Abstract
Mouse embryonic fibroblasts (MEFs) are widely used to prepare feeder layers for culturing embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) in vitro. Transportation lesions and exorbitant prices make the commercially obtained MEFs unsuitable for long term research. The aim of present study is to establish a method, which enables researchers to gain MEFs from mice and establish feeder layers by themselves in ordinary laboratories. MEFs were isolated from ICR mouse embryos at 12.5–17.5 day post-coitum (DPC) and cultured in vitro. At P2–P7, the cells were inactivated with mitomycin C or by X-ray irradiation. Then they were used to prepare feeder layers. The key factors of the whole protocol were analyzed to determine the optimal conditions for the method. The results revealed MEFs isolated at 12.5–13.5 DPC, and cultured to P3 were the best choice for feeder preparation, those P2 and P4–P5 MEFs were also suitable for the purpose. The P3–P5 MEFs treated with 10 μg/ml of mitomycin C for 3 h, or irradiated with X-ray at 1.5 Gy/min for 25 Gy were the most suitable feeder cells. Treating MEFs with 10 μg/ml of mitomycin C for 2.5 h, 15 μg/ml for 2.0 h, or irradiating the cells with 20 Gy of X-ray at 2.0 Gy/min could all serve as alternative methods for P3–P4 cells. Our study provides a reliable and economical way to obtain large amount of qualified MEFs for long term research of ESCs or iPSCs.
Keywords: Embryonic stem cell, Feeder layer, Induced pluripotent stem cell, Mitomycin C, Mouse embryonic fibroblast, X-ray
Introduction
Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) can be cultured in feeder free systems, such as in conditioned medium produced from mouse embryonic fibroblasts (MEFs) (Xu et al. 2001; Klimanskaya et al. 2005; Ludwig et al. 2006). Under more circumstances, they are plated directly on feeder layers originated from MEFs (Thomson et al. 1998; Yu et al. 2009; Hu et al. 2011), STO cells (Park et al. 2004; Takahashi and Yamanaka 2006), human embryonic fibroblasts (Kibschull et al. 2011), human placental fibroblasts (Genbacev et al. 2005), human foreskin fibroblasts (Unger et al. 2009; Skottman 2010; Strom et al. 2010), human skin fibroblasts (Richards et al. 2003; Tecirlioglu et al. 2010), amniotic mesenchymal cells (Zhang et al. 2011), etc. Human embryonic stem cells (hESCs) can even be cultured on feeder layers derived from themselves (Xu et al. 2004; Stojkovic et al. 2005; Chen et al. 2009). The usage of feeder layers is mainly based on the knowledge listed as follows: ESCs can get sufficient nutrition from blood or via intercellular exchange in vivo. Hence, besides culture media, feeder layers are often needed by ESCs or iPSCs cultured in vitro. In the culture systems, feeder layers can form more suitable microenvironment; can provide adequate nutrition or biological signals, such as collagens, laminins and heparan sulfate proteoglycans (Abraham et al. 2010; Hongisto et al. 2012); can also secret various cytokines, such as fibroblast growth factor (FGF), bone morphogenetic protein-4, activin A, transforming growth factor-β1 (Vallier et al. 2005; Prowse et al. 2007; Eiselleova et al. 2008), etc. All of these are deemed necessary for the growth of ESCs or iPSCs, and for maintaining them in an undifferentiated state during the culture period. In brief, feeder layers can mimic the real environment in vivo, to some degree, for the ESCs or iPSCs cultured in vitro.
Among the feeder cells mentioned above, MEFs are the most widely used one, despite they may potentially bring heterogenic proteins, xenogeneic materials or pathogenic microorganisms into the culture systems. The reasons of this are that MEFs can be acquired easily and seem better than human feeder cells in terms of sustaining hESCs or iPSCs (Richards et al. 2003; Eiselleova et al. 2008), though systematic comparisons are still wanted in this regard.
Nevertheless, there are several obstacles for researchers to obtain MEFs. Firstly, it’s impractical to rely on commercial way completely. Although the ready-made MEFs can be bought directly from biological companies, after long time of transportation in dry ice, the frozen fibroblasts frequently lose their attachment ability during subsequent culture. Transportation lesions are usually the principal reason behind this. From our experience, even multi-measures have been taken to enhance the protection during delivery, the received MEFs may still be difficult to form feeder layers. Actually, similar frustrating experiences are quite common among other laboratories of the same kind. These highlight that MEFs cannot be obtained steadily by purchase. Secondly, the exorbitant prices of the commercial MEFs are apparently a heavy burden for many laboratories in the long run. This imposes another block. As a consequence, it is inevitable to raise mice and isolate MEFs by the researchers themselves for a lot of laboratories, despite this will bring new workloads.
CF1 mouse is an ideal source of fibroblasts (Eiselleova et al. 2008; Jozefczuk et al. 2012), but it is not available in many situations. Moreover, its expensive price makes up another shortcoming. Some laboratories have produced feeder layers using BALB/c or Kunming White mice, and have got satisfying results. Due to the easy availability of these mice, they can be used to prepare feeder layers more conveniently than CF1. These reports provide valuable references for us, but just following suit often leads to undesirable consequences. So it is still necessary for researchers to select appropriate mouse and optimize the protocol according to their own conditions.
In the present study, we turned to ICR mouse, which was cheap enough and could be obtained at random from local animal laboratories. Through a comprehensive analysis on the key factors of the whole experimental process from MEFs isolation till feeder preparation, we developed an optimized protocol for gaining MEFs capable of preparing feeder layers, which provides a more reliable and economical way to solve the problems.
Materials and methods
Isolating mouse embryos
The adult male and female ICR mice (obtained from the Animal Laboratory in the Anhui Medical University with the permission of local Medical Ethics Committee) were put together at a ratio of 1:2. The female mice were checked every morning in the next days. The day on which vaginal plug appeared was recorded as 0.5 day post-coitum (DPC). At 12.5–17.5 DPC, the female mice were sacrificed, respectively, by cervical dislocation, and the whole mouse bodies were immersed into 75 % ethanol solution for more than 15 min for disinfection. The abdominal cavities of the female mice were opened and the whole uteri were dissected out under aseptic conditions, the embryos were separated from their placentas and rinsed with PBS without Ca2+ and Mg2+ (Gibco, Life Technologies, Carlsbad, CA, USA) for three times to remove the residual blood. The embryonic bodies were isolated and minced with sterile ophthalmic scissors in different Petri dishes.
Culture of MEFs
The minced bodies were washed with sterile PBS for three times, and treated with 0.25 % trypsin (Beyotime, Jiangsu, China) at 37 °C for 20 min, during which the bodies were pipette up and down thoroughly every 5 min to dissociate the cells, including MEFs. The MEFs were suspended with MEF medium, consisted of 90 % DMEM with GlutaMAX™-I (Gibco) and 10 % fetal bovine serum (FBS, Hyclone, Logan, UT, USA), then they were collected into falcon tubes and centrifuged at 1,000 rpm for 5 min. Washed with MEF medium for two times, the MEFs were collected in T-75 flasks (1 embryo per flask, Corning, NY, USA) and incubated for proliferation at 37 °C with 5 % CO2. The spent medium was changed with fresh MEF medium on the next day, the cells were cultivated continuously till day 3–7, during which the medium was changed routinely every 2 days.
Passaging
Having reached 80–90 % confluent, the MEFs were rinsed with PBS, and digested with 0.25 % of trypsin. After three washing steps, the MEFs were re-suspended with fresh MEF medium and distributed to new flasks for further culture.
Inactivation
At P2–P5, two inactivating approaches were carried out respectively. For the chemical inactivation method, when the MEFs had been cultured to 80–90 % confluent, mitomycin C (Biosharp, Miami, FL, USA) working solution was added into culture flasks to reach a series of terminal concentrations from 5 to 20 μg/ml, then the cells were incubated for 2–3.5 h at 37 °C with 5 % CO2. The culture medium was removed thereafter, and the MEFs were washed with PBS for five times. Subsequently, the MEFs were digested with 0.25 % of trypsin, washed with PBS for three times, and collected for immediate use or freezing storage.
The MEFs were also inactivated through irradiation method. When the fibroblasts reached 80–90 % confluence, they were placed under a medical linear accelerator (Siemens Primus M, Malvern, PA, USA) and irradiated with X-ray at 1.0–2.0 Gy/min until the total adsorbed dose reached 15–30 Gy. The irradiated MEFs were returned to cell incubator (RS Biotech Galaxy S), cultivated for another 1 h, and then they were digested with 0.25 % of trypsin. Undergone three washing steps, the MEFs were harvested for further use.
Freezing and thawing
After digestion, the inactivated MEFs were suspended with freezing medium, which consisted of 70 % DMEM with GlutaMAX™-I, 20 % FBS and 10 % DMSO (Biosharp), at a density of 1 × 106 cells/ml. Then the freezing medium was distributed to sterile freezing tubes, and the tubes were put into a Nalgene freezing container, which had been filled with 250 ml of isopropyl alcohol and placed at room temperature for more than 8 h in advance. The container was placed immediately into a −80 °C refrigerator overnight to ensure the MEFs cooled down at a rate of −1 °C/min. The next morning, the cryovials were transferred into liquid nitrogen for long term cryopreservation.
At the time of thawing, the cryovials containing the growth-arrested MEFs were taken out from the liquid nitrogen tank, briefly rolled between hands to remove the frost, and swirled gently in a 37 °C water bath. When only a small ice crystal remained, the vials were removed from the water bath. The outside of them was sprayed with 70 % ethanol before they were placed into a cell culture hood. Undergone two rinses with pre-warmed MEF medium, the cells were counted by a cytometer (Sysmex, Shanghai, China).
Feeder preparation and culture of hESCs
The growth-arrested MEFs were added into culture flasks, which had been coated with attachment factor (Gibco) previously, at a density of 2.5 × 104 cells/cm2 and incubated at 37 °C with 5 % CO2. On the next day, the MEFs formed feeder layers. The MEF medium was then replaced by hESCs medium, every 100 ml of which consisted of 79 ml of DMEM/F12 with GlutaMAX™-I (Gibco), 20 ml of knockout serum replacement (Gibco), 1 ml of non-essential amino acids solution (Gibco), 182 μl of 2-mercaptoethanol (Gibco) and 40 μl of basic FGF (Gibco). Subsequently, vials of thawed and twice washed hESCs (presented by Xiao Lei Laboratory at the Zhejiang University) were plated onto the layers, the flasks were then incubated at 37 °C with 5 % CO2. The hESCs were further cultured continuously up to ten passages for subsequent analyses.
Growth rate evaluation
In passage selecting phase, to avoid the potential influences of cell masses, the MEFs of various passages were counted manually. Then they were plated and cultured for 1–7 days, after being digested, they were counted artificially again. The growth rates were then calculated and assessed separately.
In order to avoid the potential impacts of dead and damaged cells, the MEFs just undergone inactivation were counted manually too. After being cultured for 1–7 days, they were digested and counted with a Sysmex cytometer again. The inactivating effect was then evaluated. Dead cells were manually confirmed after being stained with trypan blue (Beyotime).
Treatment of the samples
In order to select MEFs of appropriate gestational age for feeder preparation, 30 samples were isolated at 12.5–17.5 DPC (6 samples/age group) and cultured for further study. After the best chemical inactivating method had been found, another 30 samples were isolated and used to prepare feeder layers according to the optimized protocols. The supporting capacity of these layers was evaluated by 30 cultures of hESCs, which were all maintained for ten passages.
In the passage selecting phase, 36 samples were included (6 samples/passage). The proliferation rates of every passage were compared, as well as the cell states. The better passages were then chosen for further research.
In the chemical inactivating process, 12 samples were collected and treated (P2–P5, three samples/passage) using each combination of drug concentration and treating time (totally 20 methods were used for each sample). For every hopeful combination, including 10 μg/ml of mitomycin C for 3.0 h, 10 μg/ml for 2.5 h, and 15 μg/ml for 2.0 h, 12 cultures of hESCs were maintained up to ten passages, respectively, on the corresponding layers (P2–P5). Similarly, 9 MEF samples were selected (P3–P5, three samples/passage) for each irradiating combination of dose and dose rate (totally 12 combinations were utilized for each sample). For the two most promising methods, irradiating with X-ray at 1.5 Gy/min for 25 Gy and 2.0 Gy/min for 20 Gy, nine and six cultures of hESCs were maintained for ten passages on the corresponding layers separately (P3–P5 and P3–P4). As a comparison, four cultures of hESCs were maintained for five passages on feeder layers, which were established with γ-irradiated MEFs (GIBCO). The supporting capacity of the self-produced MEFs was then observed and evaluated.
Statistical analysis
The measurement data were given as mean ± SD. The differences among various passages, chemical inactivating approaches, irradiation methods were analyzed with SPSS 16.0 software using repeated measures ANOVA. P < 0.05 was considered that the difference was statistically significant.
Results
Selection of gestational age
The culturing features of the MEFs obtained from mouse embryos at 12.5–17.5 DPC were specified in Table 1. In general, short gestational age always linked to fine MEFs, with higher purity as well. Prolonged isolation led to remarkable decline in the MEFs’ quality, in terms of cell morphology, growth rate, adapting capacity against inactivation and the supporting power for hESCs. So the optimal isolation time for MEFs is 12.5–13.5 DPC. Under some circumstances, it can be treated as an acceptable substitute to isolate MEFs at 13.5–15.5 DPC, which may be more practicable for subsequent research work.
Table 1.
Culture characteristics of the MEFs with various gestational age
| Gestational age (DPC) | Output of MEFs (per embryo) | Cell morphology | Mixed cells at P0 | Growth rate at P0 | Dead cells after inactivation | Growth rate of hESCsa | Differentiated hESCsa |
|---|---|---|---|---|---|---|---|
| 12.5–13.5 | Most | Best | Some | Fastest | Some | Fast | Few |
| 13.5–14.5 | More | Better | Some | Faster | Some | Fast | Few |
| 14.5–15.5 | Many | Better | Many | Faster | Many | Fast | A few |
| 15.5–16.5 | Some | Good | More | Fast | More | Slow | Some |
| 16.5–17.5 | Some | Poor | Most | Fast | Most | Slow | Many |
aThe hESCs were all seeded on the feeder layers that derived from P3 MEFs, and had been inactivated with 10 μg/ml of mitomycin C for 3.0 h before feeder preparation
Selection of passages
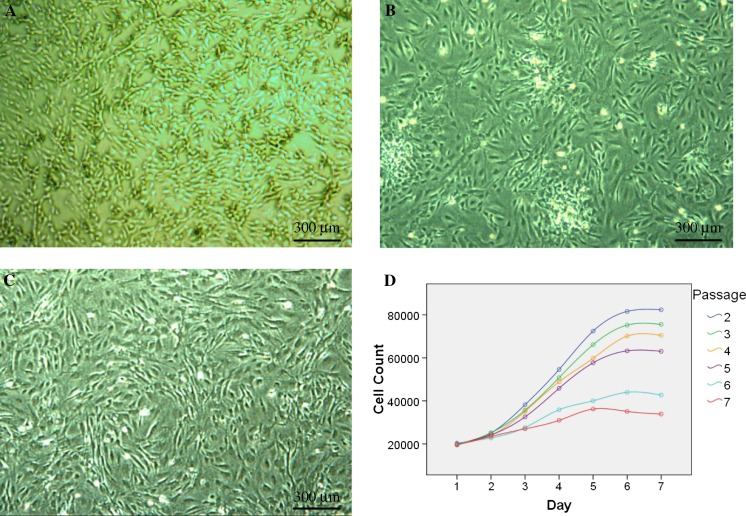
Generally speaking, the growth rates of P2–P7 MEFs declined remarkably with the rising passage number, which was more distinct between group P2–P5 and group P6–P7 (P < 0.01) (Table 2; Fig. 1). Meanwhile, the number of mixed cells in the culture vessels decreased steadily from P1 to P7. From P5, obvious catabiosis appeared. Moreover, obvious differentiation was observed in the hESCs plated on the layers of older passages. Taking all these into consideration, the MEFs of P2–P5 can all be used to prepare feeder layers. Furthermore, we specially recommend selecting P3 MEFs for the culture of high standard hESCs. Because of more unwanted cells mixed in the P0–P2 MEFs, it is appropriate to exclude P2 for preparing good quality feeder layers. These choices will be helpful to assure both the quality and the quantity of the gained MEFs.
Table 2.
Cell counts of the MEFs at different passages
| Passage* | Day1 | Day2 | Day3 | Day4 | Day5 | Day6 | Day7 |
|---|---|---|---|---|---|---|---|
| 2 | 19,587.5 ± 697.4 | 25,068.3 ± 3,232.6 | 38,212.3 ± 12,254.0 | 54,597.0 ± 19,430.6 | 72,450.3 ± 28,540.8 | 81,612.8 ± 34,182.5 | 82,375.5 ± 34,478.4 |
| 3 | 19,626.8 ± 657.2 | 25,186.8 ± 2,109.5 | 35,667.5 ± 6,854.5 | 50,817.0 ± 11,734.7 | 66,143.8 ± 10,924.4 | 75,229.0 ± 12,321.6 | 75,579.3 ± 12,488.5 |
| 4 | 19,882.3 ± 243.9 | 23,898.7 ± 1,112.8 | 35,109.3 ± 2,360.1 | 48,890.7 ± 8,514.5 | 59,813.3 ± 14,212.6 | 70,101.5 ± 18,058.4 | 70,439.8 ± 18,066.2 |
| 5 | 20,069.7 ± 695.4 | 24,209.5 ± 1,945.8 | 32,542.7 ± 6,121.1 | 45,837.2 ± 11,215.9 | 57,695.0 ± 14,679.6 | 63,262.0 ± 17,267.8 | 63,058.7 ± 17,252.3 |
| 6 | 20,413.5 ± 526.1 | 22,769.8 ± 1,989.1 | 27,610.8 ± 2,584.2 | 35,871.8 ± 5,177.3 | 40,036.7 ± 6,741.8 | 43,987.7 ± 8,168.6 | 42,689.5 ± 8,074.1 |
| 7 | 19,530.2 ± 565.3 | 23,478.0 ± 414.1 | 27,014.0 ± 1,669.2 | 30,928.0 ± 1,307.6 | 36,200.0 ± 3,187.0 | 35,071.0 ± 2,762.0 | 33,897.7 ± 3,420.0 |
Day1 represents the cell numbers per well of 12-well plates, which were counted manually just after the seeding of cells. Day2 means the corresponding data read manually on the 2nd day, and so on. Differences were considered significant in the case of P < 0.05
* P < 0.01 among different passages
Fig. 1.
Passage selection of MEFs for feeder preparation. P1 MEFs proliferated fast but with low purity (a), the feeder layers originated from inactivated P2 MEFs also showed many unwanted cells (b). The growth-arrested P3 cells could be used to prepare high standard feeder layers (c). All bright field images were taken at ×40, with an Olympus CKX41 microscope, a QIMAGING MicroPublisher 3.3 RTV camera and QCapture software. Cell numbers of each passage revealed that the expanding rate of MEFs declined from P2 to P7. The most significant difference appeared between group P2–P5 and group P6–P7 (d)
Inactivation with mitomycin C
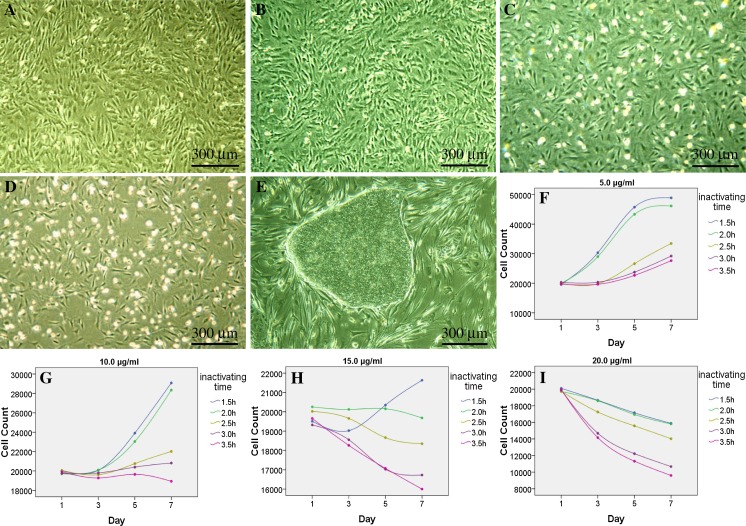
Compared with the MEFs inactivated with low concentration of mitomycin C, those treated with higher concentration usually showed slower growth rates (P < 0.01) (Table 3; Fig. 2), with decreased attachment ability. Meanwhile, the quantity of suspended cells rose steadily, and the dead MEFs increased sharply after being treated with more than 15 μg/ml of mitomycin C. Short treating time related to inadequate inactivation, while immoderate treating time could bring a large quantity of dead MEFs (P < 0.01) (Table 3; Fig. 2).
Table 3.
Cell counts of the MEFs inactivated with mitomycin C
| Concentration (μg/ml)* | Treating time (h)#,& | Day1^ | Day3^ | Day5^ | Day7^ |
|---|---|---|---|---|---|
| 5 | 1.5 | 19,555.7 ± 601.2 | 30,330.7 ± 2,330.8 | 45,756.5 ± 4,819.5 | 48,901.3 ± 5,737.6 |
| 2.0 | 19,965.2 ± 1,112.4 | 29,018.8 ± 4,136.0 | 43,323.3 ± 5,576.7 | 46,149.8 ± 5,463.3 | |
| 2.5 | 19,954.7 ± 881.4 | 19,828.8 ± 573.0 | 26,620.0 ± 739.1 | 33,470.3 ± 957.5 | |
| 3.0 | 20,333.2 ± 495.9 | 20,346.3 ± 928.7 | 23,758.0 ± 1,068.9 | 29,205.8 ± 1,458.1 | |
| 3.5 | 19,700.0 ± 410.8 | 19,643.8 ± 800.3 | 22,676.5 ± 1,142.9 | 27,646.2 ± 1,503.5 | |
| 10 | 1.5 | 20,009.2 ± 944.2 | 20,074.8 ± 2,133.6 | 23,906.0 ± 1,177.5 | 29,083.2 ± 1,161.7 |
| 2.0 | 19,697.0 ± 396.9 | 20,090.8 ± 982.9 | 23,036.2 ± 658.5 | 28,347.8 ± 1,022.9 | |
| 2.5 | 20,057.0 ± 1,230.8 | 19,611.3 ± 1,118.5 | 20,756.3 ± 1,364.7 | 22,011.8 ± 1,408.0 | |
| 3.0 | 19,759.2 ± 416.7 | 19,793.0 ± 641.1 | 20,394.2 ± 666.3 | 20,814.7 ± 994.0 | |
| 3.5 | 19,903.3 ± 763.0 | 19,278.2 ± 851.6 | 19,651.2 ± 1,119.1 | 18,932.0 ± 892.5 | |
| 15 | 1.5 | 19,506.7 ± 756.2 | 19,024.2 ± 617.7 | 20,344.2 ± 564.9 | 21,631.0 ± 908.8 |
| 2.0 | 20,251.8 ± 616.9 | 20,122.0 ± 719.9 | 20,153.3 ± 628.4 | 19,679.0 ± 965.1 | |
| 2.5 | 20,020.3 ± 927.9 | 19,654.0 ± 981.2 | 18,663.3 ± 1,004.6 | 18,348.2 ± 921.7 | |
| 3.0 | 19,319.2 ± 754.8 | 18,549.8 ± 545.7 | 17,026.0 ± 677.7 | 16,726.5 ± 517.1 | |
| 3.5 | 19,653.5 ± 595.9 | 18,260.8 ± 692.1 | 17,071.0 ± 492.0 | 16,000.7 ± 546.0 | |
| 20 | 1.5 | 20,098.3 ± 230.7 | 18,661.0 ± 272.8 | 17,149.5 ± 334.9 | 15,882.3 ± 447.9 |
| 2.0 | 19,725.7 ± 534.7 | 18,602.7 ± 546.1 | 16,949.8 ± 380.8 | 15,805.8 ± 456.5 | |
| 2.5 | 19,745.7 ± 916.8 | 17,248.3 ± 902.1 | 15,577.3 ± 913.4 | 14,027.2 ± 821.8 | |
| 3.0 | 19,885.2 ± 322.1 | 14,687.7 ± 444.3 | 12,230.3 ± 318.8 | 10,670.7 ± 346.8 | |
| 3.5 | 19,922.8 ± 527.3 | 14,156.8 ± 1,108.6 | 11,319.2 ± 761.3 | 9,597.2 ± 636.4 |
Day1 represents the cell numbers per well of a 12-well plate, which were counted manually just after the seeding of cells. Day3 means the corresponding data got by a cell counter on the 3rd day after seeding, and so on. Differences were considered significant when P < 0.05
* P < 0.01 among different concentration groups
# P < 0.01 among various treating time groups
& P < 0.01 among different combinations of concentration and treating time
^ P > 0.05 among various passage groups, so the data of P2–P5 have all been merged in current table
Fig. 2.
Inactivation effects of mitomycin C. The MEFs inactivated with 10 μg/ml of mitomycin C for 3 h (a), or with 15 μg/ml for 2.0 h (b) could both be used to prepare feeder layers, on which the hESCs grew well in an undifferentiated state (e). The MEFs treated with 15 μg/ml of mitomycin C for 2.5 h (c), or 20 μg/ml for 2.0 h (d) both brought about many suspension cells in feeder layers. All bright field images were taken at ×40, using an Olympus CKX41 microscope, a QIMAGING MicroPublisher 3.3 RTV camera and QCapture software. The MEFs inactivated with concentration series of mitomycin C for different time showed great differences in the number of attached cells during subsequent feeder preparation (f–i), treating MEFs with 10 μg/ml of mitomycin C for 3 h or 2.5 h, or 15 μg/ml for 2.0 h could all meet the demand of inactivation (a, b, e, g, h)
This suggested that ultrahigh concentration of mitomycin C or excessive long treating time could achieve the goal of arresting the growth of MEFs, but would also bring massive suspension cells, and lead to poor growth ability to the subsequently plated hESCs. Balancing the pros and cons, it will be appropriate to inactivate MEFs with 10 μg/ml of mitomycin C for 3 h routinely. In special situations, treating the MEFs with 10 μg/ml of mitomycin C for 2.5 h, or 15 μg/ml for 2.0 h can serve as alternative methods (Fig. 2).
Inactivation by X-ray irradiation
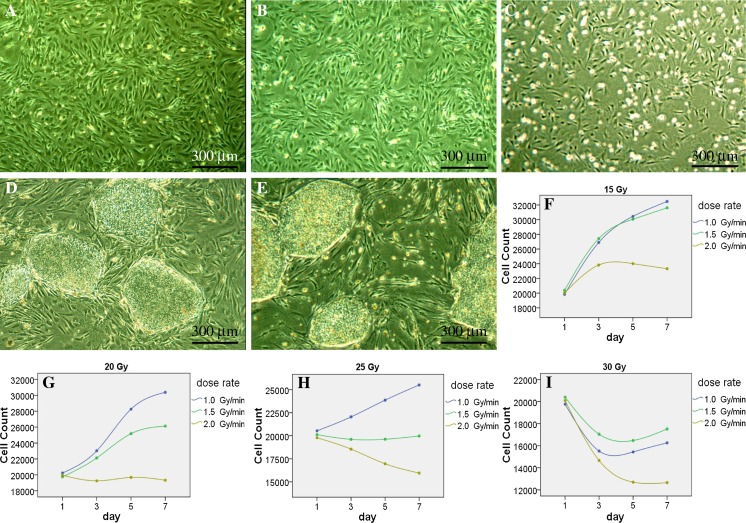
Due to low purity of P2 cells commonly appearing in chemical inactivation phase, they were excluded in irradiation experiments. P3–P5 MEFs were irradiated separately with X-ray, the results revealed that there were significant differences among the groups of various absorbed doses (P < 0.01), absorbed dose rate (P < 0.01) and passages (P < 0.01). In general, low absorbed dose or absorbed dose rate usually brought inadequate inactivation effect, high absorbed dose or absorbed dose rate often caused appropriate or even excessive inactivation effect (Table 4; Fig. 3). For the P3–P4 cells, it was perfect to be irradiated at 1.5 Gy/min for 25 Gy of absorbed dose, or at 2.0 Gy/min for 20 Gy; for P5, the optimal condition was merely at 1.5 Gy/min for 25 Gy (Table 4; Fig. 3). So it is reasonable to irradiate P3–P5 MEFs at 1.5 Gy/min for 25 Gy commonly.
Table 4.
Cell Counts of the MEFs inactivated by irradiation
| Dose (Gy)* | Dose rate (Gy/min)#,& | Passage^ | Day1 | Day3 | Day5 | Day7 |
|---|---|---|---|---|---|---|
| 15 | 1.0 | 3 | 20,073.5 ± 945.4 | 27,792.5 ± 4,085.0 | 32,189.5 ± 5,530.3 | 35,084.5 ± 6,507.5 |
| 4 | 19,852.0 ± 714.2 | 29,331.0 ± 323.9 | 34,171.5 ± 88.4 | 35,753.5 ± 232.6 | ||
| 5 | 19,551.5 ± 591.8 | 23,560.0 ± 2,199.1 | 24,987.0 ± 1,776.3 | 26,598.5 ± 2,684.9 | ||
| 1.5 | 3 | 20,896.5 ± 37.5 | 25,581.5 ± 912.9 | 29,336.5 ± 931.3 | 31,159.0 ± 1,032.4 | |
| 4 | 20,045.5 ± 1,076.9 | 27,693.0 ± 557.2 | 29,904.0 ± 421.4 | 30,953.0 ± 1,636.2 | ||
| 5 | 20,213.0 ± 616.6 | 28,993.0 ± 1,598.1 | 30,942.0 ± 2,330.6 | 32,734.0 ± 3,060.4 | ||
| 2.0 | 3 | 19,701.0 ± 130.1 | 26,147.0 ± 1,937.5 | 28,051.0 ± 1,876.7 | 28,416.5 ± 1,933.9 | |
| 4 | 20,869.0 ± 107.5 | 26,976.5 ± 163.3 | 27,241.5 ± 208.6 | 25,996.5 ± 200.1 | ||
| 5 | 19,501.0 ± 195.2 | 18,357.5 ± 198.7 | 16,745.5 ± 422.1 | 15,561.0 ± 246.1 | ||
| 20 | 1.0 | 3 | 20,302.0 ± 285.7 | 24,093.0 ± 567.1 | 32,072.0 ± 25.5 | 34,285.0 ± 397.4 |
| 4 | 20,089.0 ± 773.6 | 23,090.5 ± 5,574.1 | 28,874.5 ± 3,933.6 | 31,645.0 ± 4,881.9 | ||
| 5 | 20,318.0 ± 407.3 | 21,904.5 ± 1,109.5 | 23,833.0 ± 1,267.1 | 25,187.5 ± 2,813.6 | ||
| 1.5 | 3 | 19,722.0 ± 582.7 | 23,413.5 ± 1,502.6 | 27,653.0 ± 1,661.7 | 28,745.5 ± 2,911.2 | |
| 4 | 19,711.5 ± 37.5 | 21,665.0 ± 833.0 | 24,403.5 ± 1,017.5 | 24,942.5 ± 1,214.1 | ||
| 5 | 19,812.5 ± 502.8 | 21,306.0 ± 4,435.0 | 23,478.5 ± 4,995.7 | 24,689.0 ± 4,624.5 | ||
| 2.0 | 3 | 20,014.5 ± 178.9 | 21,600.0 ± 199.4 | 23,230.0 ± 418.6 | 23,930.0 ± 1,306.7 | |
| 4 | 19,838.5 ± 1,002.0 | 18,428.0 ± 3,477.6 | 19,365.0 ± 3,521.4 | 18,144.0 ± 3,572.3 | ||
| 5 | 20,006.5 ± 983.6 | 17,715.5 ± 2,468.5 | 16,451.0 ± 2,012.4 | 15,901.5 ± 1,777.0 | ||
| 25 | 1.0 | 3 | 20,675.0 ± 422.9 | 24,974.0 ± 86.3 | 25,920.5 ± 1,229.7 | 28,263.0 ± 502.0 |
| 4 | 20,793.0 ± 137.2 | 22,432.0 ± 899.4 | 27,501.0 ± 1,909.2 | 29,422.5 ± 176.1 | ||
| 5 | 20,123.5 ± 559.3 | 18,730.5 ± 1,685.0 | 18,160.0 ± 1,096.0 | 18,816.0 ± 1,803.1 | ||
| 1.5 | 3 | 19,862.5 ± 881.8 | 19,748.5 ± 729.0 | 20,576.0 ± 425.7 | 21,118.5 ± 484.4 | |
| 4 | 20,640.5 ± 218.5 | 20,664.5 ± 2,566.1 | 20,224.0 ± 3,289.5 | 21,041.5 ± 3,988.8 | ||
| 5 | 19,841.0 ± 181.0 | 18,418.0 ± 2,057.7 | 18,067.0 ± 2,005.4 | 17,752.0 ± 1,709.8 | ||
| 2.0 | 3 | 19,892.0 ± 427.1 | 19,822.0 ± 1,031.0 | 19,338.5 ± 685.2 | 18,049.0 ± 135.8 | |
| 4 | 20,040.0 ± 401.6 | 18,962.0 ± 3,094.3 | 17,608.5 ± 2,341.2 | 16,908.5 ± 2,868.7 | ||
| 5 | 19,424.5 ± 290.6 | 16,855.0 ± 763.7 | 13,936.5 ± 676.7 | 12,903.5 ± 441.9 | ||
| 30 | 1.0 | 3 | 19,361.0 ± 376.2 | 14,075.5 ± 1,446.0 | 15,328.0 ± 199.4 | 15,636.5 ± 57.3 |
| 4 | 19,722.5 ± 13.4 | 17,011.5 ± 324.6 | 16,768.5 ± 703.6 | 18,110.5 ± 1,727.5 | ||
| 5 | 20,218.0 ± 899.4 | 15,474.5 ± 3,428.8 | 14,213.0 ± 3,539.8 | 15,036.0 ± 4,061.6 | ||
| 1.5 | 3 | 20,756.5 ± 115.3 | 17,812.5 ± 1,949.5 | 18,356.0 ± 2,910.5 | 19,435.0 ± 3,863.6 | |
| 4 | 20,295.0 ± 646.3 | 16,943.5 ± 1,348.5 | 16,370.5 ± 1,778.4 | 17,551.0 ± 2,045.0 | ||
| 5 | 20,140.5 ± 444.8 | 16,376.5 ± 450.4 | 14,688.0 ± 445.5 | 15,563.0 ± 961.7 | ||
| 2.0 | 3 | 20,648.5 ± 432.0 | 15,291.5 ± 200.1 | 13,947.5 ± 217.1 | 14,818.5 ± 297.7 | |
| 4 | 20,292.0 ± 520.4 | 14,425.0 ± 2,259.9 | 13,129.0 ± 2,098.7 | 12,491.5 ± 1,880.2 | ||
| 5 | 19,352.5 ± 422.1 | 14,271.5 ± 840.8 | 11,024.0 ± 622.3 | 10,654.0 ± 352.1 |
Day1 represents the cell numbers per well of a 12-well plate, which were counted manually just after the seeding of cells. Day3 means the corresponding data read by a cell counter on the 3rd day, and so on. Differences were considered significant when P < 0.05
* P < 0.01 among different dose groups
# P < 0.01 among various dose rate groups
& P < 0.05 among different combinations of dose and dose rate
^ P < 0.01 between group P3–P4 and group P5
Fig. 3.
Inactivation effects of X-ray. The P3 MEFs irradiated with X-ray at 1.5 Gy/min for 25 Gy (a), or at 2.0 Gy/min for 20 Gy (b) could both be used to establish high quality feeder layers, on which the hESCs grew well in an undifferentiated state (d, e). The MEFs irradiated with X-ray at 2.0 Gy/min for 25 Gy showed excessive inactivation, with more suspension cells after feeder preparation (c). All bright field images were taken at ×40, with an Olympus CKX41 microscope and a QIMAGING MicroPublisher 3.3 RTV camera manipulated by QCapture software. Quantities of the irradiated MEFs showed significant differences among the groups of various absorbed dose and various absorbed dose rates (f–i). It is reasonable to irradiate P3–P5 MEFs at 1.5 Gy/min for 25 Gy routinely (a, d, h)
Furthermore, we plated hESCs on the feeder layers having been irradiated at 1.5 Gy/min for 25 Gy (P3–P5) or at 2.0 Gy/min for 20 Gy (P3–P4), and cultured them under ordinary conditions. They showed no visible differences with those cultured on mitomycin C treated or γ-irradiated layers, in terms of proliferation rates, morphology and differentiation states. This provided another proof that the X-ray irradiating methods can meet the demands of cultivating hESCs in common laboratories.
Discussion
MEFs have now been widely used to sustain the growth of hESCs or iPSCs in vitro, and proper preparation of feeder layers becomes a prerequisite for relevant studies. The quality of feeder layers is affected by many factors, such as trypsin concentration, digesting time, the formula of culture medium, etc. Here, we adopted the most commonly used conditions in these aspects, and focused on optimizing the most decisive factors described above.
We recommend picking the embryos at 12.5–13.5 DPC for isolation, but actually the MEFs reaped at 13.5–15.5 DPC are all acceptable for use. Considering the heavy labor load demanded in the isolation process, it is inevitable to separate a large batch of pregnant mice of the same gestational age into several batches for isolation, so acquiring MEFs at 13.5–15.5 DPC is needed in many scenarios.
Passage number is another key element for successful feeder preparation. Most researchers advised to choose P2–P3 MEFs for use because of their high expanding rate, which links to remarkable sustaining ability for ESCs. Our results indicated that P4–P5 cells are suitable for the purpose too. From our point of view, the P4–P5 MEFs can bring about more available feeder resources, and increase the practicability of the approaches.
Compared with irradiation approaches, chemical inactivation methods can be operated at random without any special equipment, so it is popular among relevant laboratories. Our results revealed the best choice is treating MEFs with 10 μg/ml of mitomycin C for 3 h, while most protocols recommended by others advised to inactivate MEFs with 10 μg/ml of mitomycin C for 2.0–2.5 h (Richards et al. 2002, 2003; Nieto et al. 2007; Jozefczuk et al. 2012; Aoshima et al. 2013). We believe the difference mainly comes from the disparate culture media, mitomycin C, the strains of mouse, etc. This also implies that it is still necessary to re-optimize the proposed protocols, according to the real conditions of a certain laboratory.
The irradiation method owns a series of unique merits: The researchers can inactivate a large amount of MEFs easily, without being afraid of bringing any contaminants, as the whole process dispenses with opening any culture vessels; meanwhile, due to nothing needs to be added, no washing steps must be carried out after irradiation, which are strictly required in chemical inactivation, so the method may remarkably reduce the workload. Previous studies usually exploited γ-ray emitted by Co60 to irradiate MEFs, this might bring heavy radioactive contamination, for the γ-ray was generated constantly regardless of whether it was needed. The X-ray used in this study was generated by an electronic linear accelerator. During the whole process, no radioactive materials were added, so it could cause very limited radioactive pollution. If problems appeared during the irradiating process, we could just suspend the X-ray generating program and solve them at will, without worrying about any harmful irradiation, for no X-ray existed at the moment. Due to no valuable references could be found, we tried to inactivate MEFs with X-ray, referring to the procedures of Co60 and the routine approaches for killing leukocytes within human blood components with X-ray prior to clinical blood transfusion. Our innovation proved for the first time that X-ray can also be utilized to inactivate MEFs. The results revealed that the best method is to irradiate P3–P5 MEFs with X-ray at 1.5 Gy/min for 25 Gy, which is noticeably lower than the proposed levels of γ-ray (Unger et al. 2009; Hongisto et al. 2012; Burt et al. 2012). These suggest that it is reasonable to replace the Co60 irradiation in the future.
Mitomycin C and γ-irradiation were usually considered to be equal in terms of inactivation effect. Some reports suggested the chemical inactivation led to reduced expression of some ligands and cytokines in MEFs, whereas similar changes did not appear in γ-irradiated feeder cells (Roy et al. 2001). Meanwhile, after mitomycin C treatment, the percentage of apoptotic or necrotic cells substantially increased (Nieto et al. 2007). These indicate that feeder cells treated with mitomycin C may be less efficient in long-term cell culture systems, and comprehensive comparisons should yet be carried out to further clear up the problem.
The factors discussed above cover all the crucial aspects of a whole protocol for the preparation of feeder layers. Compared with direct buying MEFs from market, our method enables us to obtain MEFs more steadily, reliably and economically. Compared with gaining MEFs from CF1 mice, using ICR mice brings more practicability and convenience, meanwhile it can also reduce the cost significantly. So we believe the present methods make a more favorable option for feeder preparation.
Acknowledgments
The authors thank Drs. Aidong Wu and Bing Yan at Radiotherapy Department, Affiliated Anhui Provincial Hospital, Anhui Medical University for their generous help in irradiation process; and Dr. Xiao Lei at Zhejiang University for presenting hESCs.
Conflict of interest
The authors have no conflict of interests to declare.
Abbreviations
- MEFs
Mouse embryonic fibroblasts
- ESCs
Embryonic stem cells
- hESCs
Human embryonic stem cells
- iPSCs
Induced pluripotent stem cells
- DPC
Day post-coitum
- FGF
Fibroblast growth factor
- FBS
Fetal bovine serum
References
- Abraham S, Riggs MJ, Nelson K, Lee V, Rao RR. Characterization of human fibroblast-derived extracellular matrix components for human pluripotent stem cell propagation. Acta Biomater. 2010;6:4622–4633. doi: 10.1016/j.actbio.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Aoshima K, Baba A, Makino Y, Okada Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS One. 2013;8:e77715. doi: 10.1371/journal.pone.0077715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RK, Chen YH, Verda L, Lucena C, Navale S, Johnson J, Han X, Lomasney J, Baker JM, Ngai KL, Kino A, Carr J, Kajstura J, Anversa P. Mitotically inactivated embryonic stem cells can be used as an in vivo feeder layer to nurse damaged myocardium after acute myocardial infarction: a preclinical study. Circ Res. 2012;111:1286–1296. doi: 10.1161/CIRCRESAHA.111.262584. [DOI] [PubMed] [Google Scholar]
- Chen HF, Chuang CY, Shieh YK, Chang HW, Ho HN, Kuo HC. Novel autogenic feeders derived from human embryonic stem cells (hESCs) support an undifferentiated status of hESCs in xeno-free culture conditions. Hum Reprod. 2009;24:1114–1125. doi: 10.1093/humrep/dep003. [DOI] [PubMed] [Google Scholar]
- Eiselleova L, Peterkova I, Neradil J, Slaninova I, Hampl A, Dvorak P. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol. 2008;52:353–363. doi: 10.1387/ijdb.082590le. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Krtolica A, Zdravkovic T, Brunette E, Powell S, Nath A, Caceres E, McMaster M, McDonagh S, Li Y, Mandalam R, Lebkowsk J, Fisher SJ. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil Steril. 2005;83:1517–1529. doi: 10.1016/j.fertnstert.2005.01.086. [DOI] [PubMed] [Google Scholar]
- Hongisto H, Vuoristo S, Mikhailova A, Suuronen R, Virtanen I, Otonkoski T, Skottman H. Laminin-511 expression is associated with the functionality of feeder cells in human embryonic stem cell culture. Stem Cell Res. 2012;8:97–108. doi: 10.1016/j.scr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Hu K, Yu J, Suknuntha K, Tian S, Montgomery K, Choi KD, Stewart R, Thomson JA, Slukvin II. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:e109–e119. doi: 10.1182/blood-2010-07-298331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefczuk J, Drews K, Adjaye J. Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. J Vis Exp. 2012;64:e3854. doi: 10.3791/3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibschull M, Mileikovsky M, Michael IP, Lye SJ, Nagy A. Human embryonic fibroblasts support single cell enzymatic expansion of human embryonic stem cells in xeno-free cultures. Stem Cell Res. 2011;6:70–82. doi: 10.1016/j.scr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R. Human embryonic stem cells derived without feeder cells. Lancet. 2005;365:1636–1641. doi: 10.1016/S0140-6736(05)66473-2. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Nieto A, Cabrera CM, Catalina P, Cobo F, Barnie A, Cortes JL, Barroso del Jesus A, Montes R, Concha A. Effect of mitomycin-C on human foreskin fibroblasts used as feeders in human embryonic stem cells: immunocytochemistry MIB1 score and DNA ploidy and apoptosis evaluated by flow cytometry. Cell Biol Int. 2007;31:269–278. doi: 10.1016/j.cellbi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Park SP, Lee YJ, Lee KS, AhShin H, Cho HY, Chung KS, Kim EY, Lim JH. Establishment of human embryonic stem cell lines from frozen-thawed blastocysts using STO cell feeder layers. Hum Reprod. 2004;19:676–684. doi: 10.1093/humrep/deh102. [DOI] [PubMed] [Google Scholar]
- Prowse AB, McQuade LR, Bryant KJ, Marcal H, Gray PP. Identification of potential pluripotency determinants for human embryonic stem cells following proteomic analysis of human and mouse fibroblast conditioned media. J Proteome Res. 2007;6:3796–3807. doi: 10.1021/pr0702262. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- Roy A, Krzykwa E, Lemieux R, Neron S. Increased efficiency of gamma-irradiated versus mitomycin C-treated feeder cells for the expansion of normal human cells in long-term cultures. J Hematother Stem Cell Res. 2001;10:873–880. doi: 10.1089/152581601317210962. [DOI] [PubMed] [Google Scholar]
- Skottman H. Derivation and characterization of three new human embryonic stem cell lines in Finland. In Vitro Cell Dev Biol Anim. 2010;46:206–209. doi: 10.1007/s11626-010-9286-2. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Stewart R, Przyborski S, Armstrong L, Evans J, Murdoch A, Strachan T, Stojkovic M. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- Strom S, Holm F, Bergstrom R, Stromberg AM, Hovatta O. Derivation of 30 human embryonic stem cell lines—improving the quality. In Vitro Cell Dev Biol Anim. 2010;46:337–344. doi: 10.1007/s11626-010-9308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tecirlioglu RT, Nguyen L, Koh K, Trounson AO, Michalska AE. Derivation and maintenance of human embryonic stem cell line on human adult skin fibroblast feeder cells in serum replacement medium. In Vitro Cell Dev Biol Anim. 2010;46:231–235. doi: 10.1007/s11626-010-9278-2. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Unger C, Gao S, Cohen M, Jaconi M, Bergstrom R, Holm F, Galan A, Sanchez E, Irion O, Dubuisson JB, Giry-Laterriere M, Salmon P, Simon C, Hovatta O, Feki A. Immortalized human skin fibroblast feeder cells support growth and maintenance of both human embryonic and induced pluripotent stem cells. Hum Reprod. 2009;24:2567–2581. doi: 10.1093/humrep/dep232. [DOI] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972–980. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Cai Z, Li Y, Shu J, Pan L, Wan F, Li H, Huang X, He C, Liu Y, Cui X, Xu Y, Gao Y, Wu L, Cao S, Li L. Utilization of human amniotic mesenchymal cells as feeder layers to sustain propagation of human embryonic stem cells in the undifferentiated state. Cell Reprogram. 2011;13:281–288. doi: 10.1089/cell.2010.0103. [DOI] [PubMed] [Google Scholar]