Abstract
The purpose of our study was to examine the influence of hypoxia on proliferation of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs). The mononuclear cells were separated by density gradient centrifugation from human umbilical cord blood and then, respectively, cultured under hypoxia (5 % O2) or normoxia (20 % O2). Their cell morphology, cell surface markers, β-galactosidase staining, cell growth curve, DNA cycle, and the expression of hypoxia-inducible factor-1α (HIF-1α) were evaluated. We found that hypoxia, in part via HIF-1α, improved the proliferation efficiency, and prevented senescence of hUCB-MSCs without altering their morphology and surface markers. These results demonstrated that hypoxia provides a favorable culture condition to promote hUCB-MSCs proliferation in vitro, which is a better way to obtain sufficient numbers of hUCB-MSCs for research and certainly clinical application.
Keywords: Hypoxia, Human cord blood-derived mesenchymal stem cells, Proliferation, Hypoxia-inducible factor-1α
Introduction
The multipotential capacity of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) (Lee et al. 2004), their simple isolation and culture, as well as their low probability of pathophoresis and immunity (Liu et al. 2014) make these cells the best candidates for tissue engineering. Although such cell-based therapies are becoming more prominent, they exhibit a unique in vitro expansion capacity, which however does not compensate for the large number of cells required for cell therapeutic applications (Sotirpoulou et al. 2006).
Early studies paid exquisite attention to the balance of nutrients, growth factors (Chen et al. 2009), and pH buffers (Yuan et al. 2014) used to grow cells in vitro. Very little attention was given to the oxygen concentration present in culture media. Oxygen concentrations in human body vary from 3 to 11 % (Goossens et al. 2011) and the stem cells reside in the most hypoxic areas (Roemeling-van et al. 2013; Danet et al. 2003), thus, hUCB-MSCs reside in anatomical sites that are relatively oxygen-deficient (Arikan et al. 2000). Studies in vitro have shown that culture in 2–3 % O2 stimulated human mesenchymal stem cells (hMSC) proliferation (Estrada et al. 2012). Therefore, hypoxic cultures may more accurately reflect the actual in vivo environment and may prove beneficial to hUCB-MSCs during in vitro expansion, while hUCB-MSCs are normally cultured in 20 % oxygen tension.
These observations led us to the hypothesis that hypoxia might promote the hUCB-MSCs generation. Although investigators have reported that reduced oxygen tension enhances proliferation of many stem cell types, including rat mesenchymal stem cells (MSCs) (Lennon et al. 2001), neural crest stem cells (NCSCs) (Morrison et al. 2000), embryonic stem (ES) cells, hematopoietic stem cells (HSCs), trophoblast stem cells (Keith and Simon 2007). The effect of hypoxia on the proliferation of hUCB-MSCs has not been studied. In the present study, we evaluated the influence of hypoxia on proliferation of hUCB-MSCs by examining the effects of two different oxygen levels (5 and 20 % O2) on their cell morphology, cell surface markers, β-galactosidase staining, cell growth curve, DNA cycle, and the expression of HIF-1α. We hope to develop a hypoxic cell culture system to enhance the proliferation of hUCB-MSCs.
Materials and methods
Collection of UCB
UCB units (n = 23) from full-term deliveries were collected from the unborn placenta with informed consent of the mothers. A bag system containing 17 ml of citrate phosphate dextrose (CPD) anticoagulant within the collection bag was used (Cord Blood Collection System, Eltest, Bonn, Germany) and processed within 2 h of collection. The collection was performed in accordance with the ethical standards of the local ethical committee.
Isolation and culture of mononuclear cells from UCB
Prior to the isolation of MNC, the anticoagulated cord blood was diluted 1:2 with PBS. The MNC fraction was isolated by density gradient centrifugation at 400g for 20 min at room temperature using Ficoll-Hypaque solution (Haoyang, Tianjin, China) and cell vitality was assessed by Trypan Blue exclusion. Cells were seeded at a density of 1 × 106 cell per cm2 in human umbilical cord mesenchymal stem cell growth medium (OriCellTM; Cyagen, Guangzhou, China). Flasks were maintained at 37 °C in 5 % O2/20 % O2 in humidified atmosphere. To create the hypoxic conditions the cells were cultured in a hypoxia chamber (Billups Rothenberg, Del Mar, CA, USA). Briefly, the cultures were enclosed in the chamber and purged with either 95 % air and 5 % CO2 (normoxia) or 5 % O2, 5 % CO2, and 90 % N2 (hypoxia). Cells were kept in these conditions for 5 days. Then half of the medium was changed every 3 days by removing the cells from the incubators and adding new medium previously equilibrated for 15 min in the respective incubators. The cells did not spend more than 5 min outside the incubators during medium change to prevent a possible hypoxia/reoxygenation effect. Cells in primary culture colonies were counted on an Olympus CKX41 inverted microscope.
Immunophenotyping of cultured MSC
To analyze the cell-surface expression of typical marker proteins, the MSCs were labeled with antihuman antibodies against CD44, CD105, CD29 and CD34 (BD Pharmingen, San Diego, CA, USA). Mouse isotype antibodies served as respective controls (BD Pharmingen, San Diego, USA). Ten thousand labeled sells were acquired and analyzed using a FACScan flow cytometer running CellQuest software (Becton–Dickinson).
Proliferation assay
Cell proliferation assay was performed using the Cell Counting Kit-8 (CCK8; Dojindo Laboratories, Kumamoto, Japan). According to the manufacture’s protocol, hUCB-MSCs at passage 3 under normoxic or hypoxic conditions were plated in 96 well plates at 6.25 × 103, 1.25 × 104, 2.5 × 104, 5 × 104, 1 × 105 cells ml−1, respectively, to get a standard curve relating cell density and optical density values. Cells at passage 3 were seeded in 96-well flat bottom plates at a concentration of 3,000 cells per well in 100 µl culture medium and kept for 1, 2, 3, 4, 5, 6, 7 days under normoxic or hypoxic conditions. At the endpoint, 10 µl CCK-8 was added to each well for further 2 h. Then cells number were measured as the absorbance at 450 nm using a microplate reader (TECAN, Gröbig, Austria).
The doubling time was calculated during the logarithmic phase of the growth curve. The time of population doublings was calculated using the formula:
T is the time of the logarithmic phase of the growth curve, N0 the number of cells after seeding, Nt the number of cells at the end of the logarithmic growth phase.
β-Galactosidase staining
β-Galactosidase staining was performed using a senescence-associated β-galactosidase (SA-β-gal) staining kit (Beyotime, Beijing, Jiangsu, China) following the manufacturer’s protocol. The treatment methods for the hUCB-MSCs in each group were the same as described above. The number of positive cells was counted under a phase-contrast microscope. The experiment was repeated three times in each group.
Cell cycle assay
1 × 106 cells at passage 3 under normoxic or hypoxic conditions were harvested by trypsinization (0.125 % trypsin–EDTA) and fixed in 70 % cold ethanol, respectively. After that, these cells were water-bathed with RNase A and stained with propidium iodide according to the manufacturer’s instruction (cell cycle kit, Beyotime, Jiangsu, China). Then, DNA content was assessed by flow cytometry.
Western blotting
To evaluate the influence of hypoxia on the expression of HIF-1α, the cells were cultured under hypoxia or normoxia. At passage 3, total protein was harvested from the cultured cells using a total protein extraction kit (Applygen, Beijing, China) following the manufacturer’s protocol. The protein concentration of the cells was measured with a BCA protein assay kit (Beyotime, Jiangsu, China). Cell lysate samples were separated by 10 % SDS-PAGE and then transferred to a PVDF membrane. After blocking the membrane with 5 % skim milk in Tris-buffered saline Tween-20 (TBST) for 1 h, the membranes were incubated with the following primary antibodies (diluted at 1:1,000) for 2 h at room temperature: HIF-1α (Cell Signaling Technology, Danvers, MA, USA) and β-action (Beyotime, Jiangsu, China). The membranes were washed in TBST and incubated with peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, Baltimore, MD, USA) for 2 h, washed, and developed with electrogenerated chemiluminescence (ECL) Western blotting detection reagent (7SeaPharmTech, Shanghai, China). Proteins were visualized using X-OMAT film (Kodak, Rochester, NY, USA). Densitometric analysis of the blots was performed with NIH image software.
Statistical analysis
Data were analyzed using SPSS 17.0. The variable are expressed as mean ± SD and compared with Student’s unpaired t test. Two-tailed P < 0.05 was considered statistically significant.
Results
There were 23 human umbilical cord blood samples which were used in this study, all experiments were repeated at least three times.
Cell morphology
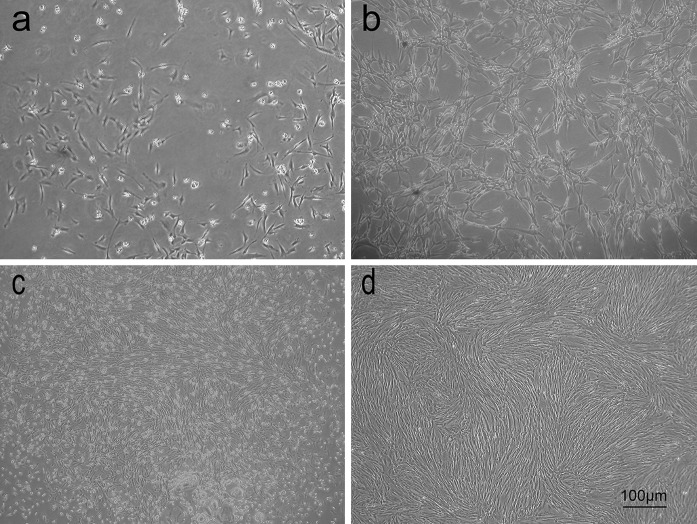
MSC isolated from human umbilical cord blood cultured in either 5 or 20 % oxygen were viewed by phase contrast microscopy. The two groups of hUCB-MSCs in primary culture formed an adherent heterogeneous cell population after 3–5 days in culture, which consisted of round and spindle shaped cells. Small colonies of fibroblast-like cells were seen after 18–20 days of primary culture. These colonies increased in size and were subcultured after 20–28 days. Colonies appeared to be larger and were more numerous in cultures maintained in hypoxia (Fig. 1c). Individual cells, however, appeared to be identical in either oxygen condition in these primary colonies. When the cells were subcultured, the heterogeneous cell populations changed into a homogeneous one with flat and fibroblast-like shape. At third passage, cells from hypoxic condition maintained their spindle morphologies, exhibited uniform cell orientation within various regions and had a better growth (Fig. 1d), while cells from normoxia showed an atypical senescent cell morphology (enlarged and flattened cell body with increased cytoplasmic granularity) (Fig. 1b).
Fig. 1.
Phase-contrast images of hUCB-MSCs show morphology changes with different passages cultured in hypoxia (5 % O2) or normoxia (20 % O2). a Normoxic cells in primary culture formed small ‘islets’, b cells of passage 3 cultured in normoxia showed a typical senescent cell morphology (enlarged and flattened cell body with increased cytoplasmic granularity), c hypoxic cells in primary culture formed big ‘islets’, d cells of passage 3 cultured in hypoxia maintained their spindle morphologies, exhibited uniform cell orientation within various regions and had a better growth within the culture
Flow cytometry analysis
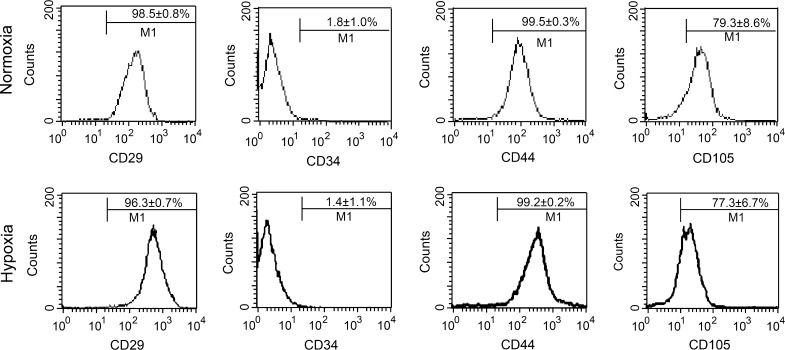
The cell surface antigen profile of hUCB-MSCs at passage 3 in hypoxia or normoxia was analyzed and compared. It was found that human cord blood-derived MSCs cultured under hypoxia expressed CD29 (96.3 ± 0.7 %), CD44 (99.2 ± 0.2 %) and CD105 (77.3 ± 6.7 %), but did not express CD34 (1.4 ± 1.1 %). Similarly, cells cultured under normoxia expressed CD29 (98.5 ± 0.8 %), CD44 (99.5 ± 0.3 %) and CD105 (79.3 ± 8.6 %), but showed little expression of CD34 (1.8 ± 1.0 %). These results indicate that the two groups of hUCB-MSCs were negative for the hematopoietic antigen CD34 and strongly positive for MSC specific surface markers such as CD44, CD29 and CD105. There was no significant difference in the percentage of CD expression between the two oxygen environments (Fig. 2).
Fig. 2.
hUCB-MSCs cultured in hypoxia or normoxia show a similar phenotype. Flow cytometry showed that hUCB-MSCs had high expression of mesenchymal stem cell markers CD44, CD29, and CD105, whereas low expression of the hematopoietic lineage markers CD 34 (n = 3 per group, P > 0.05)
Growth kinetics and β-galactosidase staining
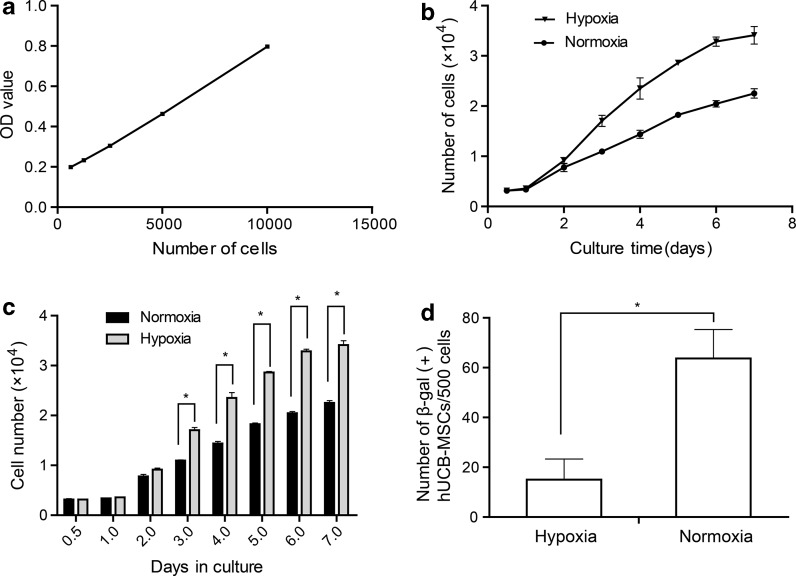
We compared the proliferation ability of passage 3 hUCB-MSCs, respectively, cultured at hypoxia and normal oxygen tension by CCK-8 assay. During the first 2 days of culture there was no significant difference in the number of cells under the two conditions indicating that hypoxia did not stimulate hUCB-MSCs to grow faster. However, from days 3 to 7, cells cultured in hypoxia had a significantly higher cell count as compared to cells cultured in normoxia (Fig. 3c, P < 0.05). Moreover, the cells cultured in normoxia showed significantly higher population doubling time (65.72 ± 1.7 h), in comparison to the cells cultured in hypoxia (49.87 ± 2.0 h) (Fig. 3b, P < 0.05). These date indicated that hypoxia promote hUCB-MSCs proliferation through the reduction of the population doubling time. In addition, senescence as assayed by the expression of SA-β-gal revealed a significant increase in normoxic cells (Fig. 3b, P < 0.05), suggesting hypoxic culture prevented hUCB-MSCs from senescence.
Fig. 3.
Growth kinetics of hUCB-MSCs of passage 3. a A standard curve relating cell density and optical density values. The number of cells was calculated using the formula: number of cells = 0.00006 × OD value + 0.1509. b The proliferation ability of hUCB-MSCs cultured in normoxia or hypoxia. The population doubling time of hUCB-MSCs cultured in normoxia was 65.72 ± 1.7 h, while that of hUCB-MSCs cultured in hypoxia was 49.87 ± 2.0 h (n = 3 per group, *P < 0.05). c Cell growth rates of hUCB-MSCs cultured in hypoxia or normoxia were statistically different after 3 days (n = 3 per group, *P < 0.05). d Cells cultured under normoxia or hypoxia were stained with β-galactosidase (β-gal). Normoxic cells increased in the percentage of β-gal expression compared with hypoxic cells (n = 3 per group, *P < 0.05)
Cell cycle analysis
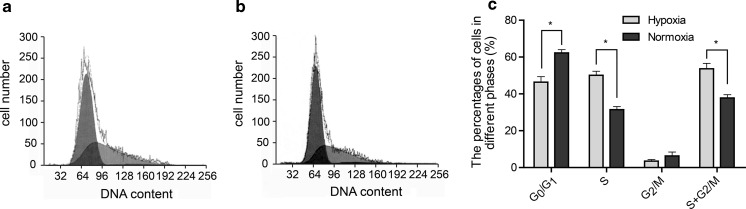
The cell cycle of hUCB-MSCs cultured in hypoxia or normoxia condition was detected with flow cytometry. The distribution of hUCB-MSCs in percentage were 51.14 ± 5.1 % at G0/G1, 1.96 ± 1.4 % at G2/M and 46.9 ± 3.8 % at S phase of cell cycle in hypoxia. In normoxia 65.67 ± 3.1 % of hUCB-MSCs were at G0/G1, 2.83 ± 3.7 % were at G2/M and 31.5 ± 2.9 % were at S phase of cell cycle. 48.86 ± 5.2 % of hUCB-MSCs cultured in hypoxia were found in S + G2/M phases compared with 34.33 ± 3.1 % in normoxia.
When cells in hypoxia were compared with those in normoxia, the proportion of cells at the G0/G1 phase was lower, and the proportion of cells at S and S + G2/M phase were higher (P < 0.05). But the proportions in G2/M phase of hUCB-MSCs cultured in the two oxygen environments were not statistically different (P > 0.05) The results imply that hypoxia promote cell proliferation, through the increase of cells in S phase and the reduction of cells in G0/G1 phase (Fig. 4c).
Fig. 4.
Cell cycle of hUCB-MSCs at passage 3 cultured in hypoxia or normoxia were examined by flow cytometry. a Cell cycles of hUCB-MSCs cultured in low oxygen tension. b Cell cycles of hUCB-MSCs cultured in normoxia. c The percentage of cells in different phases of the cell cycle is presented in the histogram. Cells in hypoxia were compared with those in normoxia, the proportion of cells at the G0/G1 phase was lower (51.14 ± 5.1 % vs 65.67 ± 3.1 %), but the proportion of cells at S (46.9 ± 3.8 % vs 31.5 ± 2.9 %) and S + G2/M (48.86 ± 5.2 % vs 34.33 ± 3.1 %) phases was higher (n = 3 per group, *p < 0.05)
Western blotting analysis
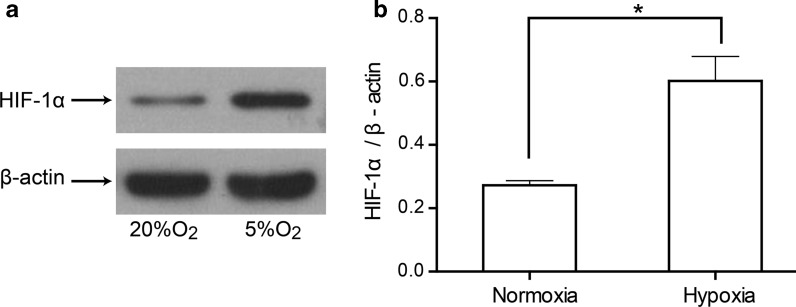
To clarify if HIF-1α was involved in the hypoxic effects on proliferation, protein expression of HIF-1α was investigated by Western blotting. After cultured under the two oxygen environments, the expression of HIF-1α in hUCB-MSCs was detected by Western blotting. As shown in Fig. 5, HIF-1α protein expression was significantly upregulated under hypoxia in hUCB-MSCs (P < 0.05).
Fig. 5.
Expression of HIF-1α in hUCB-MSCs under normoxia or hypoxia. a Expression of HIF-1α was detected by western blot. b The ratio of HIF-1α to β-actin band density values. HIF-1α protein expression was significantly upregulated under hypoxia in hUCB-MSCs (n = 3 per group, *P < 0.05)
Discussion
In present study, hUCB-MSCs were cultivated in convention oxygen (20 % O2) culture conditions and in an atmosphere of 5 % O2 that more closely approximates physiological oxygen levels (Morrison et al. 2000) in order to determine whether the two conditions differ in their effect on cell proliferation.
In this research, the morphology of hUCB-MSCs cultured in 5 % O2 condition resembles that of in 20 % O2 condition, which both showed typical fibroblast-like morphology and is characteristic of MSC. Moreover flow cytometric analysis showed that these cells exhibited the marker profile similar to MSC cultured in two different oxygen conditions. They were negative for the haematopoietic antigen CD34 and strongly positive surface for markers such as CD44, CD29 and CD105. This indicated that hypoxia maintained stemness of hUCB-MSCs. We found that cells of passage 3 maintained their spindle morphologies, exhibited uniform cell orientation within various regions and had a better growth at an oxygen level of 5 % O2, while cells from normoxia showed atypical senescent cell morphology (enlarged and flattened cell body with increased cytoplasmic granularity). In addition, senescence as assayed by the expression of SA-β-gal revealed a significant increase in normoxic cells, suggesting hypoxic culture prevented hUCB-MSCs from senescence. Consistent with our date, Tsai and colleagues have found that culturing in 1 % oxygen reduces MSC senescence (Tsai et al. 2011).
Moreover and importantly, our team also compared the proliferation ability of hUCB-MSCs cultured under two different conditions by cell growth curve and DNA cycle. Then we found that shorter population doubling time and more cells accumulating in S the phase indicating an increased proliferation rate of MSCs cultured under hypoxia, and in agreement with previous studies (Grayson et al. 2007; Mohyeldin et al. 2010). The results suggest that the hypoxic microenvironment is crucial in supporting the growth of hUCB-MSCs.
The mechanism by which reduced oxygen levels promote stem cell proliferation is uncertain and probably complex. Although several pathways have been identified to mediate hypoxic proliferation, the primary mediator of this response is HIF-1α. Wataru and colleagues have reported that HIF-1α promoted neural stem cells (NSC) proliferation (Wataru et al. 2012).
HIF-1α is a transcriptional activator mediating adaptive cellular responses to hypoxia (Semenza 2012). HIF-1α is degraded under normal tissue O2 conditions. However, under conditions of hypoxia degradation of HIF-1α is inhibited and levels increase (Semenza 2012; Roitbak et al. 2011). In the present study, western blotting demonstrated that the expression levels of HIF-1α in hUCB-MSCs were upregulated in hypoxic cells compared with normoxic cells. Consistent with our date, Ding and colleagues have found that HIF-1α modulates stem cell proliferation under hypoxia (Ding et al. 2014), while Co and colleagues did not observe significant difference in HIF-1α mRNA levels between 21 and 5 % chondrogenic culture of equine cord blood mesenchymal stromal cells in vitro (Co et al. 2014).
HIF-1α is involved in activating its downstream targets, erythropoietin (EPO), EPO receptor (EPOR) and vascular endothelial growth factor (VEGF), all of which are cytoprotective and prolong cell survival (Francis and Wei 2010). Thus, hypoxia and Hif-1α upregulation appears to be a features shared by various types of stem cells and an important regulator of their stem cell proliferation. These factors might also be upregulated by hypoxia in hUCB-MSCs, the effect of hypoxia on these factors will be explored in a future study.
A potential limitation of this study is that cultures in reduced oxygen conditions are subject to transient exposure to 20 % oxygen during media changes and/or fluctuations in incubator gas levels, which may not be representative of a true hypoxic environment. Although exposure to normoxia was kept to a minimum, the long-term effect on proliferation cultures is unknown. Furthermore, the selection of 5 % oxygen to represent a hypoxic environment was based on values used in the literature.
In conclusion, to our knowledge, this is the first study by comparing the proliferation of hUCB-MSCs under normoxic and hypoxic conditions. We found that hypoxia, in part via HIF-1α, improved the proliferation efficency, and prevented senescence of hUCB-MSCs without altering their morphology and surface markers. However, it is not well understood what are the molecular mechanisms promoting the proliferation of hUCB-MSCs and whether hypoxia increase or decrease hUCB-MSC differentiation into different mesenchymal lineages in these conditions. So further investigations are under way to determine the detailed mechanism involved in this regulation. Ultimately, we hope that the outcomes of these studies will well suit for tissue engineering, which requires large number of conditioned cells.
Acknowledgments
This study was funded by Grants from the National Natural Science Foundation (No. 31260286) of China. We are grateful to doctors and nurses of the Obstetrical Department of Maternity and child health care hospital in Zunyi for the collection of the cord blood.
References
- Arikan GM, Scholz HS, Petru E, Haeusler MC, Haas J, Weiss PA. Cord blood oxygen saturation in vigorous infants at birth: what is normal? Br J Obstet Gynaecol. 2000;107:987–994. doi: 10.1111/j.1471-0528.2000.tb10401.x. [DOI] [PubMed] [Google Scholar]
- Chen HH, Decot V, Ouyang JP, Stoltzi JF, Bensoussan D, de Isla NC. In vitro initial expansion of mesenchymal stem cells is influenced by the culture parameters used in the isolation process. Bio-Med Mater Eng. 2009;19:301–309. doi: 10.3233/BME-2009-0595. [DOI] [PubMed] [Google Scholar]
- Co C, Vickaryous MK, Koch TG. Membrane culture and reduced oxygen tension enhances cattilage matrix formation from equine cord blood mesenchymal stromal cells in vitro. Osteoarthr Cartil. 2014;22:472–480. doi: 10.1016/j.joca.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Investig. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Gao YS, Wang Y, Hu C, Sun Y, Zhang C. Dimethyloxaloylglycine increases the bone healing capacity of adipose-derived stem cells by promoting osteogenic differentiation and angiogenic potential. Stem Cells Dev. 2014;23:990–1000. doi: 10.1089/scd.2013.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada JC, Albo C, Benguria A, Dopazo A, Lopez-Romero P, Carrera-Quintanar L, Roche E, Clemente EP, Enriquez JA, Bernad A, Samper E. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19:743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovec M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Cirulation. 2011;121:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura W, Sadek HA. The cardiac hypoxic niche: emerging role of hypoxic microenvironment in cardiac progenitors. Cardiovasc Diagn Ther. 2012;2:278–289. doi: 10.3978/j.issn.2223-3652.2012.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- Liu SS, Zhang C, Zhang X, Chen XH. Human umbilical cord blood-derived stromal cells: a new source of stromal cells in hematopoietic stem cell transplantation. Crit Rev Oncol Hematol. 2014;90:93–98. doi: 10.1016/j.critrevonc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Cutlure in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemeling-van Rhijin M, Mensah FK, Korevaar SS, Leijs MJ, van Osch GJ, Ijzermans JN, Betjes MG, Baan CC, Weimar W, Hoogduijn MJ. Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front Immunol. 2013;4:203. doi: 10.3389/fimmu.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitbak T, Surviladze Z, Cunningham LA. Continuous expression of HIF-1 alpha in neural stem/progenitor cells. Cell Mol Neurobiol. 2011;31:119–133. doi: 10.1007/s10571-010-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotirpoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Kallos MS, Hunter C, Sen A. Improved expansion of human bone marrow-derived mesenchymal stem cells in microcarrier-based suspension culture. J Tissue Eng Regen Med. 2014;8:210–225. doi: 10.1002/term.1515. [DOI] [PubMed] [Google Scholar]