Abstract
Respiratory depression (RD) is a serious side effect of morphine and detrimental to effective analgesia. We reported that variants of the ATP binding cassette gene ABCC3 (facilitates hepatic morphine metabolite efflux), affect morphine metabolite clearance. In this study of 316 children undergoing tonsillectomy, we found significant association between ABCC3 variants and RD leading to prolonged postoperative care unit stay (Prolonged RD). Allele A at rs4148412 and allele G at rs729923 caused a 2.36 (95% CI=1.28–4.37, p=0.0061) and 3.7 (95% CI 1.47– 9.09, p=0.0050) times increase in odds of Prolonged RD respectively. These clinical associations were supported by increased formation clearance of morphine glucuronides in children with rs4148412 AA and rs4973665 CC genotypes in this cohort, as well as an independent spine surgical cohort of 67 adolescents. This is the first study to report association of ABCC3 variants with opioid -related RD, and morphine metabolite formation (in two independent surgical cohorts).
Keywords: morphine, ABCC3, respiratory depression, children, pharmacogenetics
INTRODUCTION
Morphine is commonly used to treat postoperative pain in children, but the provision of effective analgesia is limited by occurrence of serious side effects like respiratory depression (RD). Opioid induced RD has potentially fatal consequences and has been reported to contribute to up to 50% of postoperative respiratory failure events (1–4). Twin studies have detected significant heritability for RD (30%) from opioids (5) which indicates that genetics may play a role in determining susceptibility to RD. We recently reported that variants in certain genes in the morphine response pathway, namely, μ-opioid receptor (OPRM1), ATP-Binding Cassette ABCB1 and Fatty Acid Amide Hydroxylase (FAAH), affect morphine clinical outcomes including RD in children (6–8).
There is not much known about the effect of genes involved in morphine pharmacokinetics (PK) on morphine clinical outcomes(9) although morphine metabolism in the liver by glucuronidation to morphine 3 glucuronide (M3G) and morphine 6 glucuronide (M6G) is well described (10). The efflux of morphine glucuronides from the hepatic cell is an ATP-dependent process mediated by the ATP-Binding cassette transporters including ABCC3 (10–18). We recently demonstrated that ABCC3 variants contribute to variability in morphine, M3G and M6G pharmacokinetics (PK) (19, 20). In this study, we hypothesized that ABCC3 variants could potentially affect serious morphine clinical outcomes, namely, RD, by altering liver transport of morphine metabolites. The primary aim of the study was to identify associations between common ABCC3 genotypes and postoperative RD in children undergoing tonsillectomy, and RD causing delayed hospital discharge, as this is an economically relevant outcome associated with increased healthcare costs. We further investigated if the variants involved affected morphine’s PK in a younger Tonsillectomy cohort as well as in another independent cohort of older children and adolescents undergoing spine surgery.
Subjects and Methods
Study Design and Setting
This is a prospective, genotype blinded, clinical observational study in two independent cohorts: children undergoing outpatient adenotonsillectomy (“Tonsillectomy” cohort) and adolescents undergoing spine surgery (“Spine surgery” cohort) which are registered with clinicaltrials.gov, NCT01140724 and NCT01839461 respectively. Both studies were approved by the institutional review board and written informed consent was obtained from parents/18 year old patients and assent obtained when appropriate from children 7–17 years of age before enrollment.
Participants and Standard Anesthetic Procedures
All participants received surgery-specific standard perioperative care, including standardized surgical, anesthetic and postoperative care. In both studies, children were excluded if they or their parents were non-English speaking, allergy to morphine, had developmental delay, liver or renal diseases, or preoperative pain requiring analgesics.
Tonsillectomy cohort
Children 6 – 15 years with an American Society of Anesthesiologists (ASA) physical status 1 or 2 scheduled for tonsillectomy or adenotonsillectomy for recurrent tonsillitis, adenotonsillar hypertrophy or obstructive sleep apnea (OSA), were recruited for the study on the day of surgery. The child was considered to have OSA if he/she had a history of sleep disordered breathing with history of snoring plus respiratory pauses during sleep lasting more than 10 seconds or daytime drowsiness, or “yes” to 8 or more of the 22 questions in the Pediatric Sleep Questionnaire (PSQ)(21, 22). Anesthesia was induced using sevoflurane followed by a propofol (2 mg/kg) bolus to facilitate endotracheal intubation. Anesthesia was maintained with sevoflurane without the use of neuromuscular blockade. Patients received standard perioperative care along with one intra-operative intravenous morphine bolus dose of 0.2 mg/kg. Children with OSA received a morphine dose of 0.1 mg/kg. All children receive prophylactic ondansetron (0.1 mg/kg) and dexamethasone (0.1 mg/kg) intraoperatively. Significant postoperative pain measured with facial expression; leg movement; activity; cry; and consolability (FLACC) pain score ≥ 4/10 (25)was managed in the postoperative anesthesia care unit (PACU) with rescue doses of morphine (0.05mg/kg increments). Duration of PACU stay (time to achieve PACU discharge readiness) was defined as the duration in PACU before achieving the following discharge criteria. If a patient required more than 90 minutes to meet PACU discharge criteria following tonsillectomy, it was defined as a prolonged PACU stay.
Spine Surgery cohort
Children aged 10–18 years of age with a diagnosis of idiopathic scoliosis undergoing spine fusion were recruited. Patients received total intravenous anesthesia during the surgery with propofol and remifentanil, and morphine doses towards the end of surgery to clinically parameters (pain scores and respiratory rate). Postoperatively, they received morphine through patient controlled analgesia (PCA), managed by clinical pain service.
Clinical Outcome Measures
Metrics for opioid-related RD were recorded for each participant in the PACU for the Tonsillectomy cohort and on postoperative day 1 for the Spine Surgery cohort. In our study, we defined clinical RD as a persistent (more than a minute) oxygen desaturation <90% or respiratory rate <8 breaths per minute or oxygen desaturation <94% along with respiratory rate <10 per minute requiring supplemental oxygen to maintain SpO2 >94% in the absence of clinically obvious upper airway obstruction. Total morphine dose was total amount of morphine used (in mg/kg) intraoperatively and immediate postoperative period in PACU (Tonsillectomy cohort) and over the 1st postoperative day (Spine Surgery cohort).
Genotyping (both cohorts)
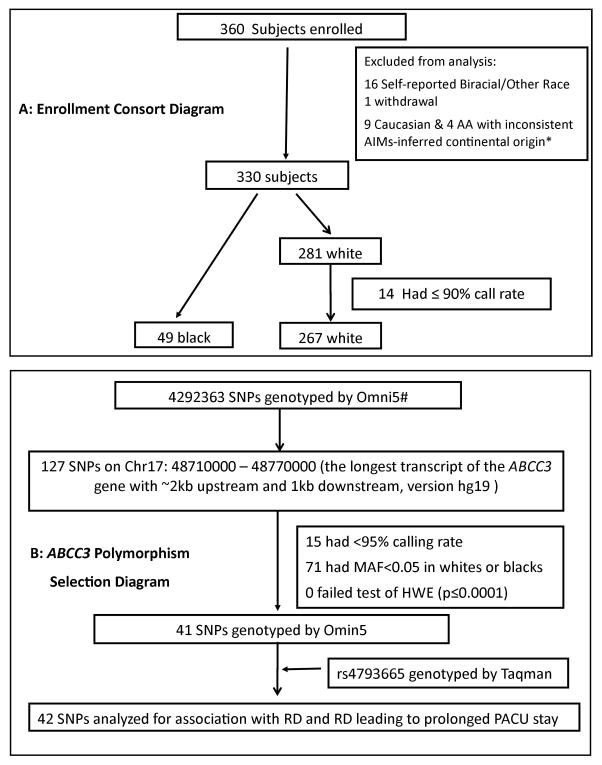
Blood was drawn in the operating room upon intravenous line placement under anesthesia for genotyping. DNA was isolated on the same day and, frozen at −20°C. One previously studied common SNP (rs4793665) was genotyped using TaqMan allelic discrimination system assays (Life Technologies, Applied Biosystems, USA). Genome-wide genotyping was performed on the Illumina Human OMNI-5 genotyping array using the iScan System (Illumina) and Infinium2 chemistry. Genotypes were called using the Gentrain2 algorithm within Illumina Genome Studio. Using PLINK, only 4 samples showed genome-wide calling rate below 95%, suggesting an overall good quality of the samples. To extract SNPs on the ABCC3 gene, we examined a region on the chromosome 17 spanning 48.71 to 48.77 Mb. This region covers the longest ABCC3 transcript, and ~2kb upstream and ~1kb downstream of ABCC3. Samples with call rates below 90% in the selected region, SNPs with call rates below 95%, minor allele frequency (MAF) lower than 0.05 in AA or Caucasians, or off HWE (p-value 0.0001) were dropped from the analyses (Figure 1B).
Figure 1. A. Enrollment consort diagram.
illustrates the flow of enrolled study participants through this clinical trial. Reasons for exclusions, enrolled and analyzed patients are reported. * Principal Component Analysis using 218 Ancestry Information Markers was done (explained in reference (7)), using Hapmap population as the reference group and principal components PC2 vs. PC1 were plotted (not shown here). The selection of subjects that did not cluster well– 9 Caucasian and 4 African American (AA) were thus excluded. B: ABCC3 Polymorphism Selection Diagram illustrates the selection of polymorphisms based on genome wide and Taqman genotyping for inclusion in genetic association analysis. SNP=Single nucleotide polymorphism. #Illumina Human OMNI-5 genotyping array using the iScan System (Illumina) and Infinium2 chemistry. RD: Respiratory depression; PACU: Postoperative Anesthesia Care Unit; SNPs: Single Nucleotide Polymorphisms
Functional consequences of SNPs above were assessed using RegulomeDB (23)(http://regulome.stanford.edu/index), with scores ranging from 1 (highly likely functional, through 2a/b (likely to affect binding of regulatory factors to DNA), to 5/6 (unlikely regulating binding).
Analysis of Genetic association with RD/Prolonged RD in the Tonsillectomy cohort
Prior to analyses, quality of the data was checked. Characteristics of the patients and properties of the SNPs were examined in African American and Caucasian children respectively. Logistic regressions were performed to analyze binary outcomes RD and Prolonged RD. Prior to evaluation of ABCC3 variants, the effects of total morphine, age, sex, BMI z scores and OSA were tested using the Statistical Analysis Software (SAS), version 9.3. To select the best fitting model, log likelihood, Akaike and Bayesian Information criterion were compared. Co-variables that significantly improved model fitting (p<0.05) were retained for subsequent genetic analyses. Using PLINK, each of the 42 SNPs was then tested in an additive model, in which the genotypes were recoded to 0, 1 and 2 according to the number of minor alleles and tested as continuous variables. To account for the population structures, we performed principal component analysis (PCA) on 218 ancestry informative markers (AIMs) as we have done with previous analyses (7, 8). A marked drop in the percentage of variation explained by PCs was observed after PC1 and 2. Therefore, the first two PCs, as well as self-reported race, were included in all the logistic models. In this study, we tested the genetic associations of 42 SNPs with two clinical outcomes.
Pharmacokinetic Sampling
Serial blood samples were obtained to quantify morphine, M3G and M6G systemic concentration. A pre-dose sample was obtained before IV morphine bolus dose from an IV line. Further samples were obtained using independent venous needle sticks 0–5 min, 10–15 min and 30–45 min after the first bolus morphine intravenous dose. For spine cohort, additional samples were drawn at 60–120 minutes, and 1–3 samples over the first 24 hours if the child had respiratory depression (as defined above).
Pharmacokinetic Analysis
Morphine and its active metabolites, M3G and M6G, were quantified in EDTA plasma using a validated liquid chromatography tandem mass spectrometry assay. Details of the analytical methods have been described elsewhere (24). The reliable limits of quantification were 0.25–1000 ng/ml (r2 > 0.99) for morphine, and 1–1000 ng/ml (r2 > 0.99) for both M3G and M6G. Total imprecision was less than 15%. The inter-day accuracy was within 85–115%.
Morphine Pharmacokinetic Model Development and Evaluation
A population pharmacokinetic model was developed for morphine using nonlinear, mixed effects modeling approach (NONMEM; version 7.2, ICON Dev. Soln., MD, USA) with PsN-Toolkit (version 3.5.3) as the interface. Data pre-processing, post-processing and visualization were performed using the statistical package R (version 2.15). A two compartment structural model, parameterized in terms of clearance (CL), central volume of distribution (V1), inter-compartmental clearance (Q), peripheral volume (V2) of distribution, was used to describe the morphine concentration–time profiles. A delay compartment was incorporated in the model to describe the delay metabolite formation. The metabolite pharmacokinetics was modeled using an additional compartment for each metabolite and was parameterized in terms of formation clearance (FCLM3G & FCLM6G), volume of distribution (VM3G & VM6G) and clearance (CLM3G & CLM6G). For further details about the PK model development for both cohorts, please see Supplementary Information section.
Pharmacokinetic-Pharmacogenetic Analysis
Potential pharmacogenetic covariate relationships were initially examined with plots of post hoc variability (η) in morphine and metabolite clearance variations with genotypes. Genetic covariate analysis was performed on selected ABCC3 SNPs found to have significant association with clinical outcomes to investigate if similar associations were observed in morphine pharmacokinetics. Genetic covariate analysis was performed using NONMEM by incorporating the genotype as categorical covariates. The significance of a genotype as a covariate in morphine PK was determined a decrease in the OFV of 3.84 (p<0.05, degree of freedom =1) between nested models.
Power analysis
To test our power to detect differences by genetic variant, we used Quanto to vary the minor allele frequency (MAF) from 0.1 to 0.5. After adjusting for multiple testing with Bonferroni (0.05/42 = 0.0012), a two-sided alpha of 0.0012 will be needed to detect significant differences. Assuming that variables are normally distributed (with a mean of 0 and a standard deviation of 1.0), we have 80% power to detect a variant that explains as little as 3% of the phenotypic variance (effect sizes 0.24 to 0.41 standard deviation units across a range of MAF).
RESULTS
Demographics and clinical characteristics
A consort diagram illustrates enrolled study subjects in the Tonsillectomy cohort (Figure 1A). Participants were primarily self-identified as Caucasian. Compared to Caucasian children, African American (AA) children were slightly heavier and had significantly higher obstructive sleep apnea (OSA) frequencies (Table 1). Although AA children required higher total morphine dose, the incidences of RD and Prolonged RD (RD leading to prolonged Post anesthesia Care Unit (PACU) stay) were comparable between AA and Caucasian children (Table 1). The spine cohort included 67 non-obese children (70% females/79% Caucasian) aged 14.6 ± 1.9 years, weighing 59.2 ± 16.6 kg.
Table 1.
Characteristics of participants in tonsillectomy cohort
Age, weight, BMI z score and intra-operative morphine requirement are shown as median and inter-quartile range (IQR), and compared between whites and blacks using Wilcoxon rank sum tests; sex, Obstructive Sleep Apnea (OSA), PACU: Postoperative Care Unit; Respiratory depression and prolonged PACU stay due to respiratory depression are shown as frequencies and proportions, and compared using Pearson’s Chi-squared tests.
BMI: Body Mass Index; BMI z scores were calculated using Center for Disease Control (CDC) growth charts.
| Whites (N=267) | Blacks (N=49) | p value | |
|---|---|---|---|
| Age (year) | 8.4 (7.2, 11.1) | 8.8 (7.1, 105) | 0.80 |
| Weight (Kg) | 33.6 (25.4, 45.3) | 34.0 (25.0, 53.8) | 0.32 |
| BMI z scores | 0.62 (−0.23, 1.59) | 0.89 (−0.01, 2.04) | 0.09 |
| Intra-operative morphine requirement (mg/kg) | 0.20 (0.17, 0.22) | 0.19 (0.15, 0.20) | 0.41 |
| Total morphine requirement (mg/kg) | 0.24 (0.20, 0.29) | 0.28 (0.20, 0.34) | 0.011 |
| Sex | 0.32 | ||
| Male | 135 (51%) | 21 (43%) | |
| OSA | 0.002 | ||
| Yes | 111 (42%) | 32 (65%) | |
| Respiratory Depression | 0.38 | ||
| Yes | 75 (28%) | 17 (35%) | |
| Prolonged PACU stay due to Respiratory Depression | 0.79 | ||
| Yes | 24 (9%) | 5 (10%) |
ABCC3 Single Nucleotide Polymorphisms (SNPs) description
A total of 127 Single Nucleotide Polymorphisms (SNPs) in the ABCC3 gene were genotyped by Illumina Human Omni 5 array techniques and one SNP by Taqman assay. Of the 128 SNPs, 42 had minor allele frequency (MAF) of 5% or more in both AA and Caucasian children (Figure 1B). Since these polymorphisms were in Hardy Weinberg equilibrium (HWE) at alpha=0.0001 level, all 42 SNPs were included in genetic association analyses.
Non-genetic covariates associated with clinical outcomes
Before testing the genetic effect, we evaluated the effects of age, sex, total morphine requirement, obstructive sleep apnea (OSA), and body mass index (BMI) z score on RD and Prolonged RD. Significant effects of total morphine dose and BMI z score were detected (p<0.05). No significant OSA effect was detected. Therefore, in subsequent genetic models in which single polymorphism associations were tested, total morphine and BMI z score were included as co-variates. As all analyses were performed with AA and Caucasian children combined, in order to reduce the risk of spurious association due to racial differences, we included race as a co-variate in all statistical modeling.
Genetic association with clinical outcomes
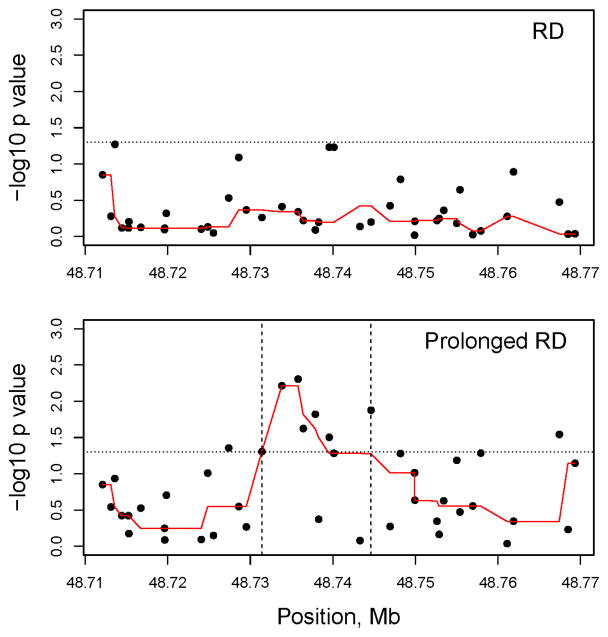
Although there was no statistically significant association between tested ABCC3 polymorphisms and RD, a clinically severe form of RD leading to prolonged stay in the PACU (Prolonged RD) had significant associations with 7 polymorphisms of ABCC3 localized to a region between 48731392 to 48744612 bp on Chromosome 17 (Table 2) (Figure 2). Two adjacent polymorphisms, rs739923 and rs4148412, showed the most significance (Table 2). For rs739923, one copy of the G allele increased odds of Prolonged RD 3.7 times (95% CI 1.47–9.09, p=0.005); for rs4148412, one copy of the A allele increased the odds 2.4 times (95%CI 1.28–4.37, p=0.0061) (Table 2). Compared to mean duration to achieve PACU discharge readiness (duration of PACU stay) for the entire Tonsillectomy cohort (n=316) of 87.1±36.7 minutes, children with high risk genotypes for Prolonged RD stayed in PACU about 50 minutes longer (p<0.05). The duration to achieve PACU discharge readiness in children with AA genotype of rs4148412 was 138.3 ± 46.8 minutes and with GG genotype of rs739923 was 154 ± 54.9 minutes.
Table 2.
Association of prolonged postoperative care unit stay due to morphine induced respiratory depression with polymorphisms in an identified critical region of ABCC3 gene
| Illumina ID | rs# | Location | Risk allele (%) | Protective allele (%) | P value association | OR (95% CI) | Putative function/ RegulomeDB score | |
|---|---|---|---|---|---|---|---|---|
| Prolonged PACU Stay due to RD | kgp9079579 | rs35364174 | 48731392 | G (0.497) | A (0.503) | 0.0496 | 1.80 (1.00, 3.24) | Intron/5 |
| rs4148412 | 48733815 | A (0.382) | G (0.618) | 0.0061 | 2.36 (1.28, 4.37) | Intron/5 | ||
| rs739923 | 48735774 | G (0.750) | A (0.250) | 0.0050 | 3.70 (1.47, 9.09) | Intron/4 | ||
| rs733392 | 48736403 | G (0.756) | A (0.244) | 0.0239 | 2.63 (1.14, 5.88) | Intron/5 | ||
| rs1978153 | 48737861 | C (0.627) | G (0.373) | 0.0152 | 2.27 (1.18, 4.35) | Intron/2b | ||
| kgp388163 | rs2301837 | 48738266 | G (0.905) | A (0.095) | 0.4262 | 1.56 (0.84, 4.55) | intron | |
| kgp3814620 | rs7216383 | 48739543 | A (0.709) | G (0.291) | 0.0316 | 2.22 (1.08, 4.76) | Intron/4 | |
| kgp2507665 | rs61479331 | 48740116 | A (0.729) | C (0.271) | 0.0524 | 2.08 (0.99, 4.35) | Intron/3a | |
| rs16949202 | 48743275 | A (0.867) | G (0.133) | 0.8366 | 1.10 (0.46, 2.63) | intron | ||
| kgp12280761 | rs886493 | 48744612 | C (0.535) | A (0.465) | 0.0134 | 2.27 (1.18, 4.35) | Intron/5 |
Note: Effects were shown as odds ratio (OR) and 95% CI for prolonged postoperative care unit (PACU) stay due to respiratory depression (RD) in the tonsillectomy cohort. OR indicated the odds ratio when minor allele (races combined) increased by one copy. Putative functional consequences of SNPs above have been also assessed using RegulomeDB (http://regulome.stanford.edu/index), with scores ranging from 1 (highly likely functional, through 2a/b (likely to affect binding of regulatory factors to DNA), to 5/6 (unlikely regulating binding).
Figure 2. Genetic association of 42 ABCC3 polymorphisms and clinical outcomes: Respiratory Depression (RD) – top panel and Prolonged Postoperative Care Unit (PACU) stay due to RD/Prolonged RD (lower panel).
The y axis shows the −log10 P values and the x axis shows the chromosomal positions of the ABCC3 polymorphisms (SNPs) on Chromosome 17. The −log10 (p values) of the single SNP association tested in additive models are plotted. The reference line (small dots) shows the −log10 (p value of 0.05) level. In both races consistently several ABCC3 SNPs between the vertical lines (region between 48731392 to 48744612 bp) showed significant association with prolonged PACU stay due to respiratory depression. The p-values were smoothed using a running median represented by the red line in both plots.
ABCC3 Single Nucleotide Polymorphisms and Morphine Pharmacokinetics
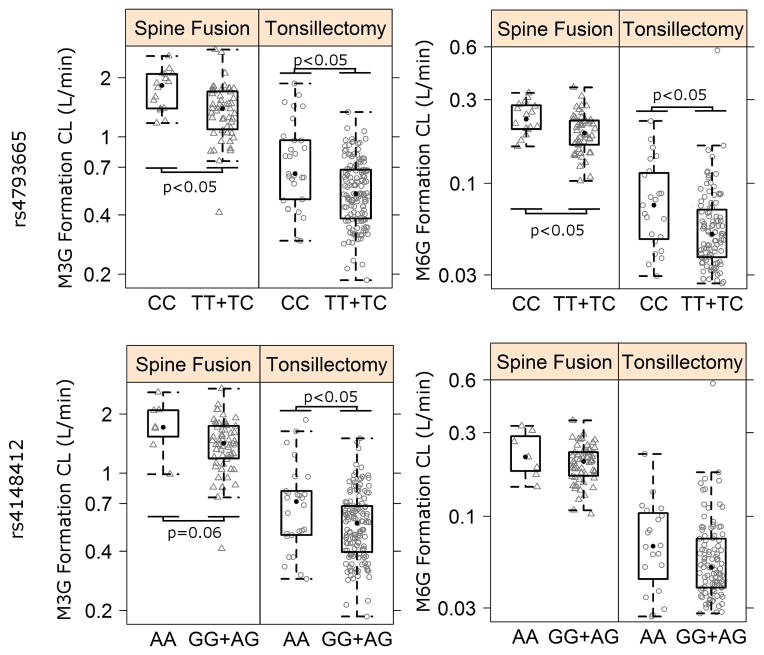
Polymorphisms identified in a particular region of the ABCC3 gene (Chr17: 48731392 to 48744612) for association with Prolonged RD, and another earlier polymorphism that we reported to have association with morphine PK, rs4793665 (19), were tested for association with morphine and metabolite formation clearances in this Tonsillectomy cohort as well as in another independent Spine Surgery cohort. ABCC3 rs4793665 CC genotype tonsillectomy and spine surgery subjects appeared to have higher mean formation clearances of M3G and M6G (FCLM3G and FCLM6G) than CT and TT genotypes combined based on variation of post-hoc individual estimates across genotypes (Figure 3). In both cohorts, rs4793665 genotypes were found to be significant covariate for M6G formation (FCLM6G; Change in Objective Function Value (dOFV) = −7.73 and −8.10 for Tonsillectomy cohort and Spine Surgery cohort respectively, Table 3) with CC genotypes having 51 % (Tonsillectomy cohort; 95% CI [4.2; 97%]) and 37% (Spine surgery cohort; 95% CI [11%; 63%]) higher formation respectively than others (Table 4). Similarly, rs4793665 CC genotypes was a significant covariate for M3G formation (FCLM3G; (dOFV) = −16.46 and −9.1 for tonsillectomy and spine surgery cohorts respectively, Table 3) with CC genotypes having 54% (95% CI (21%;100%)) in the Spine Surgery cohort and 55% 95% CI (18%;92%)) in the Tonsillectomy cohort (Table 4).
Figure 3.
Box & Whiskers plots of variation in M3G/M6G formation CL normalized to 70 kg subject with ABCC3 rs4793665 and rs4148412 genotypes in the Tonsillectomy and Spine Surgery cohorts. Statistical significant differences among genotypes are marked with p<0.05.
Table 3.
Pharmacokinetic/Pharmacogenetic association of ABCC3 SNP genotypes with morphine clearance and metabolite formation clearances in tonsillectomy and spine cohorts.
| SNP | Tests | N | Morphine* | Morphine-3-Glucuronide Formation* | Morphine-6-Glucuronide Formation* | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Illumina ID | rs# | T&A | Spine | T&A | T&A | Spine | T&A | Spine | |
|
| |||||||||
| kgp9079579 | rs35364174 | GG vs. AG+AA | 216 | 67 | −4.09 | −3.713 | −0.08 | −0.954 | −0.02 |
| rs4148412 | AA vs. AG+GG | 216 | 66 | −0.686 | −5.833 | −3.53 | −2.111 | −1.54 | |
| rs739923 | AA vs. AG+GG | 219 | 66 | −0.353 | −2.123 | −0.35 | −0.921 | −0.011 | |
| rs733392 | AA vs. AG+GG | 217 | 62 | −2.102 | −0.82 | −0.24 | −1.558 | −0.211 | |
| rs1978153 | GG vs. GC+CC | 219 | 67 | −0.817 | −0.531 | −0.06 | −0.325 | −0.81 | |
| kgp388163 | rs2301837 | AA vs. AG+GG | 216 | 67 | −1.185 | −0.095 | −0.001 | −0.008 | −0.79 |
| kgp3814620 | rs7216383 | GG vs. AG+AA | 219 | 67 | −1.19 | −2.303 | −0.004 | −1.263 | −0.301 |
| kgp2507665 | rs61479331 | CC vs. AC+AA | 219 | 67 | −1.901 | −0.801 | −0.006 | −1.263 | −0.188 |
| rs16949202 | GG vs. AG+AA | 216 | 67 | −0.109 | −0.956 | −0.07 | −0.01 | −3.278 | |
| kgp12280761 | rs886493 | AA vs. AC+CC | 218 | N/A | −0.22 | −0.011 | N/A | −0.311 | N/A |
| rs4793665 | CC vs. CT+TT | 220 | 67 | −1.727 | −16.464 | −9.102 | −7.726 | −8.096 | |
ΔOFV = OFVCov - OFVNoCov
ΔOFV < −3.84 was considered to be statistically significant and are highlighted in bold.
N = number of patients in the cohort for which the genetic data was available
Vs. = versus
T&A: Tonsillectomy Cohort; Spine: Spine Surgery Cohort
Table 4.
Key ABCC3 genotype effects on morphine clearance and metabolite formation clearances in both cohorts
| ABCC3 Polymorphism Genotypes | Study | Parameter | Morphine | Morphine-3-Glucuronide Formation | Morphine-6-Glucuronide Formation |
|---|---|---|---|---|---|
| rs4793665 CC vs CT+TT |
T&A | ΔCL# | 0.066 [−0.022;0.153] | 0.55 [0.18;0.92] | 0.51 [0.042;0.974] |
|
| |||||
| ΔOFV! | −1.73 | −16.46 | −7.73 | ||
|
| |||||
| Spine | ΔCL# | - | 0.55 | 0.37 | |
| - | [0.21;1.0] | [0.11; 0.63] | |||
|
| |||||
| ΔOFV! | - | −9.1 | −8.09 | ||
|
| |||||
| rs4148412 AA vs. GG+AG |
T&A | ΔCL# | −0.04 [−0.142;0.058] | 0.31 [−0.65;0.02] | 0.255 [−0.16;0.67] |
|
| |||||
| ΔOFV! | −0.686 | −5.833 | −2.11 | ||
|
| |||||
| Spine | ΔCL# | - | 0.45 | 0.215 | |
| - | [−0.04;0.95] | [−0.185;0.615] | |||
|
| |||||
| ΔOFV! | - | −3.53 | −1.54 | ||
ΔOFV = OFVCov - OFVNoCov; OFV=Objective Function Value
mean [95%CI]
ΔOFV < −3.84 was considered to be statistically significant (p<0.05) and are highlighted in bold.
Subjects with ABCC3 rs4148412 AA genotype had higher mean FCLM3G and FCLM6G than subjects with AG and GG genotypes combined (Figure 5). In the tonsillectomy study, rs4148412 AA genotypes were found to be significant covariate for FCLM3G (dOFV= −5.83, Table 4) with AA genotypes having 31 % (95% CI (−2%; 65%)) higher M3G formation. Similar trend (dOFV= −3.53, p =0.06) was observed in the spine study with rs4148412 AA genotypes having higher 45% (95% CI (−4%; 95%)) higher M3G formation. ABCC3 rs4148412 AA genotype subjects tended to have 25% (Tonsillectomy) and 22% (Spine surgery) higher M6G formation than others, though the association was not significant.
Though rs35364174 GG genotypes had higher (8%) morphine CL than others, there was no significant association with metabolite formations. Across both studies, no other significant genetic covariates for M3G and M6G formation (Table 3).
Putative functional consequences of SNPs
Using RegulomeDB, two of the SNPs are predicted to likely (rs1978153) or somewhat likely (rs61479331) affect binding of regulatory factors to DNA. The SNP rs4793665 was also found to have a RegulomeDB score of 2b, which makes it likely to affect binding.
DISCUSSION
In this study of 316 children undergoing tonsillectomy, we found significant associations between common polymorphisms in the ABCC3 gene and RD leading to prolonged PACU stay. Presence of allele A at rs4148412 was associated with a 2.4 fold increase in odds ratio (95% CI =1.28–4.37, p=0.0061), and allele G at rs729923 increased the odds ratio for Prolonged RD by 3.7 fold (95% CI 1.47– 9.09, p=0.0050). Correspondingly, increased formation clearances of M3G and M6G were noted in rs4148412 AA and genotypes rs4973665 CC genotypes (p<0.05) in this larger tonsillectomy cohort; a similar association was reproduced in the Spine Surgery cohort (p<0.05). In our Tonsillectomy cohort, children with genetic risk for Prolonged RD (AA genotype of ABCC3 rs4148412 and GG genotype of rs739923) stayed about 50 minutes longer in PACU compared to the entire cohort’s mean PACU stay, which implies increased PACU costs of ≈ US $392 in our hospital. This is the first time a clinically and economically relevant outcome: opioid related postoperative respiratory depression resulting in prolonged hospital stay, is associated with ABCC3 variants and simultaneously supported by variant effects on morphine metabolite formation in two independent surgical cohorts, highlighting a novel pathway with validating biological evidence.
The ABCC3 gene (also known as Multidrug Resistance Protein 3/MRP3) codes for a sinusoidal transporter with high expression in liver cells (25, 26). ABCC3 transporters are known to transport organic compounds conjugated to glucuronate (27–29). Evidence for ABCC3 mediated transport of morphine glucuronides comes from vesicular uptake experiments; insect cells overexpressing MRP3 were found to transport M3G and M6G (18) and Mrp3-null mice were unable to excrete M3G from the liver into the bloodstream resulting in 50-fold reduction in plasma M3G levels. The fact that morphine conjugation in hepatocytes is followed by transport of the metabolites across their sinusoidal membrane, where ABCC3 is located(30), prompted us to study the role of this gene in morphine outcomes and morphine-glucuronide formation clearance. To our knowledge, there are no studies evaluating ABCC3 variants and morphine effect in humans. In mice, the absence of Mrp3 was shown to decrease anti-nociceptive potency of injected M6G in MRP3 (−/−) mice and affect M6G PK (18). In humans, the antagonizing effects of M3G on morphine-induced analgesia however were noted to be weak.
In this study, we have identified a region in the ABCC3 gene located in chromosome 17 (4871392 and 48744612, spanning 13,221 bp), associated with RD leading to prolonged PACU stay. Seven of 10 SNPs located in this region are associated with this outcome in tonsillectomy patients (p<0.05). All these polymorphisms are intronic and hence are not known to change the structure of the protein. It is possible that less common exon variants are present in the region that we did not test for associations. However, upon review of the Exome Variant Server database, we did not identify any common (MAF > 5%) protein changing variants with a predicted functional effect in ABCC3. As we have mentioned in the results section and Table 2, RegulomeDB scores for rs1978153 and rs61479331 in this region and rs4793665, indicate they may have a high likelihood of affecting binding of regulatory factors to DNA. Another possible explanation arises from a limited body of knowledge regarding variations at noncoding regulatory sequences known to contribute to variable expression of genes (31, 32). It is known that the genome comprises a large number of noncoding DNA regulatory elements, including silencers, insulators, and enhancer regions, that play important regulatory roles in maintaining gene expression programs (33, 34). Enhancers have emerged as key cis-regulatory elements that can affect gene transcription independent of their orientation or distance. On examining the region closely, we find that it is close to the posttranslational modification of histone H3 (H3K27AC –acetylation of lysine 27) which is associated with both active promoters and enhancers (35, 36). Since variants that affect chromatin at distal regulatory sites frequently also direct changes in chromatin and gene expression at associated promoters (31), it is postulated that the variants at this critical region of ABCC3 affect gene expression in some yet unknown way (https://genome.ucsc.edu/cgi-bin/hgTracks?db=hg19&position=chr17%3A48731392-48744612&hgsid=422527087_473p6AuZ9wOAcJCUlay6N3C1LB4y) .
In fact, the majority of distal H3K27ac enrichment was found within introns and the activity of proximal genes was found to correlate positively with distal H3K27ac enrichment in adult liver tissues (37), providing additional support to our results and hypothesis about the mechanism.
Morphine and metabolite PK from our two independent study cohorts were well described using similar structural PK models which include a central and peripheral compartment for morphine, one distribution compartment for each metabolite and a compartment to account for the delay in the metabolite formation. Model parameters for the morphine PK model were found to be mostly similar in both tonsillectomy and Spine Surgery cohorts (Table S1). The ratio of the formation clearance of M3G relative to M6G morphine clearance estimates was found to be 7.2 in the spine surgery population and 9.6 in the tonsillectomy population. This is consistent with other reports indicating that M3G metabolite formation is 7–10 fold higher than M6G formation (38, 39). The small differences in the morphine models from the two studies could be attributed to inter-study variances arising due to differences in (a) population demographics, (b) co-medication and (c) pharmacokinetic sampling strategy.
Earlier, we showed that children with ABCC3 rs4793665 CC genotype (−211 C/T) had 46% higher M6G formation and 41% higher M3G formation indicating an increased efflux of metabolites into the blood than C/T and T/T genotypes combined(19). These previous findings were reproduced in the spine surgical cohort, where CC genotypes had 37% more M6G and 55% more M3G formation clearance. This ABCC3 SNP −211T (rs4793665) is located in the promoter region of the gene and has been reported to alter hepatic mRNA expression (40) and contribute to lower efflux of morphine glucuronides (41). Although other studies have not shown a change in mRNA expression (42), our study shows that it is associated with transformation clearance for M6G. In the current analysis we also found that ABCC3 rs4148412 AA genotype had higher M3G formation – this allele was also found to increase Prolonged RD risk by an OR of 2.36 (95% CI=1.28–4.37; p=0.0061). Prior PK studies indicate substantially higher plasma concentrations of the two metabolites (especially M3G) than those of morphine (M3G/morphine: 34; M6G/morphine: 3.9)(43) and this may be why we were unable to detect effects of this polymorphism on M6G formation. Though the genotype was observed to clearly alter the metabolite formation, no significant impact on morphine clearance was observed, which supports the fact that ABCC3 is not a transporter of morphine, and hence variants would not affect morphine clearance.
One of the limitations of the outpatient tonsillectomy population is the shorter duration of sampling for morphine and metabolite concentrations, as patients were discharged home within 1 to 2 hours after surgery. The sampling period was extended in the Spine Surgery cohort as the patients remain in the hospital after surgery. Another limitation was that we tested association for 42 polymorphisms of ABCC3 with a relatively small sample size of 316 children, so there is a possibility that some of the associated variants may have been associated by chance. However, for rs4148412, we found association with both the clinical outcome of prolonged PACU stay due to RD and with PK measures, and validation of similar PK association in an independent Spine Surgery cohort. The identification of association using 2 independent outcomes provides validation of our findings. Relatively higher doses of morphine might have contributed to overall higher incidence of RD; however children with certain ABCC3 genotypes had significantly higher M6G levels and prolonged RD with morphine. Lastly, we did not report associations with clinical outcomes with ABCC3 for the smaller spine cohort as the current sample size is not statistically robust to make meaningful conclusions. However, these are ongoing studies and will allow us to validate genetic and clinical outcomes associations in the future.
In conclusion, our pediatric tonsillectomy study demonstrates that ABCC3 variants are associated with postoperative RD leading to prolonged hospital stay. This ABCC3 genetic association with clinical outcome was supported by a consistent pharmacokinetic association in the Tonsillectomy cohort, which is further validated in an independent Spine Surgery cohort. Prolonged RD besides being a clinically significant problem, has significant potential to increase the cost of care by prolonging postoperative hospital stays. While highlighting potential economic burden, our study also offer possible solutions with preemptive identification of high risk patients and personalized analgesic selection. If children who are genetically predisposed to relatively higher risks of morphine related respiratory depression leading to prolonged hospital stay could be preemptively identified especially preoperatively, clinicians instead of intravenous morphine could potentially use alternative analgesics such as intravenous fentanyl and hydromorphone that are not transported through ABCC3 transporters. Thus, these findings are novel, clinically important and add to the knowledge of genetic factors that contribute to life-threatening central adverse effects of morphine, moving us a step closer towards safer and more efficient opioid analgesia in vulnerable patients.
Supplementary Material
Acknowledgments
This work was supported in part by USPHS Grant #UL1 RR026314 from the National Center for Research Resources and Electronic Medical Records and Genomics (eMERGE) network U01 grant #U01HG006828, NIH and with the Place Outcomes Research Award (PI: SS) and Translational Research Award (PIs: SS), Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA. The study in the spine surgical cohort was supported was supported by Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under award number K23HD082782 (PI: VC), and the Clinical Research Feasibility Funds (PI: VC) via Grant 8 UL1 TR000077 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. It was also supported by the APSF / ASA Safety Scientist Career Development Award by the Anesthesia Patient Safety Foundation (PI: Chidambaran). Additional research funding support was provided by the Department of Anesthesia, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA. No financial support except departmental salary support for the authors. This pharmacogenetic study was designed and undertaken by the authors.
Footnotes
Conflict of Interest/Disclosure
All authors listed in this manuscript have no conflicts of interest relevant to this article to disclose.
“Supplementary information is available at The Pharmacogenomics Journal’s website
Supplementary Information: This section contains text describing further the morphine and metabolite pharmacokinetic model development and evaluation and one supplementary table S1.
Author Contributions
The authors directed and had access to all the analyses and the full clinical and genetic database, wrote all drafts of the report, decided to publish the results, and attest for the accuracy and completeness of the data. Specifically, SS, VC and TF conceived of and designed the research. SS, VC, RV, JN, and TM acquired the data. SS, VC, RV, AAV, XZ and LJM analyzed and interpreted the data. XZ and JM did localization of SNPs in ABCC3 transporter and proposed mechanistic pathways. XZ and LJM did statistical analyses. VC, RV and SS drafted the initial manuscript. SS participated in funding and supervision. All authors made critical revisions to the report for important intellectual content.
References
- 1.Lotsch J, Dudziak R, Freynhagen R, Marschner J, Geisslinger G. Fatal respiratory depression after multiple intravenous morphine injections. Clinical pharmacokinetics. 2006;45(11):1051–60. doi: 10.2165/00003088-200645110-00001. [DOI] [PubMed] [Google Scholar]
- 2.Nelson KL, Yaster M, Kost-Byerly S, Monitto CL. A national survey of American Pediatric Anesthesiologists: patient-controlled analgesia and other intravenous opioid therapies in pediatric acute pain management. Anesthesia and analgesia. 2010;110(3):754–60. doi: 10.1213/ANE.0b013e3181ca749c. [DOI] [PubMed] [Google Scholar]
- 3.Fecho K, Lunney AT, Boysen PG, Rock P, Norfleet EA. Postoperative mortality after inpatient surgery: Incidence and risk factors. Therapeutics and clinical risk management. 2008;4(4):681–8. doi: 10.2147/tcrm.s2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fecho K, Jackson F, Smith F, Overdyk FJ. In-hospital resuscitation: opioids and other factors influencing survival. Therapeutics and clinical risk management. 2009;5:961–8. doi: 10.2147/tcrm.s8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angst MS, Lazzeroni LC, Phillips NG, Drover DR, Tingle M, Ray A, et al. Aversive and reinforcing opioid effects: a pharmacogenomic twin study. Anesthesiology. 2012;117(1):22–37. doi: 10.1097/ALN.0b013e31825a2a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chidambaran V, Mavi J, Esslinger H, Pilipenko V, Martin LJ, Zhang K, et al. Association of OPRM1 A118G variant with risk of morphine-induced respiratory depression following spine fusion in adolescents. The pharmacogenomics journal. 2014 doi: 10.1038/tpj.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadhasivam S, Chidambaran V, Zhang X, Meller J, Esslinger H, Zhang K, et al. Opioid-induced respiratory depression: ABCB1 transporter pharmacogenetics. The pharmacogenomics journal. 2014 doi: 10.1038/tpj.2014.56. [DOI] [PubMed] [Google Scholar]
- 8.Sadhasivam S, Zhang X, Chidambaran V, Mavi J, Pilipenko V, Mersha TB, et al. Novel associations between FAAH genetic variants and postoperative central opioid-related adverse effects. The pharmacogenomics journal. 2015 doi: 10.1038/tpj.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klepstad P, Dale O, Kaasa S, Zahlsen K, Aamo T, Fayers P, et al. Influences on serum concentrations of morphine, M6G and M3G during routine clinical drug monitoring: a prospective survey in 300 adult cancer patients. Acta anaesthesiologica Scandinavica. 2003;47(6):725–31. doi: 10.1034/j.1399-6576.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 10.Thorn CF, Klein TE, Altman RB. Codeine and morphine pathway. Pharmacogenetics and genomics. 2009;19(7):556–8. doi: 10.1097/FPC.0b013e32832e0eac. [DOI] [PubMed] [Google Scholar]
- 11.Grisk O, Schlueter T, Steinbach A, Ciecholewski S, Kloeting I, Voelker U, et al. Effects of generalized and kidney specific Mrp2 (ABCC2) deficiency on renal elimination of PAH and morphine-6-glucuronide. The FASEB Journal. 2007;21:758.11. [Google Scholar]
- 12.Huwyler J, Drewe J, Klusemann C, Fricker G. Evidence for P-glycoprotein-modulated penetration of morphine-6-glucuronide into brain capillary endothelium. British journal of pharmacology. 1996;118(8):1879–85. doi: 10.1111/j.1476-5381.1996.tb15619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of P-glycoprotein in the intestinal absorption and clinical effects of morphine. Clinical pharmacology and therapeutics. 2003;74(6):543–54. doi: 10.1016/j.clpt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Lotsch J, Schmidt R, Vetter G, Schmidt H, Niederberger E, Geisslinger G, et al. Increased CNS uptake and enhanced antinociception of morphine-6-glucuronide in rats after inhibition of P-glycoprotein. Journal of neurochemistry. 2002;83(2):241–8. doi: 10.1046/j.1471-4159.2002.01177.x. [DOI] [PubMed] [Google Scholar]
- 15.Tzvetkov MV, dos Santos Pereira JN, Meineke I, Saadatmand AR, Stingl JC, Brockmöller J. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in< i> OCT1</i> gene affect morphine pharmacokinetics after codeine administration. Biochemical pharmacology. 2013;86(5):666–78. doi: 10.1016/j.bcp.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 16.van de Wetering K, Zelcer N, Kuil A, Feddema W, Hillebrand M, Vlaming ML, et al. Multidrug resistance proteins 2 and 3 provide alternative routes for hepatic excretion of morphine-glucuronides. Molecular pharmacology. 2007;72(2):387–94. doi: 10.1124/mol.107.035592. [DOI] [PubMed] [Google Scholar]
- 17.Xie R, Hammarlund-Udenaes M, de Boer AG, de Lange EC. The role of P-glycoprotein in blood-brain barrier transport of morphine: transcortical microdialysis studies in mdr1a (−/−) and mdr1a (+/+) mice. British journal of pharmacology. 1999;128(3):563–8. doi: 10.1038/sj.bjp.0702804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelcer N, van de Wetering K, Hillebrand M, Sarton E, Kuil A, Wielinga PR, et al. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(20):7274–9. doi: 10.1073/pnas.0502530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkatasubramanian R, Fukuda T, Niu J, Mizuno T, Chidambaran V, Vinks AA, et al. ABCC3 and OCT1 genotypes influence pharmacokinetics of morphine in children. Pharmacogenomics. 2014;15(10):1297–309. doi: 10.2217/pgs.14.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda T, Chidambaran V, Mizuno T, Venkatasubramanian R, Ngamprasertwong P, Olbrecht V, et al. OCT1 genetic variants influence the pharmacokinetics of morphine in children. Pharmacogenomics. 2013;14(10):1141–51. doi: 10.2217/pgs.13.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep medicine. 2000;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 22.Chervin RD, Weatherly RA, Garetz SL, Ruzicka DL, Giordani BJ, Hodges EK, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Archives of otolaryngology--head & neck surgery. 2007;133(3):216–22. doi: 10.1001/archotol.133.3.216. [DOI] [PubMed] [Google Scholar]
- 23.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome research. 2012;22(9):1790–7. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clavijo CF, Hoffman KL, Thomas JJ, Carvalho B, Chu LF, Drover DR, et al. A sensitive assay for the quantification of morphine and its active metabolites in human plasma and dried blood spots using high-performance liquid chromatography-tandem mass spectrometry. Analytical and bioanalytical chemistry. 2011;400(3):715–28. doi: 10.1007/s00216-011-4775-z. [DOI] [PubMed] [Google Scholar]
- 25.Uchiumi T, Hinoshita E, Haga S, Nakamura T, Tanaka T, Toh S, et al. Isolation of a novel human canalicular multispecific organic anion transporter, cMOAT2/MRP3, and its expression in cisplatin-resistant cancer cells with decreased ATP-dependent drug transport. Biochemical and biophysical research communications. 1998;252(1):103–10. doi: 10.1006/bbrc.1998.9546. [DOI] [PubMed] [Google Scholar]
- 26.Konig J, Rost D, Cui Y, Keppler D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology. 1999;29(4):1156–63. doi: 10.1002/hep.510290404. [DOI] [PubMed] [Google Scholar]
- 27.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annual review of biochemistry. 2002;71:537–92. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 28.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22(47):7537–52. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 29.Zelcer N, van de Wetering K, de Waart R, Scheffer GL, Marschall HU, Wielinga PR, et al. Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. Journal of hepatology. 2006;44(4):768–75. doi: 10.1016/j.jhep.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Milne RW, Jensen RH, Larsen C, Evans AM, Nation RL. Comparison of the disposition of hepatically-generated morphine-3-glucuronide and morphine-6-glucuronide in isolated perfused liver from the guinea pig. Pharmaceutical research. 1997;14(8):1014–8. doi: 10.1023/a:1012145126847. [DOI] [PubMed] [Google Scholar]
- 31.McVicker G, van de Geijn B, Degner JF, Cain CE, Banovich NE, Raj A, et al. Identification of genetic variants that affect histone modifications in human cells. Science. 2013;342(6159):747–9. doi: 10.1126/science.1242429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaub MA, Boyle AP, Kundaje A, Batzoglou S, Snyder M. Linking disease associations with regulatory information in the human genome. Genome research. 2012;22(9):1748–59. doi: 10.1101/gr.136127.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heintzman ND, Ren B. Finding distal regulatory elements in the human genome. Current opinion in genetics & development. 2009;19(6):541–9. doi: 10.1016/j.gde.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visel A, Rubin EM, Pennacchio LA. Genomic views of distant-acting enhancers. Nature. 2009;461(7261):199–205. doi: 10.1038/nature08451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459(7243):108–12. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–37. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21931–6. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christrup LL. Morphine metabolites. Acta anaesthesiologica Scandinavica. 1997;41(1 Pt 2):116–22. doi: 10.1111/j.1399-6576.1997.tb04625.x. [DOI] [PubMed] [Google Scholar]
- 39.Hasselstrom J, Sawe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clinical pharmacokinetics. 1993;24(4):344–54. doi: 10.2165/00003088-199324040-00007. [DOI] [PubMed] [Google Scholar]
- 40.Lang T, Hitzl M, Burk O, Mornhinweg E, Keil A, Kerb R, et al. Genetic polymorphisms in the multidrug resistance-associated protein 3 (ABCC3, MRP3) gene and relationship to its mRNA and protein expression in human liver. Pharmacogenetics. 2004;14(3):155–64. doi: 10.1097/00008571-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Gradhand U, Tegude H, Burk O, Eichelbaum M, Fromm MF, Konig J. Functional analysis of the polymorphism -211C>T in the regulatory region of the human ABCC3 gene. Life sciences. 2007;80(16):1490–4. doi: 10.1016/j.lfs.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Doerfel C, Rump A, Sauerbrey A, Gruhn B, Zintl F, Steinbach D. In acute leukemia, the polymorphism -211C>T in the promoter region of the multidrug resistance-associated protein 3 (MRP3) does not determine the expression level of the gene. Pharmacogenetics and genomics. 2006;16(2):149–50. doi: 10.1097/01.fpc.0000189802.34339.a4. [DOI] [PubMed] [Google Scholar]
- 43.Sawe J, Svensson JO, Rane A. Morphine metabolism in cancer patients on increasing oral doses--no evidence for autoinduction or dose-dependence. British journal of clinical pharmacology. 1983;16(1):85–93. doi: 10.1111/j.1365-2125.1983.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.