Abstract
Accurate determination of cell number is essential for the quantitative description of biological processes. The changes should be related to a measurable reference e.g. in the case of cell culture, the viable cell number is a very valuable reference parameter. Indirect methods of cell number/viability measurements may have up to 10 % standard deviation. This can lead to undesirable large deviations in the analysis of “-omics” data as well as time course studies. Such data should be preferably normalized to the exact viable cell number at a given time to allow meaningful interpretation and understanding of the biological processes. Manual counting of cell number is very laborious and not possible in certain experimental setups. We therefore, developed a simple and reliable fluorescence based method with an accuracy of 95–98 % for the determination of the viable cell number in situ. We optimized the seeding cell densities for primary rat hepatocytes for optimal cell adhesion. This will help in efficient use of primary cells which are usually limited in availability. The method will be very useful in the application of “-omics” techniques, especially metabolome analysis where the specific rates of uptake/production of metabolites can be reliably calculated.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-014-9821-1) contains supplementary material, which is available to authorized users.
Keywords: Optimal seeding, Systems biology, Cell cultivation optimization, Hepatocytes, Fluorescence, Cell counting
Introduction
Nowadays with increasing emphasis on systems biology, the “-omics” techniques find widespread application and require quantitative description of biological phenomena. For this purpose the observed changes and differences should be related to a measurable reference value. In cell culture experiments, changes are often related to either the seeded cell number (Klingmüller et al. 2006; Niklas et al. 2009; Drobná et al. 2010), or to other experimentally determined values such as the amount of protein (Klingmüller et al. 2006; Drobná et al. 2010) or DNA within a sample or the number of nuclei (as an indicator of cell number). However, these values cannot be used as reference under certain conditions, e.g. when the cellular protein content varies over time or when the DNA content or the number of nuclei per cell is not constant e.g. in the case of hepatocytes (Guidotti et al. 2003). Furthermore, these parameters do not necessarily represent the actual viable cell number. Dead or dying cells can significantly contribute to the protein or DNA content without any influence on the metabolic signature of the culture. Therefore, a method for the determination of the viable cell number for the generation of reliable reference values is highly desired.
The ideal reference for in vitro experiments is the viable cell number. Although, the determination of the viable cell number is a standard and easy procedure in suspension cell cultures, it is much more difficult for adherent cell cultures. For robust cell types, such as most cell lines, use of trypsin and subsequent cell counting in the cell suspension is a possible way. For more sensitive cell types such as primary cells, use of trypsin for cell detachment and mechanical stress leads to cell damage. Since cells die due to such invasive process whereas they were viable when still adhered, such methods therefore do not reliably provide the viable adherent cell number.
We describe a fluorescence based method for reliable in situ determination of the viable cell number in collagen monolayer cultures of primary rat hepatocytes. The non-invasive fluorescence staining does not affect cell viability and therefore enables the maintenance of the culture and multiplexing with other assays or “-omics” analyses after cell number determination.
Material and methods
Cell isolation and Percoll enrichment
Primary rat hepatocytes were isolated from heparinised male Wistar (N = 1) or Sprague–Dawley (N = 2) rats (weighing > 250 g, purchased from Janvier, Le Genest-St-Isle, France or Harlan laboratories Inc., Indianapolis, IN, USA respectively) as previously reported (Noor et al. 2009). Viable hepatocytes were enriched by Percoll gradient centrifugation. Briefly, 20 ml of a 50 % Percoll solution [Easycoll, Biochrom, Berlin, Germany; diluted with phosphate buffered saline (PBS)] were overlaid with the cell suspension (max. 50 × 106 viable cells in a volume of 5 ml) and centrifuged at 1,000g for 20 min at 25 °C (Labofuge 400 R, Thermo Scientific, Schwerte, Germany). The supernatant was discarded; the pellet was resuspended in 50 ml PBS and centrifuged (50g; 5 min; 25 °C). After removing the supernatant, the pellet was resuspended in Williams Medium E (PAN Biotech, Aidenbach, Germany) supplemented with 15 mM HEPES (cc-pro, Oberdorla, Germany), 1 % penicillin/streptomycin (cc-pro), 50 µg/ml gentamycin (cc-pro), 10 % fetal calf serum (FCS) from PAA Laboratories, Pasching, Austria, 1.4 µM Hydrocortison (Sigma-Aldrich, Steinheim, Germany) and 1 µM insulin (cc-pro). The cell number and viability were determined using trypan blue staining (Invitrogen, Darmstadt, Germany). In all cases, the seeding cell viability was above 90 %.
Cell cultivation
Primary rat hepatocytes were seeded in different cell densities per well (2 × 105, 3 × 105, 5 × 105, 7.5 × 105 and 106) in 6-well plates (Greiner Bio-One GmbH, Frickenhausen, Germany) coated with rat tail collagen (Roche, Mannheim, Germany) in 1 ml Williams Medium E (supplemented as described above). For the concentration response curve for Calcein AM, hepatocytes were seeded at a density of 4 × 104 viable cells/well in 200 µl Williams Medium E (supplemented as described above) in a collagen coated 96-well plate. After 4 hours (h) of adherence, the medium was removed and the wells were washed with PBS at 37 °C to remove dead/unattached cells. To each well, 1 ml or 200 µl of Williams Medium E (supplemented as described above except FCS) was added in 6 or 96-well plates respectively. The cells were incubated at 37 °C with 5 % CO2 in a humidified incubator. After 12 h, the viable cell number was determined as described below. The medium and the PBS of the washing steps for the cultivation in 6-well plates were pooled and the total cell number therein was determined using a Neubauer counting chamber with trypan blue staining method.
As proliferating cell line, Hep G2 cells (DSMZ, Braunschweig, Germany) were seeded at a density of 6 × 105 cells per well in 6-well plates (Greiner Bio-One GmbH) in 1 ml Williams Medium E supplemented with 10 % FCS and 1 % penicillin/streptomycin (cc-pro). The medium was changed every 24 h.
Cell number determination
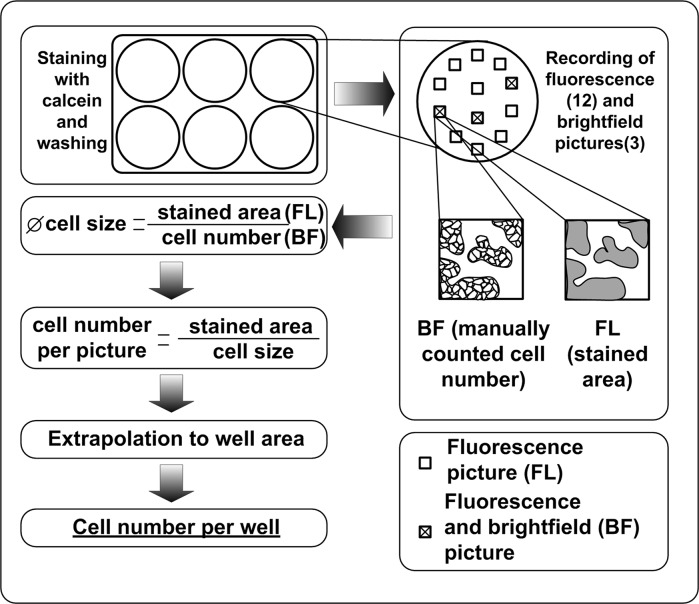
For the determination of the viable cell number, the incubation medium was removed and the cells were stained for 10 min with Calcein AM (Tebu-bio, Offenbach, Germany; 1 mg/ml in dimethyl sulfoxide) in Williams Medium E in a final concentration of 4 µg/ml. Calcein AM is an acetomethoxy derivative of calcein and is passively transported into the cells. Intracellular esterases cleave off the acetomethoxy group resulting in the generation of green fluorescent calcein which is kept inside the living cells. Since the esterases are only active in viable cells, the fluorescence is observed in viable cells only allowing an easy distinction between dead and viable cells (Bratosin et al. 2005). After washing twice with 1 ml PBS to remove unbound dye, 1 ml of Williams Medium E was added and fluorescence pictures (excitation/emission: 495 nm/515 nm) of 12 spots in each well were recorded. Bright-field pictures of three of these spots were also recorded. The cell number in the bright-field pictures was counted manually and related to the fluorescence stained area of the picture of the same spot. The stained area of every fluorescence picture was determined using ImageJ (http://rsb.info.nih.gov/ij/). The average cell size was estimated by dividing the fluorescence stained area by the manually counted cell number. This was done separately for all three spots in each well. The exact cell number in the remaining 9 spots was obtained by dividing the stained area of each spot by the average cell size in each respective well. Taking the mean cell numbers per spot and extrapolation to the area of the well, the viable cell number per well was determined. A scheme describing the procedure is depicted in Fig. 1.
Fig. 1.
Experimental procedure for the determination of the viable cell number per well in a 6-well-plate using Calcein AM staining. After staining with Calcein AM and washing with PBS, fluorescence pictures of 12 spots were recorded. Of three of these spots, bright-field pictures were also recorded. The bright-field pictures yield a manually counted cell number and the fluorescence picture provides the corresponding stained area. Relating the cell number to the stained area provides the average cell size which serves as conversion factor for the other fluorescence pictures. The cell number per picture gives the viable cell number per well via extrapolation to the area of the well
Protein extraction and quantification
After recording of the pictures of the hepatocytes, the medium was removed and 1 ml of 0.33 M NaOH was added to each well for cell lysis. The plates were incubated over night at 37 °C in a humidified incubator without CO2 supply. The protein content of the cell extracts was quantified by the Bradford assay (Bradford 1976) and the values were corrected by subtracting the background values of collagen-coated wells without cells.
Cell titer blue assay
The cell titer blue reagent (Promega, Mannheim, Germany) was diluted five fold in Wiliams Medium E and the cells were incubated with the reagent for 90 min at 37 °C. The fluorescence (excitation/emission: 545 nm/590 nm) was measured using Fluoroskan Ascent CF instrument (Labsystems, Thermo Scientific, Schwerte, Germany).
Effect of Calcein AM on hepatocytes using ATP assay
An ATP assay was carried out for the determination of the effects of Calcein AM on the hepatocytes. A concentration range from 0.001 to 250 µg/ml of Calcein AM was tested for the concentration response curve. The cells were incubated for 30 min with different Calcein AM concentrations, after which the cellular ATP content was determined using the CellTiter-Glo Kit (Promega, Mannheim, Germany). Briefly, after washing once with 200 µl PBS, the cells were incubated for 10 min with 100 µl of lysis reagent at 37 °C. The lysates (75 µl from each well) were then transferred into wells of a black 96-well plate and the luminescence was measured using a luminometer (GloMax, Promega, Mannheim, Germany).
Statistical analysis
The seeded cell number and experimentally determined cell number were compared using Student’s t test. Significance is indicated at *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
Results
Cell number
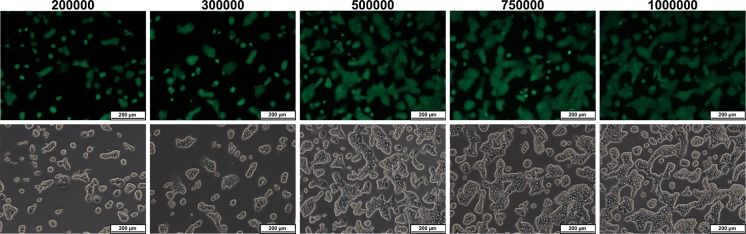
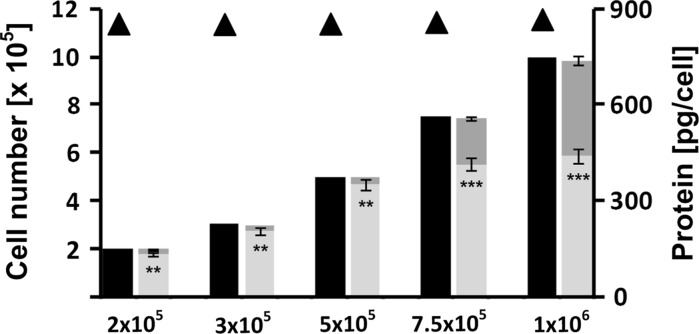
For different seeded cell numbers (2 × 105–106 viable cells per well) the actual cell numbers in situ were determined. Figure 2 shows the fluorescence and corresponding bright-field pictures for different seeded cell numbers. For the three lower seeded cell numbers (2 × 105, 3 × 105 and 5 × 105) the detected cell number was between 87 and 95 % of the initial seeded cell number. For the higher seeded cell numbers, this percentage decreased to 71 and 56 % for 7.5 × 105 and 106 seeded cells, respectively. The viable cell numbers detected in each well and the corresponding viable cell number in the pooled medium of the washing steps adds up to the initial seeded cell number (Fig. 3).
Fig. 2.
Fluorescence and corresponding bright-field pictures of wells with different initial cell seeding densities
Fig. 3.
Bar graphs represent initially seeded cell numbers (black) and corresponding experimentally determined cell numbers in the well (light grey) and in the respective pooled medium and washing steps (dark grey) for different seeding cell densities. Protein content per cell in pg determined at different initial seeding densities is shown by triangles. Error bars indicate standard deviations (N = 3; n = 9), except for the cell numbers in the pooled medium and washing steps (N = 2; n = 6)
For proliferating Hep G2 cell line, the viable cell number per well was determined every 24 h for 4 days. The doubling time was calculated to be 30 h. Furthermore, a Cell Titer Blue assay was performed every 24 h (Figure S1). The average error of the fluorescence based cell counting was 2.6 % (1.8–3.5 %). The average error of the Cell Titer Blue assay was 15.0 % (11.9–20.2 %).
Protein determination
The protein quantification was carried out after the fluorescence staining. The average protein content per rat hepatocyte cell was determined to be 864 ± 19 pg per cell after correction for the determined lost cell numbers. This value was found to be constant over the whole range of seeded cell numbers (Fig. 3).
Effect of Calcein AM on hepatocytes
We tested different Calcein AM concentrations (0.001–250 µg/ml) for their effect on the cellular ATP levels. No change in the cellular ATP levels was observed (Figure S2) at any tested Calcein AM concentration.
Discussion
We describe a reliable method for the in situ determination of the viable cell number in cultures of adherent cells with high precision. We show that seeded cell numbers up to 5 × 105 viable primary rat hepatocytes per well in a 6-well-plate ensure high fraction of adherent cells (around 90 %). On the other hand, cell seeding densities above 5 × 105 viable rat hepatocytes per well in 6-well-plates did not improve adherence showing a saturation effect. The cell number determined for every well and the cell number in the corresponding pooled washing medium for each respective well adds up to the initial seeded cell number indicating the quality and the robustness of the method with standard errors of 2–5 % (N = 3, n = 9). This includes the inaccuracies in the determination of the cell number in the cell suspension used for the seeding and the seeding process itself. In the case of most other methods of cell number determination, e.g. trypan blue exclusion method, errors up to 10 % could be expected. The method was tested in triplicates in three independent experiments carried out on different days. Inter-assay variability was found to be non-significant. In addition, our method provides an in situ and non-invasive assessment of the viable cell number in monolayer cultures, in contrast to most other methods which are either indirect (e.g. lactate dehydrogenase release), invasive (e.g. ATP or DNA quantification) or require pre-treatment for bringing the cells in suspension (usually by the use of enzymes such as trypsin). The adherent cell number is generally cell-type specific and depends on the cultivation device. In addition to the non proliferating primary rat hepatocytes, we applied the method to the proliferating Hep G2 cell line (Figure S1). The doubling time was calculated to be 30 h. The average error in cell number was less than 3 % indicating the feasibility of the method for proliferating cells. Therefore, our method can be easily adapted to other cell types and commonly used monolayer cultivation multi-well plate formats. The number of pictures recorded per well could be adjusted to represent the whole culture area. The method can be applied in temporal studies as determination of viable cell number over time is possible. This is of particular interest since the cell size often changes due to the flattening and spreading of the cells, e.g. in the case of hepatocytes. During flattening the area that is covered by one cell increases leading to an increase in the average cell size which is determined by dividing the total stained area by the manually counted cell number of the same spot. In the described method, the average cell size is determined separately for every time point and every well. The method, therefore, is perfectly suited to determine viable cell numbers over time under conditions where cell size changes with time.
Initial viability of the seeding cell suspension strongly influences the number of cells that will adhere as well as the quality of the culture. With an increasing fraction of dead cells in the cell suspension used for seeding, the fraction of viable cells that adheres will decrease not only due to space limitation but also due to the fact that dying cells release necrotic factors that may affect the viable cells. It has been reported that cultures with high seeding cell densities show a higher fraction of apoptotic cells and a higher loss of cells (Qiao and Farrell 1999). In the case of the hepatocytes, the seeding densities affect the phenotype of the cells and may therefore influence the results of the in vitro study. It is reported that low seeding cell densities favor a hepatocyte phenotype similar to the one found in regenerating liver and clearly distinct from the hepatocyte phenotype observed in healthy tissue (Koji et al. 1988). Confluent cultures result in a more differentiated phenotype and therefore better suitability for metabolism studies (Ramaiahgari et al. 2014). In the case of proliferating cells, such as mesenchymal stem cells, low seeding cell densities ensure good proliferation and expansion of the culture (Fossett and Khan 2012; Peh et al. 2013).
Determination of the optimal seeding density is highly critical in any work with primary cells since the number of available primary cells is usually limited. As such, wasting of cells by seeding unnecessary high cell numbers should be avoided. We show that an increase in the seeded viable cell number from 5 × 105 to 106 cells per well led to an increase in the detected cell number of only 25 % indicating a wasting of around 4 × 105 viable cells per well. Determination of the optimal seeding density for each cell type is therefore recommended for better experimental planning.
This fluorescence based method can be multiplexed with other assays as we show for protein quantification. The protein content per cell was found to be constant over the whole range of seeded cell numbers at the time point of the assay (12 h) in this study. This demonstrates the high robustness of the combination of cell number determination and protein quantification under conditions when cells do not increase protein production or degrade the intracellular proteins. On the other hand, the method may be especially useful under conditions where the protein content per cell is expected to increase (Li et al. 2012; Wu et al. 1993) or decrease, e.g. in autophagy under stress conditions (Hopgood et al. 1980; Liu et al. 2009). The exact determination of the cell number in combination with protein quantification enables a precise determination of the protein content per cell. This is highly valuable in proteomics analyses where protein content is sometimes used for normalization. Protein content is often used for the normalization of functional parameters such as enzyme activities. In metabolomics analysis the exact cell number can be used to calculate the specific rates of metabolites uptake/production. Exact viable cell number therefore allows quantitative description of “-omics” readouts in systems biology. Other parameters experimentally determined values such as LDH release or albumin production could also be calculated as release per cell per hour.
Using an ATP assay, we show that concentrations of Calcein AM up to 250 µg/ml do not affect the viability of the seeded cells (Figure S2, supplementary material). Cellular ATP content is a very sensitive indicator for intracellular changes (Sandker et al. 1993; Gómez-Lechón et al. 2002). Therefore, the Calcein AM staining can be assumed to be non-invasive and as such enables subsequent performance of other assays on the same culture. Many commercial fluorescence based cell counting methods on automated platforms require cells in suspension. In case of adherent cells, this will mean pretreatment of cells to bring them in suspension. As mentioned earlier, such a procedure is usually damaging to the cells. Our method in contrast, offers the advantage of allowing in situ determination of the cell number.
In summary, we developed a highly reliable and precise method for the determination of the viable cell number in situ in monolayer culture of adherent cells. This fluorescence based method eliminates inaccuracies in cell counting before seeding and the seeding process itself. Due to its non-invasive nature, the method allows multiplexing with other assays. The method can be used for the optimization of cell seeding densities thereby ensuring quality of the culture and efficient use of viable cells. This is very important in the case of primary cells where the availability is usually limited. Furthermore the results can serve as an experimentally determined reference for all other parameters analyzed in the course of an experiment over time. We therefore, show a simple method that allows quantitative analysis of biological data in mammalian cell culture for application in “-omics” methods used in systems biology approaches.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig S1 Correlation of counted cell number and fluorescence (560nm/590nm) of HepG2 cells cultured for 96 h. Sampling time points were 0 h, 24 h, 48 h, 72 h and 96 h. Error bars indicate standard deviations (n = 3). Horizontal error bars indicate standard error in fluorescence based cell counting, whereas the vertical error bars indicate the standard error in Cell titer blue assay. (TIFF 92 kb)
Fig S2 Cellular ATP content in hepatocytes after 30 minute incubation with different concentrations (0-250 µg/ml) of Calcein AM in Williams Medium E. Error bars indicate standard deviations (N = 2; n = 6). Arrow indicates the concentration of Calcein AM used in our study. (TIFF 434 kb)
Acknowledgments
This work was supported by grants from the Federal Ministry of Education and Research (BMBF, Germany) in the projects Virtual Liver Network (FKZ 315759, 315736, 0315756) and OxiSys (FKZ 0315891F). We thank Manuela Meyer from Pharmacelsus, Saarbruecken for primary rat hepatocyte isolation and Sundararajan Srinivasan, Bioinformatics department, Saarland University, for valuable support in software optimization.
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bratosin D, Mitrofan L, Palii C, Estaquier J, Montreuil J, et al. Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. Cytometry A. 2005;66A:78–84. doi: 10.1002/cyto.a.20152. [DOI] [PubMed] [Google Scholar]
- Drobná Z, Walton FS, Harmon AW, Thomas DJ, Stýblo M, et al. Interspecies differences in metabolism of arsenic by cultured primary hepatocytes. Toxicol Appl Pharmacol. 2010;245:47–56. doi: 10.1016/j.taap.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossett E, Khan WS. Optimising human mesenchymal stem cell numbers for clinical application: a literature review. Stem Cells Int. 2012;2012:465259. doi: 10.1155/2012/465259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Lechón MJ, O’Connor E, Castell JV, Jover R. Sensitive markers used to identify compounds that trigger apoptosis in cultured hepatocytes. Toxicol Sci Off J Soc Toxicol. 2002;65:299–308. doi: 10.1093/toxsci/65.2.299. [DOI] [PubMed] [Google Scholar]
- Guidotti JE, Brégerie O, Robert A, Debey P, Brechot C, Desdouets C, et al. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J Biol Chem. 2003;278:19095–19101. doi: 10.1074/jbc.M300982200. [DOI] [PubMed] [Google Scholar]
- Hopgood MF, Clark MG, Ballard FJ. Protein degradation in hepatocyte monolayers. Effects of glucagon, adenosine 3′:5′-cyclic monophosphate and insulin. Biochem J. 1980;186:71–79. doi: 10.1042/bj1860071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingmüller U, Bauer A, Bohl S, Nickel PJ, Breitkopf K, Dooley S, Zellmer S, Kern C, Merfort I, Sparna T, Donauer J, Walz G, Geyer M, Kreutz C, Hermes M, Götschel F, Hecht A, Walter D, Egger L, Neubert K, Borner C, Brulport M, Schormann W, Sauer C, Baumann F, Preiss R, MacNelly S, Godoy P, Wiercinska E, Ciuclan L, Edelmann J, Zeilinger K, Heinrich M, Zanger UM, Gebhardt R, Maiwald T, Heinrich R, Timmer J, von Weizsäcker F, Hengstler JG (2006) Primary mouse hepatocytes for systems biology approaches: a standardized in vitro system for modelling of signal transduction pathways. Syst Biol (Stevenage) 153:433–447. doi:10.1049/ip-syb:20050067 [DOI] [PubMed]
- Koji T, Nakane PK, Murakoshi M, Watanabe K, Terayama H, et al. Cell density dependent morphological changes in adult rat hepatocytes during primary culture. Cell Biochem Funct. 1988;6:237–243. doi: 10.1002/cbf.290060404. [DOI] [PubMed] [Google Scholar]
- Li SQ, Li RF, Xi SM, Hu S, Jia ZQ, Li SP, Wen XL, Song YK, Li S, Li SP, Wei FB, Chen XL (2012) Systematical analysis of impacts of heat stress on the proliferation, apoptosis and metabolism of mouse hepatocyte. J Physiol Sci 62:29–43. doi:10.1007/s12576-011-0183-6 [DOI] [PMC free article] [PubMed]
- Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, Liu Z, Cao W (2009) Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of foxO1-dependent expression of key autophagy genes by insulin. J Biol Chem 284:31484–31492. doi:10.1074/jbc.M109.033936 [DOI] [PMC free article] [PubMed]
- Niklas J, Noor F, Heinzle E. Effects of drugs in subtoxic concentrations on the metabolic fluxes in human hepatoma cell line Hep G2. Toxicol Appl Pharmacol. 2009;240:327–336. doi: 10.1016/j.taap.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Noor F, Niklas J, Muller-Vieira U, Heinzle E. An integrated approach to improved toxicity prediction for the safety assessment during preclinical drug development using Hep G2 cells. Toxicol Appl Pharmacol. 2009;237:221–231. doi: 10.1016/j.taap.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Peh GS, Toh KP, Ang HP, Seah XY, George BL, Mehta JS (2013) Optimization of human corneal endothelial cell culture: density dependency of successful cultures in vitro. BMC Res Notes 6:176. doi:10.1186/1756-0500-6-176 [DOI] [PMC free article] [PubMed]
- Qiao L, Farrell GC. The effects of cell density, attachment substratum and dexamethasone on spontaneous apoptosis of rat hepatocytes in primary culture. In Vitro Cell Dev Biol Anim. 1999;35:417–424. doi: 10.1007/s11626-999-0117-2. [DOI] [PubMed] [Google Scholar]
- Ramaiahgari SC, den Braver MW, Herpers B, Terpstra V, Commandeur JN, van de Water B, Price LS (2014) A 3D in vitro model of differentiated Hep G2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch Toxicol 88:1083–1095. doi:10.1007/s00204-014-1215-9 [DOI] [PubMed]
- Sandker GW, Weert B, Merema MT, Kuipers W, Slooff MJ, Meijer DK, Groothuis GM (1993) Maintenance of viability and transport function after preservation of isolated rat hepatocytes in various simplified University of Wisconsin solutions. Biochem Pharmacol 46:2093–2096 [DOI] [PubMed]
- Wu B, Gu MJ, Heydari AR, Richardson A. The effect of age on the synthesis of two heat shock proteins in the hsp 70 family. J Gerontol. 1993;48:B50–B56. doi: 10.1093/geronj/48.2.B50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1 Correlation of counted cell number and fluorescence (560nm/590nm) of HepG2 cells cultured for 96 h. Sampling time points were 0 h, 24 h, 48 h, 72 h and 96 h. Error bars indicate standard deviations (n = 3). Horizontal error bars indicate standard error in fluorescence based cell counting, whereas the vertical error bars indicate the standard error in Cell titer blue assay. (TIFF 92 kb)
Fig S2 Cellular ATP content in hepatocytes after 30 minute incubation with different concentrations (0-250 µg/ml) of Calcein AM in Williams Medium E. Error bars indicate standard deviations (N = 2; n = 6). Arrow indicates the concentration of Calcein AM used in our study. (TIFF 434 kb)