Abstract
Spinal cord injury is a devastating health problem that affects thousands of individuals each year. The neurons were destroyed. NT-3 is a recently discovered neurotrophin. This study sought to understand the potential involvement of MAPKs in NT-3-mediated growth inhibition of human neurons. We applied different concentrations of NT-3 and observed the growth rate of the cells and the changes in the phosphorylation state of the MAPKs ERK1/2, JNK and p38. This study discovered that NT-3-induced HNC growth was promoted primarily by phosphorylated ERK1/2, and that this phosphorylation, as well p90rskphosphorylation, was mediated by TrkC. The ERK1/2 pathway is known to play an essential role in the NT-3-mediated growth of human neurons. In conclusion, our study suggests that NT-3 promotes the growth of human neurons cells primarily through the TrkC/ERK pathway.
Keywords: NT-3, MAPKs, Growth, HNC
Introduction
It is well established that spinal cord injury (SCI) serves as a devastating health problem that affects thousands of individuals each year and causes primary and secondary injuries (Jang et al. 2014). The primary trauma causes mechanical compression, bleeding and electrolyte disturbance, finally resulting in irreversible nerve injury (Jung et al. 2014). Meanwhile, the delayed secondary impairment is made up of multiple pathophysiological processes, including ischemia, edema, hemorrhage, inflammatory responses, energy metabolism system disorder, excitotoxicity and oxidative damage, which generates reversible nerve injury (Feron et al. 2005). Besides, the secondary lesion could be modulated, and this has been considered to be a critical step for treating SCI (Feron et al. 2005; Jang et al. 2014; Kim et al. 2014).
The role of neurotrophins in neuronal plasticity, particularly that involved in the recovery processes following SCI (Wang et al. 2014) is very important. Neurotrophin 3 (NT-3) is indispensible for the development of muscle and tendon receptors (Brahimi et al. 2014). The relationship between NT-3 and the functional efficiency of proprioceptive systems has been demonstrated using NT-3 knockout mice which do not develop proper proprioceptive innervation and die shortly after birth (Tafreshi et al. 1998; Brodski et al. 2000). NT-3 shows its function via its receptor (TrkC) (Brahimi et al. 2014).
MAPKs are a family of serine/threonine kinases that play an essential role in connecting cell-surface receptors to changes in transcriptional programs (Johnson 2011). They are expressed ubiquitously and are involved in the regulation of a wide variety of critical cellular functions, including proliferation, differentiation, migration, and apoptosis (Johnson 2011). In humans, there are at least 11 members of the MAPK superfamily, which can be divided into six distinct subgroups based on sequence similarity: (a) ERK1, ERK2; (b) JNK1, JNK2, JNK3; (c) p38MAPKs. Each group of MAPKs is activated by a distinct kinase cascade in which a MAP3K or MEKK phosphorylates and activates a downstream dual-specificity MAP2K or MEK, which in turn stimulates MAPK activity through dual phosphorylation of threonine and tyrosine residues within a conserved tripeptide motif (Thr-X-Tyr). Phosphorylation of these threonine and tyrosine residues on MAPKs results in a conformational change that increases substrate accessibility and enhances catalysis (Zhou et al. 2014). Activation of ERK is through phosphorylation by MEK (MAPK/ERK kinase) 1/2 in response to various cytokines and growth factors and mediates mitogenic and antiapoptotic signals primarily, whereas members of the JNK and p38 family of MAPKs were identified originally as mediators of cellular stress and are activated by MKK4/MKK7 and MKK3/MKK6, respectively (Lei et al. 2014; Moon and Park 2014; Yao et al. 2014; Zhou et al. 2014).
A previous study showed that the NT-3 affected SCI, specially neurons. How the NT-3 affected neuron cells? In this study, the effects of NT-3 on neurons was studied. And the role of members of the MAPK superfamily in the cellular response to NT-3 was described.
Materials and methods
Unless otherwise specified, all chemicals and reagents in this study were purchased from the Sigma Chemical Company (St. Louis, MO, USA). NT-3 was purchased from Chemicon International, Temecula, CA, USA. Antibodies to IgG, GAPDH, ERK1/2, phospho-ERK1/2, p90rsk, and phospho-p90rsk1 (Ser380) were purchased from the Millipore Corporation (Billerica, MA, USA). Unless otherwise specified, the Human Neuron Cells (HNC, purchased from ScienCell, Carlsbad, CA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, New York, USA) at 38.5 °C at 5 % CO2 in humidified air.
Immunofluorescence
HNC were fixed in 3.7 % paraformaldehyde for 30 min at room temperature and permeabilized with 0.5 % Triton X-100 in PBS for 15 min, followed by blocking overnight at 4 °C in 1 % BSA in PBS with 10 % goat serum. The samples were then stained with anti-NT-3 antibody. The primary antibody binding was detected with an Alexa Fluor 488 goat anti-rabbit IgG (H + L) secondary antibody. Images were captured with a Nikon A1 confocal microscope (Nikon Co.). Experiments were performed in triplicate.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide (MTT) assay
Cells were grown in 96-well plates (1 × 103 cells/well) supplemented with NT-3. Control cells were switched from RPMI-1640 to DMEM containing 0.1 % dimethyl sulfoxide (DMSO). At 12, 24, 48 and 72 h following NT-3 treatment (0, 10, 20, 40 and 80 nM NT-3), 20 μL of MTT was added to each well to a final concentration of 0.5 %. After a 4 h incubation at 37 °C in the dark, 150 μL DMSO was added to each well for 10 min to dissolve the formazan crystals. The absorbance was measured using a microplate reader (EXL800, Cole-Parmer, Vernon Hills, IL, USA) at 490 nm. All experiments were repeated three times. The viability of the NT-3 treated cells was expressed as percentage of population growth plus the standard error of the mean (SEM) relative to that of untransfected control cells. Cell growth was calculated as follows: % growth = (mean experimental absorbance−mean control absorbance/mean control absorbance) × 100.
Western blot
The proteins that were homogenized from human neuron cells were separated by electrophoresis on 8–12 % SDS/polyacrylamide gels, and then transferred to immunoblot NC membrane. Membranes were blocked for 30 min at room temperature with PBS buffer containing 5 % fat-free milk and 0.1 % Tween20. Membranes were then incubated with primary anti-Rac1 antibody for at least 1 h at room temperature, or overnight at 4 °C. The membranes were subsequently washed three times with PBS containing 0.1 % Tween 20, incubated with peroxidase-conjugated secondary antibodies (Millipore), and developed using the ECL reagent (Pierce, Rockford, IL, USA).
siRNA design
RNA interference was used to silence the expression of ERK1/2 and TrkC in human neuron cells. ERK1/2-siRNA, TrkC-siRNA and scramble siRNA were synthesized by GenePharma Biotechnology (Shanghai, China).
Transfection of siRNA
siRNA transfection was conducted according to the protocol supplied by Invitrogen (Carlsbad, CA, USA). Briefly, 1 × 105 cells were seeded into six-well plates containing an antibiotic-free medium and incubated overnight. For each well, 5 μL siRNA were mixed with 125 μL OPTI-MEM I. The mixture was then combined with a solution of 5 μL lipofectamine in 125 μL OPTI-MEM I. After a 20-min incubation period at RT, the mixture was applied to the cells in an appropriate volume of Opti-MEM I so as to achieve a final concentration of 100 nmol/L for each siRNA. After incubation for 6 h at 37 °C, RPMI-1640 supplemented with serum was added to the wells. Cells were cultured for an additional 24 h at 37 °C before analysis.
Statistical analysis
Statistically significant differences between gene expression levels were determined using one-way ANOVA followed by a Newman–Keuls test with the GraphPad Prism 5 software (GraphPad Software, Inc., USA. http://www.graphpad.com/company/). Replicates were included in the statistical model. Differences were considered statistically significant at the 95 % confidence level (P < 0.05). Data are presented as mean ± SD.
Results
NT-3 receptor TrkC expression in HNC
To detect the expression status of NT-3 receptor TrkC in HNC, immunocytochemistry was used. Figure 1 shows TrkC was present in the HNC.
Fig. 1.
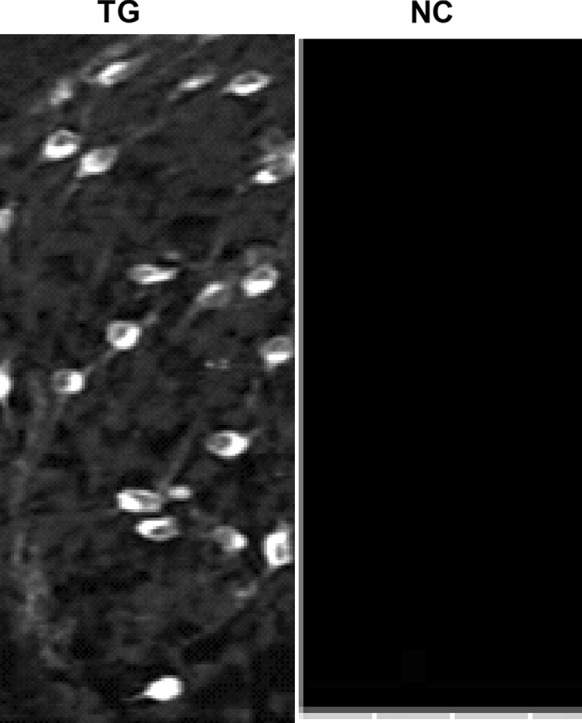
NT-3 receptor TrkC expressed in HNC. TG Test group, NC negative group
Determination of the optimal NT-3 treatment of HNC
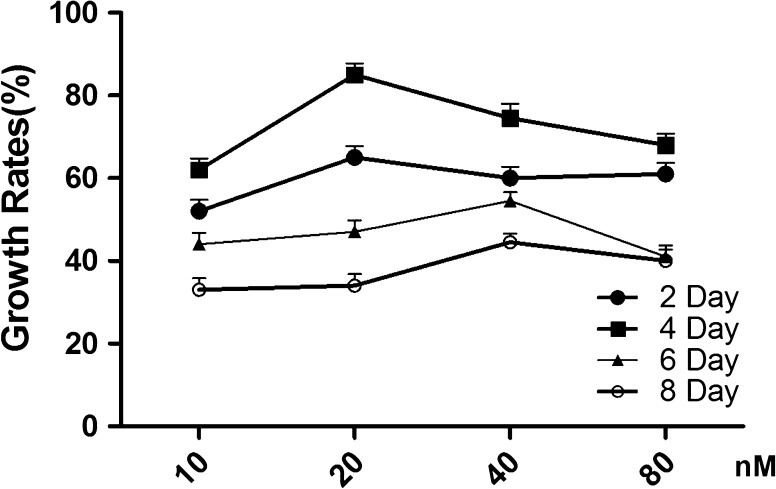
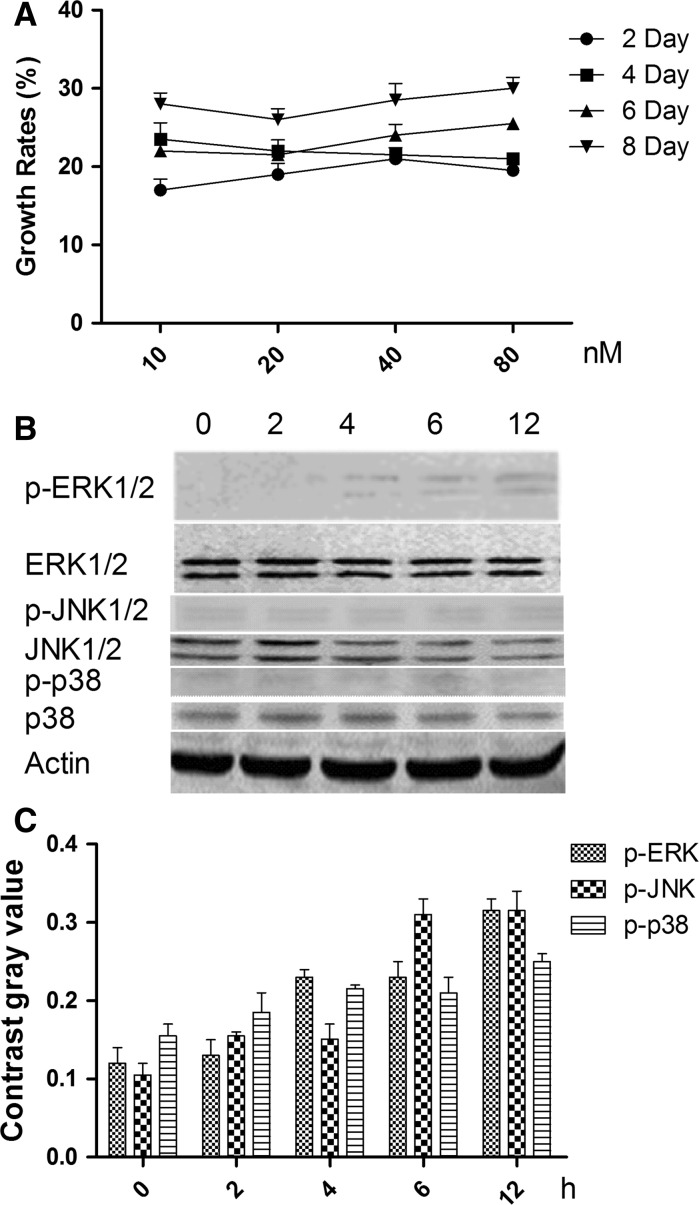
To determine the optimal concentration and duration of NT-3 treatment of HNC, NT-3 was added to the cell culture media at varying concentrations, and cell growth was monitored for 8 days. Figure 2 shows the growth rates of HNC affected by NT-3 at final concentrations of 10, 20, 40 and 80 nM on the 2nd, 4th, 6th and 8th day. The optimal concentration of NT-3 was 20 nM on the 4th day.
Fig. 2.
The growth rates at the 2nd, 4th, 6th, and 8th day in the presence of NT-3 at final concentrations of 10, 20, 40, and 80 nM.
NT-3 promotes the growth of HNC via ERK1/2 pathway
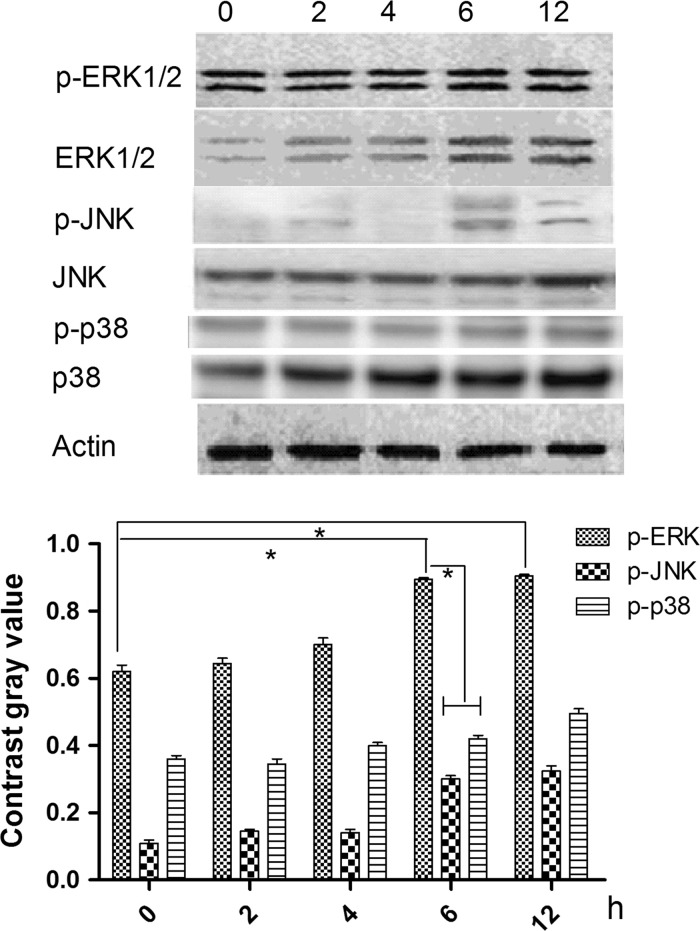
To identify the signaling pathway through which NT-3 acts on HNC, cells were treated with 20 nM NT-3 for 4 days (the optimal conditions as determined above) and the phosphorylation state of the MAPKs ERK1/2, JNK and p38 was examined 0, 2, 4, 6 and 12 h following treatment. Figure 3 shows that the ERK1/2 phosphorylation was higher than the other two proteins, after 6 h.
Fig. 3.
Effects of NT-3 with 20nM on mitogen-activated protein kinase (MAPK) activation in HNC at 0, 2, 4, 8 and 12 h. a The expression of ERK1/2, JNKs, p38 and phosphorylated ERK1/2, phosphorylated JNKs, and phosphorylated p38. b Contrast gray value of phosphorylated ERK1/2, phosphorylated JNKs, and phosphorylated p38. *There was significantly difference between two groups (P < 0.05)
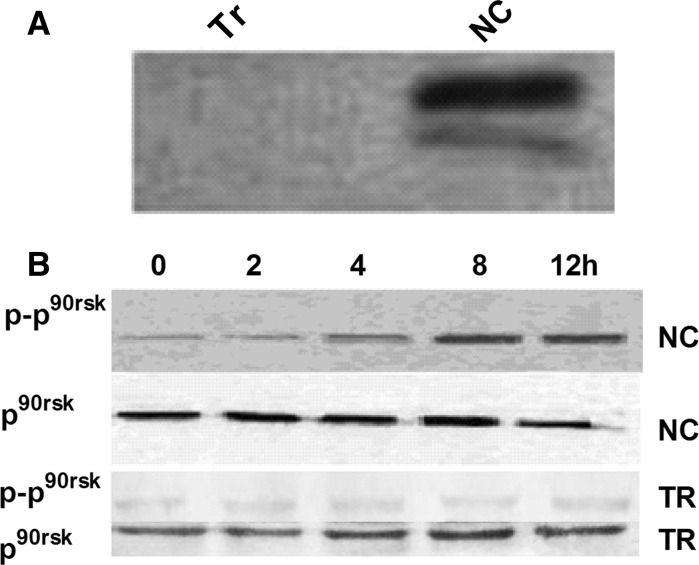
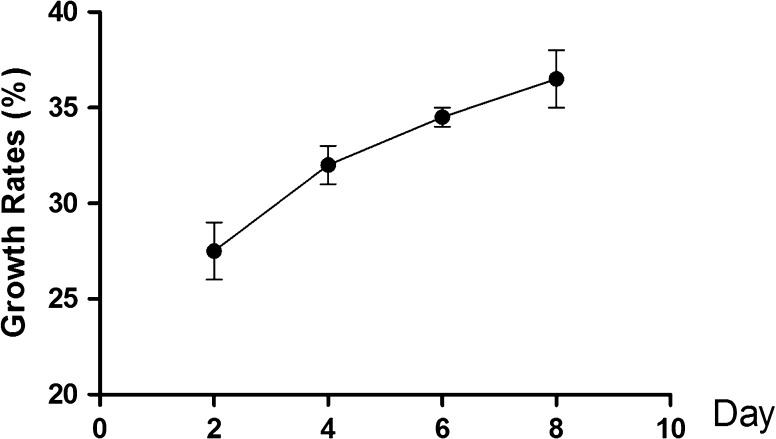
To verify that NT-3 promotes the growth of HNC via the ERK1/2 pathway, siRNA was used to inhibit ERK1/2 activity (Fig. 4). Moreover the phosphorylation of p90rsk (p−p90rsk) was also low (Fig. 4). NT-3 at 20 nM (2, 4, 6 and 8th day), did not influence the NMC growth (Fig. 5).
Fig. 4.
Expression of ERK1/2, p90rsk and phosphorylated p90rsk after ERK1/2 silencing. a Expression of ERK1/2 at protein level. b Expression of p90rsk and phosphorylated of p90rsk at protein level at different time points (0, 2, 4, 8 and 12 h)
Fig. 5.
Growth of HNC in presence of NT-3 at 20nM (2, 4, 6 and 8 day)
The effect of silenced TrkC in HNC
To certify that NT-3 exerts its function through TrkC in HNC, TrkC was silenced by siRNA, and HNC were then treated with NT-3. Figure 6a shows that TrkC protein levels were reduced, blocking the stimulation of growth by NT-3. Moreover the phosphorylation of ERK1/2 and p90rsk was also lower and showed no significant difference (Fig. 6b, c).
Fig. 6.
Effects of TrkC silenced, with NT-3 on mitogen-activated protein kinase (MAPK) activation in HNC. a Growth rates of HNC effected by NT-3 with 10, 20,40 and 80 nM at 2, 4, 6 and 8 day. b The expression of ERK1/2, JNKs, p38 and phosphorylation of ERK1/2, JNKs, p38 in protein level with NT-3 20 nM. c Contrast gray value of phosphorylation of ERK1/2, JNKs and p38 with NT-3 20 nM
Discussion
SCI is a serious trauma causing severe and often permanent disability. SCI induces primary mechanical damage and causes secondary damage to the spinal cord. Primary damage occurs by mechanical tissue disruption immediately subsequent to trauma. Secondary damage is mediated by complex cellular and molecular processes (Roloff et al. 2013). NT-3, as one of the important neurotrophic factors, modulates neuron cell growth, differentiation and survival (Brodski et al. 2000). In this study, NT-3 was added in the neuron cell culture medium. And NT-3 showed great function on cell growth, mainly through ERK1/2 pathway.
The optimal NT-3 treatment to obtain maximum growth rate was 20 nM of NT-3 for 4 days. We also demonstrated that this effect of NT-3 is mediated through TrkC, its receptor, which we showed to be expressed at high levels in HNC. Experiments in which TrkC blocked the NT-3-mediated growth provided further support for the role of TrkC.
To gain further insight into the mechanism by which NT-3 promotes HNC growth, we evaluated the activity of signaling pathways downstream of TrkC (Esteban et al. 1998). MAPKs are a super family of serine/threonine kinases that includes ERK, JNK and p38 (Zhong et al. 2014). These kinases are involved primarily in the activation of nuclear transcription factors that control cell proliferation, differentiation and apoptosis (Zhong et al. 2014). The results of our present study suggest that NT-3 promotes HNC growth via the ERK signaling pathway, and not through the activation of JNK or p38. We found that 2–12 h of NT-3 treatment was required to stimulate phosphorylation of ERK, and therefore that the stimulus is time-dependent. Furthermore, silencing both of ERK and TrkC by siRNA suppressed the NT-3-mediated promotion of HNC growth. Overall, our study suggested that the NT-3/TrkC signaling pathway attenuates HNC growth mainly through an ERK-dependent pathway. Thus, our findings suggest that NT-3 may be a prospective target for SCI therapy. Despite this promising finding, further study is necessary before any clinical application can be considered.
Acknowledgments
This work was supported by Grant No. 81450037 from the Chinese National Natural Science Foundation and baiwan project of Inner Mongolia Medical University.
References
- Brahimi F, Ko E, Malakhov A, Burgess K, Saragovi HU. Combinatorial assembly of small molecules into bivalent antagonists of TrkC or TrkA receptors. PLoS ONE. 2014;9:e89617. doi: 10.1371/journal.pone.0089617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodski C, Schnurch H, Dechant G. Neurotrophin-3 promotes the cholinergic differentiation of sympathetic neurons. Proc Natl Acad Sci USA. 2000;97:9683–9688. doi: 10.1073/pnas.160080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban I, Levanti B, Garcia-Suarez O, Germana G, Ciriaco E, Naves FJ, Vega JA. A neuronal subpopulation in the mammalian enteric nervous system expresses TrkA and TrkC neurotrophin receptor-like proteins. Anat Rec. 1998;251:360–370. doi: 10.1002/(SICI)1097-0185(199807)251:3<360::AID-AR12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- Jang JY, Lee SH, Kim M, Ryu JS. Characteristics of neuropathic pain in patients with spinal cord injury. Ann Rehabil Med. 2014;38:327–334. doi: 10.5535/arm.2014.38.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL. Defining MAPK interactomes. ACS Chem Biol. 2011;6:18–20. doi: 10.1021/cb100384z. [DOI] [PubMed] [Google Scholar]
- Jung SY, Kim DY, Yune TY, Shin DH, Baek SB, Kim CJ. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med. 2014;7:587–593. doi: 10.3892/etm.2013.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Jeong HJ, Kim MO. Changes of functional outcomes according to the degree of completeness of spinal cord injury. Ann Rehabil Med. 2014;38:335–341. doi: 10.5535/arm.2014.38.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei XF, Kim-Kaneyama JR, Arita-Okubo S, Offermanns S, Itabe H, Miyazaki T, Miyazaki A. Identification of Hic-5 as a novel scaffold for the MKK4/p54 JNK pathway in the development of abdominal aortic aneurysms. J Am Heart Assoc. 2014;3:e000747. doi: 10.1161/JAHA.113.000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Park SH. Reassembly of JIP1 scaffold complex in JNK MAP kinase pathway using heterologous protein interactions. PLoS ONE. 2014;9:e96797. doi: 10.1371/journal.pone.0096797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roloff F, Ziege S, Baumgartner W, Wewetzer K, Bicker G. Schwann cell-free adult canine olfactory ensheathing cell preparations from olfactory bulb and mucosa display differential migratory and neurite growth-promoting properties in vitro. BMC Neurosci. 2013;14:141. doi: 10.1186/1471-2202-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafreshi AP, Zhou XF, Rush RA. Endogenous nerve growth factor and neurotrophin-3 act simultaneously to ensure the survival of postnatal sympathetic neurons in vivo. Neuroscience. 1998;83:373–380. doi: 10.1016/S0306-4522(97)00385-0. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Zhang RP, Li JD. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir (Wien) 2014;156:1409–1418. doi: 10.1007/s00701-014-2089-6. [DOI] [PubMed] [Google Scholar]
- Yao YL, Ma J, Wang P, Xue YX, Li Z, Zhao LN, Li ZQ, Feng TD, Liu YH (2014) miR-101 acts as a tumor suppressor by targeting Kruppel-like factor 6 in glioblastoma stem cells. CNS Neurosci Ther 82:1345–1347 [DOI] [PMC free article] [PubMed]
- Zhong Y, Naito Y, Cope L, Naranjo-Suarez S, Saunders T, Hong SM, Goggins M, Herman JM, Wolfgang CL, Iacobuzio-Donahue CA (2014) Functional p38 MAPK identified by biomarker profiling of pancreatic cancer restrains growth through JNK inhibition and correlates with improved survival. Clin Cancer Res 89:3348–3356 [DOI] [PMC free article] [PubMed]
- Zhou Q, Gui S, Wang Y. Melatonin Inhibits the Migration of Human Lung Adenocarcinoma A549 Cell Lines Involving JNK/MAPK Pathway. PLoS ONE. 2014;9:e101132. doi: 10.1371/journal.pone.0101132. [DOI] [PMC free article] [PubMed] [Google Scholar]