Abstract
Purpose
We studied the changes in subtypes of diabetes mellitus (DM) in children and evaluated the characteristics of each group over the past 20 years. In addition, we also examined the correlation between the glycated hemoglobin (HbA1c) values at the time of diagnosis and lipid profiles.
Methods
The patients were divided into 2 groups: there were a total of 190 patients under 20 years of age firstly diagnosed with DM in Ajou University Hospital. The patients in groups I and II were diagnosed from September 1995 to December 2004 and from January 2005 to April 2014, respectively.
Results
The characteristics were compared between the 2 groups of patients. The result showed an increase in percentage of type 2 diabetes and maturity onset diabetes of the young (MODY) patients between the 2 groups. HbA1c and total cholesterol level had statistical significances to explain increasing the low-density lipoprotein cholesterol level among age, HbA1c, total cholesterol level, and z-scores of weight and body mass index (BMI) in type 2 diabetes. R-square was 0.074. However, z-score of BMI and total cholesterol level, not HbA1c, had statistical significances in type 1 diabetic patients. R-square was 0.323.
Conclusion
The increase in the proportions of both type 2 diabetes and MODY in the last 10 years needed to be reminded when diagnosing the subtypes of DM, and the dyslipidemia should be attended more as a common problem of pediatric diabetic patients.
Keywords: Type 2 diabetes mellitus, Child, Adolescent
Introduction
Type 2 diabetes in Korea and other Asian countries has gradually increased over the last 30 years1,2), which can occur in children as well as adults. A previous study showed that type 2 diabetes in children was found before 1990, but the incidence of pediatric type 2 diabetes are 5.3% (1 of 19) and 21.0% (8 of 38) in the years 1990 and 2000 in Korea, respectively3). However, there were few studies after the year 2000. Therefore, researching subtypes of diabetes mellitus (DM) have a meaning itself and can be helpful to diagnose, establish treatment plans, and investigations.
Moreover, lipid profiles of patients are ascertained in this study. Dyslipidemia is an emerging problem in Korea and Asian countries as one of the important modifiable risk factors for cardiovascular disease, in all children including diabetic patients4,5). Dyslipidemia-insulin resistance-hyperinsulinemia vicious cycle can cause inflammation, endoplasmic reticulum stress and lipotoxicity in diabetic patients and affect their progress6,7). Therefore, dyslipidemia should be attended more by clinicians, but the concern and/or eager to the treatment are less in the children than the adults.
In this study, therefore, the changes in subtypes of diabetes and their clinical and laboratory characteristics were reviewed from the viewpoint of the changes in subtypes of pediatric diabetes patients and their lipid profiles correlated with glycemic control.
Materials and methods
This was a retrospective study for ascertaining whether subtype of diabetes were really changed using the chart review and taking a look at the relationship between glucose and cholesterol level. We included all pediatric patients (under the age of 20) who firstly diagnosed diabetes during the years from September 1995 to April 2014 in Ajou University Hospital. The population was comprised of 190 children. They were divided into groups I and II, diagnosed during the years from September 1995 to December 2004 and from January 2005 to April 2014, respectively. Each of the 2 different groups was subdivided into 3 different subgroups: type 1 diabetes, type 2 diabetes, and maturity onset diabetes of the young (MODY). Patient information such as age at diagnosis, gender, height, weight, body mass index (BMI), family history of diabetes, anti-islet cell autoantibodies, serum cholesterol and triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) was collected from chart-records. Blood chemistry test was done in the morning with fasting state in the second day of admission.
Diagnostic criteria of type 1 diabetes were as follows3): (1) patients with ketoacidosis, (2) fasting serum C-peptide level below 0.6 ng/mL and (3) positive anti-islet cell antibodies or weight under 50th percentile as well as glycemic control impossible without insulin injection although the patients had fasting serum C-peptide level over 0.6 ng/mL.
Diagnostic criteria of MODY were identified in the patients having a family history with onset under 25 years of age through 3 and more consecutive generations, having nonautoimmune type, and good response to sulfonylurea. Insulin autoantibody (IAA), antiglutamic acid decarboxylase antibody (anti-GAD Ab) and islet cell antibody were measured among anti-islet cell autoantibodies. Positive response was defined as 0.9 units/mL and higher anti-GAD Ab level or 7% and higher IAA level.
Statistical analyses were conducted using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA) and P-values under 0.05 were reported as statistically significant. The comparison of continuous data and analysis of categorical variables were performed using Student t-test, and either chi-square test or Fisher exact test, respectively. Robust analysis of variance by Welch et al. was conducted for comparing among three different diabetic subtypes because homogeneity of variance between each subtype was not satisfied. Multiple regression analysis was also performed to estimate the magnitude of association between glycated hemoglobin (HbA1c) at the time of diagnosis and serum lipid level.
Results
There were 52 patients and 138 patients in groups I and II, respectively, and their basic characteristics are shown in Table 1. The values between the 2 groups such as age at diagnosis, gender, and height showed no statistically significant difference. Although weight and BMI were greater in group II than in group I, their z-scores showed no statistical significance. While 33% and 52.9% of patients in groups I and II had family history of DM, respectively, showing statistical significance (P=0.01).
Table 1. Basic characteristics.
| Characteristic | Group I (n=52) | Group II (n=138) | P-value† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Type 1 (n=44) | Type 2 (n=6) | MODY (n=2) | P-value | Total | Type 1 (n=91) | Type 2 (n=33) | MODY (n=14) | P-value | ||
| Demographic data | |||||||||||
| Age at diagnosis (yr) | 9.49±3.32 | 9.21±3.43 | 11.46±2.17 | 9.71±2.77 | 0.30 | 10.61±4.08 | 9.12±3.98 | 13.88±2.54 | 12.57±1.68 | <0.005* | 0.99 |
| Male sex | 25 (48.1) | 20 (45.5) | 3 (50.0) | 2 (100) | 0.50 | 72 (52.2) | 44 (48.4) | 21 (63.6) | 7 (50) | 0.34 | 0.20 |
| Family history of DM | 17 (33.3) | 13 (30.2) | 4 (66.7) | 0 (0) | 0.06 | 73 (52.9) | 40 (44.0) | 22 (66.7) | 11 (78.6) | <0.001* | 0.02* |
| Anthropometric data | |||||||||||
| Height (cm) | 136.95±22.23 | 134.38±22.53 | 152.07±17.66 | 141.65±14.65 | 0.18 | 143.08±25.16 | 133.43±24.93 | 162.81±12.03 | 158.57±10.96 | <0.001* | 0.23 |
| Height z-score | 0.35±0.99 | 0.24±0.98 | 0.92±1.06 | 0.92±0.25 | 0.21 | 0.49±1.20 | 0.33±1.23 | 0.74±1.11 | 0.90±1.05 | <0.001* | 0.18 |
| Weight (kg) | 35.21±0.99 | 30.53±13.58 | 54.80±9.70 | 48.00±11.31 | <0.001* | 44.92±1.20 | 31.74±15.97 | 72.62±19.14 | 58.16±14.13 | <0.001* | <0.01* |
| Weight z-score | 0.007±1.24 | -0.46±1.07 | 1.50±0.59 | 1.67±0.46 | <0.001* | 0.29±1.58 | -0.43±1.32 | 1.91±0.91 | 1.19±0.94 | <0.001* | 0.15 |
| BMI (kg/m2) | 18.16±4.19 | 16.32±3.18 | 23.74±2.21 | 23.72±0.73 | <0.001* | 20.11±6.29 | 16.72±3.68 | 28.14±4.48 | 22.95±4.51 | <0.001* | <0.01* |
| BMI z-score | -0.25±1.52 | -0.92±1.24 | 1.44±0.88 | 1.71±0.49 | <0.001* | 0.04±1.90 | -0.81±1.72 | 1.89±0.78 | 0.93±1.21 | <0.001* | 0.34 |
Values are presented as mean±standard deviation or number (%).
Group I (diagnosed from 1995 to 2004); group II (diagnosed from 1995 to 2004); MODY, maturity onset diabetes of the young; DM, diabetes mellitus; BMI, body mass index.
*P<0.05. †P-value, compared group I with group II.
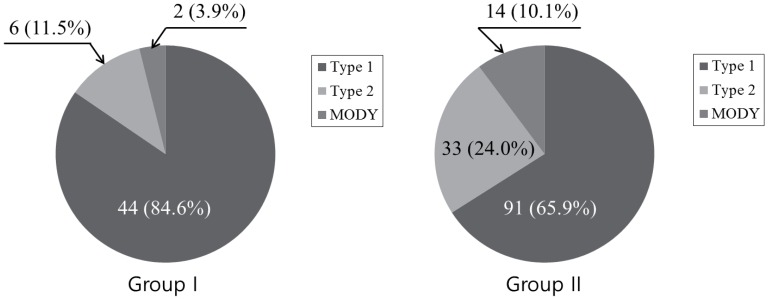
Fig. 1 shows the proportion of DM subtypes in the 2 different groups. Group I had 84.6% proportion of type 1 diabetes, 11.5% proportion of type 2 diabetes and 3.9% proportion of MODY, whereas group II had 65.9% proportion of type 1 diabetes, 24.0% proportion of type 2 diabetes and 10.1% proportion of MODY. These results showed statistical significance (P=0.04).
Fig. 1. Change of diabetes mellitus types with 2 different periods. Group I (diagnosed from 1995 to 2004); group II (diagnosed from 2005 to 2014); MODY, maturity onset diabetes of the young.
Table 2 shows the lipid levels in DM patients. There was no statistically significant difference except for triglyceride in the serum lipid levels over the last 20 years, which is consistent with the result of classification according to the subtype of DM. In multiple regression tests, HbA1c and total cholesterol level had statistical significances to explain the increase of the LDL-C level among age, HbA1c, total cholesterol level, and z-scores of weight and BMI in type 2 diabetes. R-square was 0.074. However, z-score of BMI and total cholesterol level, not HbA1c, had statistical significances in type 1 diabetic patients. R-square was 0.323.
Table 2. Glycated hemoglobin and lipid level in diabetes mellitus patients.
| Variable | Group I (n=52) | Group II (n=138) | P-value† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Type 1 (n=44) | Type 2 (n=6) | MODY (n=2) | P-value | Total | Type 1 (n=91) | Type 2 (n=33) | MODY (n=14) | P-value | ||
| HbA1c | 12.44±2.30 | 12.60±2.33 | 12.36±3.92 | 6.65±0.91 | 0.01 | 12.28±2.25 | 12.22±2.23 | 9.23±2.75 | 11.31±2.98 | <0.001 | 0.459 |
| Total cholesterol | 190.90±50.10 | 188.27±50.00 | 205.80±54.64 | 198.00±28.28 | 0.76 | 179.44±39.88 | 180.20±40.99 | 182.15±42.11 | 168.07±24.46 | 0.09 | 0.175 |
| Triglyceride | 206.11±226.24 | 216.75±237.05 | 157.4±68.33 | 129.0±0.00 | 0.15 | 146.62±159.81 | 148.55±182.18 | 154.32±109.95 | 103.55±43.60 | 0.38 | 0.009 |
| HDL | 52.90±30.16 | 49.92±12.97 | 42.60±7.76 | 44.00±0.00 | 0.15 | 45.92±13.64 | 47.34±14.83 | 41.96±10.15 | 49.44±14.95 | 0.32 | 0.066 |
| LDL | 99.02±39.32 | 94.25±38.92 | 109.8±31.95 | 131.00±0.00 | 0.23 | 107.75±35.56 | 108.51±35.97 | 112.32±32.38 | 97.2±23.02 | 0.19 | 0.394 |
Values are presented as mean±standard deviation.
Group I (diagnosed from 1995 to 2004); group II (diagnosed from 1995 to 2004); MODY, maturity onset diabetes of the young; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
†P-value, compared group I with group II.
Discussion
Over the last 10 years, in this study, the proportion of type 2 diabetes in the patients diagnosed as having DM increased approximately twice from 11.5% in group I to 23.9% in group II, which suggested that it was related to an increase in the population of obese adolescents.
In a study by Bahk and Khang8), the proportion of obese male patients among total children and adolescents increased from 3.3% to 6.9% over different periods from 1998 to 2010–2012, while the proportion of obese female patients among total children and adolescents increased from 4.7% to 7.7%. The proportions of overweight and obese male children having acanthosis nigricans, meaning insulin resistance, were 11.4% and 45.2%, respectively, while the proportions of overweight and obese female children having acanthosis nigricans were 5.9% and 43.8%, respectively9).
In the previous study, it was noticeable that the proportion of central obesity, which was related to insulin resistance and metabolic syndrome, was higher among both children and adults in Asian countries compared to those with similar weight and BMI in Western countries10,11).
This result was consistent with our practical study, which suggested that there was no statistically significant difference in z-score of weight and BMI between the 2 groups, while the number of patients diagnosed as having type 2 diabetes was remarkably increased over the last 10 years. Unfortunately, data about abdominal circumference and/or insulin resistance could not be obtained, which could have made our study more valuable.
In this study, age at diagnosis of type 2 diabetes was higher in group II (diagnosed during the period from 2005 to 2014). Type 2 diabetes was affected by puberty, which increased the insulin resistance and induced hyperinsulinemia. Both basal insulin reaction and stimulated insulin reaction increased after puberty. There were many cases suggesting that an increase in the growth hormone concentration induced insulin resistance during puberty, thereby leading to diagnosis of type 2 diabetes in mid-puberty12,13).
Generally, the type 2 diabetes patients have elevated triglycerides, low HDL-C cholesterol, and predominance of small-dense LDL-C particles, which might be related to the modulation of 3-hydroxy-3-methylglutaryl-coenzyme A-reductase. The dyslipidemia is based on inflammatory processes and on flooding of the body with energy rich substrates (glucose and/or free fatty acids) which then results in hepatic and intestinal lipoprotein overproduction leading to hypertriglyceridemia (fasting and postprandial), which in turn stimulates the secretion of apolipoprotein B (ApoB) and very LDL cholesterol (VLDL-C). In addition to high ApoB and VLDL-C, hyperinsulinemia is increased catabolism of HDL-C (resulting in low HDL-C)6,7,14,15). So, poorly controlled type 2 diabetes diabetic patient have more severe dyslipidemia.
Although few data exist on the effects of dyslipidemia on patients with type1 diabetes, some studies report that rates of dyslipidemia in the general pediatric population and in pediatric patients with well-controlled type 1 diabetes patients are similar. In contrast, in patients with type 2 diabetes, even when in good glycemic control, there are abnormalities in lipid levels, in other words, youth with type 1 diabetes also had significantly elevated Apo B levels and VLDL-C than nondiabetic youth, regardless of glycemic control16,17,18).
HbA1c reflects the concentration of serum glucose level over several weeks preceding the test. The positive correlation between HbA1c and cholesterol level in this study caused by hyperglycemic state which then result in hypertriglycemic and increased LDL-C state6,7,14,15,16,17,18).
The results of multiple regression analysis for LDL-C match to existing theory. Although factors influencing the determination of LDL-C level are variable for both types 1 and 2 diabetic patients, BMI z-score is more important consideration in the LDL-C level of type 1 diabetes patients. Whereas LDL-C level of type 2 diabetes patients affected by HbA1c level.
Nevertheless, this was a retrospective study performed at only a single tertiary medical center, which may not be representative of the population. In this study, there were some limitations such as the change in the treatment could not be analyzed during follow-up. However, the number of diabetic patients was large enough to study it as a single disease entity.
In conclusion, there was an increase in the proportions of both type 2 diabetes and clinically defined MODY in the patients diagnosed as having DM over the last 10 years. Moreover, the increase in the HbA1c at the diagnosis was related to that in total cholesterol and LDL-C levels in type 2 diabetes. Therefore, further study is needed for glycemic control of pediatric DM patients having dyslipidemia.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 2.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J, Oh J, Yu J. Autoantibody positivity and clinical characteristics of diabetes mellitus in childhood. J Korean Soc Pediatr Endocrinol. 2011;16:119–127. [Google Scholar]
- 4.Yang S, Hwang JS, Park HK, Lee HS, Kim HS, Kim EY, et al. Serum lipid concentrations, prevalence of dyslipidemia, and percentage eligible for pharmacological treatment of Korean children and adolescents; data from the Korea National Health and Nutrition Examination Survey IV (2007-2009) PLoS One. 2012;7:e49253. doi: 10.1371/journal.pone.0049253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim S, Jang HC, Park KS, Cho SI, Lee MG, Joung H, et al. Changes in metabolic syndrome in American and Korean youth, 1997-2008. Pediatrics. 2013;131:e214–e222. doi: 10.1542/peds.2012-0761. [DOI] [PubMed] [Google Scholar]
- 6.Zhu XW, Deng FY, Lei SF. Meta-analysis of Atherogenic Index of Plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes. 2015;9:60–67. doi: 10.1016/j.pcd.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Fu J, Koonen DP, Kuivenhoven JA, Snieder H, Hofker MH. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis. 2014;233:130–138. doi: 10.1016/j.atherosclerosis.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Bahk J, Khang YH. Trends in measures of childhood obesity in Korea from 1998 to 2012. J Epidemiol. 2016;26:199–207. doi: 10.2188/jea.JE20140270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y, Woo HY, Sung E, Kim CH, Kang H, Ju YS, et al. Prevalence of acanthosis nigricans in relation to anthropometric measures: community-based cross-sectional study in Korean pre-adolescent school children. Pediatr Int. 2008;50:667–673. doi: 10.1111/j.1442-200X.2008.02638.x. [DOI] [PubMed] [Google Scholar]
- 10.Lim H, Xue H, Wang Y. Association between obesity and metabolic co-morbidities among children and adolescents in South Korea based on national data. BMC Public Health. 2014;14:279. doi: 10.1186/1471-2458-14-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HM, Park J, Kim HS, Kim DH, Park SH. Obesity and cardiovascular risk factors in Korean children and adolescents aged 10-18 years from the Korean National Health and Nutrition Examination Survey, 1998 and 2001. Am J Epidemiol. 2006;164:787–793. doi: 10.1093/aje/kwj251. [DOI] [PubMed] [Google Scholar]
- 12.Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4:270–281. doi: 10.4239/wjd.v4.i6.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi KH, Hwang JS, Kim EY, Lee SH, Kim DH, Lim JS. Prevalence of insulin resistance and cardiometabolic risk in Korean children and adolescents: a population-based study. Diabetes Res Clin Pract. 2014;103:106–113. doi: 10.1016/j.diabres.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Parhofer KG. Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J. 2015;39:353–362. doi: 10.4093/dmj.2015.39.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246–1258. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz M, Giani E, Laffel L. Challenges and opportunities in the management of cardiovascular risk factors in youth with type 1 diabetes: Lifestyle and Beyond. Curr Diab Rep. 2015;15:119. doi: 10.1007/s11892-015-0692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, et al. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for Diabetes in Youth case-control study. Diabetes Care. 2009;32:416–420. doi: 10.2337/dc08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feingold KR, Grunfeld C. Diabetes and dyslipidemia. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, et al., editors. Endotext [Internet] South Dartmouth (MA): MDText.com, Inc.; c2000-2016. [cited 2016 Mar 8]. Available from: www.endotext.org. [Google Scholar]