Abstract
The efficiency of two cell types, namely adult fibroblasts, and amniotic fluid stem (AFS) cells as nuclear donor cells for somatic cell nuclear transfer by hand-made cloning in buffalo (Bubalus bubalis) was compared. The in vitro expanded buffalo adult fibroblast cells showed a typical “S” shape growth curve with a doubling time of 40.8 h and stained positive for vimentin. The in vitro cultured undifferentiated AFS cells showed a doubling time of 33.2 h and stained positive for alkaline phosphatase, these cells were also found positive for undifferentiated embryonic stem cell markers like OCT-4, NANOG and SOX-2, which accentuate their pluripotent property. Further, when AFS cells were exposed to corresponding induction conditions, these cells differentiated into osteogenic, adipogenic and chondrogenic lineages which was confirmed through alizaran, oil red O and alcian blue staining, respectively. Cultured adult fibroblasts and AFS cells of passages 10–15 and 8–12, respectively, were used as nuclear donors. A total of 94 embryos were reconstructed using adult fibroblast as donor cells with cleavage and blastocyst production rate of 62.8 ± 1.8 and 19.1 ± 1.5, respectively. An overall cleavage and blastocyst formation rate of 71.1 ± 1.2 and 29.9 ± 2.2 was obtained when 97 embryos were reconstructed using AFS cells as donor cells. There were no significant differences (P > 0.05) in reconstructed efficiency between the cloned embryos derived from two donor cells, whereas the results showed that there were significant differences (P < 0.05) in cleavage and blastocyst rates between the cloned embryos derived from two donor cell groups. Average total cell numbers for blastocyst generated using AFS cells (172.4 ± 5.8) was significantly (P < 0.05) higher than from adult fibroblasts (148.2 ± 6.1). This study suggests that the in vitro developmental potential of the cloned embryos derived from AFS cells were higher than that of the cloned embryos derived from adult fibroblasts in buffalo hand-made cloning.
Keywords: Buffalo, Adult fibroblasts, Amniotic fluid stem cells, Hand-made cloning, Embryos
Introduction
Somatic cell nuclear transfer (SCNT) through zona-free approach or hand-made cloning (HMC) has been studied in buffalo (Bubalus bubalis) in certain laboratories (Shah et al. 2008; Saha et al. 2012; Sadeesh et al. 2014). Reconstruction of embryos, however, remains one of the most difficult and demanding part of NT procedures in this species. There is some information on the comparison between in vitro culture conditions (Shah et al. 2008) and the effects of source of donor nucleus (Shah et al. 2009) on HMC in buffalo. The efficiency of obtaining live offspring from cloned buffalo embryos, however, is still very low. Therefore, much more information needs to be generated to enable large scale application of HMC technology in this species.
State of donor cells is one of the most significant factors for cloning efficiency (Kato and Tsunoda 2010). Although the quantity and quality of nevertheless unknown reprogramming factors present in oocyte determines the overall reprogramming efficiency during cloning, the degree of differentiation of donor cell also distinctly influences cloning efficiency (Rideout et al. 2000). Fetal loss and low viability were often observed in clones derived from adult cells. Berstein et al. (1996) have also proposed that cells from ear and skin tissue are less suitable for cloning due to genetic damage by ultraviolet light. Studies on the efficiency of NT with skin, kidney, gut, and muscle cells from female bovine fetuses, as well as skin, heart, kidney cells, etc. have shown that fibroblasts can support development after nuclear transfer (Kato et al. 2000). Although cloning using fibroblast nuclei offers the advantages of easy accessibility, non-invasiveness, and successful serial passages without the risk of aneuploidy, the developmental competence of fibroblasts seems to be affected not only by the genotype of the donor animal but also by the culture condition used to derive the cell lines (Heyman et al. 2002).
Many attempts have been made to establish the most competent donor cell type, especially for the mouse. Compared to somatic cells, murine embryonic stem (ES) cells give higher cloning efficiency in terms of live offspring (Wakayama et al. 1999). No significant dissimilarity was observed in the cloning efficiency of mice when somatic and NT–ES cells were compared for cloning (Wakayama et al. 2005). However, an infertile mouse was successfully cloned using NT–ES cells as donor (Mizutani et al. 2008). Although ES cells may be successful in NT in mice, this process is limited in other species where definitive ES cells have not been established so far. ES cells competent of generating germ line chimeras have not been obtained in farm animals. The pluripotent nature of these cells is not well defined and based only on expression of pluripotency markers defined for mice and human ES cells which could be ambiguous (Munoz et al. 2008). Thus investigating other somatic stem cells used in NT is necessary. Some researchers reported that use of undifferentiated donor nuclei is effective for generating cloned animals (Cheong et al. 1993; Hiiragi and Solter 2005). Compared to somatic cells, porcine stem cells give higher cloning efficiency in terms of in vitro and in vivo developmental ability of cloned embryos (Zhao and Zheng 2010). These results suggest that the undifferentiated state of donor cells may increase the cloning efficiency.
There have been only a few published reports about the results of donor cell types on the development potential of cloned buffalo embryos, since the generation of the first cloned buffalo (Shi et al. 2007). The blastocyst formation of embryos derived from cumulus cells was higher than those of embryos derived from fetal fibroblast or adult fibroblast (Shah et al. 2009). The donor cell types, adult fibroblast, fetal fibroblast or cumulus cells had similar ability to support cleavage and embryo development (Srirattana et al. 2010). The adult fibroblast derived from ear pinna and NT–ES cell-like cells derived from cloned blastocysts generated using this adult fibroblast as donor cells, when used for NT, gave almost similar cleavage and blastocyst rates (George et al. 2011). These results show that, more research is required to search a better donor cell type which can support cloned embryo production with better pregnancy rate and development to the term of cloned buffalo embryos in spite of the poor viability of buffalo NT embryos, with an exceptionally low rate of cloned buffalo calf production.
Amniotic fluid (AF) is known to contain multiple cell types derived from the developing fetus (Priest et al. 1978) of which a small percentage is believed to represent stem cell subpopulation(s), that express OCT-4, a major stem cell-specific pluripotency marker (De Coppi et al. 2007; Kim et al. 2007). Moreover, differentiation into multiple cell lineages including adipogenic, osteogenic, myogenic, endothelial, neurogenic, hepatic, and embryonic germ (EG) layers is already reported in AF cells (Tsai et al. 2004; Kim et al. 2007; Parolini et al. 2009). It has been shown that the ES cells require either feeder cells or cytokines to support their growth (Thomson et al. 1998) and frequently undergo genomic alterations and/or chromosomal aberrations during maintenance in vitro (Hanson and Caisander 2005; Maitra et al. 2005). The amniotic fluid stem (AFS) cells can be cultured without feeder cells. Apart from these, AFS cells are more easily reprogrammable than somatic cells (Li et al. 2009). The in vitro proliferative ability and expression of key pluripotency markers indicate the possibilities of using AFS cells in cloning technique for the agricultural field or for basic research.
Zhao and Zheng (2010) showed that the developmental potential of the porcine cloned embryos derived from AFS cells was higher than when derived from somatic cells. Dev et al. (2010) reported that buffalo AFS cells express many important features of pluripotent stem cells including pluripotency specific markers (OCT-4, NANOG and SOX-2). The expression of pluripotency markers in the cells suggests that AFS cells represent an intermediate stage between pluripotent ES cells stage and lineage-restricted adult stem cells. The above markers were also expressed in undifferentiated porcine AFS cells used to develop cloned porcine embryos (Zheng et al. 2009) which show that buffalo AFS cells can also be used as better donor cells for cloning experiments. The multipotent developmental characteristics of AFS cells may increase buffalo cloning efficiency, if the undifferentiated state of the donor cells affects the success rate, as observed with ES cell donors. However, no published report on production of cloned embryos using buffalo AFS cells is available. Keeping in view the aforesaid realities, the present investigation was conducted with an objective to compare the developmental rates of cloned buffalo embryos derived from AFS cells and adult fibroblast as donor cells.
Materials and methods
All chemicals, reagents, culture media were of cell culture grade and obtained from Sigma Chemicals Co. (St. Louis, MO, USA) unless otherwise indicated. Fetal bovine serum (FBS) was from Hyclone (Thermo Scientific, Wilmington, DE, USA), FBS used were from the same batch throughout the study. RNase and DNase free tips, centrifuge tubes were from Invitrogen (Carlsbad, CA, USA). Disposable 35 mm × 10 mm cell culture petri dishes, 4 wells multi dishes, six-well tissue culture plates were procured from Nunc (Roskilde, Denmark). Membrane filters (0.2 µm) were from Pall Life Sciences (Pall Corporation, Ann Arbor, MI, USA). The primers were synthesized by Sigma (P) Ltd. (Delhi, India). These studies were conducted in accordance with the guidelines laid down by the CPCSEA (Committee for the Purpose of Control and Supervision on Experiments on Animals) and with the approval from the IAEC (Institute Animal Ethics Committee).
Establishment of fibroblast cells culture
Primary ear fibroblast culture from an adult Murrah buffalo was established and prepared for cloning as reported earlier by Shah et al. (2009) with some modifications. Ear skin biopsies from adult Murrah buffalo were obtained aseptically in sterile phosphate buffered saline (PBS) with 1 % antibiotic–antimycotic solution and transferred to laboratory within 10 min. Tissue samples were washed six times with Dulbecco’s phosphate buffered saline (DPBS) and chopped into small pieces (0.5 mm). These were transferred to 25 cm2 culture flasks and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM l-glutamine, 15 % FBS, 1 % non essential amino acids, 1 % vitamins and 50 mg/ml gentamicin in a CO2 incubator (5 % CO2 in air) at 37 °C. Cells were passaged upon reaching 70–80 % confluence by partial trypsinization with 25 % EDTA and washed with cell culture medium to remove traces of the typsin—EDTA (up to passage 10–15). A half portion of the cell pellet, obtained by centrifugation (200×g, 4 °C, 5 min), was re-suspended in pre-cooled (4 °C) cryopreservation medium (DMEM supplemented with 1 % non-essential amino acids, 1 % vitamins, 1 % pen/strep/amp, 10 % (v/v) Dimethyl sulfoxide (DMSO) and 15 % FBS) stored at −80 °C overnight, and then transferred directly to liquid nitrogen (−196 °C), whereas the rest of the cells was cultured to grow further from each passage.
Immunocytochemical characterization of fibroblast cells
Immunofluorescence analyses were performed in cultured fibroblast cells based on the procedure described earlier by Fortin et al. (2010). A sterilized cover slip (35 mm2) was placed in a culture dish. The cell suspension was transferred and cultured in keratinocyte growth medium (10 % FBS, hydrocortisone and insulin at 10 μg/ml, epidermal growth factor at 10 ng/ml, and bovine pituitary extract at 60 μg/ml) until reaching approximately 70–80 % confluence. Samples were then treated with an acetone–methanol (1:1) solution for 15–20 min at room temperature and incubated in a penetrant solution (PBS with 1 % BSA and 0.1 % Tween-20) for 1 h. Subsequently, these were incubated in mouse anti-vimentin antibody solution (1:200 diluted with PBS; BM0135, BOSTER, Pleasanton, CA, USA) and mouse anti-keratin antibody solution (1:200 diluted with PBS; BM0030, BOSTER) overnight at 4 °C. On the following day, these were rinsed two to three times with PBS for 5 min each, incubated in PBS containing FITC-linked goat anti-mouse IgG (1:200 diluted; BA1101, BOSTER) for 30 min, and washed two to three times and then propidium iodide was added for nuclear staining at 5 μg/ml.
Growth kinetics
For the estimation of growth kinetics, fibroblast cells were seeded into 24-well plates (1.0 × 104 cells per well). All experiments were performed in duplicate at each time point and cell growth data were recorded until a plateau phase was reached. Determinations were made according to the method described earlier by Liu et al. (2008). The growth curve was plotted with these data, and the population doubling time (PDT) was calculated from the curve.
Collection and transportation of amniotic fluid samples
Buffalo gravid uteri at 50–100 days gestation were obtained from slaughterhouse, washed 2–3 times with isotonic saline containing 400 IU/ml penicillin and 500 µg/ml streptomycin and transported to the laboratory in a thermally insulated ice box within 4 h. The curved crown-rump (CVR) measurement was made and fetal/gestation age was estimated using Soliman equation (1975), Y = 28.66 + 4.496 X (If CVR is <20 cm), where Y is the age in days, and X is the CVR length in centimeters. Uterine incision, fetus and membranes were located, and AF was aspirated aseptically with the help of 20 ml syringe fitted with 18 gauge hypodermic needle. Amniotic fluid was collected in centrifuge tubes. The appearance and volume of fluid collected was observed.
Isolation and culture of amniotic fluid stem cells
The AFS cells were separated by centrifugation (400 g, 10 min) and washed twice with DPBS. The cell number was counted using hemocytometer and seeded at density of 103 cells/cm2 in 25 cm2 culture flask containing cell culture medium (DMEM supplemented with 15 % FBS, 1 % non-essential amino acids, 1 % vitamins, 1 % pen/strep/amp) and kept in humidified CO2 incubator at 5 % CO2 in air and 38.5 °C. Morphological features together with the shape and size of the cells, their tendency to form aggregates and their attachment to the culture flask were recorded at an interval of 24 h. The medium was replaced every 3 days. Viability of the cells was monitored by standard protocols of exclusion of trypan blue dye and the cells were counted using a hemocytometer. The cells were allowed to grow and were sub-cultured by passaging after achieving >70–80 % confluence. One half portions the cells were cryopreserved from passages 8–12 in 10 % DMSO in DMEM supplemented with 30 % FBS using the same cryopreservation protocol as described for fibroblasts, whereas rests of the cells were cultured to grow further from each passage. Passage 12 was the last time point included for characterization and differentiation studies.
Growth kinetics
For determining the growth rate of AFS cells, the same culture protocol was used as described for the fibroblast cells.
Characterization of AFS cells
Alkaline phosphatase (AP) expression
The cultured cells were screened for ES cell-like cells and alkaline phosphatase (AP) expression using AP-staining kit (Sigma Chemical Co., #86C). AFS cells were fixed in citrate–acetone–formaldehyde fixative solution for 1 min, washed three times with deionized water and incubated for 15 min at room temperature in presence of alkaline dye under dark conditions. The cells were rinsed again with de-ionized water and counter stained with neutral red and observed under inverted microscope (Nikon Inc., Tokyo, Japan) for AP staining. Cells with red stain were considered AP positive.
Expression of pluripotecny—related markers
For the analysis of mRNA expression of pluripotency—related markers (OCT-4, NANOG, SOX-2) by reverse transcription PCR (RT-PCR), total RNA was extracted from AFS cells using cell to cDNA kit (Ambion Inc, The RNA Company, Austin, TX, USA) according to the manufacturers’ protocol. The cells were washed with 200 µl ice cold PBS after which 50 µl of chilled cell lysis buffer was added and the mixture was incubated at 75 °C for 10 min in a thermal cycler. Genomic DNA was degraded by incubating the cell lysates in DNase-I at 37 °C for 30 min and the remaining activity of DNase-I was inactivated by heating at 75 °C for 5 min. For cDNA synthesis, 10 µl of the cell lysates (RNA), 4 µl dNTP mix (2.5 mM each dNTP) and 2 µl random decamer were taken in a PCR tube. The reaction mixture was mixed and incubated at 70 °C for 1 min to denature RNA for easier binding of primer in a thermal cycler. The tubes were cooled immediately on ice and remaining reverse transcriptase reagents i.e. 2 µl 10× RT buffer, 1 µl MMLV and 1 µl RNase inhibitor were added. The reaction mixture was again mixed and incubated in a thermal cycler at 42 °C for 60 min and 95 °C for 10 min to inactivate the reverse transcriptase. The synthesized cDNA was stored at −80 °C until used for amplification step.
PCR reaction was carried out in a 50 µl final volume containing 45 µl platinum PCR supermix (Invitrogen, Carlsbad, CA, USA) and 5 µl of primer (200 nM each, Sigma, St. Louis, MO, USA) and template DNA solution. A set of reaction without template cDNA was used as negative control for PCR reaction. GAPDH was used as the reference gene. The primer sequences used for GAPDH, OCT4, NANOG and SOX-2 are mentioned in Table 1. The PCR conditions were the same except for the annealing temperature (Table 1), as 94 °C for 2 min (Initial denaturation), denaturation at 94 °C for 30 s, elongation at 72 °C for 1 min (35 cycles). The amplified DNA fragments were resolved on 2 % agarose gel containing 0.5 µg/ml ethidium bromide against a 100-bp ladder and visualized under gel documentation system (Alpha Imager, Alpha Innotech, San Leandro, CA, USA). The gene-specific bands were excised and purified using AuPrep Gel Extraction kit (Life Technologies India Pvt. Ltd., Delhi, India).
Table 1.
Detail of primers used for expression of pluripotency genes
| Sr No | Gene | Primer sequence | Annealing temp (°C) | Size (bp) | Source | Accession No |
|---|---|---|---|---|---|---|
| 1 | GAPDH | CCTGCCAAGTATGATGAGA (F) | 53 | 131 | Bubalus bubalis | GU324291 |
| GAAGGTAGAAGAGTGAGTGT (R) | ||||||
| 2 | OCT-4 | GTTCTCTTTGGAAAGGTGTTC (F) | 54 | 341 | Bos taurus | AF487022 |
| ACACTCGGACCACGTCTTTC (R) | ||||||
| 3. | NANOG | GGGAAGGGTAATGAGTCCAA (F) | 56 | 211 | Bubalus bubalis | DQ487022 |
| AGCCTCCCTATCCCAGAAAA (R) | ||||||
| 4. | SOX-2 | CATGGCAATCAAAATGTCCA (F) | 54 | 215 | Bos taurus | DQ126150 |
| AGACCACGGAGATGGTTTTG (R) |
In vitro induced differentiation
For differentiation, cultured cells were detached by trypsinization, centrifuged and then cultured in lineage-specific differentiation media for 3 weeks. The cells were seeded in tissue culture grade six-well plates. For osteogenic differentiation, DMEM supplemented with 10 % FBS, 100 nM dexamethasone, 50 μM ascorbic acid and 10 mM β-glycerol phosphate was used. The differentiation of cells was assessed morphologically and stained with alizarin red which indicates the calcium mineralization in cells. To induce adipogenic differentiation, the cells were cultured in DMEM supplemented with 10 % FBS, 1 μM dexamethasone, 500 μM isobutylmethylxanthine, 60 μM indomethacin and 5 μg/ml insulin. The presence of intracellular lipid globules indicative of adipogenic differentiation was assessed by staining cells with oil red O solution on day 21. The medium was replaced twice a week. Chondrogenic differentiation was induced in confluent monolayer cultures of AFS cells using a specific chondrogenesis differentiation kit (StemPro, Invitrogen). Differentiated cells were stained with alcian blue 8GX (Sigma-Aldrich) on day 21. Non induced control cells were cultured for the same time in standard control medium (DMEM supplemented with 15 % FBS, 1 % non-essential amino acids, 1 % vitamins, 1 % pen/strep/amp).
Preparation of donor cells
Cultured adult fibroblasts and AFS cells of passages 10–15 and 8–12, respectively, were used as nuclear donors. After a minimum of 1 week of cryopreservation, one vial of cryopreserved cells was thawed at 37 °C in a water bath for approximately 15 s, then transferred into centrifuge tube and the cells were suspended in same growth medium as described for isolation and primary expansion. To remove the cryoprotectants, the contents were centrifuged twice at 200×g for 10 min, and the pellet was dissolved and cultured in a 25 cm2 culture flask with culture medium for 2–3 days before use as donor cells. A small fraction of cells was used to evaluate the cell viability with trypan blue dye exclusion method and counting live (not accepting stain) and dead (stained) cells using hemocytometer under phase contrast microscope (Nikon, Tokyo, Japan). Immediately before use, the proliferated donor cells were harvested by trypsinization and washed by centrifugation and resuspended in T20 media (T denotes HEPES modified TCM-199 supplemented with 2.0 mM l-glutamine, 0.2 mM sodium pyruvate, 50 µg/ml gentamicin and the following 20 number denotes 20 % FBS), for use as nucleus donor cells.
Collection of oocytes
Ovaries from reproductive organs of adult, apparently healthy female buffaloes collected from abattoir within 30 min of slaughter were washed three times with warm isotonic saline (35–37 °C) containing 400 IU/ml penicillin and 500 μg/ml streptomycin and transported to the laboratory within 4–6 h. Aspiration of cumulus oocyte complexes (COCs) were performed as described earlier (Chauhan et al. 1998) with some modifications. Oocytes from follicles (2–8 mm) were aspirated with 18 gauge needle attached to 10 ml syringe (Sigma Chemical Co., # Z248029) loaded with aspiration medium (TCM-199 containing 0.3 % BSA, 0.1 mg/ml glutamine and 50 μg/ml gentamicin). The oocytes were washed four to six times with the washing medium which consisted of TCM-199 with 10 % FBS, 0.09 mg/ml sodium pyruvate, 0.1 mg/ml l-glutamine and 50 μg/ml gentamicin. COCs having a compact and unexpanded cumulus mass with equal to or greater than three layers of cumulus cells and homogenous granular ooplasm were selected for in vitro maturation (IVM).
Follicular fluid collection and preparation
Follicular fluid was collected from all categories of morphologically healthy surface follicles by aspiration using 10 ml syringe with 18 gauge needle. Criteria for assessment of follicular health established earlier (Kruip and Dieleman 1982) for bovine ovaries were applied in this experiment to assess the buffalo follicles. For each collection, the follicular fluid was pooled and centrifuged twice at 3,000 rpm for 10 min. The supernatant was collected and double filtered through a 0.2 μm membrane filter. The fluid was stored in sterile 1.5 ml capacity micro centrifuge tubes at −20 °C for subsequent use in IVM.
In vitro maturation
COCs were subjected to maturation in IVM medium consisting of TCM-199 + sodium pyruvate (0.80 mM) + l-glutamine (2 mM) + 10 % FBS + 5 % follicular fluid + PMSG (20 IU/ml) + hCG (10 IU/ml) + gentamicin (50 μg/ml). The pH of the medium was adjusted to 7.4 and filtered through 0.22 μm membrane filter immediately before use. The COCs were washed several times with IVM medium and group of 15–20 COCs were placed independently in 100 μl droplets of IVM medium covered with sterilized mineral oil in 35 mm Petri dishes and cultured for 21 h under 5 % CO2 at 38.5 °C.
Preparation of recipient cytoplast and hand-made cloning (HMC)
The recipient cytoplast preparations from in vitro matured oocytes and the procedures for HMC were performed using standard protocols as described earlier (Shah et al. 2008).
Embryo culture
The activated embryos were cultured in 400 µl of Research Vitro Cleave medium (K-RVCL-50, Cook®, Brisbane, QLD, Australia) supplemented with 1 % fatty acid-free (FAF) BSA in a four well dish (15–20 embryos/well) covered with mineral oil and kept undisturbed in a humidified CO2 incubator at 38.5 °C. Embryo production rate was examined under inverted microscope (Nikon Inc., Tokyo, Japan) to record the number of cleaved embryos and blastocyst formation at 48 h post-activation (h.p.a), and 168–192 h.p.a, respectively. Blastocysts were stained with Hoechst 33342 for 1 h and the total number of their nuclei was counted as described earlier by Saikhun et al. (2004).
Experimental design and statistical analysis
The data were analyzed using SYSTAT 7.0 (SPSS Inc. Chicago, IL, USA). All values are presented as mean ± SEM unless indicated otherwise. Differences among means were analyzed by one way ANOVA after arcsine transformation of the percentage data. The differences were considered significant at P < 0.05. Two dissimilar cell types (adult fibroblasts and AFS cells) were used for determining the effect of donor cell types on developmental competence of hand-made cloned buffalo embryos. The cleavage and total blastocyst production rates were compared between these two groups.
Results
Isolation, culture and characterization of fibroblast cells
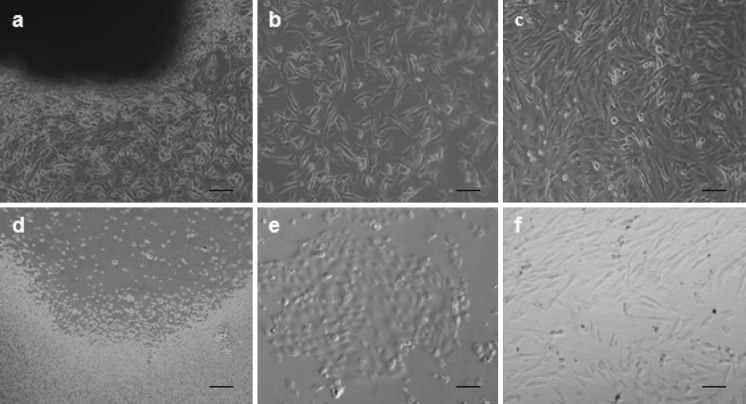
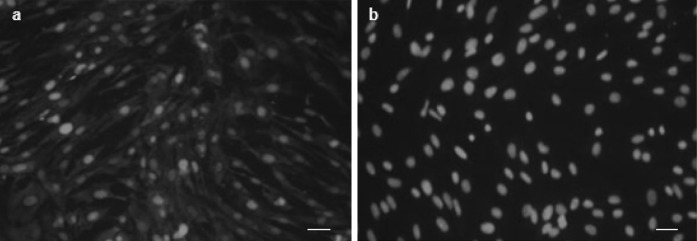
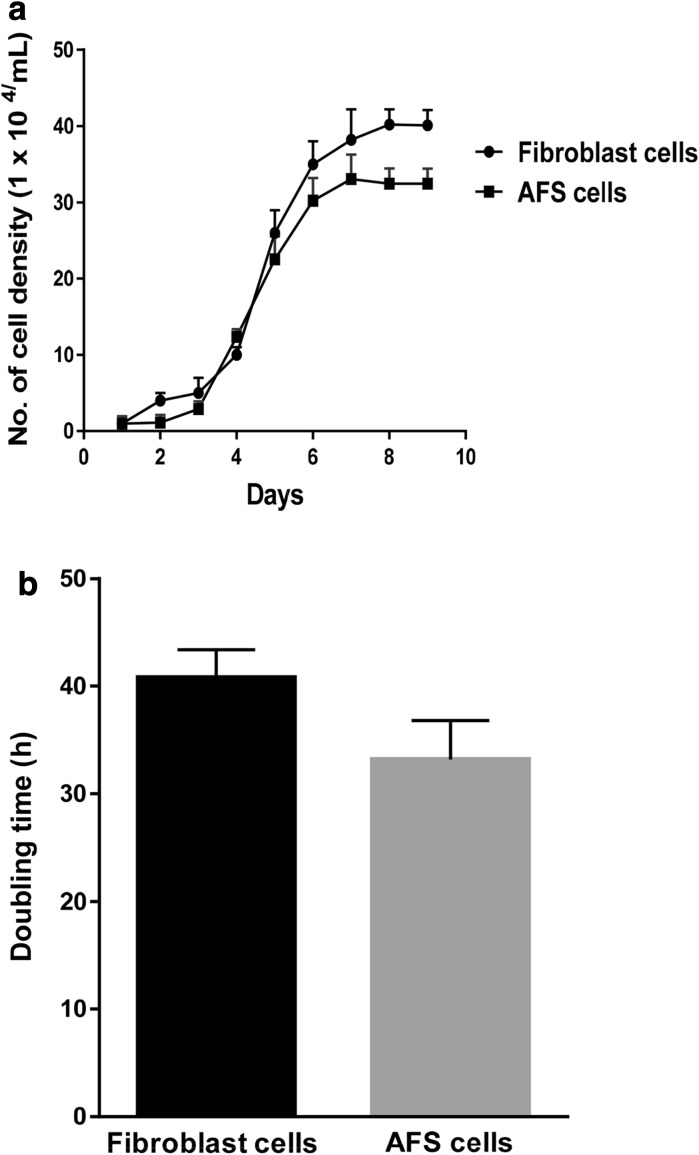
Fibroblast cells derived from buffalo ear skin could be seen migrating from the tissue pieces within 5–7 days after explanting. When time in culture was increased majority of cells adhered to the surface of the culture flasks. The attached cells expanded with spindle-shaped morphology resulting in primary cultures and were subcultures when they reached 70–80 % confluence. Representative photographs of cultured fibroblast cells are shown in Fig. 1a–c. For cell type determination, the samples were examined using fluorescence microscopy after immunofluorescent staining. Results of the fibroblast cell lines were positive for vimentin and negative for keratin (Fig. 2). Growth curve of these cells showed a typical “S”shape (Fig. 3a). An adaptive phase was apparent after cells were seeded. This phase lasted for about 2 days. Then cells entered a platform period, after which they showed a logarithmic growth period lasting 4 or 5 days. The population doubling time (PDT) was found to be 40.8 h (Fig. 3b).
Fig. 1.
Isolation and culture of adult fibrobalsts and AFS cells. a Primary explants outgrowth from ear skin tissue, b Subcultured fibroblast cells having 70–80 % confluence, c Confluent monolayer of subcultured cells of fibroblast, d Primary culture of AF cells on the day of seeding, e Attached cells with non-fibroblastic epithelial cell like phenotype, f spindle-shaped fibroblastic-like AFS cells (200 µm)
Fig. 2.
Expression of cell-specific marker in somatic cells derived from ear skin of buffalo. a Immunofluorescent staining of vimentin for fibroblasts, b negative control for vimentin (50 µm)
Fig. 3.
Cell proliferation rate (a) and population doubling time (b) of fibroblast and AFS cells
Isolation, culture and characterization of AFS cells
Following collection, all cells were spherical and variable in sizes. No anchorage was observed before 48–72 h of culturing cells. After day 5–6, morphologically different cells were observed and after 7–8 days of culturing most of the cells converted into star shaped cells which subsequently gained typical fibroblast—like shape and formed a confluent monolayer which did not change further. In addition to forming uniform cell monolayer, certain cell clumps were also observed. Starting from the second passage, the AFS cells became homogeneous exhibiting fibroblast like morphology. Initially the cells reached 75–80 % confluence after 2 weeks. However, the passaged cells exhibited higher growth rate, reaching a 95–100 % confluence after day 6 of culturing. Representative photographs of cultured AFS cells are shown in Fig. 1d–f. The growth curve of AFS cells (Fig. 3a) had the following characteristics: (1) in the first 2 days after inoculation the cells adhered to the tissue culture plates (2) on day 3, the cells entered the logarithmic growth stage (3) peak growth was on day 7 and (4) the population doubling time (PDT) was found to be 33.2 h (Fig. 3b).
Characterisation of AFS cells by alkaline phosphatase staining
Cell monolayer formed by buffalo amniotic fluid-derived stem cells showed alkaline phosphatase activity on day four of culture. The cells were found to stain positive for AP. AFS cells showed ES cell-like cells properties by AP staining. The cultured AFS cells were stained red and were considered as positive for AP expression (Fig. 4a).
Fig. 4.
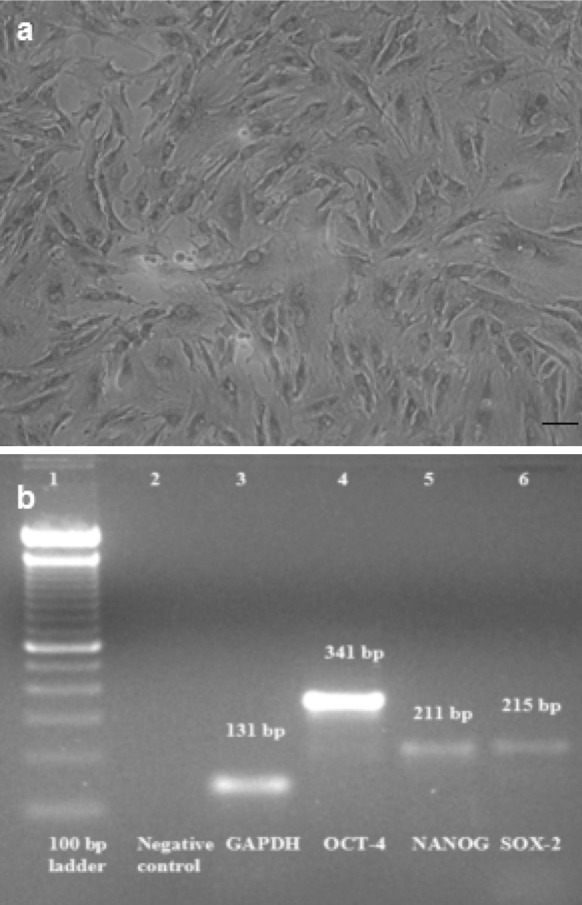
Characterization of AFS cells. a Alkaline phosphatase staining showing red colored AP positive cells (100 µm), b RT-PCR analysis of pluripotency gene expression in AFS cells, agarose gel electrophoresis of analysis RT-PCR product revealed a 341, 211 and 215 bp amplicon respectively of OCT-4, NANOG and SOX-2 genes, GAPDH has been employed as reference gene, where Lane 1 100 bp ladder 2 negative control 3 GAPDH 4 OCT-4 5 NANOG 6 SOX-2
Characterization of AFS cells for OCT-4, NANOG and SOX-2 expression by RT-PCR
The expression of OCT4, NANOG and SOX-2 genes was studied to characterize the AFS cells for stemness property. Agarose gel electrophoresis of analysis RT-PCR products revealed PCR amplicons of 341, 211 and 215 bp, respectively, of OCT-4, NANOG and SOX-2 genes in buffalo AFS cells with GAPDH (131 bp) as housekeeping gene (Fig. 4b). The gene-specific bands were purified using AuPrep gel extraction kit and got sequenced. The resulting sequences were aligned and analysed using online Basic Local Alingnment Search Tool (BLAST; National Centre for Biotechnology Information, US National Library of Medicine, Bethesda, MD, USA; http://www.ncbi.nlm.nih.gov). The OCT-4 sequence had 93 % identity with Bos taurus OCT-4 mRNA and 90 % with pig DNA sequence from clone CH242-102G9 on chromosome 7. Alignment of NANOG sequence showed 95 % homology with Bubalus bubalis homeobox transcription factor and 91 % with Bos taurus homeobox transcription factor NANOG mRNA. SOX-2 showed 98 % homology with Bos taurus SOX2 mRNA, and 96 % identity with pig DNA sequence from clone CH242-330B10 on chromosome 13.
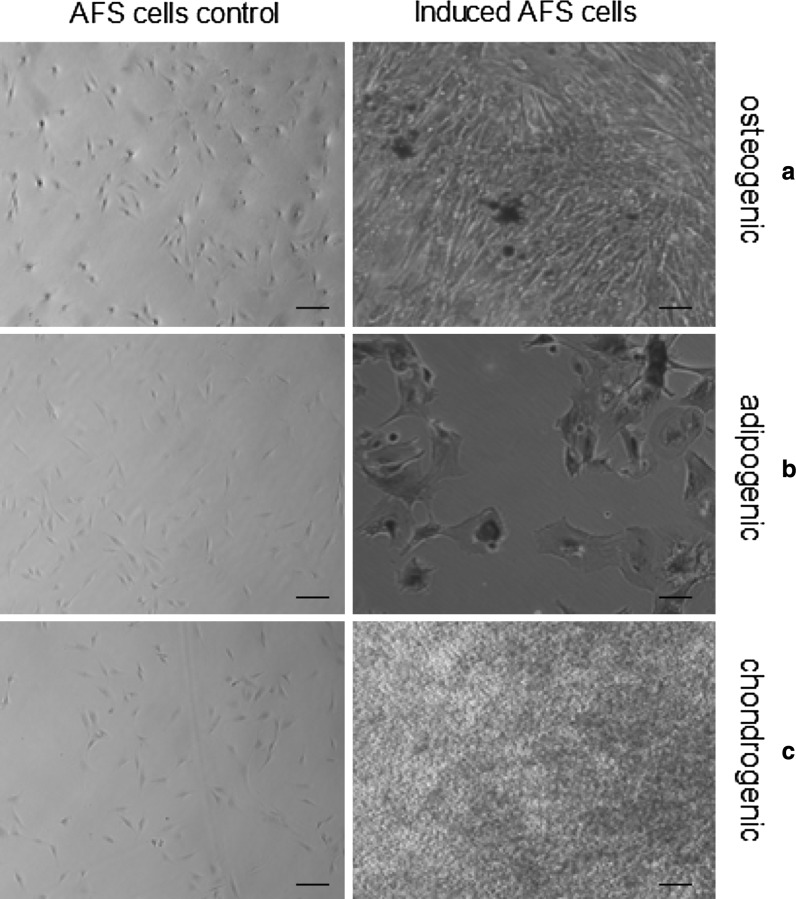
Differentiation potential of AFS cells
When AFS cells were cultured in osteogenic differentiation medium, the cells started changing morphology after 8–9 days of incubation. The cells were stained with Alizarin red on the 21st day of culture in osteogenic condition. The positive expression of Alizarin red confirmed the presence of calcium deposits in differentiated cells (Fig. 5a). When cells were cultured under adipogenic conditions, they differentiated into adipocytes and exhibited high intensity of oil red O stain in the cytoplasm of cells on the 21st day of post-induction, signifying the presence of lipid globules (Fig. 5b). The chondrogenic potential of AFS cells was evaluated by in vitro culture of these cells in a specific serum-free chondrogenic medium. The accumulation of sulfated proteoglycans was visualized by alcian blue staining on the 21st day of post-induction (Fig. 5c). Cells maintained in regular control medium did not stain positively in any of the groups.
Fig. 5.
In vitro multipotent differentiation potential of AFS cells. a Alizarin red positive osteogenic differentiated cells, b Oil red O positive adipogenic differentiated cells, c Alcian blue positive chondrogenic differentiated cells (100 µm)
Post-thaw cell viability
Viability of fibroblasts and AFS cells was 98.9 and 98.5 % before cryopreservation and post-thaw 83.8 and 85 % as assessed by trypan blue dye exclusion method. The culture behaviour and morphology of freeze–thawed cells were similar as that of fresh cells before cryopreservation.
In vitro developmental ability of hand-made cloned embryos from adult fibroblasts and AFS cells
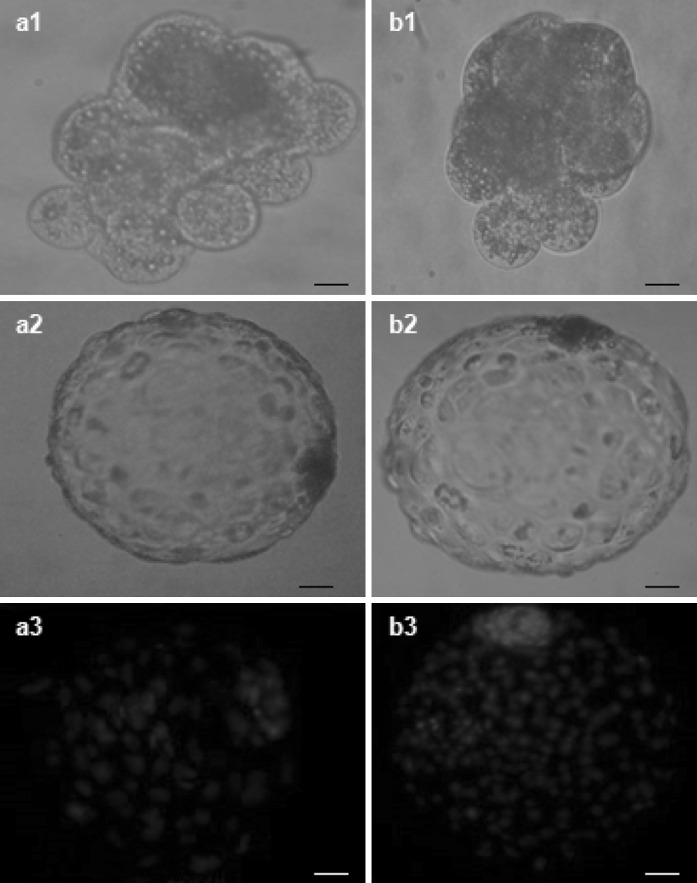
A total of 2,256 buffalo COCs with a maturation rate of 77.9 % (1,759/2,256) were used for the investigation. Of these, 1,756 COCs were used for production of hemi-cytoplasts. A total of 796 hemi-cytoplasts were used to reconstruct 191 embryos. A total of 94 embryos were reconstructed using adult fibroblast as donor cells with cleavage and blastocyst formation rates of 62.8 ± 1.8 and 19.1 ± 1.5, respectively. An overall cleavage and blastocyst formation rate of 71.1 ± 1.2 and 29.9 ± 2.2, respectively, was obtained when 97 embryos were reconstructed using AFS cell as donor cells. The results showed no significant differences (P > 0.05) in reconstruction efficiency between the cloned embryos derived from adult fibroblasts and AFS cells; whereas there were significant differences (P < 0.05) in cleavage and blastocyst rates between NT embryos derived from adult fibroblasts and AFS cells. The in vitro developmental potential of the cloned embryos derived from AFS cells was higher than that of the cloned embryos derived from adult fibroblasts. A total number of 14 blastocysts reconstructed using adult fibroblasts and AFS cells were used for determination of total number of cells. Average total cell numbers for blastocyst generated using AFS cells (172.4 ± 5.8) was significantly (P < 0.05) higher than from adult fibroblasts (148.2 ± 6.1). Representative photographs of HMC derived buffalo embryos from adult fibroblasts and AFS cells are shown in Fig. 6.
Fig. 6.
Development in vitro of buffalo hand-made cloned embryos derived from adult fibroblasts (a1–a3) and AFS cells (b1–b3). a1 and b1 8–cell stage embryo, a2 and b2 blastocyst, a3 and b3 Hoechst 33342 stained blastocyst (50 μm)
Discussion
Somatic cell nuclear transfer (SCNT) is one of the assisted reproductive techniques without adequate proficiency to facilitate large-scale commercial use. Even though many mammalian species have been cloned till date, the percentage of reconstructed oocytes that develop into normal, healthy offspring remains astonishingly low often below 1 % (Panarace et al. 2007). For successful SCNT, correct nuclear reprogramming in somatic cell genome by the recipient cytoplast is a must. The source of biological material has a great impact on the outcome of cloning experiments (Vajta 2007). As far as cloning of buffalo is concerned, reconstruction of embryos remains one of the most tricky and challenging part of NT procedures; although NT has been performed in certain laboratories. The success of obtaining live offspring from cloned buffalo embryos is still very low.
State of the donor cell is one of the most important factors for cloning efficiency. Developmental abnormalities in cloned mammals are mainly due to abnormal epigenetic reprogramming of the donor genome (Li et al. 2003). The competence of reprogramming by NT to enucleated oocytes varies significantly among the different types of cells used. Yang et al. (2007) reported that the success of nuclear reprogramming decreases as donor cells become more differentiated. Together, fetal and adult fibroblasts have been effectively used for NT, signifying that these tissues even in the older animals might enclose progenitor cells which are in a comparatively undifferentiated state and as a result, are capable of division. The success rate for cloning mice from ES cells is relatively high compared to that for differentiated cells (Wakayama et al. 1999; Rideout et al. 2000). This suggests that a cell in an undifferentiated state may be suitable donor cell in animal cloning. Although ES cells may be successful for NT in mice, this process is limited in other species where definitive ES cells have not been established. Thus investigating other somatic stem cells used in NT is necessary. At present we could not correlate this result with buffalo as reports are inadequate about the effect of donor cell types on the developmental potential of cloned embryos in this species. Shah et al. (2008) reported that the type of donor cell is an important factor influencing the production of cloned buffalo embryos using NT technique, as it affects the post-cleavage developmental competence of the reconstructed embryos. Zhao and Zheng (2010) reported that the developmental potential of the porcine cloned embryos derived from AFS cells were higher than that of the cloned embryos derived from adult fibroblasts. The possibility of using AFS cells as donors was explored in the present study in view of the very low overall cloning efficiency obtained with adult somatic cells in buffalo (Shah et al. 2009).
After culturing for few passages, purified fibroblasts were confirmed by immunofluorescent staining for vimentin and lack of keratin. This result is in agreement with Selokar et al. (2012). They reported that the somatic cells derived from the ear skin of buffalo were found to be of fibroblast origin as these expressed vimentin but not keratin. In the present work, fibroblasts of skin tissue were successfully established using adherent culture methodology. The growth curves of these cells showed a typical “S”shape and the population doubling time was found to be 40.8 h which was quite comparable with the earlier report in this species (Priya et al. 2014). Cell viability before freezing and thawing was above 80 % for these cells which indicate that ear fibroblasts have a greater tolerance to trypsin digestion and liquid nitrogen cryogenic preservation.
Recent studies of stem cell populations in amniotic fluid have postulated that the well characterized amniotic fluid is a promising alternative source of fetal stem cells (Prusa et al. 2003, 2004; In’t Anker et al. 2003, 2004). The choice of the culture medium and conditions chosen to grow buffalo AFS cells were based on reports already established for human AFS cells (De Coppi et al. 2007). However, the final selection was based on our preliminary observations on the growth of the cells in various combinations of culture media and supplements (data not shown). After 7 days of incubation, the buffalo AFS cells had morphologically different types of cells. From second passage onwards, most of the cells exhibited fibroblast-like appearance. Our results corroborated with earlier observation that fibroblast like cells were the dominant stem cells in amniotic fluid (Rho et al. 2009). These data are also in accordance with the report of Gosden (1983), who demonstrated that epithelial-like cells can be found in the beginning, while fibroblast-like cells usually appear later. These epithelial like cells are thought to be derived from the fibrous connective tissue and dermal fibroblasts (Gosden 1983).
AFS cells had a doubling time of 33.2 h, which was quite comparable to the doubling time of human (Choi et al. 2011) and porcine AFS cells (Chen et al. 2011). Another parameter that indicated the high proliferative property of the isolated cells was their growth curve characteristics. According to this calibrated curve plotted for AFS cells, there was a short lag phase implying that the cells rapidly recovered from the damage that occurred during detachment by enzymatic treatment. In the present study, strong positive expression of AP staining was demonstrated in AFS cells. The AP is a stem cell membrane marker, and elevated expression of this enzyme is associated with undifferentiated pluripotent stem cell state. Most of the pluripotent stem cells, like ES and embryonal carcinoma (EC) cells, express AP activity (Shamblott et al. 1998; Hua et al. 2009). Among the various pluripotency markers studied till date, OCT-4, NANOG and SOX-2 are one of the key regulators thought to be crucial for maintaining the gene regulatory networks for pluripotency during early mammalian embryonic development (Boyer et al. 2005; Hart et al. 2004; Yadav et al. 2010). Using species-specific primers, PCR amplicons of 341, 211 and 215 bp were observed for OCT-4, NANOG and SOX-2, respectively, in buffalo AFS cells. These results indicate that these cells were in an undifferentiated state. Ovine AF-derived stem cells were found to proliferate and express the key pluripotency markers including OCT-4 and TERT (Mauro et al. 2010). There are good numbers of reports available regarding the multilineage differentiation potential of bone marrow, adipose tissue and umbilical cord derived stem cells but very few reports are available for amniotic fluid derived stem cell differentiation potential especially of farm animal origin. The present study demonstrates that these cells, like their marrow counterparts, were able to differentiate into adipose cells in addition to osteogenic and chondrogenic lineages at par with the earlier reports from human (De Coppi et al. 2007), ovine (Mauro et al. 2010) and porcine (Chen et al. 2011) stem cells. These data also demonstrate the mesenchymal properties of the selected cell population and are thus in accordance with previous reports about the broadly pluripotent quality of amniotic fluid-derived cells (Arnhold et al. 2011). Stem cells isolated from human amniotic fluid have been reported to differentiate into the cells of mesenchymal lineages (Bossolasco et al. 2006). The multipotent developmental characteristics of AFS cells may increase buffalo cloning efficiency, if the undifferentiated state of the donor cells affects the success rate, as observed with ES cell donors. However, there are no previous published reports on production of cloned embryos using buffalo AFS cells.
Our results demonstrate the developmental potential to the blastocyst stage of cloned buffalo embryos derived from two different cell types, adult fibroblast and AFS cells. To our knowledge this is the first comparison study about the effect of adult fibroblast and AF cells in buffalo HMC. A total of 94 embryos were reconstructed using adult fibroblast as donor cells with cleavage and blastocyst production rates of 62.8 ± 1.8 and 19.1 ± 1.5, respectively. An overall cleavage and blastocyst formation rate of 71.1 ± 1.2 and 29.9 ± 2.2 was obtained when 97 embryos were reconstructed using AFS cell as donor cells. Our results suggest that adult fibroblast and AFS cells can be reprogrammed via HMC to support the development of the resultant embryos to the blastocyst stage in buffalo. However, cleavage, blastocyst formation and mean cell numbers per blastocyst for cloned buffalo embryos derived from AFS cells were higher than those derived from adult fibroblasts (P < 0.05). This reveals that AFS cells were not similar to adult fibroblasts in terms of cell cycle which may be because of the state of donor cells. In an earlier study also, significant differences in cleavage and blastocyst rates were found using AFS cells as donor cell in porcine as compared to adult fibroblast cells (Zhao and Zheng 2010). Thus, our results support the notion that the source of the donor cells may be one of the most important factors in determining the success of NT (Miyoshi et al. 2003; Powell et al. 2004). The phenomenon of epigenetic reprogramming can be made more efficient by the use of this cell type as these cells represent an intermediate stage between pluripotent ES cell stage and lineage restricted adult stem cells.
In our results, cleavage and blastocyst formation rates of HMC buffalo embryos obtained using adult fibroblast were less than those reported earlier in this species by Shah et al. (2008). Bhojwani et al. (2005) reported that the factors that probably contribute to the low level of efficiency in cloning include laboratory to laboratory variation, oocyte source and quality, donor cell types, fusion or activation methods and methods of embryo culture system. Some reports suggest that there are no clear relationships in the blastocyst formation rates among embryos derived from different donor cell types which may be due to the different cell culture systems, cloning protocols, embryo culture systems used and skills of lab personnel.
In conclusion, our study establishes that buffalo AFS cells can be derived successfully from amniotic fluid, which exhibit morphology, growth characters, osteogenic, adipogenic and chondrogenic lineages differentiation potential comparable with AFS cells of other species. The developmental potential of the cloned embryos derived from AFS cells were higher (P < 0.05) than that of the cloned embryos derived from adult fibroblast in buffalo HMC which suggests that the undifferentiated state of donor cell may increase cloning efficiency. However, the pregnancy rate, and the development to the term of cloned embryos derived from these two different cell types need further investigation.
Acknowledgments
The authors would like to thank Dr. Inderjeet Singh, Director, ICAR-Central Institute for Research on Buffaloes, for providing the necessary facilities for carrying out this work. We are also thankful to Dr. B S Punia for reviewing the manuscript. Funding support from Indian Council of Agricultural Research (ICAR), New Delhi is gratefully acknowledged.
Conflict of interest
The authors declare no conflict of interest.
References
- Arnhold S, Glüer S, Hartmann K, Raabe O, Addicks K, Wenisch S, Hoopmann M. Amniotic fluid stem cells: growth dynamics and differentiation potential after a CD-117-based selection procedure. Stem Cells Int. 2011;2011:715341. doi: 10.4061/2011/715341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstein EF, Chen YQ, Kopp JB, Fisher L, Brown DB, Hahn PJ, Robey FA, Lakkakorpi J, Uitto J. Long term sun exposure alters the collagen of the papillary dermis. J Am Acad Dermatol. 1996;34:209–218. doi: 10.1016/S0190-9622(96)80114-9. [DOI] [PubMed] [Google Scholar]
- Bhojwani S, Vajta G, Callesen H, Roschlau K, Kuwer A, Becker F, Alm H, Torner H, Kanitz W, Poehland R. Developmental competence of HMC™ derived bovine cloned embryos obtained from somatic nuclear transfer of adult fibroblast and granulosa cells. J Reprod Dev. 2005;51:465–475. doi: 10.1262/jrd.17025. [DOI] [PubMed] [Google Scholar]
- Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Buiatiotis S, Soligo D, Bosari S, Silani V, Deliliers GL, Rebulla P, Lazzari L. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–336. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan MS, Singla SK, Palta P, Manik RS, Tomer OS. Development of in vitro produced buffalo (Bubalus bubalis) embryos in relation to time. Asian Aust J Anim Sci. 1998;11:398–403. doi: 10.5713/ajas.1998.398. [DOI] [Google Scholar]
- Chen J, Lu Z, Cheng D, Peng S, Wang H. Isolation and characterization of porcine amniotic fluid-derived multipotent stem cells. PLoS One. 2011;6:e19964. doi: 10.1371/journal.pone.0019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong HT, Takahashi Y, Kanagawa H. Birth of mice after transplantation of early cell-cycle-stage embryonic nuclei into enucleated oocytes. Biol Reprod. 1993;48:958–963. doi: 10.1095/biolreprod48.5.958. [DOI] [PubMed] [Google Scholar]
- Choi SA, Lee JH, Kim KJ, Kim EY, Park KS, Park YB, Li X, Ha YN, Park JY, Kim MK. Isolation and characterization of mesenchymal stem cells derived from human amniotic fluid. Reprod Fertil Dev. 2011;23:243–253. doi: 10.1071/RDv23n1Ab291. [DOI] [Google Scholar]
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Dev K, Giri SK, Kumar A, Yadav A, Singh B, Gautam SK. Derivation, characterization and differentiation of buffalo (Bubalus bubalis) amniotic fluid derived stem cells. Reprod Dom Anim. 2010;47:704–711. doi: 10.1111/j.1439-0531.2011.01947.x. [DOI] [PubMed] [Google Scholar]
- Fortin S, Mercier LM, Camby I, Spiegl-Kreinecker S, Berger W, Lefranc F. Galectin-1 is implicated in the protein kinase Cε/Vimentin-controlled trafficking of integrin-β1 in glioblastoma cells. Brain Pathol. 2010;20:39–49. doi: 10.1111/j.1750-3639.2008.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A, Sharma R, Singh KP, Panda SK, Singla SK, Palta P, Manik R, Chauhan MS. Production of cloned and transgenic embryos using buffalo (Bubalus bubalis) embryonic stem cell-like cells isolated from in vitro fertilized and cloned blastocysts. Cell Reprogram. 2011;13:263–272. doi: 10.1089/cell.2010.0094. [DOI] [PubMed] [Google Scholar]
- Gosden CM. Amniotic fluid cell types and culture. Br Med Bull. 1983;39:348–354. doi: 10.1093/oxfordjournals.bmb.a071847. [DOI] [PubMed] [Google Scholar]
- Hanson C, Caisander G. Human embryonic stem cells and chromosome stability. APMIS. 2005;113:751–755. doi: 10.1111/j.1600-0463.2005.apm_305.x. [DOI] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Ibrahim M, Robb L. Identification, cloning and expression analysis of the pluripotency promoting NANOG genes in mouse and human. Dev Dyn. 2004;230:187–198. doi: 10.1002/dvdy.20034. [DOI] [PubMed] [Google Scholar]
- Heyman Y, Zhou Qi, Lebourhis D, Chavatte-Palmer P, Renard JP, Vignon X. Novel approaches and hurdles to somatic cloning in cattle. Cloning Stem Cells. 2002;4:47–55. doi: 10.1089/153623002753632048. [DOI] [PubMed] [Google Scholar]
- Hiiragi T, Solter D. Reprogramming is essential in nuclear transfer. Molec Repro Dev. 2005;70:417–421. doi: 10.1002/mrd.20126. [DOI] [PubMed] [Google Scholar]
- Hua J, Yu H, Liu S, Dou Z, Sun Y, Jing X, Yang C, Lei A, Wang H, Gao Z. Derivation and characterization of human embryonic germ cells: serum free culture and differentiation potential. Reprod Biomed Online. 2009;19:238–249. doi: 10.1016/S1472-6483(10)60079-X. [DOI] [PubMed] [Google Scholar]
- In’t Anker PS, Scherjon SA, Keur CK, Noort WA, Claas FHJ, Willemze R, Fibbe WE, Kanhai HHH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- In’t Anker PS, Scherjon SA, Keur CK, Groot-Swings GMJS, Claas FHJ, Fibbe WE, Kanhai HHH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tsunoda Y. Role of donor nulcei in cloning efficiency: can the ooplasm reprogram any nucleus. Int J Dev Biol. 2010;54:1623–1629. doi: 10.1387/ijdb.103203yk. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil. 2000;120:231–237. doi: 10.1530/reprod/120.2.231. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, Cho DJ, Kang SG, You J. Human amniotic fluid-derived cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruip TAM, Dieleman SJ. Macroscopic classification of bovine follicles and its validation by micromorphological and steroid biochemical procedures. Reprod Nutr Dev. 1982;22:465–473. doi: 10.1051/rnd:19820403. [DOI] [PubMed] [Google Scholar]
- Li X, Li Z, Jouneau A, Zhou Q, Renard JP. Nuclear transfer: progress and quandaries. Reprod Biol Endocrinol. 2003;1:84. doi: 10.1186/1477-7827-1-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhou J, Shi G, Ma Y, Yang Y, Gu J, Yu H, Jin S, Wei Z, Chen F, Jin Y. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum Mol Genet. 2009;18:4340–4349. doi: 10.1093/hmg/ddp386. [DOI] [PubMed] [Google Scholar]
- Liu CQ, Guo Y, Guan WJ, Ma YH, Zhang HH, Tang XX. Establishment and biological characteristics of luxi cattle fibroblast bank. Tissue Cell. 2008;40:417–424. doi: 10.1016/j.tice.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- Mauro A, Turriani M, Loannoni A, Russo V, Martelli A, Di Giacinto O, Nardinocchi D, Berardinelli P. Isolation, characterization, and in vitro differentiation of ovine amniotic stem cells. Vet Res Commun. 2010;34:25–28. doi: 10.1007/s11259-010-9393-2. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Rzucidlo SJ, Pratt SL, Stice SL. Improvements in cloning efficiencies may be possible by increasing uniformity in recipient oocytes and donor cells. Biol Reprod. 2003;68:1079–1086. doi: 10.1095/biolreprod.102.010876. [DOI] [PubMed] [Google Scholar]
- Mizutani E, Ono T, Li C, Maki-Suetsugu R, Wakayama T. Propagation of senescent mice using nuclear transfer embryonic stem cell lines. Genesis. 2008;46:478–483. doi: 10.1002/dvg.20420. [DOI] [PubMed] [Google Scholar]
- Munoz M, Rodriguez A, De Frutos C, Caamano JN, Diez C, Facal N, Gomez E. Conventional pluripotency markers are unspecific for bovine embryonic-derived cell-lines. Theriogenology. 2008;69:1159–1164. doi: 10.1016/j.theriogenology.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Panarace M, Aguero JI, Garrote M, Jauregui G, Segovia A, Cane L, Gutierrez J, Marfil M, Rigali F, Pugliese M. How healthy are clones and their progeny: 5 years of field experience. Theriogenology. 2007;67:142–151. doi: 10.1016/j.theriogenology.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Parolini O, Soncini M, Evangelista M, Schmidt D. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine. Regen Med. 2009;4:275–291. doi: 10.2217/17460751.4.2.275. [DOI] [PubMed] [Google Scholar]
- Powell AM, Talbot NC, Wells KD, Kerr DE, Pursel VG, Wall RJ. Cell donor influences success of producing cattle by somatic cell nuclear transfer. Biol Reprod. 2004;71:210–216. doi: 10.1095/biolreprod.104.027193. [DOI] [PubMed] [Google Scholar]
- Priest RE, Marimuthu KM, Priest JH. Origin of cells in human amniotic fluid cultures. Lab Invest. 1978;39:106–109. [PubMed] [Google Scholar]
- Priya D, Selokar NL, Raja AK, Saini M, Sahare AA, Nala N, Palta P, Chauhan MS, Manik RS, Singla SK. Production of wild buffalo (Bubalus arnee) embryos by interspecies somatic cell nuclear transfer using domestic buffalo (Bubalus bubalis) oocytes. Reprod Domest Anim. 2014;49:343–351. doi: 10.1111/rda.12284. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschläger M. OCT4-expressing cells in human amniotic fluid: a new source for stem cell research. Hum Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G, Hengstschläger M. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol. 2004;191:309–314. doi: 10.1016/j.ajog.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Rho GJ, Kumar BM, Balasubramanian SS. Porcine mesenchymal stem cells– current technological status and future perspective. Front Biosci. 2009;14:3942–3961. doi: 10.2741/3503. [DOI] [PubMed] [Google Scholar]
- Rideout WM, Wakayama T, Wutz A, Eggan K, Jackson-Grusby L, Dausman J, Yanagimachi R, Jaenisch R. Generation of mice from wild-type and targeted ES cells by nuclear cloning. Nat Genet. 2000;24:109–110. doi: 10.1038/72753. [DOI] [PubMed] [Google Scholar]
- Sadeesh EM, Kataria M, Balhara S, Yadav PS. Expression profile of developmentally important genes between hand-made cloned buffalo embryos produced from reprogramming of donor cell with oocytes extract and selection of recipient cytoplast through brilliant cresyl blue staining and in vitro fertilized embryos. J Assist Reprod Genet. 2014 doi: 10.1007/s10815-014-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A, Panda SK, Chauhan MS, Manik RS, Palta P, Singla SK. Birth of cloned calves from vitrified-warmed zona-free buffalo (Bubalus bubalis) embryos produced by hand-made cloning. Reprod Fertil Dev. 2012;25:860–865. doi: 10.1071/RD12061. [DOI] [PubMed] [Google Scholar]
- Saikhun J, Kitiyanant N, Songtaveesin C, Pavasuthipaisit K, Kitiyanant Y. Development of swamp buffalo (Bubalus bubalis) embryos after parthenogenetic activation and nuclear transfer using serum fed or starved fetal fibroblasts. Reprod Nutr Dev. 2004;44:65–78. doi: 10.1051/rnd:2004017. [DOI] [PubMed] [Google Scholar]
- Selokar NL, Saini M, Muzaffer M, Krishnakanth G, Saha AP, Manik RS, Chauhan MS, Palta P, Madan P, Singla SK. Roscovitine treatment improves synchronization of donor cell cycle in G0/G1 stage and in vitro development of hand-made cloned buffalo (Bubalus bubalis) embryos. Cell Reprogram. 2012;14:146–154. doi: 10.1089/cell.2011.0076. [DOI] [PubMed] [Google Scholar]
- Shah R, George A, Singh MK, Kumar D, Chauhan MS, Manik RS, Palta P, Singla SK. Hand-made cloned buffalo (Bubalus bubalis) embryos: comparison of different media and culture systems. Cloning Stem Cells. 2008;10:435–442. doi: 10.1089/clo.2008.0033. [DOI] [PubMed] [Google Scholar]
- Shah RA, George A, Singh MK, Kumar D, Anand T, Chauhan MS, Manik RS, Palta AP, Singla SK. Pregnancies established from hand-made cloned blastocyst recontstuectd using skin fibroblast in buffalo (Bubalusbubalis) Theriogenology. 2009;71:1215–1219. doi: 10.1016/j.theriogenology.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Lu F, Wei Y, Cui Y, Yang S, Wei J, Liu Q. Buffalos (Bubalus bubalis) cloned by nuclear transfer of somatic cells. Biol Reprod. 2007;77:285–291. doi: 10.1095/biolreprod.107.060210. [DOI] [PubMed] [Google Scholar]
- Soliman MK. Studies on physiological chemistry of allantoic fluid of buffalo at various periods of pregnancy. Indian Vet J. 1975;52:106–112. [Google Scholar]
- Srirattana K, Lorthongpanich C, Laowtammathro C, Imsoonthornruksa S, Ketudat-Cairns M, Phermthai T, Nagai T, Parnpai R. Effect of donor cell types on developmental potential of cattle (Bos taurus) and swamp buffalo (Bubalus bubalis) cloned embryos. J Reprod Dev. 2010;56:49–54. doi: 10.1262/jrd.09-135A. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshal VS, Jones JM. Embryonic stem cell lines derived from human blastocyst. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- Vajta G. Hand-made cloning: the future way of nuclear transfer. Trends Biotechnol. 2007;25:250–253. doi: 10.1016/j.tibtech.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Rodriguez I, Perry AC, Yanagimachi R, Mombaerts P. Mice cloned from embryonic stem cells. Proc Natl Acad Sci USA. 1999;96:14984–14989. doi: 10.1073/pnas.96.26.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama S, Mizutani E, Kishigami S, Thuan NV, Ohta H, Hikichi T, Bui HT, Miyake M, Wakayama T. Mice cloned by nuclear transfer from somatic and ntES cells derived from the same individuals. J Reprod Dev. 2005;51:765–772. doi: 10.1262/jrd.17061. [DOI] [PubMed] [Google Scholar]
- Yadav PS, Mann A, Singh V, Yashveer S, Sharma RK, Singh I. Expression of pluripotency genes in buffalo (Bubalus bubalis) amniotic fluid cells. Reprod Dom Anim. 2010;46:705–711. doi: 10.1111/j.1439-0531.2010.01733.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]
- Zhao XE, Zheng YM. Development of cloned embryos from porcine neural stem cells and amniotic fluid-derived stem cells. Animal. 2010;4:921–929. doi: 10.1017/S1751731110000121. [DOI] [PubMed] [Google Scholar]
- Zheng YM, Zhao XE, An ZX. Neurogenic differentiation of EGFP gene transfected amniotic fluid derived stem cells from pigs at intermediate and late gestational ages. Reprod Domest Anim. 2009;45:78–82. doi: 10.1111/j.1439-0531.2009.01526.x. [DOI] [PubMed] [Google Scholar]