood samples and stored at −20°C. When available, a single representative S. aureus isolate was obtained from each subject’s respiratory cultures obtained as part of routine care at both well visits as well as during acute exacerbations.
Molecular Characterization of Clinical Isolates
Initial determination of methicillin resistance in S. aureus strains was made by the VUMC clinical laboratory. For MRSA isolates, methicillin resistance was confirmed (see Supplemental Methods for details).
Genomic DNA was purified (Wizard® SV Purification System; Promega, Madison, WI) and used as the template for PCR detection of genes encoding pvl 18, the accessory gene regulator (agr) locus type19, and lukAB20. Assignment of SCCmec type by ccr and mec complex typing was performed as previously described21. Repetitive element, sequence-based polymerase chain reaction (DiversiLab System; Biomerieux, Durham, NC) was used to determine strain type and genetic relatedness between strains 22.
Serum Antibody Measurement and Functional Assays
The serum antibody response was measured against 3 specific S. aureus exotoxin antigens: α-hemolysin (Hla) and the previously demonstrated more immunogenic subunit of the bicomponent leukotoxins LukAB (LukA) and PVL (LukS-PV)13. Recombinant Hla was purchased in purified form and reconstituted per manufacturer specifications (Sigma, St. Louis, MO). LukA and LukS-PV were expressed and purified as described previously 12.
Optimal exotoxin concentration for ELISA was determined by crisscross dilution and indirect ELISA for each antigen was performed as previously described23,24. (See Supplemental Methods section for details). Total IgG for each sample was measured to ensure differences in anti-toxin titers did not simply reflect variation in total IgG. Antibody titer results are presented as geometric mean titers (GMT) for a given population unless otherwise specified. GMT for healthy control comparisons were obtained from a bank of healthy control sera and were previously published 13.
To measure the toxin-neutralization capacity of patient sera, cytotoxicity assays were employed using Human promyelocytic HL-60 cells as we have previously done13,25 (See Supplemental Methods for details). Neutralization titers of patient sera were determined by incubating toxic supernatant and PMN-like cells with serial dilutions of sera, and the titer was considered neutralizing if ≥70% of the cells were alive at that dilution.
Statistical Analysis
Wilcoxon rank-sum test and Chi-squared or Fisher’s exact tests were used, as appropriate to compare medians and proportions. Linear regression was used to evaluate the association between LukA, LukS-PV and Hla titers at disease-free baseline and history of S. aureus growth at 0-6, 6-12 or >12 months, adjusting for previous P. aeruginosa growth, baseline BMI percentile, and baseline FEV1. The analysis was repeated dichotomizing S. aureus growth as <6 months or ≥6 months. Generalized estimating equations (GEE) models were used to assess the association between antibody titers and severity of pulmonary exacerbations and to assess the association between S. aureus growth at an exacerbation and antibody titers. Cox proportional hazards models were used to estimate hazard ratios for developing a pulmonary exacerbation in subjects who were enrolled healthy. Median times to exacerbation were compared using Kaplan-Meier curves and the log-rank test. Geometric mean neutralization titers were compared using an independent t-test to make comparisons between populations, and differences were estimated via ratios of geometric means with corresponding 95% confidence intervals and p-values.
RESULTS
Clinical and Microbiologic Characteristics
Fifty subjects were enrolled in the cohort over a 12-month period (Figure 1). The cohort was comprised of pediatric subjects between 2 and 18 years of age with a median age of 11 years, included 24 females, and consisted of children with a median BMI percentile of 54.5% and median FEV1 of 95% predicted (Table 1). Clinical and microbiologic results from pulmonary exacerbations are provided in Tables 2 and 3. S. aureus isolates were obtained from 42 subjects, of which 11 (26%) were MRSA. Three of the MRSA isolates were consistent with the epidemic CA-MRSA clone in the US (SCCmec type IV, agr class I, USA300). The remaining 8 MRSA isolates were classified as SCCmec type II but were genetically diverse, belonging to USA100, 700, or 800 pulse types. All S. aureus isolates harbored the lukA gene by PCR, while only two were PVL-positive (two of the three USA300 isolates).
Figure 1. Study Overview.
Children within the target age range with approached in the Vanderbilt Cystic Fibrosis Clinic and invited to participate in the study. Subjects were recruited until 50 subjects were enrolled, a sample size calculated based on our previous studies of serologic responses to target antigens.
Table 1.
Demographics and medical history of participants at enrollment
| Characteristic | Total (N=50) a | |
|---|---|---|
| Male | 26 | (52.00) |
| Age (median, range) | 11.5 | (2-18) |
| BMI percentile (median, range) | 54.5 | (6-95) |
| FEV1 % predicted (median, range) | 95 | (37-131) |
| FVC % predicted (median, range) | 101 | (46-133) |
| Comorbidities | ||
| Pancreatic insufficiency | 46 | (92.00) |
| Asthma | 25 | (50.00) |
| Sinus Disease | 9 | (18.00) |
| CF-related diabetes | 4 | (8.00) |
| CF-related liver disease | 4 | (8.00) |
| ABPA | 1 | (2.00) |
| None | 2 | (4.00) |
| Genetic mutations (at least one copy) | ||
| Delta F508 | 48 | (96.00) |
| G542X | 3 | (6.00) |
| G551D | 1 | (2.00) |
| N1303K | 1 | (2.00) |
| W1282X | 0 | (0.00) |
| Other | 17 | (34.00) |
| History of pathogen growth | ||
| MSSA | 45 | (90.00) |
| MRSA | 25 | (50.00) |
| Pseudomonas aeruginosa | 41 | (82.00) |
| Burkholderia cepacia | 1 | (2.00) |
| Stenotrophomonas maltophilia | 11 | (22.00) |
| Haemophilus influenzae | 8 | (16.00) |
| NTM | 0 | (0.00) |
| Other | 1 | (2.00) |
: Results represented as n(%).
Table 2.
Pulmonary Exacerbations: Clinical Characteristics
| Characteristic | Total (N=73)a | Mild (N=41)a | Moderate/Severe (N=32)a |
|||
|---|---|---|---|---|---|---|
| Male b | 16 | (47) | 11 | (68.75) | 5 | (27.78) |
|
Age at baseline (years; median, interquartile range) |
11.46 | (7.53, 14.70) |
8.81 | (6.98, 12.94) |
13.08 | (10.55, 15.66) |
|
BMI percentile at baseline
(median, interquartile range) |
44 | (28, 83) | 51 | (28.5, 75.5) |
39 | (24, 87) |
|
Loss in FEV1 % predicted
from baseline (median, interquartile range) c |
7 | (2, 15) | 7 | (1, 13) | 10 | (4, 17) |
| Severity | ||||||
| Mild | 41 | (56.16) | 41 | (100.00) | ||
| Moderate | 27 | (36.99) | 27 | (84.38) | ||
| Severe | 5 | (6.85) | 5 | (15.62) | ||
| Symptoms | ||||||
| Cough | 71 | (97.26) | 40 | (97.56) | 31 | (96.88) |
| Fatigue | 48 | (65.75) | 23 | (56.10) | 25 | (78.13) |
| Fever | 9 | (12.33) | 2 | (4.88) | 7 | (21.88) |
| Weight loss | 35 | (47.95) | 13 | (31.71) | 22 | (68.75) |
| Shortness of breath | 10 | (13.70) | 2 | (4.88) | 8 | (25.00) |
| Crackles | 29 | (39.73) | 8 | (19.51) | 21 | (65.63) |
| Wheeze | 2 | (2.74) | 0 | (0.00) | 2 | (6.25) |
: Results represented as n(%) unless otherwise indicated.
: For 34 subjects (16 male, 18 female), there were 73 exacerbations; 16 subjects had only mild exacerbations and 18 subjects had at least one moderate/severe exacerbation.
: 70 exacerbations had information on FEV1.
Table 3.
Pulmonary Exacerbations: Microbiologic Characteristics
| Characteristic |
Total
(N=73)a |
Mild
(N=41)a |
Moderate/Severe
(N=32)a |
|||
|---|---|---|---|---|---|---|
| Pathogen growth | ||||||
| MSSA only | 39 | (53.42) | 25 | (60.98) | 14 | (43.75) |
| MRSA only | 12 | (16.44) | 7 | (17.07) | 5 | (15.63) |
| Pseudomonas aeruginosa only | 4 | (5.48) | 1 | (2.44) | 3 | (9.38) |
| MSSA, P. aeruginosa | 1 | (1.37) | 0 | (0.00) | 1 | (3.13) |
| MRSA, P. aeruginosa | 7 | (9.59) | 1 | (2.44) | 6 | (18.75) |
| P. aeruginosa, Stenotrophomonas
maltophilia |
1 | (1.37) | 1 | (2.44) | 0 | (0.00) |
| MSSA, S. maltophilia, Burkholderia
cepacia |
1 | (1.37) | 1 | (2.44) | 0 | (0.00) |
| MRSA, P. aeruginosa, S.
maltophilia |
1 | (1.37) | 1 | (2.44) | 0 | (0.00) |
| None | 7 | (9.59) | 4 | (9.76) | 3 | (9.38) |
| Outpatient antibioticb | ||||||
| Trimethoprim-sulfamethoxazole | 22 | (30.14) | 22 | (53.66) | 0 | (0.00) |
| Quinolones | 32 | (43.84) | 32 | (78.05) | 0 | (0.00) |
| Beta-lactams | 3 | (4.11) | 3 | (7.32) | 0 | (0.00) |
| Other | 6 | (8.22) | 6 | (14.63) | 0 | (0.00) |
| Inpatient antibioticb | ||||||
| Anti-pseudomonal beta-lactams | 29 | (39.73) | 0 | (0.00) | 29 | (90.62) |
| Carbapenem | 2 | (2.74) | 0 | (0.00) | 2 | (6.25) |
| Ceftaroline | 1 | (1.37) | 0 | (0.00) | 1 | (3.12) |
| Aminoglycosides | 29 | (39.73) | 0 | (0.00) | 29 | (90.62) |
| Vancomycin | 15 | (20.55) | 0 | (0.00) | 15 | (46.87) |
| Clindamycin | 1 | (1.37) | 0 | (0.00) | 1 | (3.12) |
| Quinolones | 4 | (5.48) | 0 | (0.00) | 4 | (12.5) |
| Trimethoprim-sulfamethoxazole | 3 | (4.11) | 0 | (0.00) | 3 | (9.37) |
| Other | 2 | (2.74) | 0 | (0.00) | 2 | (6.25) |
: Results represented as n(%).
: At some exacerbations, more than 1 antibiotic was given; thus, the sum of percentages exceeds 100%.
Approximately half of subjects (n=26) were enrolled at disease-free baseline, with the remainder being enrolled during an acute exacerbation (n=24). Of the 26 subjects enrolled at their disease-free baseline, 14 (54%) had a subsequent exacerbation during the study period.
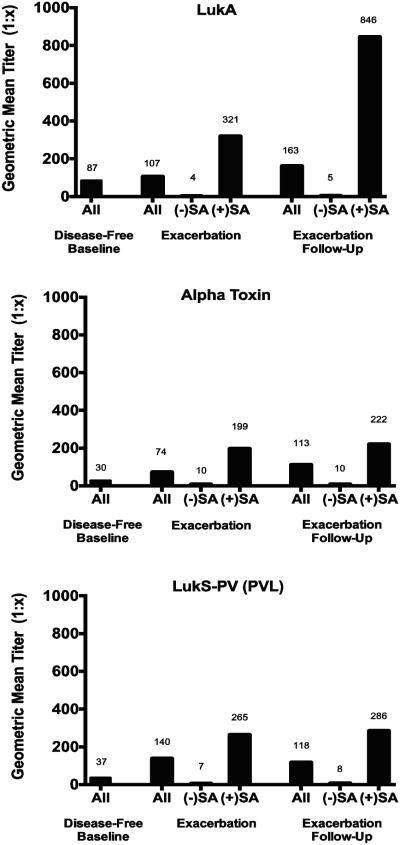
Serologic Response to Staphylococcal Leukotoxins
IgG antibodies targeting all 3 antigens of interest (LukA, Hla [alpha toxin], and LukS-PV) were present to varying degrees, based on exacerbation status and the presence of S. aureus at the time of pulmonary exacerbation (Figure 2). For each antigen, geometric mean titers were significantly higher if S. aureus was detected at the time of exacerbation. For LukA only, GMTs were significantly higher at exacerbation follow-up compared to titers during the exacerbation. These differences remained significant after controlling for the growth of other organisms (e.g. Pseudomonas aeruginosa) at the time of exacerbation. LukA titers were not correlated with total IgG values (r2 = 0.013, Figure S1), indicating that differences in titers did not merely reflect differences in total IgG.
Figure 2. Geometric mean IgG titers in serum from children with cystic fibrosis.
For those subjects that experienced pulmonary exacerbation during the study period, titers are measured during exacerbation and at follow-up (1-3 months). For LukA, alpha toxin and LukS-PV, geometric mean titers (GMT) were significantly higher if S. aureus was detected at the time of exacerbation (p<0.01 for each comparison; Mann-Whitney U test). For LukA only, GMTs were significantly higher at exacerbation follow-up compared to titers during the exacerbation (p<0.05, Wilcoxon signed-rank test).
When examining whether LukA, LukS-PV, and Hla titers at disease-free baseline differed by history of previous S. aureus growth, we found no association between these titers and history of staphylococcal growth at 0-6, 6-12 or >12 months, even after adjusting for potential confounders. Despite no association with previous growth, each toxin was an independent predictor of a higher risk of pulmonary exacerbation (p<0.05 for all 3 toxins independently) based on a Cox proportional hazard model, with 10% higher risk for every 100-unit increase in GMT for LukA (95% CI 1 – 19%), 10% for Hla (95% CI 1 – 21%), and 14% for LukS-PV (95% CI 5 – 23%).
We employed a logistic GEE model to assess whether higher antibody titers were associated with increased severity of pulmonary exacerbations. Based on a model adjusting for baseline FEV1 and baseline BMI percentile, for every increase in anti-LukA titer by 100 units, the odds of a moderate or severe exacerbation (vs. a mild exacerbation) decreased by 4% (OR: 0.96; 95% CI: 0.92 to 1.00; p=0.063).
Clinical Correlations and Predictive Capacity of LukA Serology
To assess the potential utility of a LukA titer of ≥1:160 as a marker of staphylococcal disease, we calculated an odds ratio (OR) using a GEE population averaged model. Subjects with a positive S. aureus culture during a pulmonary exacerbation were 31 times more likely to have a LukA titer ≥1:160. After adjusting for P. aeruginosa growth at time of exacerbation, baseline FEV1 and baseline BMI, we observed a 34-fold increase in the odds of having a LukA titer of ≥1:160 with a positive S. aureus culture, with a positive predictive value (PPV) of 91% (Figure 3).
Figure 3. Predictive capacity of LukAB serology in relation to detection of S. aureus during pulmonary exacerbation.
Number of samples for each given titer range are displayed. A line is arbitrarily drawn at a titer of 1:160 to demonstrate a potential cut-off value for possible predictive capacity of a positive titer. In this dataset, the presence of a positive S. aureus culture during a pulmonary exacerbation resulted in a 34-fold increase in odds of having a LukA titer ≥1:160.
Based on a linear GEE model, the LukA geometric mean titer was significantly inversely correlated with FEV1. For every unit of FEV1 decline, the LukA GMT rose by 21 units (p=0.018; 95% CI, −39.24 to −3.61). In the same model, if there was staphylococcal growth at an exacerbation, the LukA GMT was, on average, 180 units higher than if there is no S. aureus growth, though this difference was not statistically significant (p=0.71; 95% CI, −316.64 to 676.89).
Toxin Neutralization
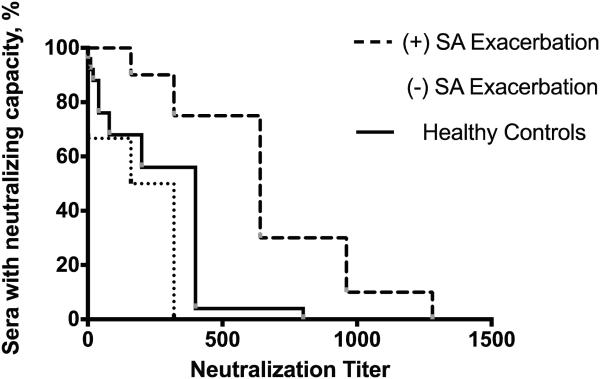
We performed a functional assessment of the LukAB antibody response by measuring the cytoprotective effect of patient sera when incubated with supernatant from S. aureus strain Newman. We have previously demonstrated that strain Newman secretes high levels of LukAB, comparatively low levels of other leukotoxins, and deletion of lukAB in strain Newman completely eliminates the cytotoxic activity 20. In this cohort, follow-up sera from subjects with S. aureus culture-positive exacerbations strongly neutralized the cytotoxicity of S. aureus supernatant (Figure 4), with a Geometric Mean Neutralization Titer (GMNT) of 584. Comparatively, sera from subjects with S. aureus-negative exacerbations neutralized with a 14-fold lower potency (GMNT of S. aureus negative sera = 42, 95% CI of the ratio 5.6 - 35). Sera from subjects with S. aureus-positive exacerbations neutralized more potently than sera from healthy controls (GMNT of healthy control sera = 152). Sera from S. aureus-negative exacerbations did not differ significantly from healthy controls in neutralization capacity. For all sera, neutralization titers correlated with serum IgG titers by ELISA, P<0.01 for both groups. Notably, there was no significant difference in neutralization titer between exacerbation and exacerbation follow-up sera, for both S. aureus-positive and S. aureus-negative exacerbations.
Figure 4. Neutralization of LukAB-mediated PMN-HL60 cytotoxicity in the presence of children with cystic fibrosis or healthy controls.
Sera obtained during exacerbations when S. aureus was isolated were significantly more potent than those in which S. aureus was not isolated (P < 0.01, independent t-test) or healthy control sera (P < 0.001, independent t-test) as measured by geometric mean neutralization titer. Neutralizing capacity did not significantly differ during and post-exacerbation.
DISCUSSION
The major findings of this study are that children with cystic fibrosis (CF) mount an immune response to the S. aureus cytotoxin LukAB during pulmonary exacerbations, and the humoral immune response to this toxin is functional in the neutralization of cytotoxicity. Moreover, the presence of a high-titer anti-LukAB response was significantly associated with culture-positive exacerbations due to S. aureus. Taken together, these findings provide strong evidence that LukAB is produced and secreted in the setting of infection in patients with CF. This is the first description of the humoral immune response to LukAB in patients with CF, and the first to demonstrate that all clinical isolates from children with CF harbored the LukAB gene. The results of this study have potential implications both for our understanding of the host response to this complex organism, as well as the potential role of anti-LukAB antibodies for the serologic assessment of infection.
To date, this study represents the largest cohort of pediatric patients with CF focused on characterizing the adaptive host response to staphylococcal infection. We observed a significant burden of staphylococcal disease in our study population. MRSA isolates recovered during the study demonstrated characteristics of both hospital-associated and community-associated strains, with no clear dominant strain identified. The bi-component leukotoxin LukSF-PV (PVL), a hallmark of CA-MRSA strains 26, 27, was rarely identified. The related toxin LukAB, however, was ubiquitously present in clinical isolates from this cohort, a novel finding for clinical isolates from patients with cystic fibrosis. Moreover, we observed that anti-LukA IgG in sera of patients with CF experiencing pulmonary exacerbations neutralized LukAB cytotoxicity in a dose-dependent manner that correlated with the anti-LukA titer.
Though S. aureus pulmonary colonization rates in patients with CF are extremely high, the reported incidence of systemic or invasive extrapulmonary infection due to S. aureus (e.g. septicemia, osteomyelitis, etc.) in CF is remarkably rare 28. Reasons for this apparent paradox are lacking, but a targeted humoral immune response – incapable of eradicating colonization, but capable of preventing disseminated or invasive disease – is one potential explanation. The data generated in this study would be supportive of this hypothesis. We noted that elevated anti-LukA titers were associated with increased exacerbation risk and declining lung function (with antibodies likely acting as a surrogate for organism burden), but higher titers were also associated with lower rates of severe exacerbations (based on clinical symptoms). This may suggest, as others have posited 29,30, that a functional anti-staphylococcal antibody response is not capable of eradication of the organism, but may reduce severity of disease.
While the anti-LukAB response was clearly functional in the neutralization of LukAB-mediated cytotoxicity, we unexpectedly found a lack of increased potency of this response in post-exacerbation follow-up sera. We previously observed an increased potency of neutralization in convalescent sera from otherwise healthy children with invasive S. aureus disease 13, presumably due to affinity maturation of IgG molecules that occurs during the convalescent period. Interestingly, this response was not seen in the sera of patients with CF. This lack of expected affinity maturation has been described previously for the humoral response to Pseudomonas aeruginosa in subjects with cystic fibrosis 31. Whether this is related to differences in the CF host, differences in the pathogen, or differential expression of antigens during colonization of the CF lung remains unclear but warrants further investigation.
The results of this study suggest there may be a potential for anti-LukA titers to serve as a biomarker of Staphylococcus aureus-related pulmonary exacerbations in pediatric patients with CF. In particular, an anti-LukA titer of ≥1:160 increased the odds of a positive S. aureus respiratory culture 34-fold, with a positive predictive value of 91%. In an era of increasing antimicrobial resistance and an increased understanding of the polymicrobial nature of pulmonary exacerbations in CF, increasing the diagnostic arsenal for identifying causative organisms would clearly be beneficial. Culture-independent diagnostics have high potential clinical utility in the care of patients with cystic fibrosis, as they remain useful even in the presence of antibiotic pre-treatment and in the absence of reliable culture specimens, and antibiotic regimens are often altered to include agents with activity against MRSA. Further studies are underway to assess the diagnostic validity of this serologic marker for S. aureus disease.
One important caveat to our study is that the presence or absence of S. aureus in culture does not necessarily define whether S. aureus is the primary pathogen causing the pulmonary exacerbation. In fact, a serologic response might be a more reliable determinant of the causative pathogen, but this is difficult to prove in the absence of a reliable gold standard for confirming the etiology. Further, our sample size was not large enough to draw statistically significant conclusions regarding correlations between clinical disease severity and specific antibody titers. Similarly, the lack of clinical isolates from all study timepoints prevented assessment of changing molecular epidemiology over time and correlation of antibody titers with various infecting strains. These questions will be a focus of future work. Finally, in vitro toxin neutralization data are not meant to serve as a surrogate marker of protection in vivo, but rather as a measure of the functional activity of anti-LukAB antibodies. Work is ongoing to assess the in vivo protective capacity of this antibody response.
Taken together, these findings provide strong evidence that LukAB is expressed by S. aureus and recognized by the human adaptive immune response in the setting of pulmonary infection in CF. We found that anti-LukAB antibodies were not only predictive of positive staphylococcal culture during exacerbation, but also functional in the neutralization of this important toxin, providing additional insights into the complex bacterial-host interactions between Staphylococcus aureus and patients with cystic fibrosis.
Supplementary Material
Figure S1: Correlation of LukA Titer to Total IgG. LukA titers from all patients were assessed as a function of the Total IgG present in the serum sample. LukA titers were not correlated with Total IgG titer (R2 = .013), indicating that differences in LukA titers were not related to differences in Total IgG.
Highlights.
Children with CF mount an immune response to the S. aureus cytotoxin LukAB.
Antibodies against LukAB were predictive of S. aureus culture during exacerbation.
LukAB was present in all clinical isolates, a novel finding for a CF cohort.
LukAB antibodies were functional in neutralization of LukAB-mediated cytotoxicity.
We found evidence of poor affinity maturation against LukAB after CF exacerbations.
Acknowledgments
Sources of Support:
This work was supported in part by grants from the National Institutes of Health and the Cystic Fibrosis Foundation:
CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences to A.D.C.; R01AI105129 to V.J.T.; CFF Center Grant C108-12 (Center ID #177) to Vanderbilt University; CFF Third Year Clinical Cystic Fibrosis Fellowship Grant CHADHA13D0 to A.D.C.
V.J.T. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: A.D.C. and I.P.T. conceived and designed the study, collected and analyzed samples, drafted the manuscript, and are guarantors of the integrity of the work as a whole. N.J.T. designed and executed the statistical analysis and drafted the statistical portions of the manuscript. N.R.S. and L.S.J. contributed extensively to laboratory analysis of samples, including ELISA and isolate characterization, and edited the manuscript. A.G.S. enrolled patients and collected samples, and revised the manuscript for content. V.J.T. provided laboratory support, critical reagents, and edited the manuscript for content. C.B.C. conceived and designed the study, provided extensive laboratory support, and revised the manuscript.
All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Prior Presentations: Data from this project were included in an oral presentation at the American Thoracic Society (ATS) 2014 International Conference in San Diego, California.
REFERENCES
- 1.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62(4):360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadori A, Antonelli A, Balteri I, Schreiber A, Bugiani M, De Rose V. Recurrent exacerbations affect FEV(1) decline in adult patients with cystic fibrosis. Respiratory medicine. 2009;103(3):407–413. doi: 10.1016/j.rmed.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Stenbit AE, Flume PA. Pulmonary exacerbations in cystic fibrosis. Current opinion in pulmonary medicine. 2011;17(6):442–447. doi: 10.1097/MCP.0b013e32834b8c04. [DOI] [PubMed] [Google Scholar]
- 4.Parkins MD, Elborn JS. Newer antibacterial agents and their potential role in cystic fibrosis pulmonary exacerbation management. The Journal of antimicrobial chemotherapy. 2010;65(9):1853–1861. doi: 10.1093/jac/dkq245. [DOI] [PubMed] [Google Scholar]
- 5.Hauser AR, Jain M, Bar-Meir M, McColley SA. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clinical microbiology reviews. 2011;24(1):29–70. doi: 10.1128/CMR.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glikman D, Siegel JD, David MZ, et al. Complex molecular epidemiology of methicillin-resistant staphylococcus aureus isolates from children with cystic fibrosis in the era of epidemic community-associated methicillin-resistant S aureus. Chest. 2008;133(6):1381–1387. doi: 10.1378/chest.07-2437. [DOI] [PubMed] [Google Scholar]
- 7.Cystic Fibrosis Foundation Patient Registry, 2012 Annual Data Report. Cystic Fibrosis Foundation; 2013. [Google Scholar]
- 8.Vanderhelst E, De Meirleir L, Verbanck S, Pierard D, Vincken W, Malfroot A. Prevalence and impact on FEV(1) decline of chronic methicillin-resistant Staphylococcus aureus (MRSA) colonization in patients with cystic fibrosis. A single-center, case control study of 165 patients. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2012;11(1):2–7. doi: 10.1016/j.jcf.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA : the journal of the American Medical Association. 2010;303(23):2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 10.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bicomponent leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Frontiers in cellular and infection microbiology. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuMont AL, Yoong P, Day CJ, et al. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(26):10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DuMont AL, Yoong P, Surewaard BG, et al. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infection and immunity. 2013;81(5):1830–1841. doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomsen IP, Dumont AL, James DB, et al. Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infection and immunity. 2014;82(3):1234–1242. doi: 10.1128/IAI.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ericsson A, Granstrom M, Mollby R, Strandvik B. Antibodies to staphylococcal teichoic acid and alpha toxin in patients with cystic fibrosis. Acta paediatrica Scandinavica. 1986;75(1):139–144. doi: 10.1111/j.1651-2227.1986.tb10170.x. [DOI] [PubMed] [Google Scholar]
- 15.Hollsing AE, Granstrom M, Strandvik B. Prospective study of serum staphylococcal antibodies in cystic fibrosis. Archives of disease in childhood. 1987;62(9):905–911. doi: 10.1136/adc.62.9.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strandvik B, Hollsing A, Mollby R, Granstrom M. Antistaphylococcal antibodies in cystic fibrosis. Infection. 1990;18(3):170–172. doi: 10.1007/BF01642107. [DOI] [PubMed] [Google Scholar]
- 17.Regelmann WE, Schechter MS, Wagener JS, et al. Pulmonary exacerbations in cystic fibrosis: young children with characteristic signs and symptoms. Pediatric pulmonology. 2013;48(7):649–657. doi: 10.1002/ppul.22658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999;29(5):1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 19.Shopsin B, Mathema B, Alcabes P, et al. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J Clin Microbiol. 2003;41(1):456–459. doi: 10.1128/JCM.41.1.456-459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumont AL, Nygaard TK, Watkins RL, et al. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol. 2011;79(3):814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo Y, Ito T, Ma XX, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. 2007;51(1):264–274. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenover FC, Gay EA, Frye S, Eells SJ, Healy M, McGowan JE., Jr Comparison of typing results obtained for methicillin-resistant Staphylococcus aureus isolates with the DiversiLab system and pulsed-field gel electrophoresis. J Clin Microbiol. 2009;47(8):2452–2457. doi: 10.1128/JCM.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi N, Hattori M, Miwa K, Nishida H. Antibodies against superantigenic exotoxins produced by Staphylococcus aureus in sera from mothers and their infants' cord blood. Am J Perinatol. 2006;23(7):413–419. doi: 10.1055/s-2006-951290. [DOI] [PubMed] [Google Scholar]
- 24.Ausubel FM. Short protocols in molecular biology : a compendium of methods from Current protocols in molecular biology. 5th Wiley; New York: 2002. [Google Scholar]
- 25.Ennaciri J, Girard D. Immune system: maturation of myeloid cells. Methods Mol Biol. 2009;550:195–203. doi: 10.1007/978-1-60327-009-0_12. [DOI] [PubMed] [Google Scholar]
- 26.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9(8):978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? The Journal of infectious diseases. 2006;194(12):1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 28.Goerke C, Wolz C. Adaptation of Staphylococcus aureus to the cystic fibrosis lung. International journal of medical microbiology : IJMM. 2010;300(8):520–525. doi: 10.1016/j.ijmm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012;30(19):2921–2927. doi: 10.1016/j.vaccine.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Fowler VG, Jr., Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(Suppl 5):66–75. doi: 10.1111/1469-0691.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciofu O, Petersen TD, Jensen P, Hoiby N. Avidity of anti-P aeruginosa antibodies during chronic infection in patients with cystic fibrosis. Thorax. 1999;54(2):141–144. doi: 10.1136/thx.54.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Correlation of LukA Titer to Total IgG. LukA titers from all patients were assessed as a function of the Total IgG present in the serum sample. LukA titers were not correlated with Total IgG titer (R2 = .013), indicating that differences in LukA titers were not related to differences in Total IgG.