Abstract
Schwann cells (SCs), the supporting cells of the peripheral nerves, are indispensable for regenerating the peripheral and central nervous system. Copious preparation of these cells in a well-defined manner is to be a privileged position. SCs cultivation is overwhelmed by contaminating fibroblasts which are often outgrowing as the predominant cell type in an in vitro culture. This study introduces a technically simple and efficient procedure for SCs isolation and enrichment based on implementing recombinant and defined supplements. Collected adult rat sciatic nerves were cultured for 10 days as in vitro predegeneration. After dissociation and plating, the medium changed to knockout serum replacement supplemented DMDM/F12 medium containing various growth factors. The whole procedure took 3 weeks and SCs purity was then evaluated through implementing specific cytoplasmic and membranous markers. The viability of enriched SCs were evaluated by MTT assay. Within 10 days, over 99 % homogenous SCs were achieved and confirmed through immunofluorescence staining and flow-cytometry for P75NTR and S100 markers, respectively. MTT data revealed that the viability and metabolic activities of purified SCs were increased in expansion medium. This study provides a technically easy and efficient method with the benefits of not utilizing bovine serum or other animal products for SCs isolation and enrichment.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-014-9810-4) contains supplementary material, which is available to authorized users.
Keywords: Schwann cell, Rat sciatic nerve, KOSR, Defined culture
Introduction
Schwann cells (SCs) are the main glial cells of the peripheral nerves. Ability on myelin formation and providing permissive environment for regeneration of axon has made SCs as one of the major candidates in cell based therapies of traumatized and/or demyelinated central and peripheral nervous system (Dai et al. 2012; Dezawa 2002; Kocsis and Waxman 2007). Over the past decades, this property has based great inclinations toward SCs in spinal cord and peripheral nerve regeneration, alone/with scaffold or together with other cells (Dai et al. 2012). However, two major obstacles remain within cultivation of SCs; copious preparation of pure cells in a rational time and getting rid of accompanying fibroblasts (FBs) released into culture from peripheral nerve connective tissue. Predegeneration before peripheral nerve dissociation is a worthy procedure which takes advantages of Wallerian degeneration and results in high cell yield and mitotically active SCs (Keilhoff et al. 1999; Kraus et al. 2010; Mauritz et al. 2004). Yet, several techniques have been introduced and implemented in different laboratories in order to separate SCs from contaminating FBs. These modifications include: repeated explantations method (Morrissey et al. 1995), combination of antimitotic treatment and antibody-mediated cytolysis which employs the use of complement (Assouline et al. 1983; Brockes et al. 1979), serum tapering (Komiyama et al. 2003), manipulating differential adhesion and detachment proprieties of SCs and FBs (Jin et al. 2008; Niapour et al. 2010), immunoselective methods through fluorescent activated cell sorting (FACS) or magnetically activated cell sorting (MACS; Vroemen and Weidner 2003), and exploiting specific biochemical diversity between SCs and EBs (Kaewkhaw et al. 2012). Requiring multiple steps, technically demanding procedures and utilizing animal products for culture are the main bottlenecks of reported systems. It is well documented that animal products is associated with several problems; the existence of uncharacterized component in the composition of animal products like sera as well as disparity between batches that may interfere the reproducibility of experiments. Possibility to transfer viruses, mycoplasms, prions or other pathogenic, immunogenic or toxic agents is another reason which minimize the usage of animal products for clinical grade application (Bonnamain et al. 2013; Wessman and Levings 1999). The aim of the current study is to describe an efficient technique for homogenous SCs culture and refinement employing recombinant and defined supplements without the use of serum or other animal-derived products using a simple schedule.
Method and materials
Cultivation and enrichment of SCs
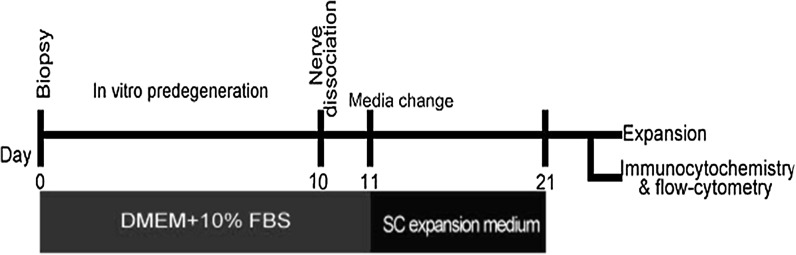
A total of ten male and female Sprague–Dawley rats (150–200 g; Pasteur Institute, Tehran, Iran) were used in this study. All animal care and experimentations were undertaken in strict compliance with the approval of Institutional Animal Ethics Committee of the Ardabil University of Medical Sciences, which follows the NIH guidelines for care and use of experimental animals. Rats were euthanized with CO2. Using aseptic techniques, sciatic nerves were exposed and cut bilaterally. Collected nerves were weighed and washed in phosphate buffer saline (PBS). Once the epineurium stripped off under stereomicroscope, nerves were cut into short segments of 2–3 mm in length. Explants were incubated in predegeneration medium containing Dulbecco’s modified eagles medium (DMEM, Invitrogen (Carlsbad, CA, SA), 12800-116) and 10 % fetal bovine serum (FBS, Invitrogen: 10270) supplemented with, 2 mM l-glutamine (Invitrogen: 25030), 100 U/ml penicillin/streptomycin (Invitrogen: 15140) for 10 days at 37 °C and 5 % CO2 (Niapour et al. 2010). After predegeneration, explants were dissociated enzymatically for 20 h. Dissociation medium consisted of DMEM, 15 % FBS plus 0.125 % collagenase type IV (Invitrogen: 17104-019) and 1.25 U dispase (Invitrogen: 17105-041) per ml. Cell suspension was then centrifuged at 300 g for 10 min. The pellet was resuspended in 10 % FBS supplemented DMEM, and plated onto poly-l-ornithine (15 mg/ml, Sigma (St. Louis, MO, USA), P4957) and laminin (1 mg/ml, Sigma, L2020) coated petri dishes at 75,000 cells/cm2. Following 24 h, the media was changed to SC expansion medium containing DMEM/F12 (Invitrogen: 31331-028), 2.5 % knockout serum replacement (KOSR) (Invitrogen: 10828-028), 2 µM forskolin (FSK, Calbiochem (San Diego, CA, USA): 344273), 10 nM heregulin (Sigma: H0786), 20 ng/ml bFGF (Sigma: F0291), 20 ng/ml EGF (Sigma: E4127) and 2 % B27 (Invitrogen: 17504). As a control, the primary cultures were continued in predegeneration medium for next 10 days. Schematic illustration of implemented method was shown in Fig. 1.
Fig. 1.
Schematic Outline of the implemented method to purify SCs. The time of predegeneration period, switching medium and following characterization have been illustrated
Characterization of SCs
Morphology
Under inverted microscope, SCs are referred to as phase bright, bi-, tri- or multipolar cells which have small cytoplasm to nucleus ratio; while FBs are well-known by a much more flattened polymorphic contour and larger rounded nuclei comparing SCs. Pictures were prepared using an inverted microscope equipped with a digital camera (Olympus DP 71, Tokyo, Japan). The total cell numbers and number of SCs were counted from six random fields (magnification 10×) by two independent investigators.
Immunofluorescent staining
SCs were fixed with 4 % paraformaldehyde for 30 min. Cells were incubated with primary antibody against p75 low affinity NGF receptor (P75LNGFR, 1:500, Abcam (Cambridge, Japan): ab6172) diluted in blocking buffer at 37 °C for 2 h. Blocking buffer consisted of 10 % goat serum (Invitrogen: PCN5000) and 1 mg/ml bovine serum albumin (BSA, Sigma: A3311) in PBS. Then, the secondary antibody, goat anti-mouse IgG conjugated-fluorescein isothiocyanate (FITC, 1:50, Chemicon International (Temecula, CA, USA); AP124F), was used for 1 h at 37 °C. Nuclear counterstaining was performed using DAPI (Sigma, D9542) for 2 min at room temperature. Images were visualized with a fluorescent microscope equipped with digital camera (Olympus DP 71). For negative controls, the primary antibody was excluded. The percentage of putative SCs among the total cells was obtained based on three independent experiments.
Flow-cytometry analysis
After antibody incubation; glial fibrillary acidic protein (GFAP, 1:200, Chemicon: MAB3402), cells were analyzed on a FACS Calibur flow-cytometer (Becton–Dickinson, Heidelberg, Germany) and Cell Quest Pro™ software. At least 10,000 events were recorded for each sample and were processed with WinMDI 2.8 software. The fraction of GFAP positive cells in the fluorescence intensity dot plot was compared to the total amount of intact cells to determine the purity of SCs and subsequent histogram was prepared.
MTT assay
Enriched SCs were trypsinized and seeded onto poly-L-ornithine/laminin (PLO/L) coated 12-multiwell plates at a density of 2 × 104 cells/well. After incubation for a week, the cell viability and metabolic activity of SCs was evaluated. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide; Sigma: M2128) was dissolved in PBS at 5 mg/ml. The stock solution was then added to the culture medium at a dilution 1:10 and incubated for 4 h at 37 °C. After discarding medium, formazan crystals were dissolved in dimethyl sulfoxide (DMSO). The absorbance was measured at 450 nm by using a microplate reader (Synergy HT, BioTek, Winooski, VT, USA). Additionally, the number of cells was counted in some parallel wells.
Statistical analysis
The data were analyzed by Student’s t test using SPSS software (SPSS15 for windows) and expressed as mean ± standard deviation (SD). Differences were considered statistically significant with P value <0.05.
Results
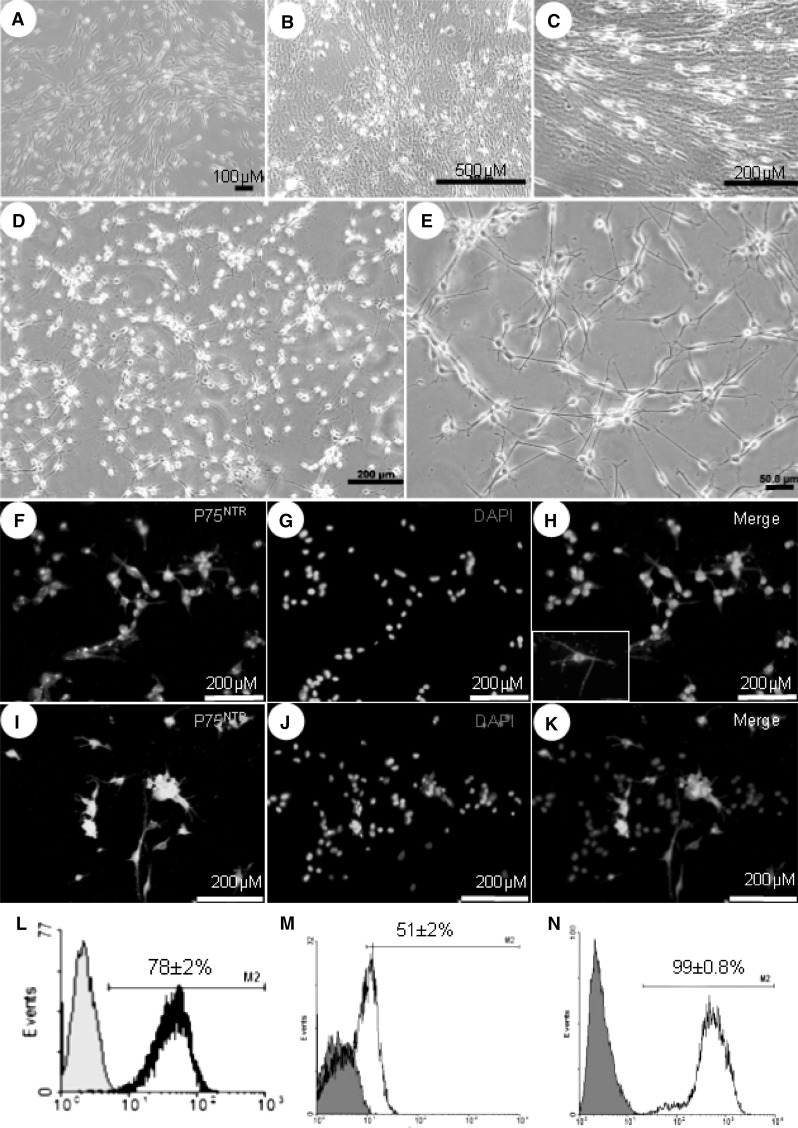
The length of harvested sciatic nerves was 2.2 ± 0.3 cm and their average weight was 10 ± 2 mg. Collected nerves were stripped off in an aseptic condition and submitted to 10-day in vitro predegeneration period (Fig. 2a–d). Predegenerated explants were dissociated and plated on PLO/L coated petri dishes. One day after primary plating, the purity of SCs was 78 ± 2 % (Fig. 3a, i). Expansion of dissociated cells within predegeneration medium resulted in heterogenic population during a week of cultivation. The number of FBs, demonstrated as a wide opaque flattened cell in culture, was rapidly increased while spindle shape SCs were shown with long directed or swirling processes in the plate (Fig. 3b, c). The later appearance indicates higher number of underlying FBs (Dreesmann et al. 2009) (Fig. 3d, e). Following 10 days culture in predegeneration medium, the SCs purity was reduced to 51 ± 2 % (Fig. 3i–k, m). When FBS was exchanged with KOSR, no significant morphological differences of the cultured cells were observed. Thus, for the rest of the study, bovine serum was substituted with KOSR. Culture of primary plated cells in SCs expansion medium came up with monotonously dispersed phase bright SCs in the plate (Fig. 3d, e), whilst FB population was diminished. Moreover, no more long-directed or swirling processes were observed in the cultures (Fig. 3d). Within 10 days, the SCs purity reached 99 ± 0.8 % as confirmed through immunofluorescence staining and flow-cytometry for P75NTR and S100 markers, respectively (Fig. 3f–h). MTT evaluation showed that the viability and metabolic activity of enriched SCs were increased after a week of cultivation in expansion medium in comparison to the beginning of the experiment; 0.81 ± 0.03 versus 0.41 ± 0.01, respectively (P < 0.05). The number of cells reached 7.81 ± 0.175 × 104 by the end of the week.
Fig. 2.
Sciatic nerve predegeneration. After cutting the skin and gluteus maximus muscle, the nerve is exposed in situ (a). Their epineurium was stripped off (b), cut into small pieces (c) and predegenerated for duration of 10 days (d)
Fig. 3.
Schwann cell culture and characterization. Sciatic explants were digested enzymatically and plated onto PLO/L coated perti dishes (a). Cultivation in predegeneration medium ,for the next week, resulted in dramatic increase in fibroblast population (b). SCs in control group acquired long processes which were aligned in a special direction (c). Culture of SCs in expansion medium led to homogenous dispersion of purified cells in the plates which is shown in (d) and with higher magnification in (e). Immunofluorecence staining for P75NTR was visualized at the end of third week in (f–h) for enriched SCs and for control group in (i–k); a little box in enriched SCs and for control group in (i–k); a little box in (h) is the same staining with a higher magnification. Flow-cytometry analysis illustrated the percentage of S100 positive cells of primary plated cells after nerve dissociation (l), control (m) and enriched SCs (n) by the end of protocol
Discussion and conclusion
SCs are characterized a considerable therapeutic promises on regenerative medicine especially in the field of spinal cord injury (Tetzlaff et al. 2011), multiple sclerosis (Jose 2002) and peripheral nerve regeneration (Dai et al. 2012). The preparation of large numbers of cells within a rational period of time is the main concern in the cell engraftment processes. Moreover, from a clinical point of view, protocols which emphasize on defined/human derived products for cell preparations prevail over methods using animal products. It has been shown that animal products increase the risk of non-human antigens expression and xeno infections (Joannides et al. 2007). Our findings establish a simple and efficient protocol to enrich the adult rat SCs without the use of serum or other animal-derived products. However, it is important to note that this is not a comparative study.
Peripheral nerve predegeneration takes advantage of Wallerian degeneration to obtain more and activated SCs in culture comparing to intact nerve culture (Niapour et al. 2010). The duration of 10 days has been implemented for predegeneration period in this research, based on other studies in which the 7–10 days in vitro predegeneration resulted in the highest SCs purity in primary cultures (Kraus et al. 2010). Furthermore, in our previous research, we also found that 10 day predegenerated nerve could retain the SCs purity for longer period after dissociation and plating (unpublished data). Bovine-derived serum was switched with recombinant serum replacement to override probable complications of animal products. Our results indicated well adjustments of both SCs and FBs with this modification. Primary plated cells were cultured with 2.5 % KOSR since our previous work and other studies have demonstrated optimal concentration of serum allowing SCs proliferation and hindering of FBs expansion (Niapour et al. 2010; Takagi et al. 2011). Given the identity of SCs, the neural crest stem cells medium was considered and adapted to SCs culture through addition of chemically defined ingredients. (Achilleos and Trainor 2012; Li et al. 2007; Martin et al. 2012). Ingredients such as bFGF and EGF are common mitogens for almost all kinds of cells including SCs (Monje et al. 2009). FSK and heregulin are SCs specific mitogens. Although, cyclic adenosine monophosphate (cAMP) elevation is toxic to most kinds of cells even the cancerous ones (Moon et al. 2012), FSK with adenylate cyclase activating property is necessary for SC culture (Fuentealba et al. 2004). Although, B27 is a commercially available supplement, lab-made alternatives containing various antioxidants and retinoic acid (RA) were reported (Chen et al. 2008). It has been previously shown that the survival rate of skin derived FBs is reduced with treatment of all trans retinoic acid (ATRA; Daly and Weston 1986). Recently, RA has been used to prevent epidural fibrosis after laminectomy (Zhang et al. 2013). Our preliminary results exhibited dramatic reduction in survival rate of the epineural FBs with even low concentrations of ATRA in comparison with untreated cells (Supporting data). Therefore, it seems that RA in B27 may play a role in the observed findings. Although the exact mechanism(s) responsible for this finding need(s) more investigation, the combination of above-mentioned components would enrich SCs in cultures in a way that over 99 % purity was obtained within 10 days of primary plating. The whole procedure, from sciatic nerve collection to desired enrichment, took 3 weeks which is comparable in simplicity of schedule and being user-friendly with respect to other available methods implying either traditionally in vitro/in vivo predegeneration (Mauritz et al. 2004) or reports omitting predegeneration periods (Kaewkhaw et al. 2012). In MTT assay, the stable tetrazolium salts are reduced to formazan dyes by dehydrogenases present in viable cells (Mosmann 1983). Therefore, the amount of formazan dye is proportional to the number of metabolically active cells in the culture. Actually, the proliferation of SCs took place during the culture process. This finding is in line with others demonstrating proliferating cells in purified cultures (Mauritz et al. 2004).
Taken together, this manuscript describes a simple and efficient technique based on implementing recombinant and well-defined components for refinement and homogenous SCs culture in vitro.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was financially supported (Grant No. 91393) by the Vice Chancellor for Research of the Ardabil University of Medical Sciences, Ardabil, Iran.
Conflict of interest
The authors have declared that no competing interests exist.
References
- Achilleos A, Trainor PA. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res. 2012;22:288–304. doi: 10.1038/cr.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assouline JG, Bosch EP, Lim R. Purification of rat Schwann cells from cultures of peripheral nerve: an immunoselective method using surfaces coated with anti-immunoglobulin antibodies. Brain Res. 1983;277:389–392. doi: 10.1016/0006-8993(83)90953-8. [DOI] [PubMed] [Google Scholar]
- Bonnamain V, et al. Human dental pulp stem cells cultured in serum-free supplemented medium. Front Physiol. 2013;4:357. doi: 10.3389/fphys.2013.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stevens B, Chang J, Milbrandt J, Barres BA, Hell JW. NS21: re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods. 2008;171:239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai LG, Huang GS, Hsu SH. Sciatic nerve regeneration by co-cultured Schwann cells and stem cells on microporous nerve conduits. Cell Transpl. 2012 doi: 10.3727/096368912X658953. [DOI] [PubMed] [Google Scholar]
- Daly TJ, Weston WL. Retinoid effects on fibroblast proliferation collagen synthesis in vitro and on fibrotic disease in vivo. JAAD. 1986;15:900–902. doi: 10.1016/S0190-9622(86)70248-X. [DOI] [PubMed] [Google Scholar]
- Dezawa M. Central and peripheral nerve regeneration by transplantation of Schwann cells and transdifferentiated bone marrow stromal cells. Anat Sci Int. 2002;77:12–25. doi: 10.1046/j.0022-7722.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- Dreesmann L, Mittnacht U, Lietz M, Schlosshauer B. Nerve fibroblast impact on Schwann cell behavior. Eur J Cell Biol. 2009;88:285–300. doi: 10.1016/j.ejcb.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Fuentealba L, Schworer C, Schroering A, Rahmatullah M, Carey DJ. Heregulin and forskolin-induced cyclin D3 expression in Schwann cells: role of a CCAAT promoter element and CCAAT enhancer binding protein. Glia. 2004;45:238–248. doi: 10.1002/glia.10325. [DOI] [PubMed] [Google Scholar]
- Jin YQ, Liu W, Hong TH, Cao Y. Efficient Schwann cell purification by differential cell detachment using multiplex collagenase treatment. J Neurosci Methods. 2008;170:140–148. doi: 10.1016/j.jneumeth.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Joannides AJ, Fiore-Hériché C, Battersby AA, Athauda-Arachchi P, Bouhon IA, Williams L, Westmore K, Kemp PJ, Compston A, Allen ND, Chandran S (2007) A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells 25:731–737 [DOI] [PubMed]
- Jose AM. Multiple sclerosis: can Schwann cells wrap it up? Yale J Biol Med. 2002;75:113–116. [PMC free article] [PubMed] [Google Scholar]
- Kaewkhaw R, Scutt AM, Haycock JW. Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nat Protoc. 2012;7:1996–2004. doi: 10.1038/nprot.2012.118. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Fansa H, Schneider W, Wolf G. In vivo predegeneration of peripheral nerves: an effective technique to obtain activated Schwann cells for nerve conduits. J Neurosci Methods. 1999;89:17–24. doi: 10.1016/S0165-0270(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG. Schwann cells and their precursors for repair of central nervous system myelin. Brain. 2007;130:1978–1980. doi: 10.1093/brain/awm161. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Nakao Y, Toyama Y, Asou H, Vacanti CA, Vacanti MP. A novel technique to isolate adult Schwann cells for an artificial nerve conduit. J Neurosci Methods. 2003;122:195–200. doi: 10.1016/S0165-0270(02)00320-5. [DOI] [PubMed] [Google Scholar]
- Kraus A, Täger J, Kohler K, Manoli T, Haerle M, Werdin F, Hoffmann J, Schaller HE, Sinis N (2010) Efficacy of various durations of in vitro predegeneration on the cell count and purity of rat Schwann-cell cultures. J Neurotrauma 27:197–203. doi:10.1089/neu.2009.0995 [DOI] [PubMed]
- Li HY, Say EH, Zhou XF. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25:2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- Martin I, Nguyen TD, Krell V, Greiner JF, Müller J, Hauser S, Heimann P, Widera D (2012) Generation of Schwann cell-derived multipotent neurospheres isolated from intact sciatic nerve. Stem Cell Rev 8:1178–1187. doi:10.1007/s12015-012-9387-2 [DOI] [PubMed]
- Mauritz C, Grothe C, Haastert K. Comparative study of cell culture and purification methods to obtain highly enriched cultures of proliferating adult rat Schwann cells. J Neurosci Res. 2004;77:453–461. doi: 10.1002/jnr.20166. [DOI] [PubMed] [Google Scholar]
- Monje PV, Rendon S, Athauda G, Bates M, Wood PM, Bunge MB. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia. 2009;57:947–961. doi: 10.1002/glia.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EY, Lee GH, Lee MS, Kim HM, Lee JW. Phosphodiesterase inhibitors control A172 human glioblastoma cell death through cAMP-mediated activation of protein kinase A and Epac1/Rap1 pathways. Life Sci. 2012;90:373–380. doi: 10.1016/j.lfs.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Morrissey TK, Bunge RP, Kleitman N. Human Schwann cells in vitro. I. Failure to differentiate and support neuronal health under co-culture conditions that promote full function of rodent cells. J Neurobiol. 1995;28:171–189. doi: 10.1002/neu.480280205. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Niapour A, Karamali F, Karbalaie K, Kiani A, Mardani M, Nasr-Esfahani MH, Baharvand H. Novel method to obtain highly enriched cultures of adult rat Schwann cells. Biotechnol Lett. 2010;32:781–786. doi: 10.1007/s10529-010-0230-z. [DOI] [PubMed] [Google Scholar]
- Takagi T, Ishii K, Shibata S, Yasuda A, Sato M, Nagoshi N, Saito H, Okano HJ, Toyama Y, Okano H, Nakamura M (2011) Schwann-spheres derived from injured peripheral nerves in adult mice–their in vitro characterization and therapeutic potential. PLoS One 6:e21497. doi:10.1371/journal.pone.0021497 [DOI] [PMC free article] [PubMed]
- Tetzlaff W, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroemen M, Weidner N. Purification of Schwann cells by selection of p75 low affinity nerve growth factor receptor expressing cells from adult peripheral nerve. J Neurosci Methods. 2003;124:135–143. doi: 10.1016/S0165-0270(02)00382-5. [DOI] [PubMed] [Google Scholar]
- Wessman SJ, Levings RL. Benefits and risks due to animal serum used in cell culture production. Dev Biol Stand. 1999;99:3–8. [PubMed] [Google Scholar]
- Zhang C, et al. All-trans retinoic acid prevents epidural fibrosis through NF-kappaB signaling pathway in post-laminectomy rats. Neuropharmacology. 2013;79C:275–281. doi: 10.1016/j.neuropharm.2013.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.