Abstract
Magnetoencephalography (MEG) is a noninvasive, silent, and totally-passive neurophysiological imaging method with excellent temporal resolution (∼1 ms) and good spatial precision (∼3-5 mm). In a typical experiment, MEG data are acquired while healthy controls and/or patients with neurologic or psychiatric disorders perform a specific cognitive task and/or receive sensory stimulation. The resulting data are generally analyzed using standard electrophysiological methods, coupled with advanced image reconstruction algorithms. To date, the total number of MEG instruments and associated users is significantly smaller than comparable human neuroimaging techniques, although this is likely to change in the near future with advances in the technology. Despite this small base, MEG research has made a significant impact on several areas of translational neuroscience, largely through its unique capacity to quantify the oscillatory dynamics of activated brain circuits in humans. This review focuses on the clinical areas where MEG imaging has arguably had the greatest impact, in regard to the identification of aberrant neural dynamics at the regional and/or network-level, monitoring of disease progression, determining how efficacious pharmacological and behavioral interventions modulate neural systems, and the development of neural markers of disease. Specifically, this review covers recent advances in understanding the abnormal neural oscillatory dynamics that underlie Parkinson's disease, autism spectrum disorders, HIV-associated neurocognitive disorders, cerebral palsy, attention-deficit/hyperactivity disorder, cognitive aging, and posttraumatic stress disorder. MEG imaging has had a major impact on how clinical neuroscientists understand the brain basis of these disorders, and its translational influence is rapidly expanding with new discoveries and applications emerging continuously.
Keywords: MEG, oscillation, ADHD, autism, HAND, HIV, PTSD, Parkinson's, cerebral palsy, aging, motor, gamma, beta, alpha, connectivity
Introduction
Magnetoencephalography (MEG) is a neurophysiological recording method that is primarily utilized in human studies of systems-level brain function. Although the first MEG measurements occurred more than 40 years ago,1 the instrumentation and analytical sophistication of the field have dramatically increased over the past decade.2-5 At present, MEG is the only noninvasive high-resolution imaging technique that does not rely on vascular responses (i.e., it measures the neurophysiology directly) and the only functional brain imaging method to offer both high spatial (2-5 mm) and temporal resolution (< 1 ms; see Figure 1).5 Another unique advantage of MEG over other noninvasive methods is that it does not require a reference electrode, which greatly simplifies the interpretation and facilitates network modeling and connectivity analyses.
Figure 1. Typical Magnetoencephalography (MEG) System.
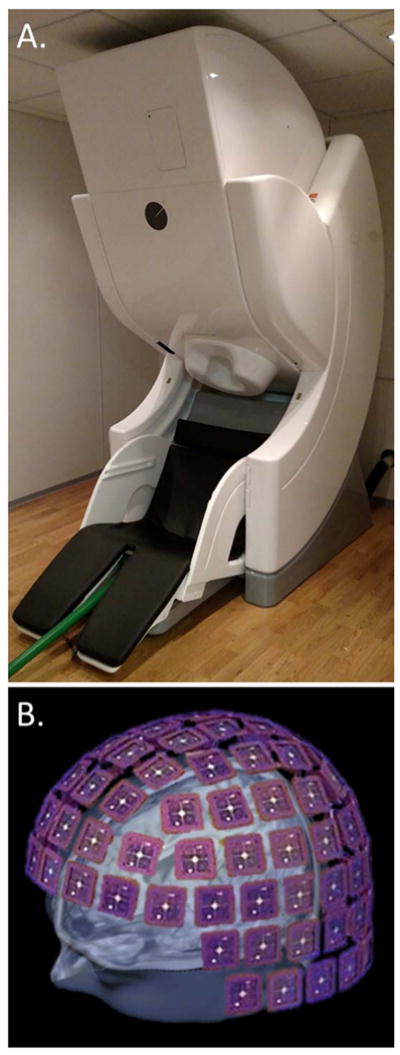
(A) A modern whole-head MEG system equipped with 306 cortical sensors. MEG instruments are housed in magnetically-shielded rooms to reduce interference from the environment's ambient magnetic fields. (B) Encased within the MEG helmet is a large array of magnetic sensors that are immersed in liquid helium. This helium endows the sensor array, and associated elements, with superconducting properties which are needed to measure the ultra-minute magnetic fields, but also creates a significant maintenance cost as the Helium has to be replenished periodically. In (B), the array of the 306-sensor instrument shown in (A) is depicted. Each square represents a sensor chip (102 total) and is comprised of 3 independent MEG sensors, with each sensor having a different sensitivity to components of the magnetic fields.
The physical signal quantified in MEG is comprised of the summated magnetic fields produced by ionic currents within active neural regions. Just as electric current passing through power lines produces magnetic fields, ionic currents within brain tissue also produces magnetic fields according to the same physical principles (e.g., “the right-hand rule” of magnetism).6 However, these neuromagnetic fields are ultra-minute (10-15 T) relative to other magnetic fields that are typically encountered (e.g., the earth's static field is about 10-5 T), thus MEG recordings are normally carried out in magnetically-shielded rooms using special sensors and/or noise-cancellation software.2-6 As per the physiological basis of these ionic currents, MEG is primarily sensitive to dendritic currents in populations of pyramidal neurons in the neocortex.6-7 However, other cortical neurons also contribute to the signal, as do neural cells in subcortical and cerebellar regions to a lesser extent.7 The orientation, depth (i.e., distance from the sensors), and inherent synchronicity of the neural population also affects the strength of MEG signals, although modern high-density MEG systems are generally sensitive to neural responses across the brain.7-8 Importantly, in MEG, signal measurement occurs at the sensor-level and typical systems are equipped with a large number of sensors (e.g., 300+) arranged in a helmet-like array (see Figure 1). Consequently, it is necessary to transform the sensor/channel measurements into source space (i.e., anatomical coordinates), and this is generally accomplished using head models and optimization algorithms such as minimum-norm estimation, beamforming methods, and/or related source estimation techniques.2-6 This process is often termed MEG source reconstruction.
This review focuses on emerging applications of MEG imaging in translational neuroscience. Some of the most exciting and rapidly-growing research areas for MEG will be covered, including the identification and characterization of neural mechanisms underlying autism, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and HIV-associated neurocognitive disorders. Several MEG groups are also using the technique's dynamic mapping capabilities to examine working memory processing in aging, aberrant brain circuits in Parkinson's disease, and the dynamics of motor control in healthy participants and those with cerebral palsy. We will review these areas and the most rapidly evolving application, which is likely the use of MEG to characterize therapeutic effects of experimental and FDA-approved pharmacotherapies and behavioral interventions. To limit redundancy, we will review these “pharmaco-MEG” studies in the section focusing on the target disease (e.g., the effect of stimulants in ADHD). Of note, this review is not limited to studies that were published within a specific time range, although the number of MEG studies per year has increased substantially over the past 15 years and this trend is reflected in this review and Figure 2. In addition, we will not cover the accepted clinical applications of MEG, which in the United States currently includes functional mapping of eloquent cortices (e.g., language-related regions) in neurosurgical candidates, and the localization of epileptic foci in patients with intractable/refractory epilepsy. There are many good review papers on the role of MEG in epilepsy and functional mapping prior to tumor resection, and we encourage the interested reader to peruse those publications.9-13
Figure 2. Number of MEG Publications per Year.
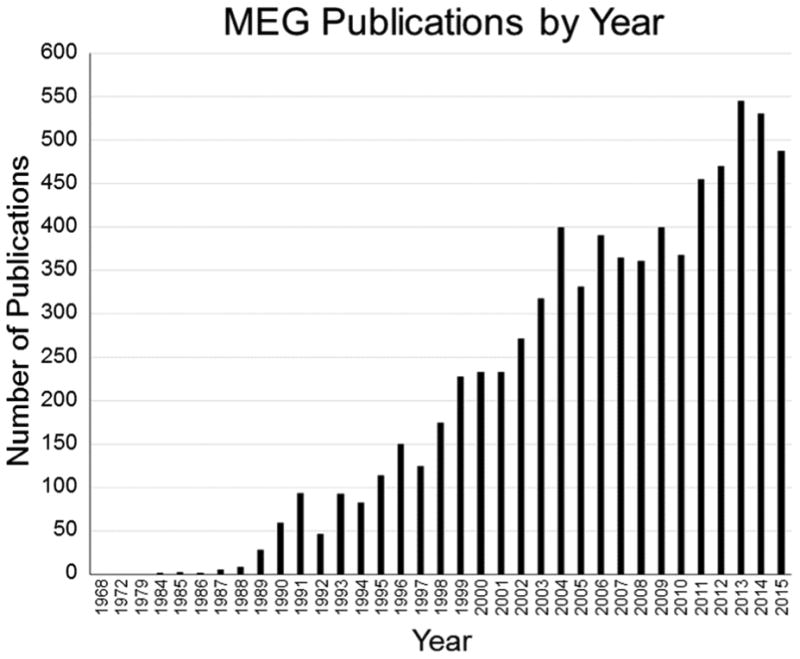
Since David Cohen published the first MEG article in 1968, the number of MEG publications per year has steadily increased, rising sharply over the past 15 to 20 years. This figure is based on a PubMed search conducted in early 2016 using the term “magnetoencephalography.”
Before beginning, a few words about the interpretation and nature of MEG results are probably in order. First, with the exception of resting-state recordings, MEG experiments almost always use event-related designs and are laid out very similar to invasive electrophysiology studies in monkeys and rats. Basically, there is a baseline period with no stimulation, followed by the presentation of a stimulus at time 0.0 seconds (s), which leads to an “active” period where the brain's response to the stimulus occurs. This series of events constitutes one trial, and in the typical MEG experiment there are 80-100 trials per condition, per participant.2 The MEG signals are recorded continuously throughout the experiment and these trials are aggregated (offline) in each participant to increase the signal-to-noise ratio of the brain response. In interpreting the signals, it is the relative response (active compared with baseline) that is of paramount interest, because neural cells spontaneously fire and cortical regions are constantly undergoing electrical fluctuations that are seemingly unrelated to ongoing processing. Second, over the past decade, there has been an increasing focus on population-level neural oscillatory activity.14 In oscillatory analyses, neural activity in discrete frequency bands, including delta (1-4 Hz), theta (4-7 Hz), alpha (8-14 Hz), beta (14-26 Hz), gamma (30-50 Hz), and high gamma (> 50 Hz) are examined separately to quantify rhythmic changes in the underlying neural population during cognitive processing.14 Note that most MEG studies focus on one or two specific frequency bands, based on their hypotheses, and that the precise definition of a band (e.g., alpha) is often determined statistically for a given study; for example, the definition of alpha may vary from 8-12 Hz to 8-14 Hz in different studies. Examining such frequency bands separately enhances the signal-to-noise ratio of each, and enables identification of high-frequency neural activity that is often of lower amplitude. These high-frequency responses (i.e., gamma) are widely thought to be critical for interregional communication across active brain networks,15 and thus characterizing them is of major importance for understanding overall brain function. For clarity, oscillatory analyses rely on largely the same experimental design, except that trials are often longer, with the relative response again being of primary importance (i.e., active period compared with pre-stimulus baseline). As will become apparent, the application of oscillatory analysis methods to MEG signals and subsequent source reconstruction is a powerful method for identifying the dynamics of brain function (see Figure 3),16 and has allowed major discoveries in several distinct areas of clinical cognitive neuroscience.
Figure 3. MEG Oscillations and Dynamics.
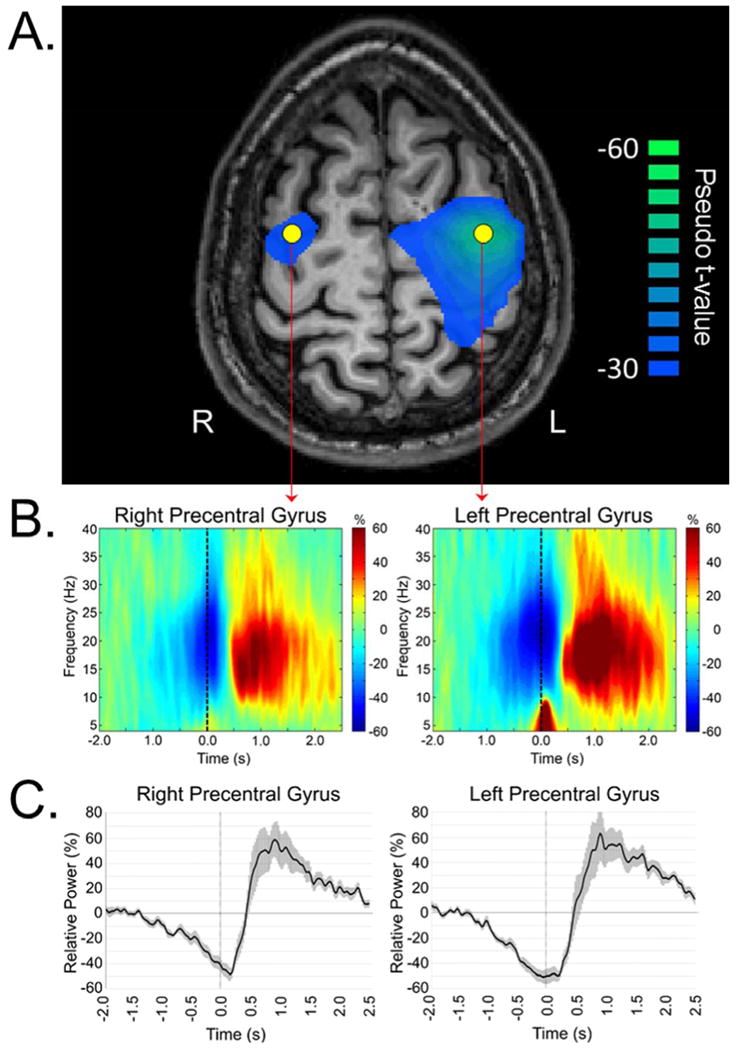
(A) Example output from a MEG source reconstruction, using a beamforming algorithm, of beta oscillatory activity during the planning stage of a right-hand movement (-0.45 s to 0.0 s; 0.0 s = movement onset) in healthy individuals. To more precisely examine the dynamics, “virtual sensors” can be extracted from any voxel in the brain and these data have the same temporal precision as the original recording (e.g., 1 ms). In this example, virtual sensors were extracted from the peak voxel in each hemisphere, which corresponded to the bilateral primary motor cortices (yellow dots). (B) Time-frequency spectrograms of oscillatory power (relative to baseline) for the voxels extracted from left and right primary motor cortices. For each spectrogram, frequency (in Hz) is shown on the y-axis, while time (in s) is shown on the x-axis. Color bars reflect positive (red) and negative (blue) values relative to the baseline period, which was -2.0 to -1.2 s before motor onset (movement onset = 0.0 s). Negative activity represents an event-related desynchronization (ERD), whereas the positive activity constitutes an event-related synchronization (ERS). (C) Time course of beta activity (16-26 Hz) for each peak voxel. For each voxel time series, relative power (in percentage from baseline) is shown on the y-axis, while time (in s) is shown on the x-axis. Error bars (shaded) reflect standard error of the mean (SEM).
Finally, a brief overview of MEG terminology and metrics is probably necessary to help facilitate understanding of the key aspects of studies discussed below, as some terms in the field are rather counterintuitive. Critically, the most common metric is amplitude or power of the neural signal, and this can expressed for a particular voxel (i.e., cube of tissue) in the brain, a whole brain region, or at the sensor-level. Pseudo-t is another common MEG unit; in these maps, each pseudo-t value reflects the noise-normalized differential power between an active period and a baseline period for a specific voxel. Other common units include the phase of the signal, based on a sensor-level or voxel time series, as well as an array of functional connectivity measures that are computed using the voxel time series data (typically). Such connectivity measures are thought to reflect inter-regional interactions, and common metrics include coherence, phase-locking factor, phase-lag index, and many other indices. Lastly, for oscillatory analyses, two other common terms are event-related desynchronization (ERD) and event-related synchronization (ERS). An ERD refers to a decrease in band-limited power or amplitude relative to a baseline, whereas as an ERS refers to an increase in band-limited power or amplitude. ERD and ERS responses are typically expressed in percentage units, reflecting the percent increase or decrease relative to the baseline, and can be computed at the sensor or voxel level.
Autism Spectrum Disorders (ASD)
Autism spectrum disorders (ASD) is one of the most active areas of MEG psychiatric and neurological research, and findings to date have provided significant insight into the underlying pathophysiology. Given the volume of literature, we will focus on studies of auditory processing and the resting-state, as there are several recent reviews that focus more broadly on the electrophysiology of autism.17-19
Many MEG studies have shown that participants with ASD have significant auditory processing abnormalities. These studies can be largely divided into two separate categories, consisting of timing deficits in the main auditory response and high-frequency gamma (30-50 Hz) aberrations. Starting with the gamma findings, Wilson et al. were first to report changes in the gamma-band in response to auditory stimuli in autism.20 They observed reduced steady-state gamma responses in children and adolescents with ASD compared with typically-developing controls, and this effect was more pronounced in the left auditory cortices.20 Reduced auditory gamma has also been shown in adults with ASD, and reduced phase-locking in the gamma range has been reported for both children and adults with ASD in MEG studies.21-23 Interestingly, both the early auditory gamma response and the steady-state response are impaired in unaffected first-degree relatives of persons with ASD, suggesting that these findings might be useful as endophenotypes.21,24 While the findings of gamma abnormalities have emerged more recently, latency differences in auditory processing have been appreciated for some time. In the standard paradigm, auditory tones (e.g., 1 kHz) are presented using an oddball paradigm to encourage attention to the stimuli, and in the analysis the focus is on the 100 ms latency response (the M100). Multiple MEG studies have shown delayed peak latencies in participants with ASD compared with age matched controls,22,25-26 and a recent study connected this delayed latency in ASD to gamma abnormalities during the pre-stimulus baseline.23 Thus, these two categories of deficits may be on the eve of convergence. Finally, one recent study showed that an effective behavioral intervention for adolescents with ASD modulates gamma abnormalities, which supports a direct connection between these neural responses and the observed symptomatology.27
In regard to resting-state activity, an early MEG study of children with ASD showed elevated delta, theta, alpha, and high-frequency activity compared with typically-developing children in many regions of the brain, but that only alpha frequency responses in the parietal and anterior temporal regions correlated with symptom severity.28 More recent studies have suggested that fewer regions are involved in resting-state ASD aberrations, and that interregional connectivity differences may be critical.29-32 For example, Edgar and colleagues investigated a large group of ASD and typically-developing children and found that alpha activity was stronger in the sensorimotor and parietal association cortices of the ASD group, and that alpha power in these regions correlated with scores on a social responsiveness scale.30 Group differences were not observed in other areas, although the well-known correlation between peak occipital alpha frequency and age was notably missing in children with ASD.30 Connectivity studies using MEG have found hyper-connectivity among frontal, temporal, and subcortical regions in the beta and gamma frequencies of children with ASD, including an orbitofrontal, subcortical, and temporal network implicated in social cognition.31 The same study also found that parietal and occipital regions were hypo-connected to most brain regions in the theta and alpha ranges in the ASD compared to typically-developing group, and that children with ASD had altered network topology at both global and local levels.31 Finally, another recent MEG study showed that the direction of abnormality (i.e., hyper- or hypo-connectivity) in ASD was strongly dependent on the precise frequency band and whether the network included a region in the frontal lobes, with some networks showing opposite effects (hyper/hypo) depending on the precise frequency range being examined.32 The findings of Kitzbichler and colleagues32 were of major importance, as they provided critical insight to the seemingly contradictory findings of hyper- and hypo-connectivity in ASD, which extend beyond the MEG literature and involve numerous functional MRI (fMRI) and electroencephalography (EEG) studies.
Despite these breakthroughs, the neuronal mechanisms that underlie ASD are not fully understood, as much remains to be discovered. One critical question is how the well-established deficits in auditory processing are related to the resting-state findings and the symptomatology of ASD. While sensory processing abnormalities are an accepted component of ASD, the overall symptom profile is much broader and includes a key social component. Thus, identifying the basic links amongst these neural alterations and symptoms will be critical for future MEG studies and help move the field towards defining a MEG-based neural marker of ASD.
Attention-Deficit/Hyperactivity Disorder (ADHD)
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurobehavioral disorder in children and adolescents and, for about 65% of affected children, at least some impairing symptoms will persist into adulthood.33-34 While there are only a handful of MEG studies that have investigated ADHD, almost all have taken a pharmaco-MEG approach and evaluated the same patients before and after stimulant-based medications, which are by far the most common treatment for children and adults with ADHD.34-35 MEG is uniquely suited for such investigations, as caffeine and other stimulants are robust vasoconstrictors that are known to modulate the fMRI signal through vascular effects alone (i.e., caffeine can alter the fMRI signal through vascular changes that are independent of neuronal function),36-39 whereas MEG measures the neural signal directly and thus is immune to vascular biases.2-4
One early MEG study of ADHD focused on somatosensory processing and found that adults with ADHD had reduced alpha and beta oscillatory responses in the somatosensory cortices following electrical stimulation of the median nerve relative to matched controls.40 Such findings suggest that the basic sensory interface is altered in ADHD, and this group proposed that such deficits likely influence higher-order processing.40 Another study used a classic oddball paradigm and found evidence of impaired engagement of the ventral visual pathway in adults with ADHD.41 They argued that the stimulus-driven reorienting system was defective in ADHD. Several other ADHD studies have focused uniquely on high-frequency gamma activity due to its proposed role in network-level communication and connectivity.15,42-45 One used a long duration (i.e., minutes) time estimation task and found that un-medicated adults with ADHD were impaired at judging time, and had significantly reduced gamma activity relative to healthy controls across a network of brain regions that included prefrontal cortices.46 Participants in this study were also scanned in the medicated state, and the results indicated significantly improved performance in the time estimation task, but persistent deficits in gamma activity in most brain regions.46 A related study of sensory processing investigated gamma activity in the primary auditory cortices, and reported reduced auditory gamma in the un-medicated state, along with significantly increased gamma following medication.47 Interestingly, medicated patients did not statistically differ from controls in this study, which may suggest that stimulant medications act to normalize connectivity via modulating local gamma activity. Related findings have emerged from MEG studies of attention in patients with ADHD. For example, one study of auditory attention found that stimulants modulate gamma activity in frontal and parietal areas,48 whereas another found hyper-connectivity between prefrontal and auditory cortices in un-medicated adults with ADHD, which was significantly decreased following stimulant administration.49 One other study focused on alpha differences during visual attention and found that decreased alpha lateralization correlated with impaired performance in patients with ADHD.50
Finally, there have also been several MEG studies of resting-state activity in ADHD. One focused on the default-mode network and found that un-medicated adults with ADHD had reduced neural activity compared to demographically-matched controls in the medial prefrontal cortices, but not other nodes in the network.51 This study also found that administering stimulant medications significantly increased 8-14 Hz alpha activity in the medial prefrontal area, and decreased inattention symptoms.51 A later pharmaco-MEG study of the default-mode network found an array of connectivity differences between un-medicated patients with ADHD and controls that varied by frequency, including hypo-connectivity between medial prefrontal and posterior cingulate cortices, and hyper-connectivity between posterior regions of the default mode network (see Figure 4).52 This study also found that, following stimulant administration, all connectivity differences in the default mode network dissipated and there were no significant connectivity differences between the two groups.52 This finding was particularly important as it suggests that ADHD medications may normalize connectivity in active brain networks, and thus reconciles data from other studies that focused on the local amplitude of neural activity and found decreased symptoms but residual brain abnormalities following medication delivery. While future studies are certainly needed, the current data supports abnormal functional connectivity in ADHD and that stimulant medications may normalize connectivity and suppress symptoms by modulating local high-frequency gamma activity.
Figure 4. Functional Connectivity in the Default-Mode Network of Adults with and without ADHD.
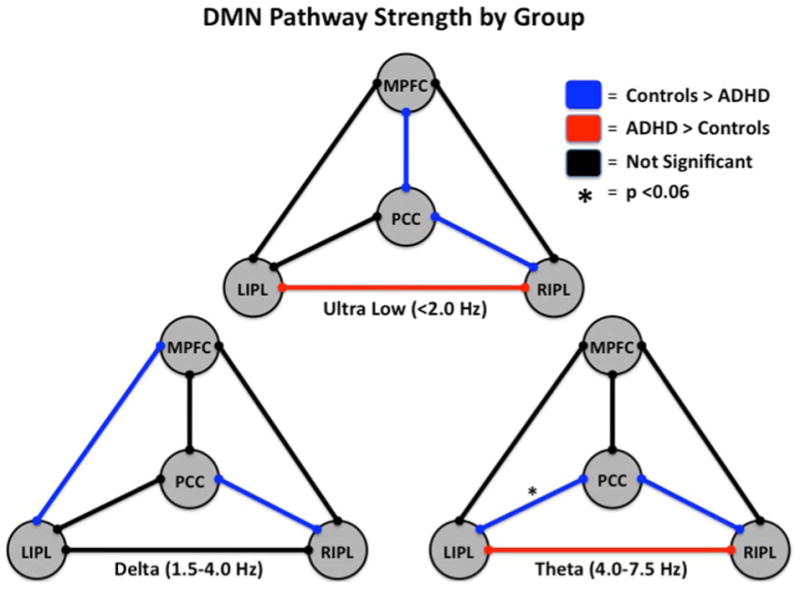
Three models of the default-mode network (DMN) showing group differences and similarities in functional connectivity along particular pathways at distinct frequencies of neuronal activity are shown. See Figure Legend (upper right) for definition of pathway colors. In the ultra-low frequency range, adults without ADHD showed stronger coupling between the medial prefrontal cortices (MPFC) and posterior cingulate cortices (PCC) and the PCC and right inferior parietal (RIPL) areas, whereas those with ADHD showed stronger connectivity between the RIPL and left inferior parietal (LIPL) nodes. In the delta band, controls showed increased connectivity between the MPFC and LIPL regions and the PCC and RIPL regions relative to un-medicated patients with ADHD. Theta frequency neuronal activity was more strongly coupled between the PCC and the RIPL and LIPL areas in adults without ADHD relative to their un-medicated peers with ADHD, while the latter group showed stronger coupling between the RIPL and LIPL cortical areas. Interestingly, the increased functional connectivity in the PCC-RIPL pathway of adults without ADHD was consistent throughout all three frequency ranges of neuronal coupling. Following medication administration, all differences in connectivity between adults with and without ADHD dissipated and were no longer significant, indicating that stimulants increased connectivity along some pathways and decreased it along others.52
Although the collection of MEG studies in this area have provided critical insight, one major area a ADHD symptomatology has yet to be systematically examined. Essentially, response variability is a hallmark feature of ADHD and the unique characteristics of MEG imaging make it an ideal modality for identifying the neural mechanisms. One particularly attractive hypothesis is that response variability is related to current gamma levels in active cortical regions, and that the abnormally reduced gamma in ADHD cannot reliably sustain interregional communication during active cognitive processing. This hypothesis could be tested with MEG and future studies should consider this, and alternative mechanisms, in investigations that identify the precise neural dynamics that underlie response variability in ADHD.
Post-Traumatic Stress Disorder (PTSD)
Posttraumatic stress disorder (PTSD) is a complex diagnosis involving re-experiencing, avoidance, negative mood and cognition, and arousal symptoms.53 The lifetime prevalence of PTSD in the United States is roughly 7-9%,54-55 but prevalence rates among recent combat veterans returning from wars in Iraq and Afghanistan has been estimated at 13% to 22%.56-57 Greater combat exposure has been generally associated with greater PTSD symptomatology.56-57
While MEG studies of PTSD are historically rare, there has been a significant wave of studies over the past few years as interest and funding in this area has sharply increased. Probably the most common findings involve an advanced analysis method that looks at the overall pattern of synchronous neural interactions, with the long-term goal of identifying a MEG biomarker for PTSD.58-62 These studies have shown that the right parieto-occipital and temporal cortices miscommunicate with other brain regions in PTSD.58-62 Moreover, a recent study using this method in over 200 participants showed that global neural interactions were significantly down-modulated with increasing lifetime trauma scores in resilient veterans, but not those with PTSD, and that this effect was strongest in small networks in the right superior temporal gyrus.62 Studies using more conventional methods have also found aberrant right hemispheric activity in PTSD, including increased slow wave activity in right frontal cortices during rest63 and increased right prefrontal activity during the processing of aversive relative to positive and neutral images.64 Enhanced processing of trauma stimuli (i.e., combat-related words) in veterans with PTSD compared with non-PTSD veterans was reported in a later study, although the main neural difference was in visual cortices.65 However, a recent study demonstrated aberrant processing of non-threatening touch (i.e., an air-puff delivered to a finger) in veterans with PTSD compared to those without, with significant differences in primary somatosensory areas and the right prefrontal cortices.66 This finding suggests aberrant sensory responses may be a more general phenomenon that is not tied directly to the valence of the stimuli, and again implicates right hemispheric cortices. Of note, this group also found that the magnitude of neural activity in prefrontal and somatosensory cortices was correlated with symptom severity, indicating that those with the most abnormal response had the most severe symptoms.66 A new study of verbal working memory from our laboratory reached a similar conclusion, as veterans with PTSD exhibited normal responses during both encoding and maintenance operations in left hemispheric language regions, but disturbed responses in right inferior frontal, right supramarginal and superior temporal cortices throughout the majority of encoding and maintenance periods (see Figure 5).67 As with other studies, these right hemispheric responses correlated with PTSD symptom severity, and we propose that such activity in homologue areas reflects compensatory processing in veterans with PTSD.67 In a follow-up study, we showed that attention training treatment sharply reduced PTSD symptom severity and largely normalized neural activity in these same brain regions during verbal working memory processing.68
Figure 5. Dynamics of Working Memory Dysfunction in Posttraumatic Stress Disorder (PTSD).
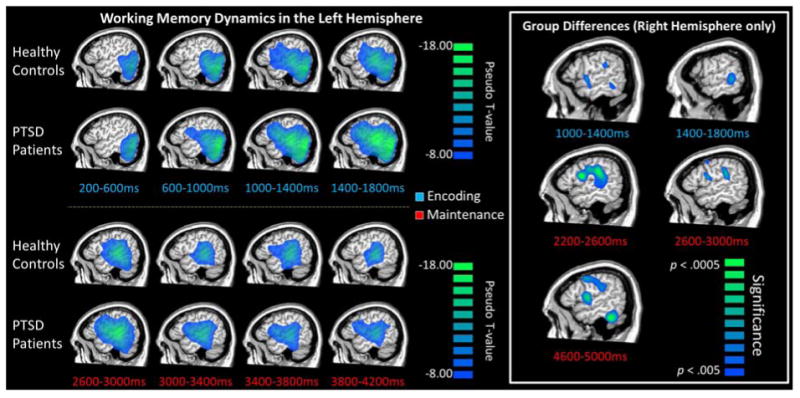
Group mean functional images (beamformer, pseudo-t scale) for controls and veterans with PTSD are displayed in the sagittal orientation for select time windows of the encoding (top) and maintenance (bottom) periods. Note that pseudo-t values reflect the differential power per voxel between an active period (shown below image) and a pre-stimulus baseline period. (Left) Both groups exhibited strong alpha-beta oscillatory responses in the left hemispheric fronto-temporal cortices throughout the encoding phase, which narrowed to include only the alpha band during maintenance. Interestingly, there were no group differences in these left fronto-temporal circuits in any time period. (Right) In contrast, veterans with PTSD exhibited significantly stronger oscillatory responses in multiple right hemispheric brain regions during the encoding and maintenance periods relative to demographically-matched controls. The critical regions included the right inferior frontal gyrus (homologue of Broca's area), right supramarginal gyrus, and right temporal cortices (p < 0.005, corrected). Note that right hemispheric group differences were observed in other time periods (not shown) as well.67
Finally, several studies have examined functional connectivity in veterans with PTSD, and these studies have found abnormal long range hyper-connectivity in the high gamma band of patients with PTSD during rest,69 as well as theta hyper-connectivity in the right parietal cortices of veterans with PTSD during a mental flexibility task.70 In both cases, the degree of aberrant connectivity was correlated with cognitive outcome variables and symptom scales.69-70 Interestingly, a recent study from the same group showed that veterans with PTSD have increased inter-network synchronization in the gamma and high gamma range relative to those without PTSD, and that symptom severity was correlated with gamma activity in the salience network.71
PTSD is definitely one of the fastest growing research areas for MEG, and this is likely to continue as the method is ideal for studies of brain dynamics. MEG studies to date have identified altered dynamics across widespread cortical regions and networks in patients with PTSD (mostly veterans), although findings of right hemispheric abnormalities involving frontal, prefrontal, and parietal cortices are by far the most common. Some of these findings appear to be very consistent and may hold promise as MEG-based biomarkers, which would be a significant breakthrough as distinguishing PTSD from other disorders is not trivial. Studies that connect MEG markers and clinical symptom scales are particularly needed as the field moves in this direction.
HIV-Associated Neurocognitive Disorders (HAND)
The advent of combination antiretroviral therapy shifted the nature of HIV-infection from a terminal illness to a chronic manageable condition, with a life expectancy that has gradually approached that of seronegative persons.72-73 However, HIV-infected patients remain at a significantly elevated risk of developing HIV-associated neurocognitive disorders (HAND), with 35-70% of all patients exhibiting at least subtle impairments on tests of neuropsychological function.74-81 Neuropsychological studies of HAND have documented aberrations across a broad range of functional domains (e.g., attention, fine motor), but the basic pathophysiology remains unresolved. Furthermore, despite its high prevalence, there are no diagnostic tests or biomarkers that can precisely detect HAND, and diagnoses are currently made by exclusion of other factors.
MEG studies in this area have tended to focus on identifying the effects of HIV-infection on brain function and the neural processes that underlie HAND. While only a few MEG studies have been conducted to date, their findings have provided significant insight. For example, an MEG study of motor control demonstrated that HIV-infected patients had reduced beta responses (14-28 Hz) prior to movement onset in the supplementary motor area and bilateral primary motor cortices (stronger on the side contralateral to movement) relative to demographically-matched controls.82 HIV-infected patients also had increased activity in bilateral prefrontal cortices, which likely reflected compensatory processes serving movement planning, as significant correlations were observed between the amplitude of such activity and performance on the Trail Making Test (A+B).82 An MEG study of visual attention also found abnormal activity in the right prefrontal cortices, as well as the frontal eye-fields of HIV-infected older adults compared with matched uninfected controls.83 Consistent with the motor control study, the magnitude of neural responses in these areas correlated with neuropsychological performance in the HIV-infected patients.83 In regard to resting-state activity, MEG studies have shown deficits in functional connectivity in HIV-infected patients, especially involving right temporo-parietal and occipital cortices,84 and reduced resting beta activity in superior parietal regions, posterior cingulate, supplementary motor cortices, and the paracentral lobule.85 Finally, a recent multimodal study from our laboratory examined local gray matter volume and tactile processing in older HIV-infected and uninfected participants. We found reduced neural responses in the left postcentral gyrus (contralateral to stimulation) shortly after onset of the tactile stimulus in HIV-infected patients, along with increased alpha activity in the prefrontal cortices.86 HIV-infected patients also had reduced gray matter in parahippocampal, lingual, and other areas, as well as a region of the left postcentral gyrus that spatially coincided with the abnormal MEG response (see Figure 6).86 This latter finding suggests that some of the functional MEG aberrations observed in HIV disease may reflect alterations in the underlying neural structure, whereas others are more purely functional in nature (e.g., prefrontal cortices). Of particular clinical interest, we computed sensitivity and specificity indices for HAND using the peak amplitude values of the two main MEG findings (i.e., left postcental gyrus and prefrontal cortices) in conjunction, and found that the sensitivity and specificity for HAND were over 85%.86 These findings strongly suggest that MEG metrics may serve as powerful classifiers in the context of HAND and HIV-associated brain dysfunction. Furthermore, another recent MEG study reported excellent test-retest reliability after ∼24 weeks in both HIV-infected and uninfected participants,87 which of course is a critical property for any biomarker of disease.
Figure 6. Structural-Functional Deficits in HIV-infected Older Adults.
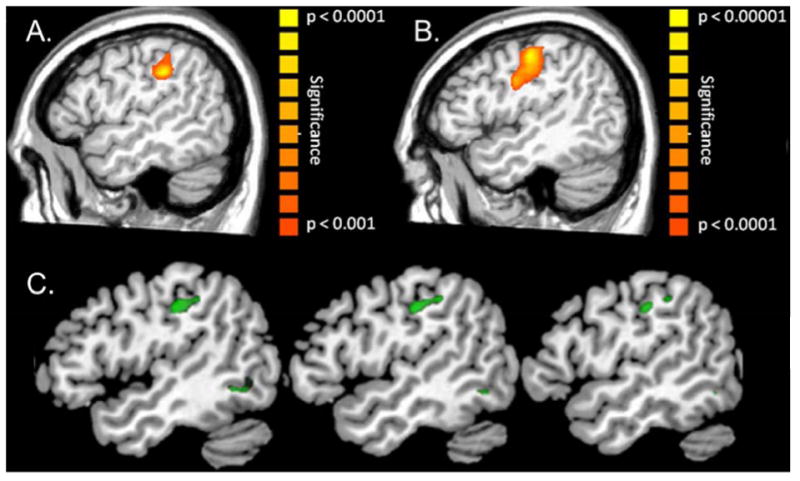
(A) HIV-infected patients had weaker activity in the left postcentral gyrus following tactile stimulation to the right hand compared to (B) demographically-matched uninfected participants (p < 0.01, corrected). (C) The same HIV-infected patients also had reduced gray matter volume relative to uninfected controls in the left postcentral gyrus and several other brain regions (green areas; p < 0.001, corrected). This area of reduced gray matter in the left postcentral gyrus spatially overlapped with the maximal MEG response, suggesting that aberrations in brain structure contribute to at least some functional MEG abnormalities in HIV-infected patients. Of note, this study found abnormal MEG responses in the prefrontal cortices of HIV-infected patients as well, and no structural deficits were observed in this region.86
In summary, MEG studies of the neural alterations associated with HIV-infection are rare, but have increased substantially over the past three years. These studies have repeatedly implicated the prefrontal cortices, right more often than left, as a key dysfunctional hub across several different tasks. These data and those reflecting altered sensory processing in HIV-infection provide clear support for the utility of MEG in identifying and characterizing the pathophysiology of interest, and the technique's potential as a future test for HAND. Future studies should examine the impact of disease duration (time since diagnosis), substance abuse, and aging, as these factors may be particularly important in understanding the pathophysiology.
Parkinson's Disease (PD)
Parkinson's disease (PD) is the second most common neurodegenerative disorder in the world, it is progressive and has an overall prevalence of 1-2% in the United States.88-90 There is a wealth of literature that describes the motor abnormalities commonly observed in patients with PD,91-95 which include muscle rigidity, instability, hypo- or bradykinesia (i.e., lack of, or slowness of movement), and resting tremor. While the exact mechanisms are unknown, these symptoms likely arise from the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc).96
Some of the most important MEG studies of patients with PD have involved recording MEG simultaneously with local-field potentials (LFP) from within the subthalamic nucleus (STN) during deep-brain stimulation (DBS) surgery in patients with severe PD.97-102 These simultaneous MEG-LFP recordings have allowed conclusions about how the subcortical pathophysiology transcends and affects the cortex. One of the most common findings from this work has been pathological synchrony between neurons within the STN, as well as between STN and cortical motor neurons, both of which are normalized by DBS and/or dopamine replacement therapy. This hyper-synchrony is frequency specific, with tremor-related alterations being found predominantly in the theta (4-7 Hz) and alpha bands (8-12 Hz), corresponding to the frequency of Parkinsonian tremor and its harmonic, while more general motor deficits are related to beta (14-30 Hz) and gamma (> 30 Hz) synchrony. For example, Hirschmann and colleagues found that during isometric contraction, coherence between the STN and the primary motor cortex was dominant in the beta rhythm, and that this synchrony was significantly diminished and motor symptoms improved following administration of dopaminergic medication.102 These studies, coupled with knowledge from purely invasive recordings (for a review, see 103), support a model of subcortico-cortical oscillatory deviations in PD, where neural oscillations become hyper-synchronous in the STN and eventually impair motor function by engulfing other structures within the motor network into a state of excessive hyper-synchronization.104
While important in their own right, studies that have employed simultaneous LFP-MEG methodology have at least two major limitations. First, due to the invasive nature of LFP recordings, comparisons involving a healthy control sample are simply not possible. Secondly, patients who are eligible for DBS surgery generally exhibit very severe symptoms and thereby are representative of only a small percentage of the overall PD population. Thus, MEG studies of patients with mild-to-moderate PD provide a nice complement, and offer broader implications as to the role of oscillations in PD.105-115 One such study found increased beta connectivity between the primary motor cortices and supplementary motor area during isometric contraction, which correlated with measures of symptom severity and was reduced following administration of dopaminergic medication.106 Another study from the same group found widespread resting-state synchrony between the primary motor cortices, premotor region, supplementary motor area, and parietal regions in theta and alpha frequencies that was directly related to tremor amplitude in patients with PD.107 Recent work from our laboratory confirmed that un-medicated patients with PD have pathologically high synchrony between the primary motor cortices, and further showed that these patients have significantly diminished beta amplitude relative to demographically-matched controls during the resting-state.112 Interestingly, administration of dopaminergic medication selectively ameliorated these differences in beta amplitude.112
Work from our laboratory was also the first to utilize the temporal precision of MEG to systematically investigate the effects of PD on motor physiology before, during, and after transient movement.113 Essentially, prior to movement onset, there is a desynchronization in the beta band, termed the peri-movement beta ERD, which starts approximately 0.5 s before motor onset and continues throughout movement execution. Following movement termination, there is a strong resynchronization in the beta band known as the post-movement beta rebound (PMBR) response.113-121 The PMBR reaches its maximum amplitude 0.5 to 1.0 s after the termination of movement, and continues for about 1.0 s before returning to baseline levels. These responses are at least partially spatially distinct; the peri-movement beta ERD generally involves the bilateral primary sensorimotor cortices, supplementary motor area, parietal lobe and the cerebellum, whereas the PMBR has been reported in the sensorimotor cortices, supplementary motor area, premotor, and medial prefrontal regions.113-121 The temporal and spatial precision of MEG is optimal for studying this pattern of responses in health and in patients with movement disorders such as PD. By exploiting these optimal properties, we showed that the peri-movement beta ERD response in the primary motor cortices was selectively aberrant in patients with PD compared to controls (see Figure 7).113 Since then, other labs have identified and selectively modulated the peri-movement beta ERD response in PD.122-123 Of note, one of these studies demonstrated differences in the millisecond time course of the peri-movement beta ERD, and suggested that patients with PD exhibit neural dynamics reflective of a reactive, rather than proactive, movement planning process.122 This and other supporting evidence suggests that this motor-related oscillatory response may serve as a biomarker of symptom severity and disease progression.
Figure 7. Aberrant Beta Oscillations in Parkinson's Disease (PD).
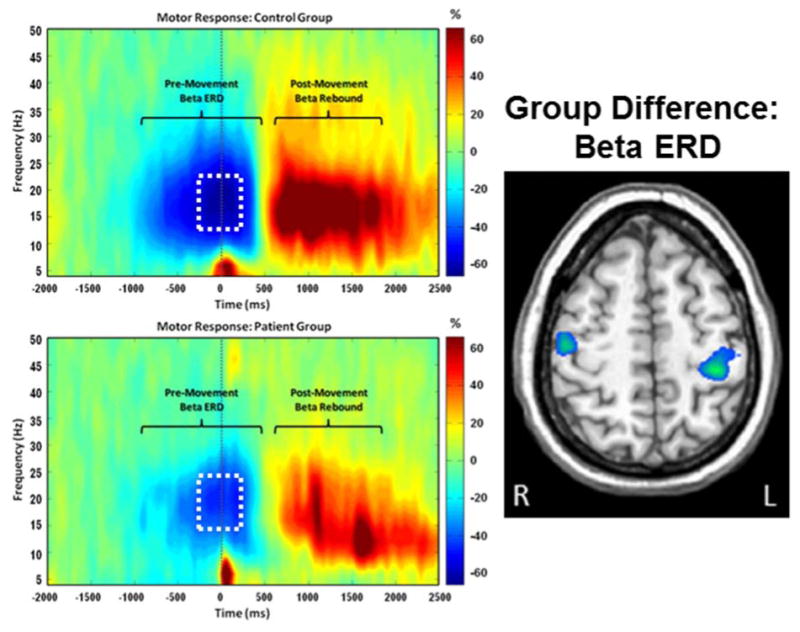
(Left) Average time-frequency spectrograms from a MEG sensor near the sensorimotor cortex are shown for healthy controls (top panel) and patients with PD (bottom panel). Time (in ms) is denoted on the x-axis, with 0 ms defined as movement onset. Frequency (in Hz) is shown on the y-axis. The typical pattern of peri-movement beta desynchronization (ERD) and post-movement beta rebound (PMBR) during a right-hand movement task, expressed as percent difference from baseline, can be discerned in each group. The time period within the white box (-300 to 200 ms) was subjected to source reconstruction analyses. These analyses revealed that peak group differences in the beta ERD response (right) were within the motor hand knob region of the left precentral gyrus and extended onto the adjacent postcentral gyrus (p = .035, corrected). In both cases, responses were weaker in patients with PD. Trending differences were also seen in the right precentral gyrus near the hand knob region (p < .005, uncorrected; p = .107, corrected).113 Abnormalities in beta activity appear to be a central feature of PD and have been observed in the cortex in MEG studies and in the subthalamic nucleus during deep brain stimulation (DBS) surgeries.
While future studies are certainly needed, MEG studies in PD have provided critical insight into how this disease affects brain physiology, especially within the subcortical-cortical motor network. One major question in the field is how specific these neural abnormalities are to PD, as the symptom profile of PD overlaps with several different disorders (e.g., progressive supranuclear palsy). MEG studies showing that the neurophysiological aberrations are unique to PD will be exceptionally important, and establish a foundation for developing disease specific markers that could potentially be used to identify patients for future clinical trials.
Cerebral Palsy (CP)
Cerebral palsy (CP) is one of the most prevalent pediatric neurologic conditions diagnosed in the United States,124 with almost four out of every 1000 children being affected. It results from a defect or insult to the immature developing brain and,125 although the neurologic damage does not progressively worsen, it often results in neuromuscular impairments that limit the child's mobility. The total lifetime economic burden to our society is estimated to be about one million dollars per child, with a major portion of these funds directed at interventions that are aimed at improving the child's mobility.126 MEG studies in this area have primarily focused on identifying the contribution of central mechanisms (i.e., the brain) to the observed motor deficits in children and adolescents with CP.
Early MEG studies in this area focused on the primary somatosensory response evoked by electrical stimulation of the tibial nerve. These studies showed that children with CP had sharply reduced response amplitudes compared to typically-developing controls in the contralateral somatosensory cortices, especially for the earliest component.127 A later study showed that body-weight supported treadmill training improved lower extremity motor function, and modulated these same MEG somatosensory responses in children with CP.128 More recent work in this area has examined the oscillatory dynamics of somatosensory processing, and these studies have shown that children with CP have abnormal neural synchronization within sensorimotor cortices following tactile stimulation of the foot.129-130 Specifically, children with CP exhibited decreased synchronization in the theta-alpha range (4-14 Hz) in response to tactile stimulation, whereas typically-developing controls showed robust increases in theta-alpha synchronization in the same medial sensorimotor cortices.129 Interestingly, the strength of neural synchronization in the somatosensory cortices, induced by tactile stimulation, has also been connected to ankle strength, step length, and walking speed in children with CP, which suggests a direct connection between these sensory processing measures and motor performance.130 A related study of particular interest showed that theta-alpha synchronization in the somatosensory cortices was normal in children with CP following tactile stimulation of the hand.131 Since the children in this study did not have upper extremity impairments, these findings may suggest that theta-alpha synchronization is selectively disturbed in CP and is only abnormal in neural regions that serve severely impaired limbs.
Finally, one recent MEG study examined motor control of the knee joint in children and adolescents with CP.132 This groundbreaking study precisely quantified movement onset and examined the oscillatory dynamics of motor planning, execution, and termination. Their findings showed that patients with CP had significantly stronger beta ERD responses during the motor planning stage compared to typically-developing controls, along with significantly weaker gamma responses during movement execution.132 These investigators proposed that the stronger beta ERD reflected increased planning activity, as patients tried to compensate for their motor impairments, and that the reduced gamma response indicated that this planning was not fully successful, and ultimately cascaded into the execution and termination stages.132 These findings may suggest a specific therapeutic target for designing new physical therapy interventions.132-133 While this study of motor activity in CP provided critical data, much remains to be discovered and MEG will certainly be involved. Understanding the neural mechanisms underlying motor impairments will be strongly advanced if the temporal dynamics of neuronal circuits can be quantified in real time along with the movement parameters. Furthermore, the silent nature of MEG and other unique properties makes it an ideal technique for studying children and adolescents with CP, as these participants are easily startled and often have spasms that make fMRI scanning more tenuous. Future studies will need to identify how the severity of CP affects the neuronal aberrations reported in previous studies, and decipher how age and other factors modulate these findings.
Healthy Aging
Healthy aging is associated with declines in sensory, perceptual, and cognitive processes.134-135 The neurophysiological changes that underlie these declines are not fully understood, and MEG studies in this area have generally focused on identifying and characterizing these changes in the context of sensory stimulation and/or demanding cognitive tasks. Findings from other modalities (e.g., fMRI) suggest that older adults typically utilize greater neural resources relative to younger adults, which may reflect compensatory mechanisms136 or neural dedifferentiation.137
Perhaps the most commonly studied aging effects in the MEG literature pertain to sensory and perceptual operations, with aging being associated with deficits in such processes. Several MEG studies have examined the neurophysiological underpinnings of healthy aging using auditory processing paradigms, and have shown that older adults generally demonstrate a stronger amplitude response relative to younger adults in superior temporal regions shortly after the onset of an auditory stimulus (∼50ms). This accentuated early neural response has been demonstrated in response to complex sounds,138 phonetic sounds,139-140 spoken words,141 pitch,142-143 and tones.139,144-145 Interestingly, similar age-related differences have been reported in somatosensory MEG studies utilizing median nerve stimulation, with older adults exhibiting an increased early cortical response in the primary somatosensory cortices,146-151 especially contralateral to the stimulated nerve.146-150 Of note, neural responses at later latencies are typically more comparable in strength and do not generally show aging effects in auditory or somatosensory modalities, although there have been exceptions.138,141 Together these studies suggest that early hyper-responsivity to sensory stimulation in the elderly may underlie age-related behavioral deficits in sensory processing. In addition, the latency of these responses and later components is generally delayed in older relative to younger adults, and this may underlie the age-related slowing of sensory and perceptual processes commonly seen.
A handful of MEG resting-state studies have also examined the neural underpinnings of aging. Recent studies have found that global low frequency delta/theta (0.5-6.5 Hz) activity decreased with increasing age, and that such reductions in bilateral temporal and parietal regions were associated with reduced cognitive performance.152-153 MEG studies that have focused on network-level activity have also found reduced delta (2-4 Hz) network size and larger beta/gamma (>16 Hz) network size in older compared with younger adults, with increased beta/gamma connectivity in bilateral temporal lobes being associated with worse performance on visuoconstructive tasks.154-155 Taken together, stronger low-frequency oscillations in the resting-state are associated with better executive performance in the elderly, while increased beta/gamma connectivity in the temporal cortices during rest may be deleterious and contribute to neural dedifferentiation in the elderly.
Finally, MEG has been used to investigate age-related differences in the spatiotemporal dynamics underlying higher-order cognitive processes, with working memory perhaps being the most popular. For example, Proskovec and colleagues reported widespread neural activity in left fronto-temporal regions of both young and older adults during the encoding and maintenance periods of a verbal working memory task, but only older adults also engaged the right prefrontal cortices during task performance.156 Results for the retrieval phase of working memory were reported by Aine et al.157 Notably, no age-related differences in working memory performance were observed in either of these studies.156-157 Together, these studies support the compensation hypothesis of cognitive aging.136 Essentially, older adults recruited more neural resources during working memory performance across encoding, maintenance, and retrieval periods compared with younger adults, while performance was relatively equal between groups. Interestingly, the Proskovec study also found strong alpha (9-12 Hz) synchronization in the occipital cortices during memory maintenance in both age groups, although this response occurred earlier and encompassed more cortical tissue in older adults (Figure 8).156 Occipital alpha synchronization is believed to reflect inhibition of the dorsal visual stream during memory maintenance, which would aid in blocking incoming visual distractions and help preserve memory representations in more anterior cortical regions.158-160 Thus, the earlier and more widespread increase in occipital alpha activity in older adults likely reflects an additional compensatory mechanism.
Figure 8. Healthy Aging Modulates the Dynamics of Working Memory Processing.
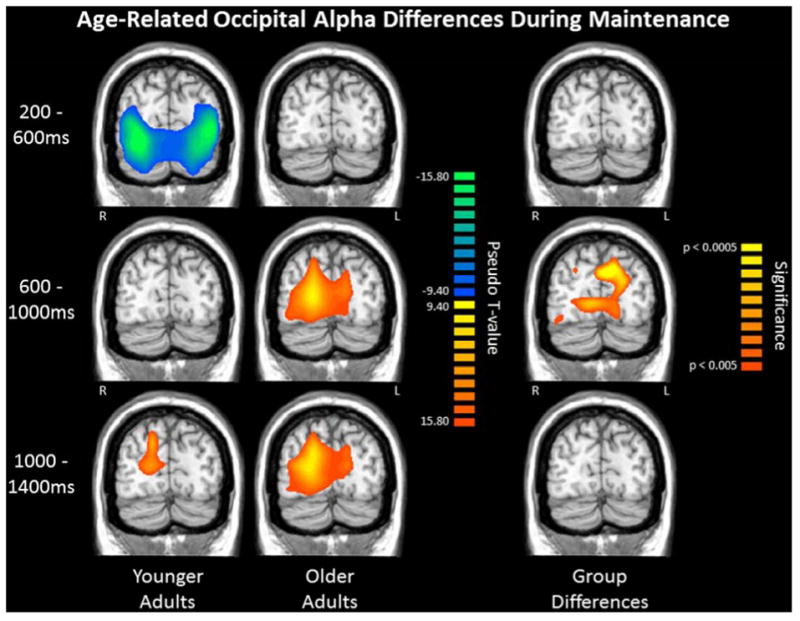
Group mean beamformer images (pseudo-t) for younger (Left) and older (Middle) adults, with each row depicting a different time period of the early maintenance phase (0.0 ms = offset of encoding grid and beginning of memory maintenance). Note that pseudo-t units indicate the differential power per voxel between the active period (far left) and a pre-stimulus baseline period. (Right) Significant group differences (p < .005, corrected) in alpha oscillatory activity in parieto-occipital cortices as a function of time. Younger adults showed a shift from a bilateral decrease in alpha activity in the occipital cortices to a right-lateralized increase in alpha activity in parieto-occipital areas. Conversely, older adults demonstrated a progressive increase in alpha activity across bilateral occipital cortices, with the increases in alpha occurring earlier and involving more cortical tissue in older adults relative to younger adults.156 Such alpha synchronization is thought to decrease or inhibit visual input and thereby aid in maintaining memory representations.
In summary, MEG is an ideal tool for examining the mechanisms of cognitive aging, as its spatiotemporal precision allows age-related delays and slowing in the system to be accurately characterized. While most early investigations focused on sensory processing, MEG studies of higher-order processes are now appearing in the literature and these data will eventually help the field distinguish early signs of dementia from the neuronal signatures of healthy aging. A solid understanding of age-related changes will also enable identification of accelerated cognitive aging, and lead to more controlled neuroimaging investigations of aging subtypes in the future.
Conclusions & Future Directions
MEG is an advanced neurophysiological imaging tool with excellent spatiotemporal precision, which makes it an ideal method for studying the brain dynamics that underlie neurologic and psychiatric disorders. These unique properties have enabled MEG imaging to make a significant impact on clinical cognitive neuroscience, despite the relative rarity and emergent status of the technique. Specifically, MEG studies have identified aberrations in the auditory system of patients with ASD, including deficient high-frequency gamma activity and deviations in response timing. Several groups are now working to develop an auditory-based MEG biomarker. MEG studies of ADHD have quantified how stimulant medications affect brain function in these patients, and identified connectivity deficits and gamma abnormalities across multiple brain regions. Notably, these studies have suggested that stimulants may normalize connectivity by modulating local gamma activity, which would be consistent with models of how gamma activity affects network-level neural interactions. The role of MEG in understanding PTSD is rapidly expanding, with the early findings indicating that patients (mostly veterans) have altered oscillatory dynamics across multiple cortical regions, with right frontal and parietal areas being among the most commonly implicated. MEG studies of HIV-infection and HAND have also highlighted deficits in the right prefrontal cortices, although the most exciting findings in this area are likely the sensitivity and specificity data, as well as the reliability data, showing that MEG metrics may serve as a diagnostic biomarker. In studies of PD, MEG has already had a huge impact in quantifying beta activity across the motor network, both in a surgical context (DBS) and in more mild cases of PD. Several recent studies have started to dissect how beta oscillations in the motor circuit separately affect motor planning, execution, and termination stages, which will ultimately lead to understanding how beta activity deficits interact with motor symptoms. MEG studies of children with CP are also expanding rapidly, as the silent MEG recording environment is ideal for this population. Critical findings thus far have included beta abnormalities during motor planning, as well as major somatosensory processing deficits. Finally, MEG studies of cognitive aging have shown considerable promise, as the excellent temporal resolution of MEG is ideal for discerning age-related changes in neural timing. Findings so far include early sensory processing deficits, as well as strong compensatory activity in right hemispheric homologue regions during higher-order cognitive tasks. MEG markers of healthy versus pathological aging are also under development, although much work remains to be completed in this arena.
The use of MEG has grown rapidly over the past decade, especially in the past few years, and this is very likely to continue through the next decade, with the increasing focus on brain dynamics in clinical cognitive neuroscience, and dedicated funding opportunities like President Obama's Brain Initiative in the United States and the European Union's Human Brain Project. This growth will include efforts to develop new MEG-based biomarkers of disease, as well as markers that can be used to monitor disease progression and therapeutic responses to treatment (i.e., pharmaco-MEG). In the future, MEG may see action in the early stages of drug development to ascertain where drugs are primarily acting. This seems especially likely for disorders where certain brain areas are known to underlie key aspects of the symptomatology, such as the frontal and prefrontal cortices in ADHD. Thus, MEG will likely appear in many new venues in the coming years, as it continues to benefit from the scientific community's growing focus on translational research. Lastly, new innovative MEG sensor designs are now emerging,161 and these hold promise for significantly improving the spatial resolution of MEG (< 1 mm) while lowering instrumentation costs. If successful, the number of MEG sites and the pace of discovery could rapidly expand.
Acknowledgments
This work was supported by NIH grant R01 MH103220 (TWW), NSF grant #1539067 (TWW), the Shoemaker Prize from the University of Nebraska Foundation (TWW), a Kinman-Oldfield Award for Neurodegenerative Research from the University of Nebraska Foundation (TWW), and a grant from the Nebraska Banker's Association (TWW). The Center for Magnetoencephalography at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Drs. Wilson and Heinrichs-Graham, Ms. Proskovec, and Mr. McDermott have read the journal's policy on conflicts of interest and have no potential conflicts of interest to disclose. In addition, all authors have read the journal's authorship agreement and fulfilled all criteria for authorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen DS. Magnetoencephalography: Evidence of magnetic fields produced by alpha rhythm currents. Science. 1968;161:784–786. doi: 10.1126/science.161.3843.784. [DOI] [PubMed] [Google Scholar]
- 2.Wilson TW. Noninvasive neurophysiological imaging with magnetoencephalography. In: Xiong H, Gendelman HE, editors. Current laboratory methods in neuroscience research. New York: Springer Press; 2014. pp. 293–311. [Google Scholar]
- 3.Supek S, Aine CJ, editors. Magnetoencephalography: From signals to dynamic cortical networks. Verlag: Springer Press; 2014. [Google Scholar]
- 4.Hansen PC, Kringelbach ML, Salmelin R, editors. MEG: An introduction to the methods. New York: Oxford University Press; 2010. [Google Scholar]
- 5.Hari R, Salmelin R. Magnetoencephalography: From SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. Neuroimage. 2012;61(2):386–96. doi: 10.1016/j.neuroimage.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 6.Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography -Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65(2):413–495. [Google Scholar]
- 7.Murakami S, Okada Y. Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. J Physiol. 2006;575:925–936. doi: 10.1113/jphysiol.2006.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillebrand A, Barnes GR. A quantitative assessment of the sensitivity of whole-head MEG to activity in the adult human cortex. Neuroimage. 2002;16:638–650. doi: 10.1006/nimg.2002.1102. [DOI] [PubMed] [Google Scholar]
- 9.Bagić A. Look back to leap forward: The emerging new role of magnetoencephalography (MEG) in nonlesional epilepsy. Clin Neurophysiol. 2015 May 15; doi: 10.1016/j.clinph.2015.05.009. [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- 10.Stufflebeam SM. Clinical magnetoencephalography for neurosurgery. Neurosurg Clin N Am. 2011;22(2):153–167. vii–viii. doi: 10.1016/j.nec.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stufflebeam SM, Tanaka N, Ahlfors SP. Clinical applications of magnetoencephalography. Hum Brain Mapp. 2009;30(6):1813–1823. doi: 10.1002/hbm.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefan H, Rampp S, Knowlton RC. Magnetoencephalography adds to the surgical evaluation process. Epilepsy Behav. 2011;20(2):172–177. doi: 10.1016/j.yebeh.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N, Stufflebeam SM. Clinical application of spatiotemporal distributed source analysis in presurgical evaluation of epilepsy. Front Hum Neurosci. 2014;8:62. doi: 10.3389/fnhum.2014.00062. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen O, Spaak E, Zumer JM. Human brain oscillations: From physiological mechanisms to analysis and cognition. In: Supek S, Aine CJ, editors. Magnetoencephalography: From signals to dynamic cortical networks. Verlag: Springer Press; 2014. pp. 359–403. [Google Scholar]
- 15.Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolić D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoffelen JM, Gross J. Studying dynamic neural interactions with MEG. In: Supek S, Aine CJ, editors. Magnetoencephalography: From signals to dynamic cortical networks. Verlag: Springer Press; 2014. pp. 359–403. [Google Scholar]
- 17.Rojas DC, Wilson LB. γ-band abnormalities as markers of autism spectrum disorders. Biomark Med. 2014;8(3):353–68. doi: 10.2217/bmm.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McFadden KL, Rojas DC. Electrophysiology of autism. In: Fitzgerald Michael., editor. Recent Advances in Autism Spectrum Disorders – Volume II. 2013. InTech. [DOI] [Google Scholar]
- 19.Rojas DC. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J Neural Transm. 2014;121(8):891–905. doi: 10.1007/s00702-014-1216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol Psychiatry. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojas D, Maharajh K, Teale P, Rogers S. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8(1):66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TPL, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biological Psychiatry. 2010;68(12):1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W, Cannon KM, Monroe JF, Cornew L, Qasmieh S, Liu S, Welsh JP, Levy SE, Roberts TP. Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. J Autism Dev Disord. 2015;45(2):395–405. doi: 10.1007/s10803-013-1904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rojas DC, Teale PD, Maharajh K, et al. Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Mol Autism. 2011;2(1):11. doi: 10.1186/2040-2392-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gage NM, Siegel B, Roberts TP. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Brain Res Dev Brain Res. 2003;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, Woldoff S, Qasmieh S, Gandal M, Schmidt GL, Zarnow DM, Levy SE, Edgar JC. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging bio-marker for autism. Autism Res. 2010;3:8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Hecke AV, Stevens S, Carson AM, et al. Measuring the Plasticity of Social Approach: A Randomized Controlled Trial of the Effects of the PEERS Intervention on EEG Asymmetry in Adolescents with Autism Spectrum Disorders. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1883-y. [DOI] [PubMed] [Google Scholar]
- 28.Cornew L, Roberts TP, Blaskey L, Edgar JC. Resting-state oscillatory activity in autism spectrum disorders. J Autism Dev Disord. 2012;42(9):1884–1894. doi: 10.1007/s10803-011-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman JI, Liu S, Bloy L, Blaskey L, Roberts TP, Edgar JC. Alpha-to-gamma phase-amplitude coupling methods and application to autism spectrum disorder. Brain Connect. 2015;5(2):80–90. doi: 10.1089/brain.2014.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar JC, Heiken K, Chen YH, Herrington JD, Chow V, Liu S, Bloy L, Huang M, Pandey J, Cannon KM, Qasmieh S, Levy SE, Schultz RT, Roberts TP. Resting-state alpha in autism spectrum disorder alpha associations with thalamic volume. J Autism Dev Disord. 2015;45(3):795–804. doi: 10.1007/s10803-014-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye AX, Leung RC, Schäfer CB, Taylor MJ, Doesburg SM. Atypical resting synchrony in autism spectrum disorder. Hum Brain Mapp. 2014;35(12):6049–6066. doi: 10.1002/hbm.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitzbichler MG, Khan S, Ganesan S, Vangel MG, Herbert MR, Hämäläinen MS, Kenet T. Altered development and multifaceted band-specific abnormalities of resting state networks in autism. Biol Psychiatry. 2015;77(9):794–804. doi: 10.1016/j.biopsych.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 34.Center for Disease Control and Prevention. Mental health in the United States: Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder – United States. MMWR. 2005;54(34):842–847. [PubMed] [Google Scholar]
- 35.Coghill D, Seth S. Osmotic, controlled-release methylphenidate for the treatment of ADHD. Expert Opin Pharmacother. 2006;7:2119–2138. doi: 10.1517/14656566.7.15.2119. [DOI] [PubMed] [Google Scholar]
- 36.Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Dietary caffeine consumption modulates fMRI measures. Neuroimage. 2002;17(2):751–757. [PubMed] [Google Scholar]
- 37.Addicott MA, Yang LL, Peiffer AM, et al. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum Brain Mapp. 2009;30(10):3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honey G, Bullmore E. Human pharmacological MRI. Trends Pharmacol Sci. 2004;25(7):366–374. doi: 10.1016/j.tips.2004.05.009. Review. [DOI] [PubMed] [Google Scholar]
- 39.Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Relationship between caffeine-induced changes in resting cerebral perfusion and blood oxygenation level-dependent signal. AJNR Am J Neuroradiol. 2004;24(8):1607–1611. [PMC free article] [PubMed] [Google Scholar]
- 40.Dockstader C, Gaetz W, Cheyne D, Wang F, Castellanos FX, Tannock R. MEG event-related desynchronization and synchronization deficits during basic somatosensory processing in individuals with ADHD. Behav Brain Funct. 2008;12:4–8. doi: 10.1186/1744-9081-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helenius P, Laasonen M, Hokkanen L, Paetau R, Niemivirta M. Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia. 2011;49(7):1889–1896. doi: 10.1016/j.neuropsychologia.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 45.Bartos M Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 46.Wilson TW, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW. Estimating the passage of minutes: Deviant oscillatory frontal activity in medicated and un-medicated ADHD. Neuropsychology. 2013;27(6):654–665. doi: 10.1037/a0034032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson TW, Wetzel MW, White ML, Knott NL. Gamma-frequency neuronal activity is diminished in adults with attention-deficit/hyperactivity disorder: A pharmaco-MEG study. J Psychopharmacol. 2012;26(6):771–777. doi: 10.1177/0269881111430731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franzen JD, Wilson TW. Amphetamines modulate prefrontal γ oscillations during attention processing. Neuroreport. 2012;23(12):731–735. doi: 10.1097/WNR.0b013e328356bb59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinrichs-Graham E, Franzen JD, Knott NL, White ML, Wetzel MW, Wilson TW. Pharmaco-MEG evidence for attention related hyper-connectivity between auditory and prefrontal cortices in ADHD. Psychiatry Res. 2014;221(3):240–245. doi: 10.1016/j.pscychresns.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ter Huurne N, Onnink M, Kan C, Franke B, Buitelaar J, Jensen O. Behavioral consequences of aberrant alpha lateralization in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(3):227–233. doi: 10.1016/j.biopsych.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Wilson TW, Franzen JD, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Hum Brain Mapp. 2013;34(3):566–574. doi: 10.1002/hbm.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franzen JD, Heinrichs-Graham E, White ML, Wetzel MW, Knott NL, Wilson TW. Atypical coupling between posterior regions of the default-mode network in attention-deficit/hyperactivity disorder: A pharmaco-MEG study. J Psychiatry Neurosci. 2013;38(5):333–340. doi: 10.1503/jpn.120054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 54.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–622. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kilpatrick DG, Resnick HS, Milanik ME, et al. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress. 2013;26(5):537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seal K, Bertenthal D, Miner C, et al. Bringing the War Back Home: Mental Health Disorders Among 103,788 US Veterans Returning From Iraq and Afghanistan Seen at Department of Veterans Affairs Facilities. Archives of Internal Medicine. 2007;167:476–482. doi: 10.1001/archinte.167.5.476. [DOI] [PubMed] [Google Scholar]
- 57.Seal K, Metzler T, Gima K, et al. Trends and Risk Factors for Mental Health Diagnoses Among Iraq and Afghanistan Veterans Using Department of Veterans Affairs Health Care, 2002–2008. American Journal of Public Health. 2009;99(9):1651–1658. doi: 10.2105/AJPH.2008.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engdahl B, Leuthold AC, Tan HRM, Lewis SM, Winskowski AM, Dikel TN, Georgopoulos AP. Post-traumatic stress disorder: a right temporal lobe syndrome? J Neural Eng. 2010;7(6):066005. doi: 10.1088/1741-2560/7/6/066005. [DOI] [PubMed] [Google Scholar]
- 59.Georgopoulos AP, Tan HRM, Lewis SM, Leuthold AC, Winskowski AM, Lynch JK, Engdahl B. The synchronous neural interactions test as a functional neuromarker for post-traumatic stress disorder (PTSD): a robust classification method based on the bootstrap. J Neural Eng. 2010;7(1):16011. doi: 10.1088/1741-2560/7/1/016011. [DOI] [PubMed] [Google Scholar]
- 60.James LM, Belitskaya-Lévy I, Lu Y, Wang H, Engdahl BE, Leuthold AC, Georgopoulos AP. Development and application of a diagnostic algorithm for posttraumatic stress disorder. Psychiatry Res. 2015;231(1):1–7. doi: 10.1016/j.pscychresns.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Anders SL, Peterson CK, James LM, Engdahl B, Leuthold AC, Georgopoulos AP. Neural communication in posttraumatic growth. Exp Brain Res. 2015;233(7):2013–2020. doi: 10.1007/s00221-015-4272-2. [DOI] [PubMed] [Google Scholar]
- 62.James LM, Engdahl BE, Leuthold AC, Lewis SM, Van Kampen E, Georgopoulos AP. Neural network modulation by trauma as a marker of resilience: differences between veterans with posttraumatic stress disorder and resilient controls. JAMA Psychiatry. 2013;70(4):410–418. doi: 10.1001/jamapsychiatry.2013.878. [DOI] [PubMed] [Google Scholar]
- 63.Kolassa IT, Wienbruch C, Neuner F, Schauer M, Ruf M, Odenwald M, Elbert T. Altered oscillatory brain dynamics after repeated traumatic stress. BMC Psychiatry. 2007;7:56. doi: 10.1186/1471-244X-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adenauer H, Pinösch S, Catani C, Gola H, Keil J, Kissler J, Neuner F. Early processing of threat cues in posttraumatic stress disorder-evidence for a cortical vigilance-avoidance reaction. Biol Psychiatry. 2010;68(5):451–458. doi: 10.1016/j.biopsych.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 65.Todd RM, MacDonald MJ, Sedge P, Robertson A, Jetly R, Taylor MJ, Pang EW. Soldiers with Posttraumatic Stress Disorder See a World Full of Threat: Magnetoencephalography Reveals Enhanced Tuning to Combat-Related Cues. Biol Psychiatry. 2015 May 27; doi: 10.1016/j.biopsych.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Badura-Brack AS, Becker KM, McDermott TJ, Ryan TJ, Becker MM, Hearley AR, Heinrichs-Graham E, Wilson TW. Decreased somatosensory activity to non-threatening touch in combat veterans with posttraumatic stress disorder. Psychiatry Res. 2015;233(2):194–200. doi: 10.1016/j.pscychresns.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Khanna MM, Heinrichs-Graham E, Wilson TW. Male veterans with PTSD exhibit aberrant neural dynamics during working memory encoding: A MEG study. Journal of Psychiatry and Neuroscience. doi: 10.1503/jpn.150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Bar-Haim Y, Pine DS, Abend R, Khanna MM, Heinrichs-Graham E, Wilson TW. Attention training improves aberrant neural dynamics during working memory encoding in male veterans with PTSD. Psychological Medicine. doi: 10.3758/s13415-016-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunkley BT, Doesburg SM, Sedge PA, Grodecki RJ, Shek PN, Pang EW, Taylor MJ. Resting-state hippocampal connectivity correlates with symptom severity in post-traumatic stress disorder. Neuroimage Clin. 2014;5:377–384. doi: 10.1016/j.nicl.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunkley BT, Sedge PA, Doesburg SM, Grodecki RJ, Jetly R, Shek PN, Taylor MJ, Pang EW. Theta, mental flexibility, and post-traumatic stress disorder: connecting in the parietal cortex. PLoS One. 2015;10(4):e0123541. doi: 10.1371/journal.pone.0123541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunkley BT, Doesburg SM, Jetly R, Sedge PA, Pang EW, Taylor MJ. Characterising intra- and inter-intrinsic network synchrony in combat-related post-traumatic stress disorder. Psychiatry Res. 2015 doi: 10.1016/j.pscychresns.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis. 2016;26:17–25. doi: 10.1097/QCO.0b013e32835ba6b1. [DOI] [PubMed] [Google Scholar]
- 74.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: A review. [Review] Neuropsychol Rev. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- 76.Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24:275–283. doi: 10.1097/WCO.0b013e32834695fb. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2001;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 80.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 81.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: Prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 82.Wilson TW, Heinrichs-Graham E, Robertson KR, Sandkovsky U, O'Neill J, Knott NL, Fox HS, Swindells S. Functional brain abnormalities during finger-tapping in HIV-infected older adults: a magnetoencephalography study. J Neuroimmune Pharmacol. 2013;8(4):965–74. doi: 10.1007/s11481-013-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson TW, Fox HS, Robertson KR, Sandkovsky U, O'Neill J, Heinrichs-Graham E, Knott NL, Swindells S. Abnormal MEG oscillatory activity during visual processing in the prefrontal cortices and frontal eye-fields of the aging HIV brain. PLoS One. 2013;8(6):e66241. doi: 10.1371/journal.pone.0066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becker JT, Bajo R, Fabrizio M, Sudre G, Cuesta P, Aizenstein HJ, et al. Functional connectivity measured with magnetoencephalography identifies persons with HIV disease. Brain Imaging Behav. 2012;6:366–373. doi: 10.1007/s11682-012-9149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Becker KM, Heinrichs-Graham E, Fox HS, Robertson KR, Sandkovsky U, O'Neill J, Swindells S, Wilson TW. Decreased MEG beta oscillations in HIV-infected older adults during the resting state. J Neurovirol. 2013;19(6):586–594. doi: 10.1007/s13365-013-0220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson TW, Heinrichs-Graham E, Becker KM, Aloi J, Robertson KR, Sandkovsky U, White ML, O'Neill J, Knott NL, Fox HS, Swindells S. Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV-infected older adults. Hum Brain Mapp. 2015;36(3):897–910. doi: 10.1002/hbm.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Becker JT, Fabrizio M, Sudre G, Haridis A, Ambrose T, Aizenstein HJ, et al. Potential utility of resting-state magnetoencephalography as a biomarker of CNS abnormality in HIV disease. J Neurosci Methods. 2012;206:176–182. doi: 10.1016/j.jneumeth.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson's disease in the community? J Neurol Neurosurgy Psychiatry. 2002;73:529–534. doi: 10.1136/jnnp.73.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2012;34:143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 91.Bartels AL, Balash Y, Gurevich T, Schaafsma JD, Hausdorff JM, Giladi N. Relationship between freezing of gait (FOG) and other features of Parkinson's: FOG is not correlated with bradykinesia. J Clin Neurosci. 2003;10:584–588. doi: 10.1016/s0967-5868(03)00192-9. [DOI] [PubMed] [Google Scholar]
- 92.Dewey RB, Jr, Taneja A, McClintock SM, Cullum CM, Dewey RB, 3rd, Bernstein I, Husain MM. Motor symptoms at onset of Parkinson disease risk for cognitive impairment depression. Cogn Behav Neurol. 2012;25:115–120. doi: 10.1097/WNN.0b013e31826dfd62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fahn S. The history of dopamine and levodopa in the treatment of Parkinson's disease. Mov Disord. 2008;23(Suppl 3):S497–508. doi: 10.1002/mds.22028. [DOI] [PubMed] [Google Scholar]
- 94.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K, Parkinson Study G. Levodopa and the progression of Parkinson's disease. New Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 95.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 96.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 97.Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- 98.Litvak V, Eusebio A, Jha A, et al. Movement-related changes in local and long-range synchronization in Parkinson's disease revealed by simultaneous magnetoencephalography and intracranial recordings. J Neurosci. 2012;32:10541–10553. doi: 10.1523/JNEUROSCI.0767-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirschmann J, Ozkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson's disease. NeuroImage. 2011;55:1159–1168. doi: 10.1016/j.neuroimage.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 100.Hirschmann J, Hartmann CJ, Butz M, Hoogenboom N, Ozkurt TE, Elben S, Vesper J, Wojtecki L, Schnitzler A. A direct relationship between oscillatory subthalamic nucleus-cortex coupling and rest tremor in Parkinson's disease. Brain. 2013;136:3659–3670. doi: 10.1093/brain/awt271. [DOI] [PubMed] [Google Scholar]
- 101.Bock A, Kuhn AA, Trahms L, Sander TH. Validity of subthalamic-cortical coherency observed in patients with Parkinson's disease. Biomed Engin. 2013;58:157–164. doi: 10.1515/bmt-2012-0023. [DOI] [PubMed] [Google Scholar]
- 102.Hirschmann J, Ozkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Differential modulation of STN-cortical cortico-muscular coherence by movement levodopa in Parkinson's disease. NeuroImage. 2013;68:203–213. doi: 10.1016/j.neuroimage.2012.11.036. 2013. [DOI] [PubMed] [Google Scholar]
- 103.Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol. 2005;116:2510–2519. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 104.Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 2004;24:9240–9243. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salenius S, Avikainen S, Kaakkola S, Hari R, Brown P. Defective cortical drive to muscle in Parkinson's disease and its improvement with levodopa. Brain. 2002;125:491–500. doi: 10.1093/brain/awf042. [DOI] [PubMed] [Google Scholar]
- 106.Pollok B, Kamp D, Butz M, Wojtecki L, Timmermann L, Sudmeyer M, Krause V, Schnitzler A. Increased SMA-M1 coherence in Parkinson's disease - Pathophysiology or compensation? Exp Neurol. 2013;247:178–181. doi: 10.1016/j.expneurol.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 107.Pollok B, Makhloufi H, Butz M, Gross J, Timmermann L, Wojtecki L, Schnitzler A. Levodopa affects functional brain networks in Parkinsonian resting tremor. Mov Disord. 2009;24:91–98. doi: 10.1002/mds.22318. [DOI] [PubMed] [Google Scholar]
- 108.Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- 109.Bosboom JL, Stoffers D, Stam CJ, van Dijk BW, Verbunt J, Berendse HW, Wolters E. Resting state oscillatory brain dynamics in Parkinson's disease: an MEG study. Clin Neurophysiol. 2006;117:2521–2531. doi: 10.1016/j.clinph.2006.06.720. [DOI] [PubMed] [Google Scholar]
- 110.Stoffers D, Bosboom JL, Deijen JB, Wolters E, Stam CJ, Berendse HW. Increased cortico-cortical functional connectivity in early-stage Parkinson's disease: an MEG study. NeuroImage. 2008;41:212–222. doi: 10.1016/j.neuroimage.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 111.Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Berendse HW, Stam CJ. Slowing of oscillatory brain activity is a stable characteristic of Parkinson's disease without dementia. Brain. 2007;130:1847–1860. doi: 10.1093/brain/awm034. [DOI] [PubMed] [Google Scholar]
- 112.Heinrichs-Graham E, Kurz MJ, Becker KM, Santamaria PM, Gendelman HE, Wilson TW. Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson's disease: a pharmaco-magnetoencephalography study. J Neurophysiol. 2014;112:1739–1747. doi: 10.1152/jn.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heinrichs-Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres-Russotto D, Hutter-Saunders JA, Estes KA, Meza JL, Mosley RL, Gendelman HE. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson's disease. Cereb Cortex. 2014;24:2669–2678. doi: 10.1093/cercor/bht121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gaetz W, Macdonald M, Cheyne D, Snead OC. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. NeuroImage. 2010;51:792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 115.Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp. 2006;27:213–229. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. NeuroImage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol. 2011;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010;73:75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heinrichs-Graham E, Wilson TW. Coding complexity in the human motor circuit. Hum Brain Mapp. 2015 Sep 25; doi: 10.1002/hbm.23000. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson TW, Heinrichs-Graham E, Becker KM. Circadian modulation of motor-related beta oscillatory responses. Neuroimage. 2014;102(2):531–539. doi: 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson TW, Fleischer A, Archer D, Hayasaka S, Sawaki L. Oscillatory MEG motor activity reflects therapy-related plasticity in stroke patients. Neurorehabil Neural Repair. 2011;25(2):188–193. doi: 10.1177/1545968310378511. [DOI] [PubMed] [Google Scholar]
- 122.te Woerd ES, Oostenveld R, de Lange FP, Praamstra P. A shift from prospective to reactive modulation of beta-band oscillations in Parkinson's disease. NeuroImage. 2014;100:507–519. doi: 10.1016/j.neuroimage.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 123.te Woerd ES, Oostenveld R, Bloem BR, de Lange FP, Praamstra P. Effects of rhythmic stimulus presentation on oscillatory brain activity: the physiology of cueing in Parkinson's disease. NeuroImage Clin. 2015;9:300–309. doi: 10.1016/j.nicl.2015.08.018. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Christensen D, Van Naarden BK, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, Benedict RE, Kirby RS, Wingate MS, Fitzgerald R, Yeargin-Allsopp M. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 2014;56:59–65. doi: 10.1111/dmcn.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yeargin-Allsopp M, Van Naarden BK, Doernberg NS, Benedict RE, Kirby KS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002:a multisite collaboration. Pediatrics. 2008;121(3):547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 126.Center for Disease Control and Prevention. Economic costs associated with mental retardation cerebral palsy hearing loss, and vision impairment--United States, 2003. MMWR. 2004;53:57–59. [PubMed] [Google Scholar]
- 127.Kurz MJ, Wilson TW. Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy. Neurosci Lett. 2011;490(1):1–5. doi: 10.1016/j.neulet.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 128.Kurz MJ, Wilson TW, Corr B, Volkman KG. Neuromagnetic activity of the somatosensory cortices associated with body weight-supported treadmill training in children with cerebral palsy. J Neurol Phys Ther. 2012;36(4):166–172. doi: 10.1097/NPT.0b013e318251776a. [DOI] [PubMed] [Google Scholar]
- 129.Kurz MJ, Heinrichs-Graham E, Arpin DJ, Becker KM, Wilson TW. Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. J Neurophysiol. 2014;111(3):573–579. doi: 10.1152/jn.00553.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kurz MJ, Heinrichs-Graham E, Becker KM, Wilson TW. The magnitude of the somatosensory cortical activity is related to the mobility and strength impairments seen in children with cerebral palsy. J Neurophysiol. 2015;113(9):3143–3150. doi: 10.1152/jn.00602.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW. Children with cerebral palsy have uncharacteristic somatosensory cortical oscillations after stimulation of the hand mechanoreceptors. Neuroscience. 2015;305:67–75. doi: 10.1016/j.neuroscience.2015.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev Med Child Neurol. 2014;56(11):1072–1077. doi: 10.1111/dmcn.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valvano J. Neurophysiological evidence for motor planning limitations in children with cerebral palsy. Dev Med Child Neurol. 2014;56(11):1035–1036. doi: 10.1111/dmcn.12534. [DOI] [PubMed] [Google Scholar]
- 134.Salthouse TA, Babcock RL, Shaw RJ. Effects of adult age on structural and operational capacities in working memory. Psychol Aging. 1991;6:118–127. doi: 10.1037//0882-7974.6.1.118. [DOI] [PubMed] [Google Scholar]
- 135.Park DC, et al. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- 136.Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- 137.Park J, et al. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. J Neurosci. 2012;32:2154–2158. doi: 10.1523/JNEUROSCI.4494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alain C, McDonald KL. Age-related differences in neuromagnetic brain activity underlying concurrent sound perception. J Neurosci. 2007;27:1308–1314. doi: 10.1523/JNEUROSCI.5433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matilainen LE, Talvitie SS, Pekkonen E, Alku P, May PJ, Tiitinen H. The effects of healthy aging on auditory processing in humans as indexed by transient brain responses. Clin Neurophysiol. 2010;121(6):902–911. doi: 10.1016/j.clinph.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 140.Cheng CH, Baillet S, Hsiao FJ, Lin YY. Effects of aging on the neuromagnetic mismatch detection to speech sounds. Biol Psychol. 2015;104:48–55. doi: 10.1016/j.biopsycho.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 141.Aine CJ, Adair JC, Knoefel JE, et al. Temporal dynamics of age-related differences in auditory incidental verbal learning. Cogn Brain Res. 2005;24(1):1–18. doi: 10.1016/j.cogbrainres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 142.Kovacevic S, Qualls C, Adair JC, et al. Age-related effects on superior temporal gyrus activity during an auditory oddball task. Neuroreport. 2005;16(10):1075–1079. doi: 10.1097/00001756-200507130-00009. [DOI] [PubMed] [Google Scholar]
- 143.Cheng CH, Lin YY. The effects of aging on lifetime of auditory sensory memory in humans. Biol Psychol. 2012;89(2):306–312. doi: 10.1016/j.biopsycho.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 144.Ross B, Fujioka T, Tremblay KL, Picton TW. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J Neurosci. 2007;27(42):11172–11178. doi: 10.1523/JNEUROSCI.1813-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ross B, Snyder JS, Aalto M, et al. Neural encoding of sound duration persists in older adults. Neuroimage. 2009;47(2):678–687. doi: 10.1016/j.neuroimage.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 146.Huttunen J, Wikstrom H, Salonen O, Ilmoniemi RJ. Human somatosensory cortical activation strengths: comparison between males and females and age-related changes. Brain Res. 1999;818(2):196–203. doi: 10.1016/s0006-8993(98)01215-3. [DOI] [PubMed] [Google Scholar]
- 147.Stephen JM, Ranken D, Best E, et al. Aging changes and gender differences in response to median nerve stimulation measured with MEG. Clin Neurophysiol. 2006;117(1):131–143. doi: 10.1016/j.clinph.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 148.Hagiwara K, Ogata K, Okamoto T, et al. Age-related changes across the primary and secondary somatosensory areas: an analysis of neuromagnetic oscillatory activities. Clin Neurophysiol. 2014;125(5):1021–1029. doi: 10.1016/j.clinph.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 149.Zappasodi F, Pasqualetti P, Tombini M, et al. Hand cortical representation at rest and during activation: gender and age effects in the two hemispheres. Clin Neurophysiol. 2006;117(7):1518–1528. doi: 10.1016/j.clinph.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 150.Goto S, Fujisawa Y, Uemura J, Yamada S, Hoshiyama M, Hirayama M. Disinhibitory shift of recovery curve of somatosensory-evoked response in elderly: A magnetoencephalographic study. Clin Neurophysiol. 2015;126(6):1228–1233. doi: 10.1016/j.clinph.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 151.Cheng CH, Chan PY, Baillet S, Lin YY. Age-Related Reduced Somatosensory Gating Is Associated with Altered Alpha Frequency Desynchronization. Neural Plast. 2015;2015:302878. doi: 10.1155/2015/302878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vlahou EL, Thurm F, Kolassa IT, Schlee W. Resting-state slow wave power, healthy aging and cognitive performance. Sci Rep. 2014;4:5101. doi: 10.1038/srep05101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leirer VM, Wienbruch C, Kolassa S, Schlee W, Elbert T, Kolassa IT. Changes in cortical slow wave activity in healthy aging. Brain Imaging Behav. 2011;5(3):222–228. doi: 10.1007/s11682-011-9126-3. [DOI] [PubMed] [Google Scholar]
- 154.Schlee W, Leirer V, Kolassa S, Thurm F, Elbert T, Kolassa IT. Development of large-scale functional networks over the lifespan. Neurobiol Aging. 2012;33(10):2411–2421. doi: 10.1016/j.neurobiolaging.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 155.Schlee W, Leirer V, Kolassa IT, Weisz N, Elbert T. Age-related changes in neural functional connectivity and its behavioral relevance. BMC Neurosci. 2012;13:16. doi: 10.1186/1471-2202-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Proskovec AL, Heinrichs-Graham E, Wilson TW. Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Hum Brain Mapp. doi: 10.1002/hbm.23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aine CJ, Woodruff CC, Knoefel JE, et al. Aging: compensation or maturation? NeuroImage. 2006;32(4):1891–1904. doi: 10.1016/j.neuroimage.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Current Biology. 2012;22:1969–1974. doi: 10.1016/j.cub.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 159.Jiang H, van Gerven MA, Jensen O. Modality-specific Alpha Modulations Facilitate Long-term Memory Encoding in the Presence of Distracters. J Cogn Neurosci. 2015;27:583–592. doi: 10.1162/jocn_a_00726. [DOI] [PubMed] [Google Scholar]
- 160.Tuladhar AM, ter Huurne N, Schoffelen JM, Maris E, Oostenveld R, Jensen O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapp. 2007;28:785–792. doi: 10.1002/hbm.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alem O, Benison AM, Barth DS, Kitching J, Knappe S. Magnetoencephalography of epilepsy with a microfabricated atomic magnetrode. J Neurosci. 2014;34(43):14324–14327. doi: 10.1523/JNEUROSCI.3495-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


