Abstract
Mesenchymal stem cells (MSCs) offer promise as therapeutic aid in the repair of tendon and ligament injuries in race horses. Fetal adnexa is considered as an ideal source of MSCs due to many advantages, including non-invasive nature of isolation procedures and availability of large tissue mass for harvesting the cells. However, MSCs isolated from equine fetal adnexa have not been fully characterized due to lack of species-specific markers. Therefore, this study was carried out to isolate MSCs from equine umbilical cord blood (UCB) and characterize them using cross-reactive markers. The plastic-adherent cells could be isolated from 13 out of 20 (65 %) UCB samples. The UCB derived cells proliferated till passage 20 with average cell doubling time of 46.40 ± 2.86 h. These cells expressed mesenchymal surface markers but did not express haematopoietic/leucocytic markers by RT-PCR and immunocytochemistry. The phenotypic expression of CD29, CD44, CD73 and CD90 was shown by 96.36 ± 1.28, 93.40 ± 0.70, 73.23 ± 1.29 and 46.75 ± 3.95 % cells, respectively in flow cytometry, whereas, reactivity against the haematopoietic antigens CD34 and CD45 was observed only in 2.4 ± 0.20 and 0.1 ± 0.0 % of cells, respectively. Osteogenic and chondrogenic differentiation could be achieved using established methods, whereas the optimum adipogenic differentiation was achieved after supplementing media with 15 % rabbit serum and 20 ng/ml of recombinant human insulin. In this study, we optimized methodology for isolation, cultural characterization, differentiation and immunophenotyping of MSCs from equine UCB. Protocols and markers used in this study can be employed for unequivocal characterization of equine MSCs.
Keywords: Mesenchymal stem cell, Umbilical cord blood, Characterization, Differentiation, Horse
Introduction
Mesenchymal stem cells (MSCs) represent an archetype of multipotent somatic stem cells that hold promise for application in equine regenerative medicine. Equine MSCs have been isolated from different post-natal tissues including, bone marrow (Violini et al. 2009), adipose tissue (Braun et al. 2010; de Mattos et al. 2009) and peripheral blood (Dhar et al. 2012; Spaas et al. 2013). However, age-dependent decline in absolute number and invasive procedures involved (Stenderup et al. 2003) limited the utility of adult tissues as a source of MSCs. Equine fetal adnexa, such as umbilical cord matrix (Lovati et al. 2011), umbilical cord blood (Koch et al. 2007; Schuh et al. 2009), amnion (Lange-Consiglio et al. 2012), placenta (Carrade et al. 2011b) and amniotic fluid (Lovati et al. 2011; Gulati et al. 2013) are rich, safe and non-invasive sources of MSCs. Among these sources umbilical cord blood (UCB) derived MSCs are considered superior due to their greater proliferation and differentiation potential, delayed senescence and immune tolerance properties (Carrade et al. 2011a). These cells also express markers associated with embryonic phenotypes (Reed and Johnson 2008) indicating their more primitive nature with broader differentiation capacities (Moretti et al. 2010).
Although many workers have reported isolation of MSCs from equine UCB (De Schauwer et al. 2012; Koch et al. 2007; Schuh et al. 2009), a complete characterization has been lacking, which is in sharp contrast to the detailed guidelines described for the unequivocal characterization of human MSCs. Their functional and cultural characteristics (population doubling time and plating efficiency) are not well studied. Moreover, immunophenotypic characterization of UCB-MSCs has not been undertaken, primarily due to non-availability of equine-specific monoclonal antibodies (mAbs) (De Schauwer et al. 2012; Rozemuller et al. 2010).
The capacity of tri-lineage differentiation is one of the hallmarks of MSCs (Dominici et al. 2006). Using standard induction media, equine UCB-MSCs could be induced to differentiate towards osteocytes, chondrocytes, adipocytes (De Schauwer et al. 2012; Koch et al. 2007; Schuh et al. 2009), tenocytes (Mohanty et al. 2014) and hepatocytes (Reed and Johnson 2008). However, adipogenic differentiation of equine UCB-MSCs has yielded variable results and needs further optimization (Giovannini et al. 2008; Vidal et al. 2006). Therefore, in this study we evaluated the cultural characteristics of equine UCB-derived MSCs and optimized methodology for immunophenotyping and tri-lineage differentiation.
Materials and methods
Unless otherwise specified, all chemicals and cell culture media used for mesenchymal stem cell isolation and culture were procured from the Sigma Chemicals Co. (St. Louis, MO, USA) and tissue culture flasks and dishes from Corning (Corning, NY, USA).
Collection of UCB, isolation and culture of MSCs
UCB was collected from thoroughbred mares (n = 20) in an organized farm at Hisar, Haryana (India) in a blood collection bag containing citrate–phosphate dextrose adenine (CPDA) as the anticoagulant and transported at 4 °C to the laboratory immediately. Mesenchymal stem cells were isolated by following the method described by Koch et al. 2007. Briefly mononuclear cells were separated using Histopaque-1077. The cells were then treated with RBC lysis buffer for 5 min at 4 °C followed by two washings with Dulbecco’s phosphate buffer saline (DPBS). The cells were suspended in one ml of mesenchymal stem cell growth medium containing low-glucose DMEM supplemented with 15 % of foetal bovine serum (FBS), MEM non-essential amino acid (1 %), vitamin (1 %), penicillin (100 IU/ml), streptomycin (0.1 mg/ml) and l-glutamine (2 mM). Live cells were counted by trypan blue dye (0.4 %) exclusion method using haemocytometer and were seeded at 105 cells/cm2 in 25 cm2 tissue culture flasks. Incubation was done at 38.5 °C in humidified atmosphere containing 5 % CO2. The first medium was changed after 24 h and thereafter every 3rd day. The cell growth and morphology was observed under inverted microscope (IX51, Olympus, Tokyo, Japan). The cells were sub-cultured at 80 % confluency.
Alkaline phosphatase (AP) staining
UCB-MSCs were grown in six-well plates and stained using AP staining kit following the manufacturer’s instructions. Equine fibroblast cells prepared from adult horse ear pinna served as negative control for AP staining.
Growth kinetics
The colony forming unit assay was performed as per the method described by Lovati et al. (2011), with slight modification. UCB-MSCs were seeded (300 cells/cm2) in the growth medium in 60-mm tissue culture dish and allowed to grow for 5 days by incubating at 5 % CO2, 38.5 °C and 90 % humidity. Cells were then fixed with methanol for 30 min and stained with Giemsa stain for 15 min. Colonies consisting of more than 16–20 nucleated cells, were counted and data were reported as plating efficiency (PE %), calculated as number of colonies/number of seeded cells × 100. The experiment was done in triplicate with three different MSCs samples.
The proliferation capacity of UCB-MSCs was evaluated at passage P1–P8 in triplicates from three different donors. In each passage, 5 × 103 cells/cm2 were cultured in 25 cm2 tissue culture flask. At 80 % confluency, cells were trypsinized and the number of viable cells was counted by the trypan blue dye exclusion method. The population doublings (PD) were obtained according to the formula CD = ln (Nf/Ni)/ln 2, PD = CT/CD, where Ni represents initial seeded cells, Nf is the final number of cells harvested, CT is the culture time (in hour) and CD is the cell doubling number.
UCB-MSCs were plated at a density of 1.8 × 104 into six-well tissue culture dishes. Every 2 days, over the 10 days of culture, cells from one well of each plate were trypsinized and counted. All experiments were performed in triplicate. At each time point cell growth curve was plotted to show increase in cell concentration.
Reverse transcription polymerase chain reaction (RT-PCR)
The expression of mesenchymal cell surface marker genes (CD29, CD44, CD73, CD90 and CD105) and hematopoietic/leukocytic marker genes (CD14, CD34 and CD45) was assessed by RT-PCR in the cells at passage 3. The RNA was isolated using RNeasy kit (Qiagen, Milan, Italy) as per manufacturer’s protocol. The first strand cDNA was synthesized by using revert aid first strand cDNA synthesis kit (Fermentas, Hanover, MD, USA) in a 20 μl reaction volume, using 1 μg RNA, 10 mM dNTPs, 0.2 μg oligo dT primers, 20 units of RiboLock RNAase inhibitor and 200 units of M-MuLV reverse transcriptase H. The PCR amplification was done using primers specific for each gene (Table 1) separately in 25 μl volume. The amplification reaction mixture consisted of 1× PCR buffer, 0.2 mM of each dNTP, 0.5 μM of each gene specific forward and reverse primers, 1.5 mM MgCl2, 1.25 U of Taq DNA Polymerase, and 3 μl of cDNA. An initial 5 min denaturation step at 94 °C was followed by 35 cycles including denaturation at 94 °C for 30 s, gene specific annealing temperature (Table 1) for 30 s and elongation at 72 °C for 30 s followed by final elongation step at 72 °C for 10 min. The amplified PCR products were resolved in 2 % electrophoresis through agarose gel containing ethidium bromide (0.5 µg/ml) and visualized in Syngene GBox gel documentation system (Cambridge, CB4 1TF, UK).
Table 1.
Details of primers used for RT-PCR analysis
| Marker | Primer sequence (5′–3′) | Amplicon size (bp) | Annealing Temp. (°C) | Accession no. |
|---|---|---|---|---|
| GAPDH | F: CAAGGTCATCCATGACAACTTTG | 496 | 58 | NM_001163856.1 |
| R: GTCCACCACCCTGTTGCTGTAG | ||||
| CD29 | F: CTTATTGGCCTTGCATTGCT | 169 | 58 | XM.005606848 |
| R: TTCCCTCGTACTTCGGATTG | ||||
| CD44 | F: ATCCTCACGTCCAACACCTC | 165 | 58 | NM_001085435.1 |
| R: CTCGCCTTTCTTGGTGTAGC | ||||
| CD90 | F: TGCGAACTCCGCCTCTCT | 93 | 60 | EU881920.1 |
| R: GCTTATGCCCTCGCACTTG | ||||
| CD73 | F: GGGATTGTTGGATACACTTCAAAAG | 90 | 60 | XM 001500115.2 |
| R: GCTGCAACGCAGTGATTTCA | ||||
| CD105 | F: AAGAGCTCATCTCGAGTCTG | 338 | 56 | XM_003364145.1 |
| R: ATGCTCAGGGATCATTGGGG | ||||
| CD34 | F: CACTAAACCCTCTACATCATTTTCTCCTA | 101 | 60 | XM 001491596 |
| R: GGCAGATACCTTGAGTCAATTTCA | ||||
| CD45 | F: TGATTCCCAGAAATGACCATGTA | 101 | 60 | AY114350.1 |
| R: ACATTTTGGGCTTGTCCTGTAAC | ||||
| CD14 | F: TTGATCTCAGCTGCAACAGG | 303 | 56 | NM_001081927.1 |
| R: CAGAGGGTCGGTTGGTTAAGAC | ||||
| Osteocalcin | F: TGAAGACCAGTATCCTGATGC | 174 | 60 | XM_001496174.1 |
| R: GCTGACTTGTTTCCTGACTG | ||||
| RUNX2 | F: CGTGCTGCCATTCGAGGTGGTGG | 351 | 58 | XM_001502519.3 |
| R: CCTCAGAACTGGGCCCTTTTTCAG | ||||
| Collagen 2α1 | F: GGAGACTACTGGATTGACCC | 451 | 62 | NM_001081764.1 |
| R: CCCACTTACCGGTGTGTTTC | ||||
| PPAR-γ | F: AAGAGCAGAGCAAAGAGGTG | 457 | 62 | XM_001492430.1 |
| R: GGGCTTCACATTCAACAAAC | ||||
| Adiponectin | F: GGAGACAGCTACTCCCCAAGAT | 187 | 58 | NC_009146.2 |
| R: GTCCAGTCTTACCTCTCAAACCT |
Immunocytochemistry and flow cytometry
A total of six surface markers were analyzed by immunocytochemistry and flow cytometry, the mesenchymal cell markers CD29 (Integrin β-1), CD44 (H-CAM), CD73 (ecto-5′-nucleotidase), CD90 (Thy-1), and the haematopoietic markers CD34 and CD45 (LCA).
Immunocytochemistry was performed as described by Lovati et al. (2011) with some modifications. UCB-MSCs at P3 were seeded in triplicate at 5 × 103 cells/cm2 in eight well glass chamber slide (SPL biosciences Ltd., Pocheon-si, Gyeonggi-do, Korea) and incubated in 5 % CO2 incubator at 38.5 °C overnight for attachment. The cells were rinsed briefly with rinse buffer consisting of DPBS with 0.2 % bovine serum albumin (BSA) followed by fixation with 4 % paraformaldehyde for 20 min. To avoid nonspecific binding cells were incubated in blocking buffer (3 % FBS in DPBS) for 30 min. After washing three times with rinse buffer, cells were incubated with 1:50 dilution of each murine monoclonal antibody (BD Biosciences, San Diego, CA, USA) separately against CD29 (clone TS2/16), CD44 (clone IM7), CD73 (clone 5F/B9), CD90 (clone 5E10), CD34 (clone 8G12) and CD45 (clone 2D1) for 1 h at 37 °C in a moist chamber. After three washings, cells were incubated with anti-mouse FITC conjugated IgG/IgM, at 1:100 dilution in blocking buffer for 1 h at 37 °C in dark. After three washings, cells were visualized for expression of MSC markers under the fluorescence microscope (1X51, Olympus) using suitable filter. Adult horse peripheral blood mononuclear cells served as positive control for CD34 and CD45 antibodies.
For flow cytometry, UCB-MSCs were trypsinized and re-suspended at 106 cells/ml in culture medium. The cells were pelleted and fixed with 4 % paraformaldehyde at 4 °C followed by two washings with washing buffer (0.2 % BSA in PBS containing 0.01 % sodium azide). Cells (approximately 105) in triplicate tubes were incubated with 1:50 dilution of mouse anti-human CD29-FITC (eBioscience, San Diego, CA, USA), CD44-FITC (eBioscience), CD73-FITC (BD Pharmingen, San Diego, CA, USA), CD90-PE (BD Pharmingen), CD45-FITC (BD Bioscience) and CD34-PE (BD Bioscience) antibodies for 45 min in dark at room temperature. Cells were washed three times and re-suspended in washing buffer. For all tubes, at least 10,000 cells were analyzed using FACS Calibur (BD Biosciences, San Diego, CA, USA). The data were analyzed with FACSDiva software (BD Biosciences). All data were corrected for non-specific binding using isotype-matched negative controls.
In vitro trilineage differentiation
Trilineage differentiation was performed in triplicate on three independent MSC samples at passage 3 and 5. The cells were re-suspended in MSC growth medium at a density of 3 × 103 cells/cm2 in six-well tissue culture dishes and incubated at 5 % CO2, 38.5 °C. On attaining 80 % confluency, the cells in triplicate wells were incubated in respective differentiation media for 28 days with medium changes every 3rd day. The cells cultured in MSC culture medium containing 2 % fetal bovine serum for 28 days served as undifferentiated control.
For osteogenic differentiation, the differentiation medium consisted of low glucose DMEM supplemented with 10 % FBS, 1 % penicillin/streptomycin, 100 nM dexamethasone, 10 mM β-glycerophosphate and 0.05 mM l-ascorbic acid-2-phosphate. The cells were stained with Von Kossa and Alizarin red S stain on day 14, 21 and 28. The expression of Osteocalcin and Runx2 genes was confirmed by RT-PCR using gene specific primers (Table 1).
For chondrogenic differentiation, the medium comprising high glucose DMEM supplemented with 1 % ITS-Prepix, dexamethasone (1 µM), ascorbic acid-2-phosphate (0.1 µM), l-proline (40 µg/ml), sodium pyruvate (1 mM) and human recombinant transforming growth factor β3 (TGFβ3) at 10 ng/ml was used. The differentiated cells were stained with 1 % Alcian blue (in 3 % acetic acid, pH 2.5) on day 14, 21 and 28 and expression of Collagen 2αI gene was observed by RT-PCR using primers (Table 1).
For adipogenic differentiation, the cells were cultured in adipogenic induction medium for 72 h followed by culture in adipogenic maintenance medium for 24 h. Adipogenic induction medium consisted of low-glucose DMEM supplemented with 10 % FBS, 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methyl-xanthine (IBMX), 0.2 mM indomethacin, 1 % penicillin/streptomycin and different concentrations (10–20 µg/ml) of recombinant human insulin, rabbit serum (10–20 %). Adipogenic maintenance medium contained same ingredients as adipogenic induction medium except IBMX. The differentiated cells were stained with Oil-Red-O (0.5 % in isopropanol) followed by counter staining with Harris’ haematoxyline for 1 min. RNA isolated from the cells was tested by RT-PCR for expression of PPAR-γ and Adiponectin genes using gene specific primers (Table 1).
Statistical analysis
The SPSS 17.0 software (IBM, Windows Version) was used for the statistical analysis. All data were presented as mean ± SE. Data were analyzed by one-way ANOVA using Duncan’s multiple range test (DMRT) at 0.05 % level of significance.
Results
Isolation and propagation of cells
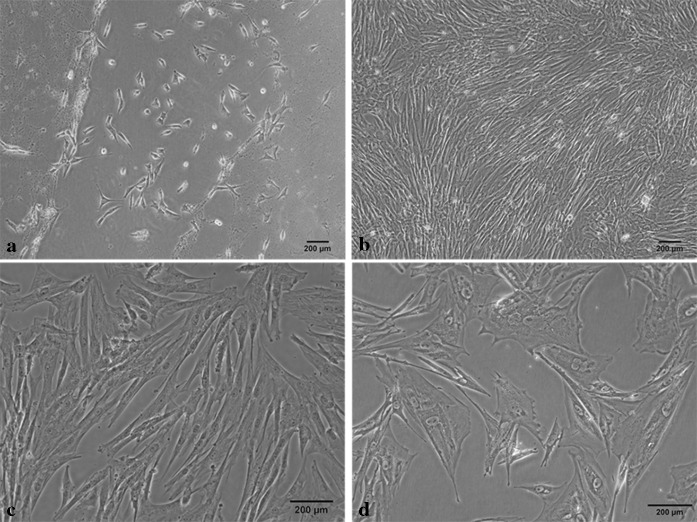
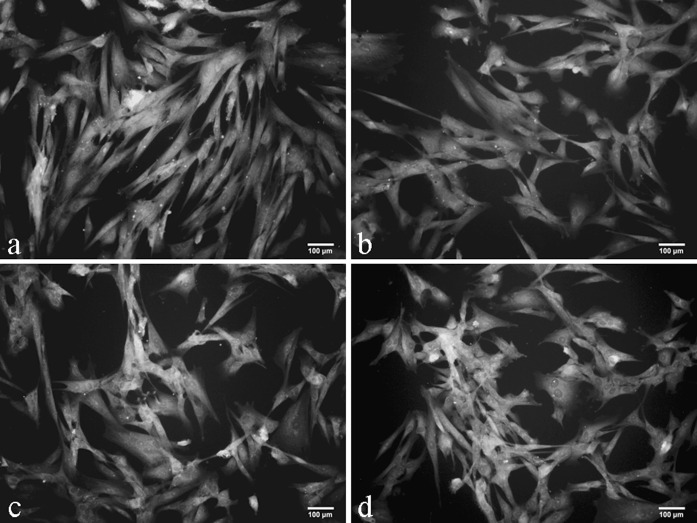
About 165 ml (range 130–200 ml) of UCB was recovered from thoroughbred mares (n = 20) during full term foaling. No sample had signs of coagulation or hemolysis. The mononuclear cells were separated by histopaque density gradient method and were seeded at the rate of 1 × 105 cells/cm2 in 25 cm2 tissue culture flasks in medium containing low glucose DMEM supplemented with 15 % fetal bovine serum for isolation of MSCs. Plastic-adherent spindle-shaped colonies were observed in 13 of 20 UCB samples, with isolation frequency of 65 %. Primary colonies were observed as early as 6 days post-seeding (range 6–20 days) and 80 % cell confluency was reached by 30 days post-seeding. The isolated cells presented endothelioid and fibroblastoid morphologies but on subculture, there was predominance of fibroblast-like cells at passage 1 and after passage 2, UCB derived cells showed morphologically homogeneous population of fibroblast-like cells (Fig. 1).
Fig. 1.
Morphology of equine umbilical cord blood derived cells. a Primary colony exhibiting a mesenchymal stem cells-like shape with a flat polygonal morphology. b Monolayer of rapidly expanding adherent spindle-shaped fibroblastoid cells at passage 10. c Sub-confluent monolayer of expanding adherent spindle-shaped fibroblastoid cells at passage 15. d The UCB-derived cells at passage 20 showing change in morphology from fibroblastoid to irregular shape and increased cell size
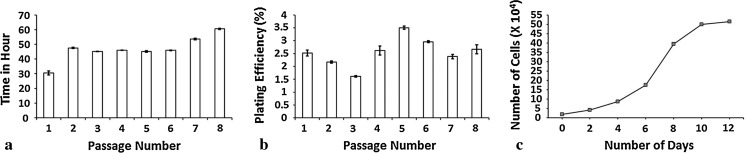
The mean population-doubling time (PD) during initial 8 passages was 46.40 ± 2.86 h. The lowest (p ≤ 0.05) population doubling time was observed in P3 (45.16 ± 0.01 h) and the highest in P8 (58.53 ± 0.44 h) (Fig. 2). The mean plating efficiency during initial 8 passages was 2.57 ± 0.16 %. The highest plating efficiency (p ≤ 0.05) was observed in passage 5 (3.50 ± 0.06 %) and the lowest in passage 8 (2.16 ± 0.26 %). The calibrated growth curve at passage 4 was ‘S’ shaped with a short lag phase (Fig. 2). Undifferentiated cells proliferated till passage 20 before showing growth arrest. Subsequently, the morphology of these cells changed from fibroblastoid to irregular shape with increased cell size, decreased proliferation rate, increased passage time and finally stopped dividing.
Fig. 2.
Cultural characteristics of isolated UCB-MSCs (n = 3) plotted at different passages. a Population doubling times. b Plating efficiency. c Growth curve at passage 4. Data is presented as mean ± SE at p < 0.05
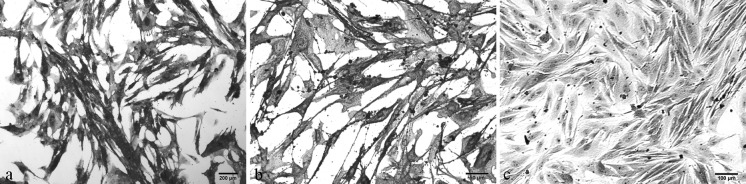
The UCB-derived cells showed heterogeneous population at initial passage (P0 and P1), some cells were alkaline phosphatase (AP) positive (blue), while others were AP-negative (red). In subsequent passages, cell population became more homogenous, showing positive AP staining. Equine fibroblast cells prepared from adult horse ear pinna were negative for AP staining (Fig. 3).
Fig. 3.
Alkaline phosphatase (AP) staining of equine UCB-MSCs. a Heterogenous population of both AP positive (blue) and AP negative (red) cells at passage 1, b Homogenous population of AP positive (stained blue) MSCs at passage 5, c Equine fibroblast cells were AP-negative (stained red). (Color figure online)
Gene expression
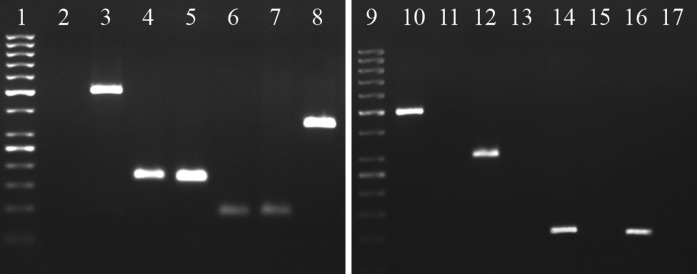
RT-PCR analysis of undifferentiated passage 3 cells showed that UCB-derived cells expressed mesenchymal stem cell markers, viz, CD29, CD44, CD73, CD90 and CD105, but did not express hematopoetic/leukocytic makers (CD34, CD45 and CD14) (Fig. 4).
Fig. 4.
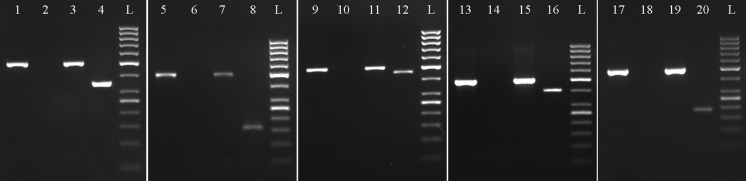
RT-PCR analysis for expression of mesenchymal and haematopoietic surface genes by equine UCB-MSCs. The cells showed the expression of mesenchymal markers CD29 (lane 4), CD44 (lane 5), CD73 (lane 6), CD90 (lane 7) and CD105 (lane 8). The markers CD14, CD34 and CD45 were not expressed by UCB-MSCs (lanes 13, 15 and 17) while were expressed by equine PBMCs included as positive control (lanes 12, 14 and 16), respectively. Lanes 1 and 9: 50 bp ladder; 2 and 11: Non template control; 3 and 10: GAPDH (RNA internal control)
Expression of cell surface markers
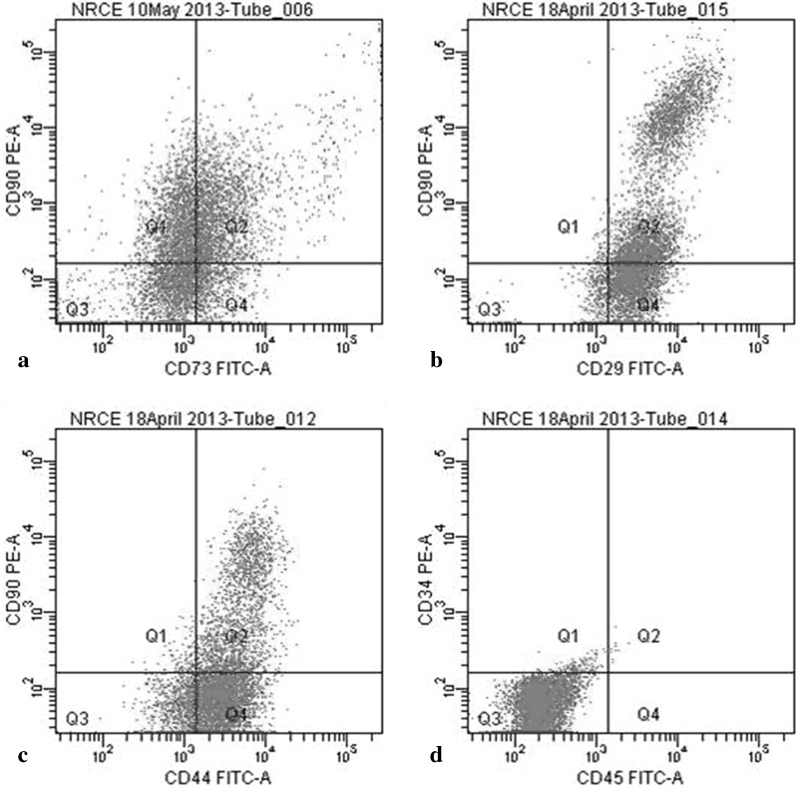
Immunocytochemistry of UCB-derived cells indicated that the cells were positive for CD29, CD44, CD73 and CD90, while negative for CD34 and CD45 proteins (Fig. 5). Flow cytometry analysis showed that equine UCB-MSCs were CD29pos, CD44pos, CD73pos, CD90pos, CD34neg and CD45neg. The findings indicated that CD29 and CD44 were expressed by 96.36 ± 1.28 and 93.4 ± 0.70 % of UCB-derived cells, while CD73 and CD90 were expressed by 73.23 ± 1.29 and 46.75 ± 3.95 % cells, respectively. Reactivity against the haematopoietic antigens CD45 and CD34 was observed only in 0.1 ± 0.0 and 2.4 ± 0.20 % of cells, respectively (Fig. 6).
Fig. 5.
Expression of cell surface markers in equine UCB-MSCs by immunostaining. The cells were stained with antibodies directed against a CD29, b CD44, c CD73, d CD90 visualized under fluorescent microscope
Fig. 6.
Flow cytometry analysis of equine UCB-MSCs for expression of surface markers. Plots are showing: cells stained with CD73 and CD90 (a), CD29 and CD90 (b), CD44 and CD90 (c) and CD34 and CD45 (d)
Osteogenic differentiation
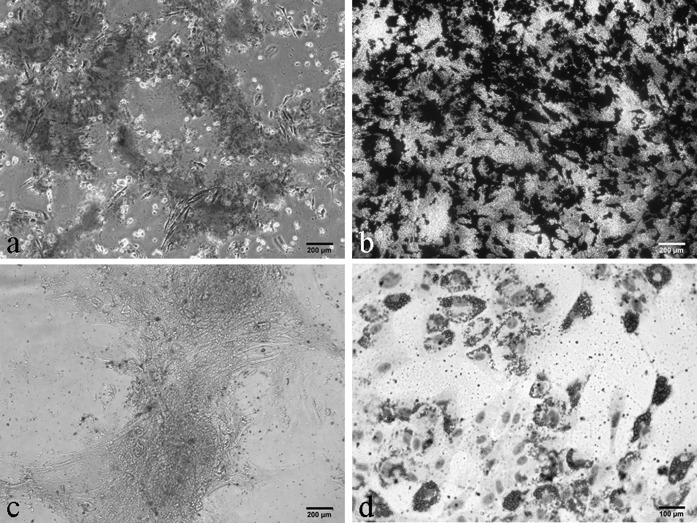
The UCB-MSCs induced to osteogenic differentiation showed extensive extra cellular calcium deposition as demonstrated by positive Alizarin red S staining and von Kossa staining (Fig. 7) after 14 days of induction, in comparison to undifferentiated control cells. On day 21 post-induction, the cells were more intensely stained. Differentiated UCB-MSCs showed expression of osteogenic lineage specific transcription factors i.e. Runx2 (351 bp) and Osteocalcin (174 bp) by RT-PCR, while undifferentiated control UCB-MSCs did not express these genes (Fig. 8).
Fig. 7.
Cytochemical staining of differentiated equine UCB-MSCs at passage 3. a Alizarin red S and b Von Kossa staining after osteogenic differentiation showing matrix mineralization with phosphate and calcium, c Alcian blue staining after chondrogenic differentiation showing marked deposition of glycosaminoglycans in the matrix, d Oil red O-stain after induction of adipogenic differentiation, showing cytoplasmic neutral triglycerides droplets. (Color figure online)
Fig. 8.
RT-PCR analysis of differentiated UCB-MSCs showing expression of differentiation marker genes. RUNX2 and osteocalcin, col2α1 and PPAR-γ and adiponectin genes were expressed by UCB-MSCS on differentiation to osteocytes, chondrocytes and adipocytes (lanes 4, 8, 12, 16 and 20) but not in undifferentiated control cells (lanes 2, 6, 10, 14 and 18), respectively. GAPDH gene was expressed by both undifferentiated control RNA (lanes 1, 5, 9, 13 and 17) and by differentiated cells (lanes 3, 7, 11, 15 and 19), L: 50 bp DNA ladder
Chondrogenic differentiation
On exposure of equine UCB-MSCs to chondrogenic induction medium, the cells showed marked deposition of glycosaminoglycans (GAG) in the matrix by day 21, which was observable after Alcian blue staining (Fig. 7). The cells formed cell aggregates in cultures on day 14, which further transformed into spherical masses by day 21. The cells maintained in regular control medium showed no detectable deposition of GAGs. The expression of Collagen 2αI (451 bp) gene by RT-PCR confirmed chondrogenic induction in these cells compared to untreated cells (Fig. 8).
Adipogenic differentiation
UCB-derived MSCs could not be induced to adipogenic differentiation using standard adipogenic induction medium as described for studies in human. Three different concentrations of rabbit serum (10, 15 and 20 %) and insulin (10, 15 and 20 μg/ml) were tested in this study. Optimum differentiation was achieved after supplementing adipogenic induction medium with 15 % rabbit serum and 20 μg/ml of recombinant human insulin. The induction of adipogenic differentiation resulted in differentiation of the cells to adipocytes by day 10 compared with non-induced control cells, as demonstrated by Oil Red O staining (Fig. 7). During adipogenic differentiation, a distinct ring of dark coarse granules around the cell periphery was observed initially by day 3, which developed into fat globules by day 10 with accumulation of intra-cytoplasmic vacuoles of Oil red O staining neutral triglycerides droplets. The adipogenic induction was further confirmed by the presence of mRNAs for both PPAR-γ (457 bp) and Adiponectin (187 bp) by RT-PCR after 10 days of induction (Fig. 8).
Discussion
The UCB-MSCs have been recognized as important source of MSCs in equines (Iacono et al. 2012; Koch et al. 2007; Schuh et al. 2009). However, MSCs isolated from equine umbilical cord are not fully characterized due to lack of species-specific markers. Thus, the aim of this study was to reflect upon its cultural characteristics, to study the expression of cell surface mesenchymal markers using cross-reacting antibodies and to understand their trilineage differentiation capacity.
In our study, we reported the isolation frequency of 65 % for equine UCB-MSCs using histopaque density gradient method, which was more than reported by Koch et al. (2007) (57 %). This isolation percentage was less than reported by Schuh et al. (2009) and Iacono et al. (2012) (75 %). Isolation frequency ranging between 57 and 80 % has been reported previously (De Schauwer et al. 2011; Koch et al. 2007; Schuh et al. 2009). The plastic adherent cells during first passage were heterogeneous, with endothelioid and fibroblastoid morphologies. The different morphologies might reflect mixture of true mesenchymal stem cells with unrestricted somatic stem cells (Koch et al. 2007).
Mean population doubling time for initial 8 passages in the present study was lower than that reported for UCB-MSCs (Iacono et al. 2012) whereas the plating efficiency (2.57 ± 0.16 %) during initial 8 passages was higher than those reported for MSCs isolated from amniotic fluid, umbilical cord matrix and bone marrow (Lovati et al. 2011). In human medicine, a lower doubling time (DT) and therefore, a greater proliferative activity has been reported for cells isolated from umbilical cord matrix and from cord blood compared to bone marrow-derived MSCs (Karahuseyinoglu et al. 2007). This behaviour could reflect the more primitive nature of cells isolated from fetal adnexa compared to those obtained from bone marrow (Weiss and Troyer 2006). These observations suggested that equine UCB-MSCs are of primitive nature and have higher clonogenicity. This is further substantiated by the observation that these cells had short lag phase implying their rapid recovery from the damage occuring during detachment by enzymatic treatment. AP positive staining demonstrated their high phosphatase activity, a unique feature of undifferentiated stem cells (Lange-Consiglio et al. 2012; Yadav et al. 2011). Further, these cells could be serially passaged up to 20 passages, demonstrating their better self renewal potential than reported previously (Schuh et al. 2009).
The MSC specific cell surface markers like CD29, CD44, CD73, CD90 and CD 105 were expressed by undifferentiated equine UCB-MSCs as revealed in RT-PCR analysis. There was lack of expression of leukocytic or hematopoietic markers i.e. CD34, CD45 and CD14. Similar gene expression results for CD73, CD90, CD105 and lack of CD45 transcript has been reported in adipose tissue (AT) MSCs (Braun et al. 2010) and bone marrow (BM) MSCs (Ranera et al. 2011). Lange-Consiglio et al. (2013) also demonstrated the expression of CD29, CD44 and CD105 by equine bone marrow and amnion derived mesenchymal stem cells.
The findings of gene expression studies were further confirmed by demonstration of proteins on cell surfaces by immunocytochemistry and flow cytometry. A limited cross-reactivity of mAbs between species has been demonstrated in an earlier study (Ibrahim et al. 2007), in which only 14 out of 379 mAbs against human cluster of differentiation (CD) molecules showed cross-reactivity with equine leukocytes. In the present study, a panel of monoclonal antibodies for immunophenotypic characterization of UCB-MSCs was selected based on cross-reaction studies (De Schauwer et al. 2012; Lange-Consiglio et al. 2012; Ranera et al. 2011). Immunocytochemistry of undifferentiated equine UCB-MSCs showed the expression of MSC positive cell surface markers CD29, CD44, CD73, CD90 and absence of expression of haematopoetic markers CD34 and CD45. The results of the present study are in accordance with a previous report (Ranera et al. 2011) for expression of CD73, CD90, CD105 and lack of CD45 in MSCs derived from equine adipose tissue and bone marrow derived MSCs. However, variable results for CD34 expression by equine bone marrow and amniotic membrane derived MSCs have been reported previously (Lange-Consiglio et al. 2012; Ranera et al. 2011). The haematopoietic marker CD34 was not registered at P1, but was present at P5 for amniotic membrane derived MSCs (Lange-Consiglio et al. 2012). Contrary to this finding, the expression of the haematopoietic CD34 marker was observed in adipose tissue derived MSCs till P3 (Ranera et al. 2011).
In flow cytometry, CD29, CD44, CD73 and CD90 were expressed by 96.36 ± 1.28, 93.4 ± 0.70, 73.23 ± 1.29 and 46.75 ± 3.95 % of UCB-MSCs, respectively, in the present study. Although similar level of expression of CD29 and CD44 has been reported in equine UCB-MSCs by De Schauwer et al. (2012), the cells in their study did not express CD73. This might be due to the fact that the CD73 clones used by De Schauwer (AD2, 7G2, 4G4 and 496406) did not cross-react with equine CD73. In our study also, clone AD2 did not react with equine MSCs (data not shown), while clone 5F/B9 reacted with 73.23 ± 1.29 % of UCB-MSCs. Therefore, a set of cross-reactive clones as identified in this study and by De Schauwer et al. (2012) may be employed for unequivocal characterization of equine MSCs till a single specific marker for equines is established.
Osteogenic differentiation was observed as early as day 14 post-induction by specific staining. Earlier reports indicate osteogenic differentiation of UCB-MSCs by day 21 (De Schauwer et al. 2012; Koch et al. 2007; Schuh et al. 2009). In addition, we also demonstrated the expression of Runx2 (transcription factor for early osteogenesis) and Osteocalcin (a non-collagenous protein specific for late osteoblast) on day 14 post-induction of differentiation (Declercq et al. 2005).
The UCB-MSCs could be differentiated to chondrocyte lineage by day 21 post-induction with the cell aggregates transforming into spherical masses, which were stained positively with Alcian blue staining. Similar results have been reported previously in monolayer cultures (Iacono et al. 2012) and in pellet cultures (De Schauwer et al. 2011; Koch et al. 2007; Schuh et al. 2009). The findings were further confirmed by expression of Collagen 2αI gene by RT-PCR as reported previously (Reed and Johnson 2008).
Induction of adipogenic differentiation of equine MSCs has been reported to be more difficult compared to other farm animals and human MSCs (Koch et al. 2007; Vidal et al. 2006; Giovannini et al. 2008). In our study, adipogenic induction medium was formulated by supplementation of dexamethasone, indomethacin, 3-isobutyl-1-methylxanthine (IBMX), different concentrations of insulin (10–20 µg/ml) and rabbit serum (10–20 %) in low glucose DMEM. The optimum adipogenic differentiation was observed on supplementation of insulin (20 µg/ml) and rabbit serum (15 %). We observed that by increasing the insulin concentration to 20 µg/ml, better results were obtained than those reported earlier for equine MSCs (Lovati et al. 2011; Schuh et al. 2009). The differentiation process was further confirmed by expression of PPAR-γ and adiponectin gene by RT-PCR analysis. PPAR-γ is an adipogenic specific transcription factor that stabilizes the metabolic function of differentiated adipocytes (Lee et al. 2004; Wang et al. 2004) and adiponectin is the most abundant protein in adipose tissue which promotes insulin sensitivity, lipid accumulation and adipocyte differentiation, (Fu et al. 2005) and its expression confirms adipogenesis (Csaki et al. 2007).
Conclusion
Our findings established that UCB is a rich source of MSCs and UCB-MSCs in equines have lower population doubling time and higher plating efficiency indicating their high proliferative capacity than MSCs from adult sources. We identified a panel of cell surface MSC positive markers by immunocytochemistry and flow cytometry for identification of these cells which could be used for characterization of UCB-MSCs. We were able to refine the methodology for adipogenic differentiation of UCB-MSCs. The findings establish that UCB is a non-invasive and safe source of MSCs, which may be used for tissue engineering and regenerative medicine in equines.
Acknowledgments
We thank National Research Centre on Equines (NRCE), Hisar for infrastructure support. Financial support to Niharika Mohanty from Indian Council of Agricultural Research (ICAR) and to Baldev R Gulati from Department of Biotechnology, Government of India is duly acknowledged. The authors wish to thank Equine Breeding Stud, Hisar, Haryana, India for providing access for sampling. The assistance in flow cytometry by BD-FACS academy, New Delhi, India is duly acknowledged.
References
- Braun J, Hack A, Weis-Klemm M, Conrad S, Treml S, Kohler K, Walliser U, Skutella T, Aicher WK. Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells. Am J Vet Res. 2010;71:1228–1236. doi: 10.2460/ajvr.71.10.1228. [DOI] [PubMed] [Google Scholar]
- Carrade DD, Affolter VK, Outerbridge CA, Watson JL, Galuppo LD, Buerchler S, Kumar V, Walker NJ, Borjesson DL. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 2011;13:1180–1192. doi: 10.3109/14653249.2011.602338. [DOI] [PubMed] [Google Scholar]
- Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, Librach F, Buerchler S, Friedman MS, Walker NJ, Borjesson DL. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy. 2011;13:419–430. doi: 10.3109/14653249.2010.536213. [DOI] [PubMed] [Google Scholar]
- Csaki C, Matis U, Mobasheri A, Ye H, Shakibaei M (2007) Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem Cell Biol 128:507–520 [DOI] [PubMed]
- Declercq HA, Verbeeck RM, De Ridder LI, Schacht EH, Cornelissen MJ (2005) Calcification as an indicator of osteoinductive capacity of biomaterials in osteoblastic cell cultures. Biomaterials 26:4964–4974 [DOI] [PubMed]
- de Mattos CA, Alves AL, Golim MA, Moroz A, Hussni CA, de Oliveira PG, Deffune E. Isolation and immunophenotypic characterization of mesenchymal stem cells derived from equine species adipose tissue. Vet Immunol Immunopathol. 2009;132:303–306. doi: 10.1016/j.vetimm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- De Schauwer C, Meyer E, Cornillie P, De VS, Van de Walle GR, Hoogewijs M, Declercq H, Govaere J, Demeyere K, Cornelissen M, Van SA (2011) Optimization of the isolation, culture, and characterization of equine umbilical cord blood mesenchymal stromal cells. Tissue Eng Part C Methods 17:1061–1070 [DOI] [PubMed]
- De Schauwer C, Piepers S, Van de Walle GR, Demeyere K, Hoogewijs MK, Govaere JL, Braeckmans K, Van SA, Meyer E (2012) In search for cross-reactivity to immunophenotype equine mesenchymal stromal cells by multicolor flow cytometry. Cytometry A 81:312–323 [DOI] [PubMed]
- Dhar M, Neilsen N, Beatty K, Eaker S, Adair H, Geiser D. Equine peripheral blood-derived mesenchymal stem cells: isolation, identification, trilineage differentiation and effect of hyperbaric oxygen treatment. Equine Vet J Equine Vet J. 2012;44:600–605. doi: 10.1111/j.2042-3306.2011.00536.x. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy Position Statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Fu Y, Luo N, Klein RL. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- Giovannini S, Brehm W, Mainil-Varlet P, Nesic D. Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation. 2008;76:118–129. doi: 10.1111/j.1432-0436.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- Gulati BR, Kumar R, Mohanty N, Kumar P, Somasundaram RK, Yadav PS (2013) Bone morphogenetic protein-12 induces tenogenic differentiation of mesenchymal stem cells derived from equine amniotic fluid. Cells Tissues Organs 198:377–389 [DOI] [PubMed]
- Iacono E, Brunori L, Pirrone A, Pagliaro PP, Ricci F, Tazzari PL, Merlo B. Isolation, characterization and differentiation of mesenchymal stem cells from amniotic fluid, umbilical cord blood and Wharton’s jelly in the horse. Reproduction. 2012;143:455–468. doi: 10.1530/REP-10-0408. [DOI] [PubMed] [Google Scholar]
- Ibrahim S, Saunders K, Kydd JH, Lunn DP, Steinbach F. Screening of anti-human leukocyte monoclonal antibodies for reactivity with equine leukocytes. Vet Immunol Immunopathol. 2007;119:63–80. doi: 10.1016/j.vetimm.2007.06.034. [DOI] [PubMed] [Google Scholar]
- Karahuseyinoglu S, Cinar O, Kilic E. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007;7:26. doi: 10.1186/1472-6750-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange-Consiglio A, Corradetti B, Bizzaro D, Magatti M, Ressel L, Tassan S, Parolini O, Cremonesi F. Characterization and potential applications of progenitor-like cells isolated from horse amniotic membrane. J Tissue Eng Regen Med. 2012;6:622–635. doi: 10.1002/term.465. [DOI] [PubMed] [Google Scholar]
- Lange-Consiglio A, Corradetti B, Meucci A, Perego R, Bizzaro D, Cremonesi F (2013) Characteristics of equine mesenchymal stem cells derived from amnion and bone marrow: in vitro proliferative and multilineage potential assessment. Equine Vet J 45:737–744 [DOI] [PubMed]
- Lee OK, Kuo TK, Chen W, Lee K, Hsieh S, Chen T. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Lovati AB, Corradetti B, Lange CA, Recordati C, Bonacina E, Bizzaro D, Cremonesi F. Comparison of equine bone marrow-, umbilical cord matrix and amniotic fluid-derived progenitor cells. Vet Res Commun. 2011;35:103–121. doi: 10.1007/s11259-010-9457-3. [DOI] [PubMed] [Google Scholar]
- Mohanty N, Gulati BR, Kumar R, Gera S, Kumar P, Somasundaram RK, Kumar S. Immunophenotypic characterization and tenogenic differentiation of mesenchymal stromal cells isolated from equine umbilical cord blood. In Vitro Cell Dev Biol. 2014;50:338–348. doi: 10.1007/s11626-013-9729-7. [DOI] [PubMed] [Google Scholar]
- Moretti P, Hatlapatka T, Marten D, Lavrentieva A, Majore I, Hass R, Kasper C. Mesenchymal stromal cells derived from human umbilical cord tissues: primitive cells with potential for clinical and tissue engineering applications. Adv Biochem Eng Biotechnol. 2010;123:29–54. doi: 10.1007/10_2009_15. [DOI] [PubMed] [Google Scholar]
- Ranera B, Lyahyai J, Romero A, Vazquez FJ, Remacha AR, Bernal ML, Zaragoza P, Rodellar C, Martin-Burriel I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol. 2011;144:147–154. doi: 10.1016/j.vetimm.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Reed SA, Johnson SE. Equine umbilical cord blood contains a population of stem cells that express Oct4 and differentiate into mesodermal and endodermal cell types. J Cell Physiol. 2008;215:329–336. doi: 10.1002/jcp.21312. [DOI] [PubMed] [Google Scholar]
- Rozemuller H, Prins HJ, Naaijkens B, Staal J, Buhring HJ, Martens AC. Prospective isolation of mesenchymal stem cells from multiple mammalian species using cross-reacting anti-human monoclonal antibodies. Stem Cells Dev. 2010;19:1911–1921. doi: 10.1089/scd.2009.0510. [DOI] [PubMed] [Google Scholar]
- Schuh EM, Friedman MS, Carrade DD, Li J, Heeke D, Oyserman SM, Galuppo LD, Lara DJ, Walker NJ, Ferraro GL, Owens SD, Borjesson DL. Identification of variables that optimize isolation and culture of multipotent mesenchymal stem cells from equine umbilical-cord blood. Am J Vet Res. 2009;70:1526–1535. doi: 10.2460/ajvr.70.12.1526. [DOI] [PubMed] [Google Scholar]
- Spaas JH, De SC, Cornillie P, Meyer E, Van SA, Van de Walle GR. Culture and characterisation of equine peripheral blood mesenchymal stromal cells. Vet J. 2013;195:107–113. doi: 10.1016/j.tvjl.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, Gimble JM. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 2006;36:613–622. doi: 10.1111/j.1532-950X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol. 2009;10:29. doi: 10.1186/1471-2121-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s Jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav PS, Mann A, Singh V, Yashveer S, Sharma RK, Singh I. Expression of pluripotency genes in buffalo (Bubalus bubalis) amniotic fluid cells. Reprod Domest Anim. 2011;46:705–711. doi: 10.1111/j.1439-0531.2010.01733.x. [DOI] [PubMed] [Google Scholar]