Abstract
The use of food additives has increased enormously in modern food technology but they have adverse effects in human healthy. The aim of this study was to investigate the DNA damage of some food additives such as citric acid (CA), benzoic acid (BA), brilliant blue (BB) and sunset yellow (SY) which were investigated in human male germ cells using comet assay. The sperm cells were incubated with different concentrations of these food additives (50, 100, 200 and 500 μg/mL) for 1 h at 32 °C. The results showed for CA, BA, BB and SY a dose dependent increase in tail DNA%, tail length and tail moment in human sperm when compared to control group. When control values were compared in the studied parameters in the treatment concentrations, SY was found to exhibit the highest level of DNA damage followed by BB > BA > CA. However, none of the food additives affected the tail DNA%, tail length and tail moment at 50 and 100 μg/mL. At 200 μg/mL of SY, the tail DNA% and tail length of sperm were 95.80 ± 0.28 and 42.56 ± 4.66, for BB the values were 95.06 ± 2.30 and 39.56 ± 3.78, whereas for BA the values were 89.05 ± 2.78 and 31.50 ± 0.71, for CA the values were 88.59 ± 6.45 and 13.59 ± 2.74, respectively. However, only the highest concentration of the used food additives significantly affected the studied parameters of sperm DNA. The present results indicate that SY and BB are more harmful than BA and CA to human sperm in vitro.
Keywords: Food additives, Tail length, Sperm, Comet assay, Benzoic acid
Introduction
Food additives are used widely for various purposes, including preservation, coloring, and sweetening. They are added to stop or delay nutritional losses due to microbiological, enzymatic or chemical changes of foods and to prolong the shelf life and quality of foods (Zengin et al. 2011). Recently, food additives have attracted the attention as potential causes of various human diseases; they might be among the factors responsible for the outbreak of cancer, hepatic and nephritic failures, and mutagenic potential (Tanaka 2007; Turkoglu 2007; Demir et al. 2008). There are many studies that showed the genotoxicity of different food additives in different cell lines (Macioszek and Kononowicz 2004; Sarıkaya and Solak 2003; Yılmaz et al. 2008a; Mpountoukas et al. 2008).
Citric acid (E-330) (CA) is used as a flavouring agent and preservative in food, especially in biscuit, cake, prepared soup, cheese and cheese products, baby food, chewing gum, fizzy lemonade, margarine, meat, fish, and beverages, in particular, soft drinks (Turkoglu 2007).
Benzoic acid (E-210) (BB) is commonly used as an antimicrobial substance in many food products, at concentrations ranging between 150 and 1,000 mg/kg, like fruit juice, syrup, pickle, ketchup, margarine, biscuit, waffle, cake and cream to preserve these substances from yeast, mould and bacteria effects (Sarıkaya and Solak 2003).
Brilliant Blue FCF (BB) has a wide range of applications in industries and consumer products either alone or in combination with other dyes, to produce various shades of blue, green, and violet. It is used to dye textiles, leathers, plastics, papers, printing inks, paints, and household products. In some countries, BB is applied in food products, beverages, cosmetics, and drugs (Lucova et al. 2013).
Sunset yellow (SY) is a synthetic coloring dye belonging to azo compounds. The dye is isolated as dried sodium salt (Solomon 1996), and may be added to orange squash, orange jelly, marzipan, swiss roll, apricot jam, citrus marmalade, lemon curd, sweets, hot chocolate mix, packet soups, snack chips, trifle mix, breadcrumbs, cheese sauce mix and soft drinks (Sayed et al. 2012).
Living organisms have a genome that is subject to the constant action of exogenous or endogenous agents that can affect the chemical integrity of DNA by altering the information contained therein. Different changes are responsible for genomic instability, and these changes play an important role in carcinogenesis (Jefford and Irminger-Finger 2006; Pereira et al. 2012).
Genetic Toxicology is an area which covers mainly the study of mutagenicity, carcinogenicity and teratogenicity. In this area there are several tests for biomonitoring, genotoxicity and mutagenesis and one such test used to evaluate mutagenic potential is the comet assay. This test constitutes a simple, rapid, sensitive and inexpensive assay for detecting DNA damage (Tice et al. 2000). The comet assay is used to detect mutations. Furthermore, it verifies genomic lesions that, after processing, can result in mutation. Unlike mutations, lesions detected by comet assay are subject to correction. The technique entails the immersion of viable cells in agarose, lysing the cell membrane by detergents and alkaline salts, and subsequent electrophoresis. With electrophoresis under alkaline conditions, cells with damaged DNA have a higher rate of migration toward the anode due to single strand breaks or alkali labile sites, double simulating the appearance of a comet (head and tail) (Pereira et al. 2012; Pavão et al. 2007).
Presently, no validated in vitro methods exist for the assessment of male reproductive toxicity (Bremer et al. 2005) and the introduction of a battery of alternative tests to be integrated to provide detailed information on the hazard of compounds to the mammalian reproductive cycle is urgently needed (Villani et al. 2010). For CA, BA, BB and SY food additives, application of the comet assay was assessed whether the used concentrations of food additives would be likely to induce DNA damage after exposure time in male germ cells. Moreover, there are currently no data available in the literature related to research on the DNA damage of food additives on this cell. In this context, the aim of the present work was to verify the possible induction of DNA damage in human sperm using the comet test.
Materials and methods
Chemicals
Benzoic acid (BA) was obtained from Sigma (St. Louis, MO, USA), and citric acid (CA) was obtained from Merck (Darmstadt, Germany). BB, SY and RPMI 1640 were bought from Sigma–Aldrich (Seelze, Germany). Proteinase K was obtained from Roche Diagnostics (Mannheim, Germany). All other reagents and chemicals used were of analytical grade.
Preparation of sperm samples
Semen samples were obtained from six 30-year-old healthy males according to WHO criteria (WHO 2000) (sperm number: 64 × 106 per mL; motility: 72 %; sperm morphology: abnormal forms: 25 %).
10 μL sperm were treated for 1 h at 32 °C with the different concentrations of some food additives (50, 100, 200 and 500 μg/mL) and were resuspended in RPMI-1640 for analysis (Anderson et al. 1994, 2003). After the incubation, sperm were centrifuged with RPMI 1640 at 1,348 g for 5 min, and then the supernatant was removed and resuspended in PBS.
Treated and non-treated cells were suspended in low melting point agarose (0.65 %), and 75 μL of suspension was quickly layered over slides which were precoated with normal melting point agarose (0.65 %), immediately covered with a cover slip and the slides were placed at +4 °C for 10–15 min. After solidification, the coverslip was gently removed and immersed in cold lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH 10, in which 10 % DMSO, 1 % Triton X-100, and 0.05 mg/mL proteinase K were added) at 4 °C for 1 h. The slides were removed and placed on a horizontal gel electrophoresis platform covered with electrophoresis buffer (300 mM NaOH, 1 mM EDTA pH 13). Then, they were left in the solution for 20 min to allow the unwinding of the DNA (Ozkan et al. 2009). Electrophoresis was run at 25 V for 20 min at 4 °C. All procedural steps were performed under yellow light conditions to minimize additional DNA damage. The slides were then placed vertically in a neutralizing tank and gently washed three times for 5 min with neutralizing buffer (0.4 M Tris–HCl buffer, pH 7.5). Ethidium bromide (10 mg in 50 mL of distilled water) was dispensed directly onto slides and covered with a cover slip.
Assesment of sperm DNA damage by image analysis
All measurement data were analyzed using BS 200 ProP with software image analysis (BS 200 ProP, BAB Imaging System, Ankara, Turkey). The DNA comets were evaluated by measuring the tail DNA%, tail length and tail moment of 50 comets. All experiments were performed at least three times, each with two parallel slides per data point. A higher percentage tail DNA% and tail lenght indicated a higher level of DNA damage (Behravan et al. 2011).
Statistics
Differences between groups were evaluated by one-way analysis of variance (ANOVA) followed by the Tukey test. Values were expressed as mean ± standard error of the mean (SEM). Statistical significance was accepted at the p < 0.05 level.
Results
DNA damaging effects of food additives on sperm cells are shown in Table 1 and Figs. 1–7. The percentage of tail DNA%, tail length and tail moments was significantly (p < 0.05) increased in CA, BA, BB and SY treatment group with increasing concentrations of food additives (Figs. 1–4; Table 1).
Fig. 2.
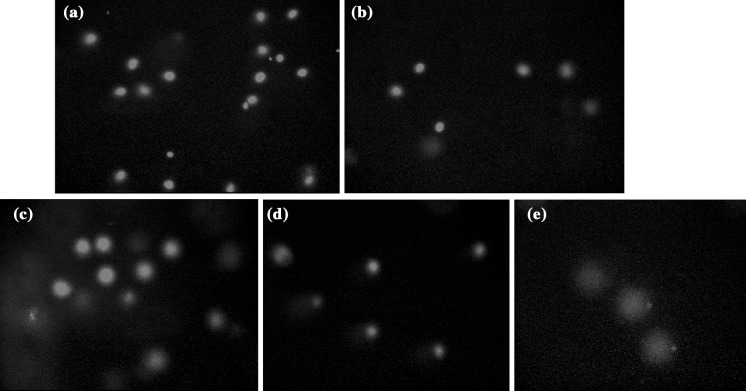
DNA damaging activity of benzoic acid (BA) in isolated human sperm cells. a Control group, b 50 μg/mL BA treatment group, c 100 μg/mL BA treatment group, d 200 μg/mL BA treatment group, e 500 μg/mL BA treatment group
Fig. 3.
DNA damaging activity of brilliant blue (BB) in isolated human sperm cells. a Control group, b 50 μg/mL BB treatment group, c 100 μg/mL BB treatment group, d 200 μg/mL BB treatment group, e 500 μg/mL BB treatment group
Fig. 6.
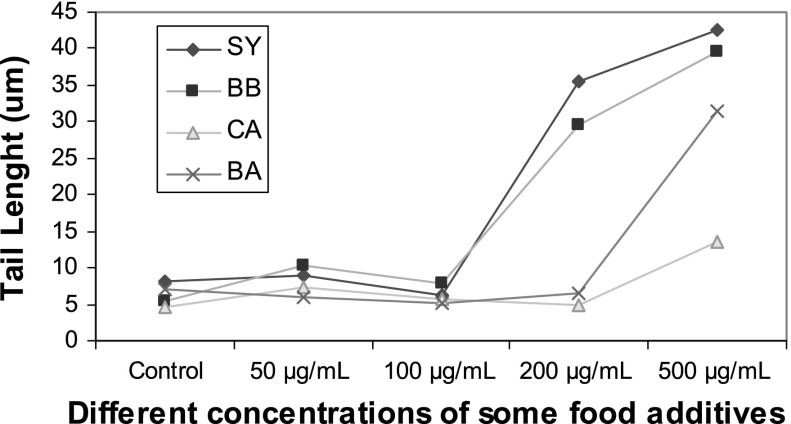
Effects of different concentrations of food additives (0–500 μg/mL) on comet tail length in human
Table 1.
Estimated mean values of tail DNA%, tail length and tail moment of comets by image analysis upon treatment of human sperm cells with different concentrations of food additives (0–500 μg/mL)
| Different exposure concentrations of some food additives (0–500 μg/mL) | Tail DNA% Mean ± SE |
Tail length Mean ± SE |
Tail moment Mean ± SE |
|---|---|---|---|
| For citric acid (CA) | |||
| Control | 57.90 ± 3.28 | 4.61 ± 0.34 | 2.67 ± 0.35 |
| 50 μg/mL | 61.98 ± 5.10 | 7.28 ± 1.05 | 4.51 ± 0.66 |
| 100 μg/mL | 69.24 ± 6.41 | 5.66 ± 0.56 | 3.90 ± 00.7 |
| 200 μg/mL | 69.91 ± 1.51 | 5.01 ± 0.28 | 3.51 ± 0.27 |
| 500 μg/mL | 88.59 ± 6.45a,b,c,d | 13.59 ± 2.74a,b,c,d | 12.13 ± 3.31a,b,c,d |
| For benzoic acid (BA) | |||
| Control | 65.90 ± 6.70 | 7.12 ± 0.74 | 4.70 ± 0.78 |
| 50 μg/mL | 67.78 ± 2.40 | 6.07 ± 0.34 | 4.11 ± 0.08 |
| 100 μg/mL | 72.14 ± 0.57 | 5.09 ± 0.35 | 3.67 ± 0.28 |
| 200 μg/mL | 76.14 ± 7.40 | 6.47 ± 1.40 | 4.99 ± 1.46 |
| 500 μg/mL | 89.05 ± 2.78 a,b,c,d | 31.50 ± 0.71a,b,c,d | 28.06 ± 1.50a,b,c,d |
| For brilliant blue (BB) | |||
| Control | 66.12 ± 12.98 | 5.34 ± 1.05 | 3.62 ± 1.32 |
| 50 μg/mL | 69.56 ± 3.87 | 13.33 ± 1.53 | 9.27 ± 1.11 |
| 100 μg/mL | 73.91 ± 1.22 | 7.93 ± 0.74 | 5.86 ± 0.61 |
| 200 μg/mL | 90.03 ± 2.86a,b,c | 29.45 ± 6.27a,b,c | 26.61 ± 6.49a,b,c |
| 500 μg/mL | 95.06 ± 2.30a,b,c | 39.56 ± 3.78a,b,c | 37.56 ± 2.68a,b,c |
| For sunset yellow (SY) | |||
| Control | 56.59 ± 5.34 | 8.09 ± 3.12 | 4.61 ± 1.91 |
| 50 μg/mL | 58.34 ± 3.04 | 9.06 ± 1.48 | 5.29 ± 0.95 |
| 100 μg/mL | 66.45 ± 1.32 | 6.31 ± 1.14 | 4.19 ± 0.08 |
| 200 μg/mL | 95.80 ± 0.28 a,b,c | 35.44 ± 8.24a,b,c | 33.94 ± 7.79a,b,c |
| 500 μg/mL | 96.56 ± 2.01 a,b,c | 42.56 ± 4.66a,b,c | 41.05 ± 3.91a,b,c |
Significance at p < 0.05
aComparision of control with the other groups
bComparision of 50 μg/mL treatment group with the 100, 200 and 500 μg/mL treatment groups
cComparision of 100 μg/mL treatment group with the 200 and 500 μg/mL treatment groups
dComparision of 200 μg/mL treatment group with the 500 μg/mL treatment group
Fig. 1.
DNA damaging activity of citric acid (CA) in isolated human sperm cells. a Control group, b 50 μg/mL CA treatment group, c 100 μg/mL CA treatment group, d 200 μg/mL CA treatment group, e 500 μg/mL CA treatment group
Fig. 7.
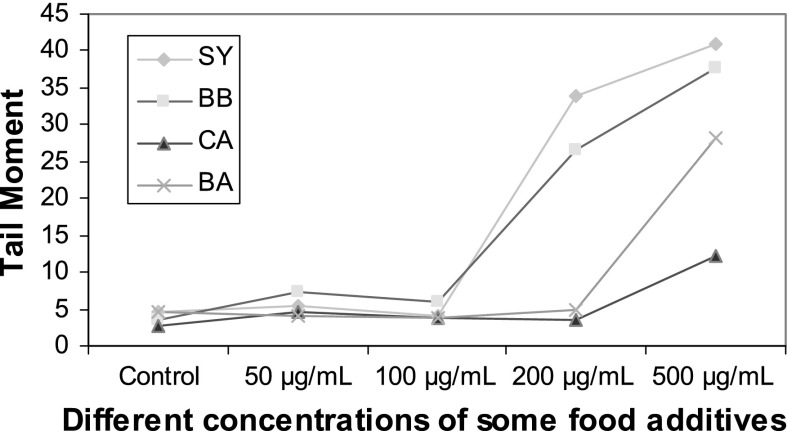
Effects of different concentrations of food additives (0–500 μg/mL) on comet tail moment in human
Fig. 4.
DNA damaging activity of sunset yellow (SY) in isolated human sperm cells. a Control group, b 50 μg/mL SY treatment group, c 100 μg/mL SY treatment group, d 200 μg/mL SY treatment group, e 500 μg/mL SY treatment group
CA, BA, BB and SY at 0, 50 and 100 μg/mL did not reveal any statistically significant increases in tail DNA%, tail length and tail moment (Figs. 5–7). BA and CA at 500 μg/mL were found to produce significant increases in tail DNA%, tail length and tail moment when incubated with sperm cells (p < 0.05). In addition, significant increases were found at concentrations of SY and BB at 200 and 500 μg/mL suggesting their DNA damaging effects (p < 0.05).
Fig. 5.
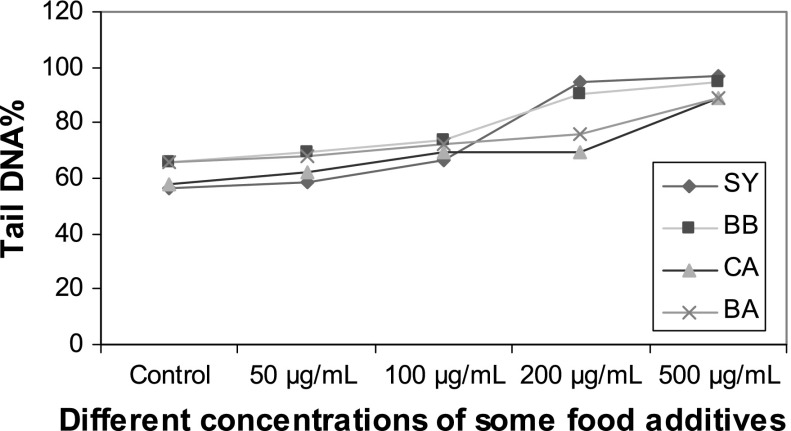
Effects of different concentrations of food additives (0–500 μg/mL) on comet tail DNA% in human
Treatment with 200 μg/mL of CA, BA, BB and SY showed a maximum increase of 69.91 ± 1.51, 76.14 ± 7.40, 90.03 ± 2.86 and 95.80 ± 0.28 in tail DNA%, respectively. An amount of 200 μg/mL of BA and SY showed a statistically significant increase (p < 0.05) when compared with control, or the addition of CA and BA.
The highest value of 96.56 ± 2.01 for tail DNA% was exhibited by sperm treated with 500 μg/mL of SY whereas treatment with BB, BA and CA at the same concentrations showed a maximum increase of 95.06 ± 2.30, 89.05 ± 2.78 and 88.59 ± 6.45, respectively.
Discussion
To evaluate the genotoxic effects induced by physical and chemical agents, various test systems have been described in bacteria, in mammalian cells, and in plants (Macioszek and Kononowicz 2004; Yılmaz et al. 2008a; Prival et al. 1991; Arslan et al. 2008; Mamur et al. 2010). Chromosomal aberrations (CAs), sister-chromatid exchanges (SCEs), and micronuclei (MN) tests and comet assay are widely used in cultured human peripheral lymphocytes (Macioszek and Kononowicz 2004; Mamur et al. 2010; Rencuzogullari et al. 2001; Yuzbasioglu et al. 2006; Yılmaz et al. 2008b). Comet assay may be used for laboratory investigations as a method to detect the impact of potential germ cell mutagens directly on sperm DNA in vitro.
More chemicals eliciting different mechanisms of DNA damage and response should be tested to define the applicability domain of the test. In addition, for some of the compounds, primary DNA lesions induced under the specific experimental test conditions should be qualitatively and quantitatively characterized. One area of research that has been studied as a cause of male infertility is the integrity of sperm DNA which is affected by exogenous and endogenous factors. Indeed, it has been estimated that 10,000 oxidation hits occur to the DNA of each cell per day within the human body and more than 35 different forms of oxidized bases are found in DNA in vitro (Ames et al. 1993; Halliwell 2000; Babazadeh et al. 2010). Genotoxicity data of the used food additives on human sperm cell in vitro studied outside their normal biological context are very rare. Thus, in this study the DNA damage of CA, BA, BB, SY used as food additives in human sperm was examined by using the comet assays.
The use of food additives in various products is increasing (Sayed et al. 2012). Natural or synthetic food additives have been used as coloring, curing, and/or sweetening agents in many food stuffs (Sayed et al. 2012). Although epidemiological studies of food additives are important for the assessment of toxicological risk to humans, they are difficult because exposure cannot be accurately assessed. Thus, risk assessment largely depends on laboratory toxicity studies (Sasaki et al. 2002; Yılmaz et al. 2009). There are no data about toxicity of food additives on human male germ cells. Therefore, sperm cells were treated with concentrations of food additives of 50, 100, 200 and 500 μg/mL (the amount used in foods) in this study.
Several studies suggested that certain types of azo dyes, including SY, exhibit mutagenic effects (Surjyo and Anisur 2005; King-Thom et al. 2006; Tsuboy et al. 2007; Oliveira et al. 2010). The toxicity and carcinogenicity of SY in mammalian system may result either via interactions of intact molecules with cytosolic receptors (Lubet et al. 1983; Collier et al. 1993; Seesuriyachan et al. 2007) or via the formation of free radicals and arylamines azoreduction (Nony et al. 1980; Pearce et al. 2003). Sayed et al. (2012) reported on genotoxic effects induced by a synthetic food additive, SY, in mice. The results showed that SY had genotoxic effects as indicated by increased frequency of SCE’s, by CAs in both somatic and germ cells, and by increased morphological sperm abnormalities and DNA fragmentation. SY has been shown to cause allergic or intolerance reactions in certain people, particularly those with a pre-existing sensitivity to aspirin, but no mutagenic effect was reported (Health Canada, Food Additive Dictionary 2007). In the present study, the genotoxicity of SY observed was comparatively higher than that of other additives. When the damage was evaluated in terms of the percentage tail DNA, the highest value of 96.56 ± 2.01 was exhibited by sperm treated with 500 μg/mL of SY.
Mahmoud (2006) has evaluated the toxic effects of the synthetic dye BB in rats. The used concentration of the synthetic dye BB was mostly attributable to hepatocellular damage, renal failure and decrease in spermatogenesis process. BB showed no evidence of mutagenicity when tested in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538 with or without metabolic activation. BB has been tested in vivo in a mouse micronucleus assay and comet assays. In all cases, no micronucleus induction or DNA damage were observed. Based on these data, BB is not of concern with respect to genotoxicity (EFSA-Panel 2010). In case of sperm, the data obtained in terms of tail DNA% at the evaluated doses of BB of 200 and 500 μg/mL revealed statistically significant results in this study.
BA is commonly used as an antimicrobial substance in many food products. Sarıkaya and Solak (2003) have reported that BA significantly decreases the life period and increases somatic mutation and recombination (SMART) in Drosophila melanogaster. Yılmaz et al. (2008a) have reported that BA significantly increased CAs and decreased the mitotic index in the Allium sativum root tips. In another study, Yılmaz et al. (2009) reported that BA significantly increased CAs, SCEs, and micronucleus frequency in human lymphocytes. They indicated that BA was a weak genotoxic agent, especially at lower concentrations, in human lymphocyte cultures. In this study, the results showed that BA had a DNA-damaging effect especially at the highest concentration in human male germ cells but similar effects were not obtained at concentrations of 50, 100, and 200 μg/mL of BA.
In several in vitro and in vivo tests CA was not mutagenic (HERA 2005). Navarro-Escobar et al. (2010) observed that CA solution was cytotoxic at a concentration of 15 %. Murine fibroblast cells were exposed to CA at increasing concentrations for 3 h at 37 °C. The results showed that exposure to 2.5 and 5 % NaOCl and 8.5 % CA resulted in a significant cytotoxic effect (Marins et al. 2012) but it did not induce genetic damage in vitro. In other study, CA posses clastogenic and cytotoxic activity in cultured human lymphocytes, especially at high concentration. CA decreased the replication index, especially at the highest concentration compared with control however, this decrease was not significant. In this study, sperm treated with 50, 100, and 200 μg/mL of CA also exhibited low percentage tail DNA and tail length. However, the DNA damage to sperm was not significantly altered in the low concentration groups after treatment with the compounds, but only the highest concentration of CA caused DNA damage in sperm cells.
The modern technological advancement in food industry has resulted in the use of a variety of food additives alone or in combination (Kiple and Ornelas 2000). The comet assay can be usefully applied in risk assessment of human germ cell mutagens. The present study clearly demonstrates a statistically significant increase in the DNA damage induced by various food additives. According to our study results have implications for man in terms of risk assessment and such damaging effects to germ cells need more detailed studies in view of the use of food additives.
Acknowledgments
The author would like to thank to Esra GUVEN for helping me to prepare this study.
Conflict of interest
The author declared no conflicts of interest.
References
- Ames BN, Shigenaga MK, Gold LS. DNA lesions, inducible DNA repair, and cell division: three key factors in mutagenesis and carcinogenesis. J Environ Health Perspect. 1993;101:35–44. doi: 10.1289/ehp.93101s535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Yu TW, Phillips BJ, Schmezer P. The effect of various antioxidants and other modifying agents on oxygen–radical-generated DNA damage in human lymphocytes in the comet assay. Mutat Res. 1994;307:261–271. doi: 10.1016/0027-5107(94)90300-X. [DOI] [PubMed] [Google Scholar]
- Anderson D, Schmid TE, Baumgartner A, Cemeli-Carratala E, Brinkworth MH, Wood JM. Oestrogenic compounds and oxidative stress (in human sperm and lymphocytes in the Comet assay. Mutat Res. 2003;544:173–178. doi: 10.1016/j.mrrev.2003.06.016. [DOI] [PubMed] [Google Scholar]
- Arslan M, Topaktas M, Rencuzogullari E. The effects of boric acid on sister chromatid exchanges and chromosome aberrations in cultured human lymphocytes. Cytotechnology. 2008;56:91–96. doi: 10.1007/s10616-007-9094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babazadeh Z, Razavi S, Tavalaee M, Deemeh MR, Shahidi M, Nasr-Esfahani MH. Sperm DNA damage and its relation with leukocyte DNA damage. Reprod Toxicol. 2010;29:120–124. doi: 10.1016/j.reprotox.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Behravan J, Mosafa F, Soudmand N, Taghiabadi E, Razavi BM, Karimi G. Protective effects of aqueous and ethanolic extracts of Portulaca oleracea L. aerial parts on H2O2-induced DNA damage in lymphocytes by comet assay. Acupunct Meridian Stud. 2011;4:193–197. doi: 10.1016/j.jams.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Bremer S, Balduzzi D, Cortvrindt R, Daston G, Eletti B, Galli A, Huhtaniemi I, Laws S, Lazzari G, Liminga U, Smitz J, Spano M, Themmen A, Tilloy A, Waalkens-Behrends I (2005) The effects of chemicals on mammalian fertility. The report and recommendations of ECVAM Workshop 53-the first strategic workshop of the EU ReProTect Project. Altern Lab Anim 33: 391–416 [DOI] [PubMed]
- Collier SW, Strom JE, Bronaugh RL. Reduction of azo dyes during in vitro percutaneous absorption. Toxicol Appl Pharmacol. 1993;118:73–79. doi: 10.1006/taap.1993.1011. [DOI] [PubMed] [Google Scholar]
- Demir E, Kocaoglu S, Kaya B. Genotoxicity testing of four benzyl derivatives in the Drosophila wing spot test. Food Chem Toxicol. 2008;46:1034–1041. doi: 10.1016/j.fct.2007.10.035. [DOI] [PubMed] [Google Scholar]
- EFSA-panel on food additives and nutrient sources added to food (ANS) (2010) Scientific opinion on the re-evaluation of brilliant blue FCF (E 133) as a food additive. European Food Safety Authority (EFSA), Parma. EFSA J 8, pp 1853
- Halliwell B. Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come? Am J Clin Nutr. 2000;72:1082–1087. doi: 10.1093/ajcn/72.5.1082. [DOI] [PubMed] [Google Scholar]
- Health Canada, Food Additive Dictionary (2007) http://www.hc-sc.gc.ca/fn-an/securit/addit/ diction/index_e.html
- HERA (2005) Citric acid and salts. Edition 1.0 pp. 1–6
- Jefford CE, Irminger-Finger I (2006) Mechanisms of chromosome instability in cancers. Crit Rev Oncol Hematol 59(1):1–14 [DOI] [PubMed]
- King-Thom C, Ssu-Ching C, Larry DC. Review of the Salmonella typhimurium mutagenicity of benzidine, benzidine analogues, and benzidine-based dyes. Mutat Res. 2006;612:58–76. doi: 10.1016/j.mrrev.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Kiple KF, Ornelas KC. Contemporary food related issues. United Kingdom: The Cambridge University Press ; 2000. pp. 1667–1676. [Google Scholar]
- Lubet RA, Conolly G, Kouri RE, Nebert DW, Bigelow SW. Biological effects of the Sudan dyes: role of the cytosolic receptor. Biochem Pharmacol. 1983;32:3053–3058. doi: 10.1016/0006-2952(83)90248-4. [DOI] [PubMed] [Google Scholar]
- Lucova M, Hojerova J, Pazourekova S, Klimova Z. Absorption of triphenylmethane dyes brilliant blue and patent blue through intact skin, shaven skin and lingual mucosa from daily life products. Food Chem Toxicol. 2013;52:19–27. doi: 10.1016/j.fct.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Macioszek VK, Kononowicz AK. The evaluation of the genotoxicity of two commonly used food colors: quinoline yellow (E 104) and brilliant black BN (E 151) Cell Mol Biol Lett. 2004;9:107–122. [PubMed] [Google Scholar]
- Mahmoud NH. Toxic effects of the synthetic food dye brilliant blue on liver, kidney and testes functions in rats. J Egypt Soc Toxicol. 2006;34:77–84. [Google Scholar]
- Mamur S, Yuzbasıoglu D, Unal F, Yılmaz S. Does potassium sorbate induce genotoxic or mutagenic effects in lymphocytes? Toxicol In Vitro. 2010;24:790–794. doi: 10.1016/j.tiv.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Marins JSR, Sassone LM, Fidel SR, Ribeiro DA. In Vitro genotoxicity and cytotoxicity in murine fibroblasts exposed to EDTA, NaOCl, MTAD and Citric Acid. Braz Dent J. 2012;23:527–533. doi: 10.1590/S0103-64402012000500010. [DOI] [PubMed] [Google Scholar]
- Mpountoukas P, Vantarakis A, Sivridis E, Lialiaris T. Cytogenetic study in cultured human lymphocytes treated with three commonly used preservatives. Food Chem Toxicol. 2008;46:2390–2393. doi: 10.1016/j.fct.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Navarro-Escobar E, Gonzalez-Rodriguez M, Ferrer-Luque P. Cytotoxic effects of two acid solutions and 2.5 % sodium hypochlorite used in endodontic therapy. Med Oral Patol Oral Cir Bucal. 2010;15:90–94. doi: 10.4317/medoral.15.e90. [DOI] [PubMed] [Google Scholar]
- Nony CR, Bowman MC, Carins T, Lowry LK, Tolos WP. Metabolism studies of an azo dye and pigment in the hamster based on analysis of the urine for potentially carcinogenic aromatic amine metabolites. J Anal Toxicol. 1980;4:132–140. doi: 10.1093/jat/4.3.132. [DOI] [PubMed] [Google Scholar]
- Oliveira GA, Ferraz ER, Chequer FM, Grando MD, Angeli JP, Tsuboy MS, Marcarini JC, Mantovani MS, Osugi ME, Lizier TM, Zanoni MV, Oliveira DP. Chlorination treatment of aqueous samples reduces, but does not eliminate, the mutagenic effect of the azo dyes Disperse Red 1, Disperse Red 13 and Disperse Orange 1. Mutat Res. 2010;703:200–208. doi: 10.1016/j.mrgentox.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Ozkan D, Yuzbasıoglu D, Unal F, Yılmaz S, Aksoy H. Evaluation of the cytogenetic damage induced by the organophosphorous insecticide acephate. Cytotechnology. 2009;59:73–80. doi: 10.1007/s10616-009-9195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavão PRG, Gontijo AMMC, Ribeiro DA, Salvadori DMF (2007) Ausência de efeito genotóxico induzido por esteróides anabolizantes em indivíduos fisiculturistas. Rev Bras Educ Fís Esp 215–10
- Pearce CI, Lloyd JR, Guthrie JT. The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigments. 2003;58:179–196. doi: 10.1016/S0143-7208(03)00064-0. [DOI] [Google Scholar]
- Pereira LLS, Marcussi S, Sátiro LC, Pereira CA, Andrade LF, Davide LC, Santos CD. Application of comet assay to assess the effects of white bean meal on DNA of human lymphocytes. Braz J Pharm Sci. 2012;48:103–108. doi: 10.1590/S1984-82502012000100012. [DOI] [Google Scholar]
- Prival JM, Simmon FV, Mortelmans EK. Bacterial mutagenicity testing of 49 food ingredients gives very few positive results. Mutat Res. 1991;260:321–329. doi: 10.1016/0165-1218(91)90017-G. [DOI] [PubMed] [Google Scholar]
- Rencuzogullari E, Ila HB, Kayraldiz A, Topaktas M. Chromosome aberrations and sister chromatid exchanges in cultured human lymphocytes treated with sodium metabisulfite, a food preservative. Mutat Res. 2001;490:107–112. doi: 10.1016/S1383-5718(00)00142-X. [DOI] [PubMed] [Google Scholar]
- Sarıkaya R, Solak K. Benzoik asit’in Drosophila melanogaster’de somatik mutasyon ve rekombinasyon testi ile genotoksisitesinin araştırılması. GU Gazi Egitim Fakultesi Dergisi. 2003;23:19–32. [Google Scholar]
- Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K, Taniguchi K, Tsuda S. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res. 2002;519:103–109. doi: 10.1016/S1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Sayed HM, Fouad D, Ataya FS, Hassan NHA, Fahmy MA. The modifying effect of selenium and vitamins A, C, and E on the genotoxicity induced by sunset yellow in male mice. Mutat Res. 2012;744:145–153. doi: 10.1016/j.mrgentox.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Seesuriyachan P, Takenaka S, Kuntiya A, Klayraung S, Murakami S, Aoki K. Metabolism of azo dyes by Lactobacillus casei TISTR1500 and effects of various factors on decolourization. Water Res. 2007;41:985–992. doi: 10.1016/j.watres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Solomon TW. Organic chemistry. 6. New York: Wiley; 1996. [Google Scholar]
- Surjyo JB, Anisur RK. Cytotoxic and genotoxic effects of the azo-dye p-dimethylaminoazobenzene in mice: a time-course study. Mutat Res. 2005;587:1–8. doi: 10.1016/j.mrgentox.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Inhibitory effects of xanthone on paraquat-and-NaNO(2)-induced genotoxicity in cultured cells. J Toxicol Sci. 2007;32:571–574. doi: 10.2131/jts.32.571. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Iyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–221. doi: 10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tsuboy MS, Angeli JPF, Mantovani MS, Knasmuller S, Umbuzeiro GA, Ribeiro LR. Genotoxic, mutagenic and cytotoxic effects of the commercial dye CI Disperse Blue 291 in the human hepatic cell line HepG2 (2000) Toxicol In Vitro. 2007;211:650–655. doi: 10.1016/j.tiv.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Turkoglu S. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat Res. 2007;62:14–64. doi: 10.1016/j.mrgentox.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Villani P, Spanò M, Pacchierotti F, Weimer M, Cordelli E. Evaluation of a modified comet assay to detect DNA damage in mammalian sperm exposed in vitro to different mutagenic compounds. Reprod Toxicol. 2010;30:44–49. doi: 10.1016/j.reprotox.2009.10.015. [DOI] [PubMed] [Google Scholar]
- WHO . World health organization laboratory manual for the examination of human semen and sperm–cervical mucus interaction. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Yılmaz S, Unal F, Aksoy H, Yuzbasıoglu D, Celik M. Cytogenetic effects of citric acid and benzoic acid on Allium chromosomes. Fresenius Environ Bull. 2008;17:1029–1037. [Google Scholar]
- Yılmaz S, Unal F, Yuzbasıoglu D, Aksoy H. Clastogenic effects of food additive citric acid in human peripheral lymphocytes. Cytotechnology. 2008;56:137–140. doi: 10.1007/s10616-008-9137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yılmaz S, Unal F, Yuzbasıoglu D. The in vitro genotoxicity of benzoic acid in human peripheral blood lymphocytes. Cytotechnology. 2009;60:55–61. doi: 10.1007/s10616-009-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzbasioglu D, Celik M, Yılmaz S, Unal F, Aksoy H. Clastogenicity of the fungicide afugan in cultured human lymphocytes. Mutat Res. 2006;604:53–59. doi: 10.1016/j.mrgentox.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Zengin N, Yuzbasıoglu D, Unal F, Yılmaz S, Aksoy H. The evaluation of the genotoxicity of two food preservatives: sodium benzoate and potassium benzoate. Food Chem Toxicol. 2011;49:763–769. doi: 10.1016/j.fct.2010.11.040. [DOI] [PubMed] [Google Scholar]