Abstract
Placental insufficiency leads to intrauterine growth restriction (IUGR) and a lifelong risk of developing type 2 diabetes. Impaired islet development in the growth restricted fetus, including decreased β-cell replication, mass, and insulin secretion, is strongly implicated in the pathogenesis of later life type 2 diabetes. Currently, standard medical management of a woman with a pregnancy complicated by placental insufficiency and fetal IUGR is increased fetal surveillance and indicated preterm delivery. This leads to the dual complications of IUGR and preterm birth – both of which may increase the lifelong risk for type 2 diabetes. In order to develop therapeutic interventions in IUGR pregnancies complicated by placental insufficiency and decrease the risk of later development of type 2 diabetes in the offspring, the mechanisms responsible for impaired islet development in these cases must be determined. This review focuses on current investigations testing the hypothesis that decreased nutrient supply to the IUGR fetus inhibits an intra-islet hepatocyte growth factor – vascular endothelial growth factor A (HGF – VEGFA) feed forward signaling pathway and that this is responsible for developmental islet defects.
Keywords: Intrauterine growth restriction, insulin, hepatocyte growth factor, vascular endothelial cell growth factor, pancreatic islet, pregnancy
1. Intrauterine Growth Restriction and the Pancreatic Islet
1.1. Clinical Aspects of Intrauterine Growth Restriction (IUGR)
Placental insufficiency and intrauterine growth restriction (IUGR) complicate approximately 4-8% of pregnancies (Platz and Newman, 2008). IUGR is best defined as a failure of the fetus to reach its genetic potential size. The distinction between IUGR patients and those that are simply small for gestational age (SGA) based on their genetic make-up has important implications for short and long term morbidities (Von Beckerath et al., 2013). There are a variety of causes of IUGR, including maternal and placental diseases, as well as idiopathic cases. In the majority of cases, however, decreased placental nutrient and oxygen transfer to the fetus are important pathophysiological features. Current medical management includes close fetal monitoring and delivery of the fetus when the risks associated with continued development in a compromised intrauterine environment are felt to be greater than the risks of induced preterm birth (Zeitlin et al., 2000). This leads to serious complications for the baby including increased perinatal mortality as well as serious short and long term morbidities (Rosenberg, 2008). One of the most worrisome long term consequences of IUGR is an increased risk for developing type 2 diabetes later in life (Hales and Barker, 2001). The development of type 2 diabetes requires both insulin resistance and an inability of the pancreatic β-cell to compensate for this insulin resistance by increasing insulin secretion (Kasuga, 2006). Therefore, the development of the pancreatic islet and β-cell has been the subject of intense investigation (Green et al., 2010). Despite this, gaps in our understanding of the pathogenesis of IUGR limit specific therapies available for women whose pregnancies are complicated by placental insufficiency. Nutritional strategies have produced variable results, including some that actually increased the severity of fetal growth restriction and were associated with higher fetal mortality rates (Brown et al., 2011; Rush et al., 1980). Development of better therapies holds the promise of improving fetal growth, prolonging pregnancy, and decreasing the risk in the offspring of developing adult diseases like diabetes later in life. This review will highlight some of the recent work aimed at understanding the pathophysiology of impaired islet development during IUGR which might inform the design of these improved therapies.
1.2. Placental Insufficiency and Impaired Pancreatic Islet Development
In humans, severe placental insufficiency results in persistently decreased oxygen and nutrient transfer to the fetus, including amino acids and notably leucine, as early as the end of the second and beginning of the third trimester. In fact, the degree of impairment in leucine transfer to the fetus directly correlates with the severity of fetal growth restriction in cases of placental insufficiency (Jansson et al. 1998, 2002; Paolini et al., 2001; Marconi et al., 1999). Severe placental insufficiency also results in decreased fetal glucose stimulated insulin secretion when tested during percutaneous umbilical cord blood sampling (Nicolini et al., 1990), and reduced pancreatic islet vascularity and β-cell mass (Van Assche et al., 1977). This contrasts with a morphological analysis of pancreases from less severely growth restricted patients in which no difference was found in β-cell mass (Beringue et al., 2002). The differences between the Van Assche and Beringue studies highlight the important distinction between IUGR fetuses who fail to reach their genetic growth potential due to placental insufficiency from those who are simply small for gestational age based on their genotype (Von Beckerath et al., 2013). This distinction is even more critical to consider when one realizes that there are genetic mutations which result in decreased fetal insulin secretion, decreased fetal growth, and a higher risk for developing diabetes in later life. Loss of function mutations in the gene encoding glucokinase is an important example of such a mutation (Spyer et al., 2001).
1.3. Animal Models to Study Impaired Pancreatic Islet Development during Placental Insufficiency
While we know some of the structural and functional islet defects that result from IUGR, in humans the mechanisms underlying impaired islet development in IUGR remain unknown. In order to determine these mechanisms, animal models must be used. One such model organism is the pregnant sheep, in which several models of placental insufficiency have been developed (Green et al., 2010; Morrison, 2008). Of these sheep models, one of the best characterized in terms of impaired islet development also is the most severe sheep placental insufficiency model of IUGR available (PI-IUGR) (Green et al., 2010). Experimental placental insufficiency and fetal growth restriction in the PI-IUGR model is established by exposing pregnant sheep to elevated ambient temperatures beginning on approximately days 35-42 of gestation (normal sheep gestation is approximately 148 days) and ending between 105-115 days of gestation. Impaired placental function is evident as early as days 95-105 (Limesand et al., 2013; de Vrijer et al., 2006). Decrements in umbilical blood flow, oxygen and nutrient transfer to the fetus, and fetal anabolic hormone concentrations become more prominent as gestation progresses and are associated with asymmetric fetal growth restriction (de Vrijer et al,. 2004; Limesand et al., 2007; Brown et al., 2012; Galan et al., 2005; Regnault et al., 2007).
Pregnant sheep, and this model in particular, offer several advantages for determining the mechanism by which placental insufficiency impairs fetal islet development and function of the β-cell. These advantages include both similarities to human placental insufficiency and technical features which allow for complex in vivo and in vitro fetal studies (Green et al., 2010; Jansson et al., 1998; Paolini et al., 2001; Van Assche et al., 1977; Nicolini et al., 1990; Barry and Anthony, 2008; Barry et al., 2008; Brown et al., 2012; Limesand et al., 2005 2006; Ross et al., 1996; Rozance et al., 2015; Thorn et al., 2013). Development of the human and sheep fetal endocrine pancreas is similar. The transition of pancreatic progenitor cells into more differentiated endocrine cells with mature secretory products, formation of pancreatic iselts, and replication of existing endocrine cells within the islet occur at the same gestational stages in both human and sheep fetuses (Green et al., 2010). In addition to developmental similarities, technical advantages include the ability to isolate fetal islet endothelial cells to 95% purity (Rozance et al., 2015). For the current review the most important similarities between human IUGR and our sheep PI-IUGR model are decreased nutrient supply to the fetus including amino acids and leucine, decreased fetal insulin concentrations and glucose stimulated insulin secretion, and decreased islet size and β-cell mass (Green et al., 2010; Limesand et al., 2005 2006; Ross et al., 1996; Rozance et al., 2015; Thorn et al., 2013). Interestingly, while pancreatic islets are smaller and β-cell insulin content is lower, isolated pancreatic islets from PI-IUGR fetal sheep actually secrete a higher fraction of the cellular insulin present when tested in vitro compared to normal fetal sheep (Limesand et al., 2005, 2006). In fact, in the in vivo condition hypoxemia and hypercatecholinemia in the fetus play a major role in the suppression of insulin secretion in the PI-IUGR fetus and once the adrenergic signaling is blocked the PI-IUGR islets actually become hyper responsive to glucose stimulation (Rozance et al., 2009; Leos et al., 2010). Fetal hypoxia and hypercatecholinemia in pregnancies complicated by fetal growth restriction (Greenough et al., 1990) might set up the formerly IUGR fetus for increased postnatal insulin secretion leading to apparent hyperinsulinemic hypoglycemia in a subset of these patients once normoxia is established and catecholamine concentrations return to normal (Arya et al., 2013).
In fact, unlike the fairly consistent finding of decreased fetal insulin concentrations and secretion in IUGR, studies of insulin concentrations in the early postnatal period are quite variable (Collins and Leonard 1984; Collins et al., 1990; Bazaes et al., 2003; Hoe et al., 2006; Wang et al., 2007). As formerly IUGR infants age the incidence of increased adiposity and insulin resistance increases (Hofman et al., 1997; Ong et al., 2000; Mericq et al., 2005). This complicates interpretation of insulin secretion measurements in children and young adults who experienced IUGR, because of the hyperbolic relationship between insulin secretion and insulin sensitivity (Kahn et al.; 1993). However, studies that accounted for insulin sensitivity showed impaired insulin secretion in children and young adults who had IUGR during fetal life (Li et al., 2001; Jensen et al., 2002).
2. Hepatocyte Growth Factor (HGF) and Vascular Endothelial Cell Growth Factor A (VEGFA) in the Pancreatic Islet
2.1. HGF and VEGFA in Normal Islet Development
There has been increasing interest in the role of the pancreatic vasculature and paracrine signaling between the β-cell and endothelial cells. The paracrine signals most studied in this context are hepatocyte growth factor (HGF) produced by the endothelial cell and vascular endothelial growth factor A (VEGFA) produced by the β-cell, though they are often studied in isolation from each other. Genetic manipulations that increase islet HGF or VEGFA signaling also increase adult islet vascularity, β-cell mass, and/or insulin secretion (Dai et al., 2003; Garcia-Ocana et al., 2000, 2001; Lammert et al., 2001). When islet HGF or VEGFA signaling is decreased, adult animals develop reduced islet vascularity, β-cell mass, insulin secretion, glucose intolerance, and diabetes (Dai et al., 2005; Kamba et al., 2006; Lammert et al., 2003; Brissova et al., 2006; Mellado-Gil et al., 2011; Reinert et al., 2013). Most currently available literature regarding islet HGF and VEGFA signaling does not directly address the pathogenesis of impaired islet development during placental insufficiency. Furthermore, published studies primarily used genetic and pharmacological inhibition or activation of HGF and VEGFA signaling, resulting in an “all or none” phenotype. The genetic manipulations were all performed in mice and have not been extended to models of placental insufficiency. Furthermore, very few studies have focused on islet HGF and VEGFA signaling in the pathogenesis of impaired islet development contributing to the fetal origins of diabetes following IUGR.
2.2. IUGR and Islet HGF and VEGFA
Two models of IUGR have been used to test the impact of impaired placental function and reduced nutrient and oxygen supply to the fetus on the islet vasculature and islet VEGFA, both in pregnant rats. In one model fetal growth restriction was produced by ligating the uterine arteries near the end of gestation and in the other fetal growth restriction was produced by restricting maternal protein intake during pregnancy. In both of these models, decreased fetal and/or neonatal islet vascularity and VEGFA were observed (Boujendar et al.,2003; Ham et al., 2009). Furthermore, these decrements could be corrected or prevented. Decreased islet VEGFA and vascularity following bilateral uterine artery ligation could be corrected by treatment with a Glucagon Like Peptide-1 analog in the neonatal period (Ham et al., 2009). In the low protein diet model of fetal growth restriction, decreased islet VEGFA and vascularity could be prevented with maternal dietary taurine supplementation (Boujendar et al.,2003).
In order to first establish the importance of fetal endothelial cell secretion of HGF for stimulating isolated fetal sheep islet function, we isolated fetal sheep endothelial cells and then used these cells to condition cell culture media (Figure 1A). Isolated fetal sheep pancreatic islets were then incubated overnight in this conditioned media and the insulin content of the islets was determined. Compared to non-conditioned media, incubation in the endothelial cell-conditioned media resulted in higher islet insulin contents. This effect was blocked by the addition of a specific inhibitory anti-HGF antibody, demonstrating the necessity of HGF signaling for this islet response (Figure 1A,B). Furthermore, incubation of fetal islets in non-conditioned media with supplemental HGF also resulted in higher islet insulin contents, demonstrating that HGF also is sufficient for this islet response (Figure 1A) (Rozance et al., 2015).
Figure 1. Hepatocyte Growth Factor is a necessary and sufficient component of endothelial cell conditioned media to increase islet insulin concentrations.
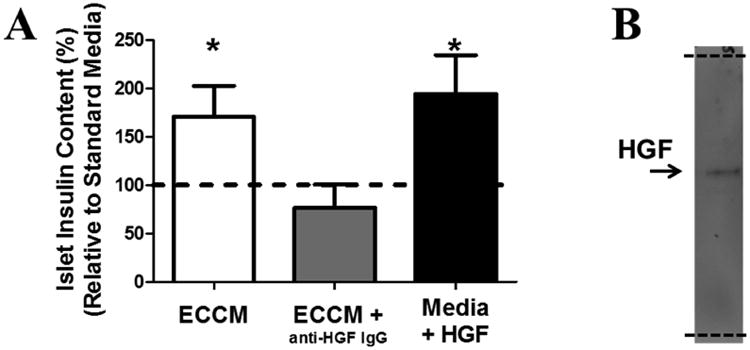
A) Islets from 4 fetuses were divided into endothelial cell (EC) conditioned media (ECCM), ECCM + an inhibitory anti-HGF IgG antibody, and non-conditioned media + HGF (100 ng/mL). Following an overnight incubation the insulin content of the islets was determined and compared to non-conditioned media (dashed line). All conditions included non-specific IgG. * P<0.05. B) Western blotting with the anti-HGF antibody on ECCM demonstrates specificity with a single band (arrow). The entire length of the membrane is shown (indicated by dashed lines). (Rozance et al., 2015)
In our severe sheep PI-IUGR model of placental insufficiency, fetal islet vessel density and β-cell mass were reduced at day 103-105 of gestation, after placental insufficiency is established (Rozance et al., 2015; Limesand et al., 2013). At day 130-135 of gestation the decrement in β-cell mass is greater (Limesand et al., 2005, 2013). However, despite significantly smaller islets, islet vascularity is decreased in proportion to islet size, such that islet vessel density is equivalent to controls (Rozance et al., 2015; Limesand et al., 2005). This finding demonstrates that reduced vessel density in the islets of PI-IUGR fetuses at day 103-105 of gestation results in the down-regulation of islet growth in order to match the vascular supply available.
In addition to determining islet morphology in the PI-IUGR sheep fetus, we also interrogated fetal islet HGF and VEGFA. Similar to the two rodent models of IUGR described above, VEGFA is significantly decreased in PI-IUGR islets (Figure 2). Thus, the primary signal for islet vascular growth is decreased in the islets of three different animal models of IUGR. We also found that both secreted and cellular HGF concentrations were lower in fetal islet endothelial cells isolated from PI-IUGR animals compared to controls (Figure 2) (Rozance et al., 2015). In order to determine the functional significance of decreased islet endothelial cell HGF production in PI-IUGR, we incubated freshly isolated normal fetal sheep islets overnight in media conditioned by isolated control and PI-IUGR islet endothelial cells. Islets incubated in control endothelial cell-conditioned media had higher insulin content than islets incubated in non-conditioned media. But islets incubated in PI-IUGR endothelial cell-conditioned media had insulin contents that were equivalent to islets incubated with non-conditioned media (Figure 3) (Rozance et al., 2015). These experimental observations reveal functional consequences of decreased HGF secretion by the PI-IUGR fetal islet endothelial cell for the adjacent fetal β-cell which limit insulin production; a hallmark of impaired islet development in the PI-IUGR fetus (Limesand et al., 2006).
Figure 2. VEGFA and HGF are lower in PI-IUGR islets and isolated islet endothelial cells, respectively.
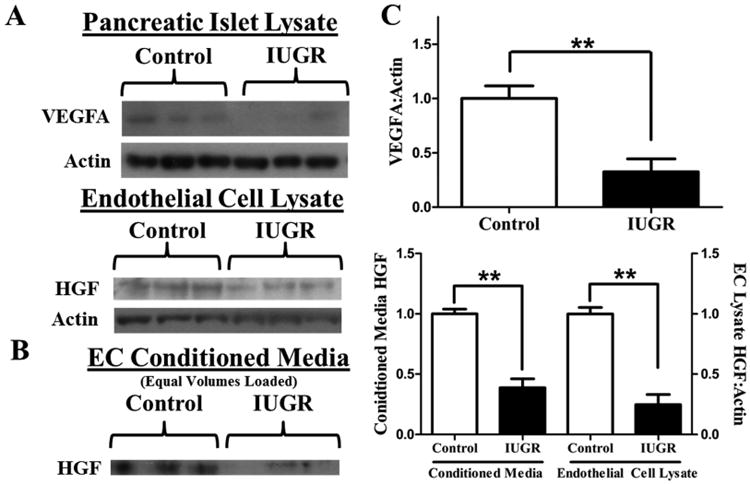
A) Representative western blots for VEGFA and HGF in pancreatic islet lysates and pancreatic islet endothelial cell lysates, respectively, after loading equal amounts of protein. B) Representative western blot for HGF in EC conditioned media after loading equal volumes of media. C) Quantitative analysis of islet lysate (control, n=10; PI-IUGR, n=4) and islet EC lysates and conditioned media (control, n=3; PI-IUGR, n=4). ** P<0.01. (Rozance et al., 2015)
Figure 3. Media conditioned by isolated PI-IUGR islet ECs does not increase islet insulin content.
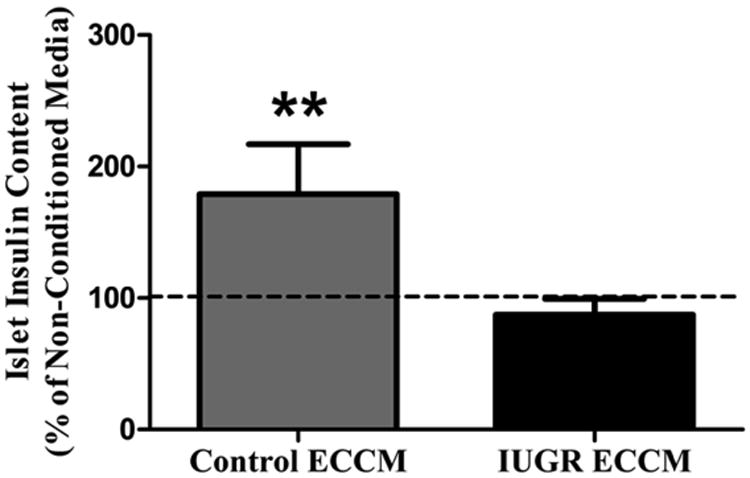
Islets from 4 fetuses were divided into non-conditioned media and media conditioned by ECs isolated from control (Control ECCM) or PI-IUGR (IUGR ECCM) fetal islets. Following an overnight incubation the islet insulin content was determined and compared to non-conditioned media (dashed line). ** P<0.01. (Rozance et al., 2015)
What remains to be demonstrated is which phenotypic characteristic, or combination of characteristics, in PI-IUGR is responsible for islet defects in HGF and VEGFA production and if this can be reversed after onset of fetal growth restriction and islet dysfunction. It is possible that that islet HGF-VEGFA signaling is a nutrient and/or hormone sensitive feed forward signaling loop (Figure 4). This would explain how HGF-VEGFA signaling is decreased in experimental models of placental insufficiency. Insulin and IGF-1 are anabolic fetal growth factors that could act as potential stimulators of islet HGF-VEGFA signaling, because both stimulate VEGFA production in different adult organ systems and cells (Lu et al., 1999; Warren et al., 1996). IGF-1 is particularly important to investigate as a mechanism, because both circulating and pancreatic IGF-1 concentrations are lower in the PI-IUGR model of fetal growth restriction (Brown et al., 2012; Chen et al., 2012). While insulin concentrations also are low in IUGR, fetal mice with a genetic knock out of both insulin genes actually have β-cell hyperplasia and more vascularized islets than controls (Duvillié et al., 2002).
Figure 4. Intra-islet HGF-VEGFA signaling.
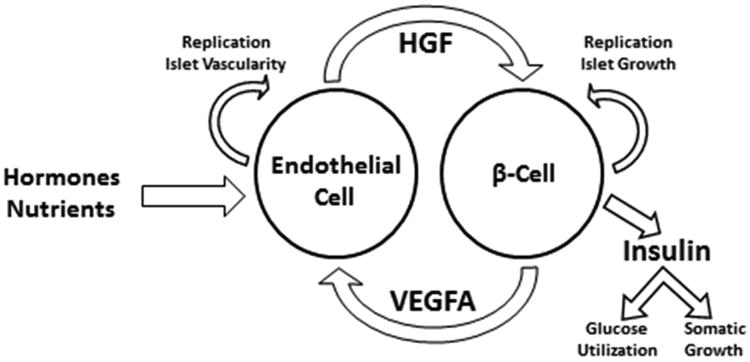
A nutrient sensitive islet HGF-VEGFA feed forward signaling loop in the fetus is particularly important to test, as it would not only provide a mechanism for the matching of islet growth and vascular supply; but it also would explain how islet growth is integrated with and regulated by the fetal nutrient supply. Given insulin's role as a fetal somatic growth factor (Fowden, 1992), this also would imply that the islet endothelial cell acts in conjunction with the pancreatic β-cell as a fetal “nutrient sensor”, linking nutrient supply to signals for somatic growth.
Amino acids are likely candidate nutrient substrates for initiating or stimulating signaling through this signaling loop. Published work in hepatic cells have demonstrated that leucine stimulation of the mammalian target of rapamycin (mTOR) is essential for producing HGF secretion (Tomiya et al., 2002, 2007). mTOR directly phosphorylates and activates the transcription factor STAT3 leading to increased HGF expression (Kim et al., 2009; Laplante and Sabatini, 2013; Onda et al., 2002; Wojcik er al., 2006). The presence of a feed forward islet HGF-VEGFA signaling loop has not been definitively demonstrated. However, there is evidence for VEGFA stimulated HGF production in adult islet endothelial cells and HGF stimulated VEGFA production in numerous cell types, though not in the pancreatic β-cell (Lin et al., 2012; Matsumura et al., 2013; Moriyama et al., 1998; Van et al,, 1998; Johansson et al., 2006).
Importantly, in the PI-IUGR islet the potential for increased signaling through the HGF-VEGFA pathways exists. PI-IUGR islets have normal expression of the HGF receptor cMET and the PI-IUGR islet endothelial cells actually have increased expression of the VEGFA receptor, VEGFR2 (Figure 5) (Rozance et al., 2015). Therefore, if provided appropriate signals, including restoration of a normal fetal amino acid supply, PI-IUGR islet HGF-VEGFA signaling might increase and result in a normalization of pancreatic islet size, vascularity and function.
Figure 5. cMET and VEGFR2 are the same or higher in PI-IUGR islets and isolated islet ECs.
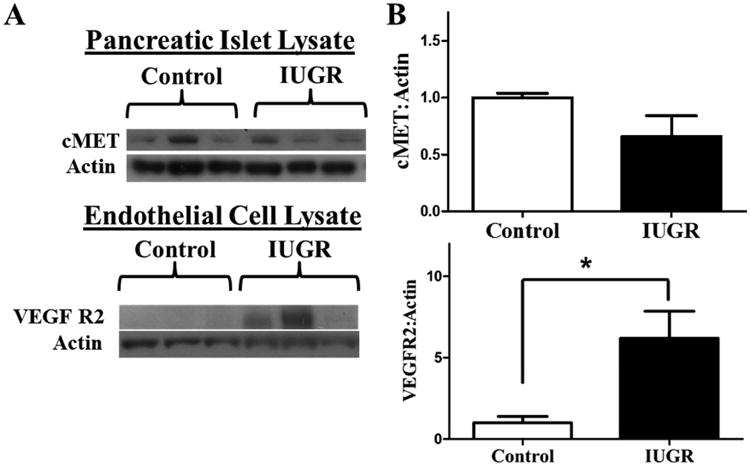
A) Representative western blot for cMet and VEGFR2 in pancreatic islet lysates and pancreatic islet endothelial cell lysates, respectively. B) Quantitative analysis of islet lysate (control, n=10; PI-IUGR, n=4) and islet EC lysates (control, n=3; PI-IUGR, n=4). * P<0.05. (Rozance et al., 2015)
3. Conclusions
Strategies to treat or prevent human IUGR and the associated complications have produced variable results, including preventative strategies with high maternal protein supplementations that actually increased the severity of fetal growth restriction and were associated with higher fetal mortality rates (Brown et al., 2011). These studies, however, were performed prior to robust animal experiments. It is fundamental, therefore, to understand how the IUGR fetus responds to novel strategies in animal models and determine the mechanisms responsible for such responses prior to developing new treatment strategies to test in human pregnancies. Testing the role of these novel strategies on islet growth, development, and function will be important to determine the mechanisms responsible for improvements in fetal growth. This is because of the role that the fetal pancreatic islet plays in sensing the fetal nutrient supply and secreting appropriate amounts of the fetal anabolic growth factor, insulin. This approach will allow for the design of the most specific therapeutic interventions that will likely be safer for the IUGR fetus than previously used strategies.
Highlights.
Severely growth restricted fetuses have impaired insulin secretion, islet size, and islet vascularity.
These features of growth restricted fetuses may underlie part of their increased risk for developing type 2 diabetes as adults.
Islet HGF and VEGFA signaling represent a potential mechanism linking islet growth and vascularity.
In cases of placental insufficiency, decreased pancreatic islet HGF and VEGFA signaling are potentially responsible for impaired insulin secretion, islet size, and islet vascularity.
Acknowledgments
This work was supported by a Pilot and Feasibility Award from the Center for Women's Health Research, University of Colorado; as well as NIH Grants R01 DK088139 and K08 HD060688 (PJR, PI; WWH, Co-I)), and NIH T32 HD007186 (WWH PI and PD) and NIH K12 HD068372 (WWH, PD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or NICHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arya VB, Flanagan SE, Kumaran A, Shield JP, Ellard S, Hussain K, Kapoor RR. Clinical and molecular characterisation of hyperinsulinaemic hypoglycaemia in infants born small-for-gestational age. Arch Dis Child Fetal Neonatal Ed. 2013;98:F356–358. doi: 10.1136/archdischild-2012-302880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology. 2008;69:55–67. doi: 10.1016/j.theriogenology.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol. 2008;32:225–230. doi: 10.1053/j.semperi.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Bazaes RA, Salazar TE, Pittaluga E, Pena V, Alegria A, Iniguez G, Ong KK, Dunger DB, Mericq MV. Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics. 2003;111:804–809. doi: 10.1542/peds.111.4.804. [DOI] [PubMed] [Google Scholar]
- Béringue F, Blondeau B, Castellotti MC, Bréant B, Czernichow P, Polak M. Endocrine pancreas development in growth-retarded human fetuses. Diabetes. 2002;51:385–391. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- Boujendar S, Arany E, Hill D, Remacle C, Reusens B. Taurine supplementation of a low protein diet fed to rat dams normalizes the vascularization of the fetal endocrine pancreas. J Nutr. 2003;133:2820–2825. doi: 10.1093/jn/133.9.2820. [DOI] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. Pancreatic Islet Production of Vascular Endothelial Growth Factor-A Is Essential for Islet Vascularization, Revascularization, and Function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal Amino Acid Supplementation During Pregnancy for Intrauterine Growth Restriction. Frontiers in Bioscience. 2011;S3:428–444. doi: 10.2741/s162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW., Jr Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab. 2012;303:E352–E364. doi: 10.1152/ajpendo.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Rozance PJ, Hay WW, Jr, Limesand SW. Insulin-like growth factor and fibroblast growth factor expression profiles in growth-restricted fetal sheep pancreas. Exp Biol Med (Maywood) 2012;237:524–529. doi: 10.1258/ebm.2012.011375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JE, Leonard JV. Hyperinsulinism in asphyxiated and small-for-dates infants with hypoglycaemia. Lancet. 1984;2(8398):311–313. doi: 10.1016/s0140-6736(84)92685-0. [DOI] [PubMed] [Google Scholar]
- Collins JE, Leonard JV, Teale D, Marks V, Williams DM, Kennedy CR, Hall MA. Hyperinsulinaemic hypoglycaemia in small for dates babies. Archives of Disease in Childhood. 1990;65:118–1120. doi: 10.1136/adc.65.10.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Li Y, Yang J, Liu Y. Hepatocyte growth factor preserves beta cell mass and mitigates hyperglycemia in streptozotocin-induced diabetic mice. J Biol Chem. 2003;278:27080–27087. doi: 10.1074/jbc.M211947200. [DOI] [PubMed] [Google Scholar]
- Dai C, Huh CG, Thorgeirsson SS, Liu Y. Beta-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am J Pathol. 2005;167:429–436. doi: 10.1016/s0002-9440(10)62987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrijer B, Regnault TR, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol Endocrinol Metab. 2004;287:E1114–1124. doi: 10.1152/ajpendo.00259.2004. [DOI] [PubMed] [Google Scholar]
- de Vrijer B, Davidsen ML, Wilkening RB, Anthony RV, Regnault TR. Altered placental and fetal expression of IGFs and IGF-binding proteins associated with intrauterine growth restriction in fetal sheep during early and mid-pregnancy. Pediatr Res. 2006;60:507–512. doi: 10.1203/01.PDR.0000242364.78002.71. [DOI] [PubMed] [Google Scholar]
- Duvillié B, Currie C, Chrones T, Bucchini D, Jami J, Joshi RL, Hill DJ. Increased islet cell proliferation, decreased apoptosis, and greater vascularization leading to beta-cell hyperplasia in mutant mice lacking insulin. Endocrinology. 2002;143:1530–1537. doi: 10.1210/endo.143.4.8753. [DOI] [PubMed] [Google Scholar]
- Fowden AL. The role of insulin in fetal growth. Early Hum Dev. 1992;29:177–181. doi: 10.1016/0378-3782(92)90135-4. [DOI] [PubMed] [Google Scholar]
- Galan HL, Anthony RV, Rigano S, Parker TA, de Vrijer B, Ferrazzi E, Wilkening RB, Regnault TR. Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol. 2005;192:272–279. doi: 10.1016/j.ajog.2004.05.088. [DOI] [PubMed] [Google Scholar]
- Garcia-Ocana A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- Garcia-Ocana A, Vasavada RC, Cebrian A, Reddy V, Takane KK, Lopez-Talavera JC, Stewart AF. Transgenic overexpression of hepatocyte growth factor in the beta-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50:2752–2762. doi: 10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol. 2010;205:211–224. doi: 10.1677/JOE-09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A, Nicolaides KH, Lagercrantz H. Human fetal sympathoadrenal responsiveness. Early Hum Dev. 1990;23:9–13. doi: 10.1016/0378-3782(90)90124-2. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Ham JN, Crutchlow MF, Desai BM, Simmons RA, Stoffers DA. Exendin-4 normalizes islet vascularity in intrauterine growth restricted rats: potential role of VEGF. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181a282a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe FM, Thornton PS, Wanner LA, Steinkrauss L, Simmons RA, Stanley CA. Clinical features and insulin regulation in infants with a syndrome of prolonged neonatal hyperinsulinism. Journal of Pediatrics. 2006;148:207–212. doi: 10.1016/j.jpeds.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Hofman PL, Cutfield WS, Robinson EM, Bergman RN, Menon RK, Sperling MA, Gluckman PD. Insulin resistance in short children with intrauterine growth retardation. Journal of Clinical Endocrinology and Metabolism. 1997;82:402–406. doi: 10.1210/jcem.82.2.3752. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- Jensen CB, Storgaard H, Dela F, Holst JJ, Madsbad S, Vaag AA. Early differential defects of insulin secretion and action in 19-year-old caucasian men who had low birth weight. Diabetes. 2002;51:1271–1280. doi: 10.2337/diabetes.51.4.1271. [DOI] [PubMed] [Google Scholar]
- Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet Endothelial Cells and Pancreatic {beta}-Cell Proliferation: Studies in Vitro and during Pregnancy in Adult Rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Yoon MS, Chen J. Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J Biol Chem. 2009;284:35425–35432. doi: 10.1074/jbc.M109.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2010;298:E770–778. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Johnson MS, Goran MI. Effects of low birth weight on insulin resistance syndrome in caucasian and African–American children. Diabetes Care. 2001;24:2035–2042. doi: 10.2337/diacare.24.12.2035. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1297–R1305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated Insulin Release and Storage in Fetal Sheep Pancreatic Islets with Intrauterine Growth Restriction. Endocrinology. 2006;147:1488–1497. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW., Jr Reductions in insulin concentrations and beta-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol Endocrinol Metab. 2013;304:E516–E523. doi: 10.1152/ajpendo.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YM, Huang YL, Fong YC, Tsai CH, Chou MC, Tang CH. Hepatocyte growth factor increases vascular endothelial growth factor-A production in human synovial fibroblasts through c-Met receptor pathway. PLoS One. 2012;7:e50924. doi: 10.1371/journal.pone.0050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Amano S, Miyamoto K, Garland R, Keough K, Qin W, Adamis AP. Insulin-induced vascular endothelial growth factor expression in retina. Invest Ophthalmol Vis Sci. 1999;40:3281–3286. [PubMed] [Google Scholar]
- Marconi AM, Paolini CL, Stramare L, Cetin I, Fennessey PV, Pardi G, Battaglia FC. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr Res. 1999;46:114–119. doi: 10.1203/00006450-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Matsumura A, Kubota T, Taiyoh H, Fujiwara H, Okamoto K, Ichikawa D, Shiozaki A, Komatsu S, Nakanishi M, Kuriu Y, Murayama Y, Ikoma H, Ochiai T, Kokuba Y, Nakamura T, Matsumoto K, Otsuji E. HGF regulates VEGF expression via the c-Met receptor downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine cells. Int J Oncol. 2013;42:535–542. doi: 10.3892/ijo.2012.1728. [DOI] [PubMed] [Google Scholar]
- Mellado-Gil J, Rosa TC, Demirci C, Gonzalez-Pertusa JA, Velazquez-Garcia S, Ernst S, Valle S, Vasavada RC, Stewart AF, Alonso LC, Garcia-Ocana A. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic beta-cell death and accelerates the onset of diabetes. Diabetes. 2011;60:525–536. doi: 10.2337/db09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, Soto N, Iniguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia. 2005;48:2609–2614. doi: 10.1007/s00125-005-0036-z. [DOI] [PubMed] [Google Scholar]
- Morrison JL. Sheep Models of Intrauterine Growth Restriction: Fetal Adaptations and Consequences. Clin Exp Pharmacol Physiol. 2008;35:730–743. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Kataoka H, Hamasuna R, Yokogami K, Uehara H, Kawano H, Goya T, Tsubouchi H, Koono M, Wakisaka S. Up-regulation of vascular endothelial growth factor induced by hepatocyte growth factor/scatter factor stimulation in human glioma cells. Biochem Biophys Res Commun. 1998;249:73–77. doi: 10.1006/bbrc.1998.9078. [DOI] [PubMed] [Google Scholar]
- Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–430. doi: 10.1055/s-2007-1004939. [DOI] [PubMed] [Google Scholar]
- Onda H, Crino PB, Zhang H, Murphey RD, Rastelli L, Gould Rothberg BE, Kwiatkowski DJ. Tsc2 null murine neuroepithelial cells are a model for human tuber giant cells, and show activation of an mTOR pathway. Mol Cell Neurosci. 2002;21:561–574. doi: 10.1006/mcne.2002.1184. [DOI] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental Transport of Leucine, Phenylalanine, Glycine, and Proline in Intrauterine Growth-Restricted Pregnancies. J Clin Endocrinol Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- Platz E, Newman R. Diagnosis of IUGR: traditional biometry. Semin Perinatol. 2008;32:140–147. doi: 10.1053/j.semperi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta. 2007;28:714–723. doi: 10.1016/j.placenta.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C, McGuinness OP, Powers AC. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62:4154–4164. doi: 10.2337/db13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. The IUGR newborn. Semin Perinatol. 2008;32:219–224. doi: 10.1053/j.semperi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol. 1996;270:E491–E503. doi: 10.1152/ajpendo.1996.270.3.E491. [DOI] [PubMed] [Google Scholar]
- Rozance PJ, Limesand SW, Barry JS, Brown LD, Hay WW., Jr Glucose replacement to euglycemia causes hypoxia, acidosis, and decreased insulin secretion in fetal sheep with intrauterine growth restriction. Pediatr Res. 2009;65:72–78. doi: 10.1203/PDR.0b013e318189358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozance PJ, Anderson M, Martinez M, Fahy A, Macko AR, Kailey J, Seedorf GJ, Abman SH, Hay WW, Jr, Limesand SW. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes. 2015;64:555–564. doi: 10.2337/db14-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush D, Stein Z, Susser M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics. 1980;65:683–97. [PubMed] [Google Scholar]
- Spyer G, Hattersley AT, Sykes JE, Sturley RH, MacLeod KM. Influence of maternal and fetal glucokinase mutations in gestational diabetes. Am J Obstet Gynecol. 2001;185:240–241. doi: 10.1067/mob.2001.113127. [DOI] [PubMed] [Google Scholar]
- Thorn SR, Brown LD, Rozance PJ, Hay WW, Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes. 2013;62:65–73. doi: 10.2337/db11-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiya T, Inoue Y, Yanase M, Arai M, Ikeda H, Tejima K, Nagashima K, Nishikawa T, Fujiwara K. Leucine stimulates the secretion of hepatocyte growth factor by hepatic stellate cells. Biochem Biophys Res Commun. 2002;297:1108–1111. doi: 10.1016/s0006-291x(02)02339-2. [DOI] [PubMed] [Google Scholar]
- Tomiya T, Nishikawa T, Inoue Y, Ohtomo N, Ikeda H, Tejima K, Watanabe N, Tanoue Y, Omata M, Fujiwara K. Leucine stimulates HGF production by hepatic stellate cells through mTOR pathway. Biochem Biophys Res Commun. 2007;358:176–180. doi: 10.1016/j.bbrc.2007.04.093. [DOI] [PubMed] [Google Scholar]
- Van BE, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84:751–753. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- von Beckerath AK, Kollmann M, Rotky-Fast C, Karpf E, Lang U, Klaritsch P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol. 2013;208:130.e1–6. doi: 10.1016/j.ajog.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Wang X, Cui Y, Tong X, Ye H, Li S. Glucose and lipid metabolism in small-for-gestational-age infants at 72 hours of age. Journal of Clinical Endocrinology and Metabolism. 2007;92:681–684. doi: 10.1210/jc.2006-1281. [DOI] [PubMed] [Google Scholar]
- Warren RS, Yuan H, Matli MR, Ferrara N, Donner DB. Induction of vascular endothelial growth factor by insulin-like growth factor 1 in colorectal carcinoma. J Biol Chem. 1996;271:29483–8. doi: 10.1074/jbc.271.46.29483. [DOI] [PubMed] [Google Scholar]
- Wojcik EJ, Sharifpoor S, Miller NA, Wright TG, Watering R, Tremblay EA, Swan K, Mueller CR, Elliott BE. A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene. 2006;25:2773–2784. doi: 10.1038/sj.onc.1209306. [DOI] [PubMed] [Google Scholar]
- Zeitlin J, Ancel PY, Saurel-Cubizolles MJ, Papiernik E. The relationship between intrauterine growth restriction and preterm delivery: an empirical approach using data from a European case-control study. BJOG. 2000;107:750–758. doi: 10.1111/j.1471-0528.2000.tb13336.x. [DOI] [PubMed] [Google Scholar]


