Abstract
Mesenchymal stem cells (MSCs) have shown great therapeutic potential in clinical trials; however, loss of pluripotency due to culture senescence is a major factor limiting their application. Understanding the physiology of stem cell self-renewal and stemness, and identifying the molecules that regulate these processes, are critical to future advances in tissue and organ regeneration. The Krüppel-like factor (Klf) family are key transcription factors implicated in self-renewal of embryonic stem cells. Here we identify Klf2 as a crucial transcription factor in undifferentiated human bone marrow stromal cells (hBMSCs), as indicated by gene expression in three culture media. To investigate the role of Klf2 in detail, an overexpression study using a lentiviral system in hBMSCs was performed. After Klf2 overexpression, cell proliferation was increased. The expression of pluripotency-associated genes, including Oct4, Nanog, and Rex1, was also upregulated by Klf2 overexpression. In addition, quantitative RT-PCR indicated a lower level of expression of differentiation related genes in Klf2 overexpressing cells as compared to control cells. Our results identify a functionally conserved role for Klf2 in hBMSCs, in which its expression is biologically important for stemness and self-renewal. These results are the first to show a role for Klf2 in the proliferation and pluripotency of hBMSCs.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-014-9837-6) contains supplementary material, which is available to authorized users.
Keywords: Klf2, hBMSCs, Pluripotency, Proliferation, Self-renewal
Introduction
Mesenchymal stem cells (MSCs) are highly attractive tools in the field of tissue engineering and regenerative medicine, as they have the ability to self-renew and to differentiate into several lineages; i.e., chondrocytes, osteocytes, and adipocytes. However, loss of proliferation and pluripotent potential upon subsequent expansion in vitro hampers their clinical application (Banfi et al. 2000; Bonab et al. 2006). Diverse modified forms of traditional media have been reported to optimize culture conditions for the undifferentiated proliferation of MSCs, adding growth factors, cytokines, or serum substitutes such as platelet solutes (Tamama et al. 2006; Bieback et al. 2009; Esposito et al. 2009). However, the effects of growth factors in the cultivation of MSCs are various and difficult to control. Thus, understanding the physiology and regulatory molecules important for stem cell self-renewal and stemness is essential to establishing a strategy for undifferentiated proliferation of MSCs. Few studies, however, have identified the specific transcription factors that modulate proliferation and control the expression of those genes that determine the cell fate of MSCs, such as Oct4, Nanog, and Rex1 (Liu et al. 2009; Seo et al. 2009; Bhandari et al. 2010).
The Krüppel-like factors (Klfs) are a family of evolutionarily conserved zinc finger transcription factors that regulate multiple biological processes, including proliferation, apoptosis, differentiation, and development (McConnell et al. 2007; Pearson et al. 2008). Klfs can also control self-renewal and pluripotency of human embryonic stem cells (hESCs), by regulating key pluripotency genes (Bruce et al. 2007; Jiang et al. 2008; Chan et al. 2009). Klf2, Klf4, and Klf5 were reported to integrate into the Nanog transcriptional network and to regulate the self-renewal of ESCs (Jiang et al. 2008; Chan et al. 2009). Bruce et al. showed that Klf2 and Klf4 are co-localized with Oct4 in ESC nuclei and regulate the maintenance of ESC pluripotency (Bruce et al. 2007). Klf4 is also one of the original four factors shown to convert fibroblasts to induced pluripotent stem cells (iPSCs), which have many characteristics of embryonic stem cells (Takahashi and Yamanaka 2006). While many studies have identified the role of Klf genes in ESC fate, a function for Krüppel-like factors in the context of mesenchymal stem cells has not been reported, particularly in regulating pluripotency and proliferation of MSCs.
In this study, using three culture media described previously (Zhu et al. 2010), we determined whether Krüppel-like factors were present in cultured human bone marrow stromal cells (hBMSCs), and detected significant induction of Klf2 in hBMSCs cultured with our medium M1. We then focused on Klf2, and tested the hypothesis that it exerts a positive effect on the multipotency and proliferation of hBMSCs.
Materials and methods
Cultivation of hBMSCs
With the approval of our hospital Ethics Committee, hBMSCs were obtained from iliac crest marrow aspirates collected from healthy donors for iliac crest bone transplantation, following informed consent. The mononuclear cells were obtained using Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO, USA) density-gradient centrifugation at 2,500 rpm for 18 min. The cells were washed twice with phosphate-buffered saline (PBS; Gibco, Grand Island, NY, USA) and seeded on plates at a density of 2 × 106/cm2 in three media, respectively, in a humidified atmosphere of 5 % CO2 at 37 °C. The culture medium was renewed every 3–4 days until non-adherent cells were removed. When the adherent cells formed colonies and grew to 80 % confluence, half of the cells harvested were used for RT-PCR, and half were subcultured until passage 4.
The three media used for cultivation of hBMSCs were described previously (Zhu et al. 2010). In brief, Medium 1 (M1) was composed of 58 % low-glucose (1 g/l) Dulbecco’s modified Eagle’s medium (DMEM; Gibco), 40 % MCDB-201 medium (Sigma-Aldrich), 1× insulin transferrin selenium (Sigma-Aldrich), 2 × 10–8 M dexamethasone (Sigma-Aldrich), 10–4 M ascorbic acid 2-phosphate (Sigma-Aldrich), 2 % gentamicin (Pan-Biotech GmbH, Aidenbach, Germany), 2 % fetal calf serum (FCS; Biochrom AG, Berlin, Germany), 10 ng/ml epidermal growth factor (Strathmann Biotec AG, Hamburg, Germany), 10 ng/ml platelet-derived growth factor-BB (Strathmann), 100 U penicillin, and 1,000 U streptomycin (Gibco). Medium 2 (M2) was high-glucose (4.5 g/l) DMEM, supplemented with 15 % FCS, 1 % (5 × 10–5 M) β-mercaptoethanol (β-ME; Serva, Heidelberg, Germany), 1 % nonessential amino acids (Gibco), and 2 % gentamicin. Medium 3 (M3) was a traditional culture medium, containing low-glucose (1 g/l) DMEM, 10 % FCS, and 2 % gentamicin.
Lentivirus infection and stable transductions
Replication-defective lentivirus encoding the complete Klf2 open reading frame (LV-Klf2) and a lentivirus vector encoding green fluorescent protein (LV-GFP) were constructed by Shanghai GenePharma Co. (Shanghai, China). hBMSCs cultured in Medium 3 (traditional culture medium) were collected in the logarithmic phase and seeded at 6.5 × 103 per cm2 (~50 % confluence), 24 h prior to infection. Cells were then treated with lentivirus at a multiplicity of infection (MOI) of 200. Polybrene (Sigma) was added to the culture medium to a final concentration of 5 μg/ml. The culture medium was replaced with fresh medium 24 h after infection. Puromycin (2 μg/ml) was added to the medium to select stable lines. As controls, hBMSCs were treated with LV-GFP (Mock) or without lentivirus (normal control; NC).
Western blotting
Cells were lysed with RIPA buffer supplemented with a complete protease-inhibitor cocktail (Roche, Nutley, NJ, USA). Lysates were incubated on ice for 30 min, followed by centrifugation at 12,000×g for 10 min at 4 °C and supernatant collection. The protein concentrations of samples were determined using the Bio-Rad Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA, USA), following the manufacturer’s instructions. Protein samples (20 µg) were subjected to 10 % SDS–polyacrylamide gel electrophoresis and transferred onto a Hybond-PVDF membrane (Amersham Biosciences, Piscataway, NJ, USA). Proteins were detected using primary antibodies recognizing Klf2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and GAPDH (MAB374, Chemicon, Temecula, CA, USA). Primary antibodies were detected with appropriate secondary horseradish peroxidase–conjugated antibodies (Zymed Laboratories Inc., South San Francisco, CA, USA) and visualized by enhanced chemiluminescence detection (Amersham Biosciences). The relative intensities of each protein band were normalized to that of control cells, and quantified using the Quantity One software (version 4.6.5, Bio-Rad Inc.).
Proliferation assays
To measure cell growth after infection, two proliferation assays were performed. Cell Counting Kit-8 (Dojindo Molecular Technologies Inc., San Diego, CA, USA) was used according to the manufacturer’s protocol. Briefly, cells were harvested and duplicate aliquots were placed into 96-well plates; 10 μl of Cell Counting Kit-8 solution (CCK-8) was added to each well. After incubation for 1 h at 37 °C, the A450 was measured using a Beckman Coulter DTX 880 plate reader. In addition, the population doubling level was calculated for each group harvest directly from the cell count, using the formula log N/log 2. To yield the cumulative doubling levels, the population doubling level for each passage was calculated and then added to the population doubling levels of the previous passages.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from hBMSCs from passages 0 to 4 using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Synthesis of cDNA was carried out using 0.5-µg samples of total RNA, oligo-dT primers, and a QuantiTect Reverse Transcription kit (Qiagen) to prepare samples for reverse transcriptase-polymerase chain reaction (RT-PCR). Gene expression was analyzed by qPCR using a QuantiTect SYBR Green PCR Kit (Qiagen). The primers for each gene are listed in Table 1. The expression of target genes was normalized to that of β-actin. Quantitative Real-time RT-PCR was performed on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA).
Table 1.
Primer sequences for real-time PCR
| Gene | Primer sequence (5′–3′) | Product size | Accession number |
|---|---|---|---|
| Klf2 | F: AAGACCTACACCAAGAGTTCGCATC R: TCTGAGCGCGCAAACTTCC |
110 | NM_016270.2 |
| Klf5 | F: ACGCATCCACTACTGCGATTACC R: AACCTCCAGTCGCAGCCTTC |
132 | NM_001730.3 |
| Klf4 | F: AAGAGTTCCCATCTCAAGGCACA R:GGGCGAATTTCCATCCACAG |
91 | NM_004235.4 |
| Oct4 | F: CTTGCTGCAGAAGTGGGTGGAGGAA R: CTGCAGTGTGGGTTTCGGGCA |
169 | NM_001159542.1 |
| Nanog | F: GAACTCTCCAACATCCTGAACCTC R: CCTTCTGCGTCACACCATTGC |
127 | NM_024865.2 |
| Rex1 | F: GGAATGTGGGAAAGCGTTCGT R: CCGTGTGGATGCGCACGT |
152 | NM_174900.3 |
| ON | F: CGAGCTGGATGAGAACAACA R: AAGTGGCAGGAAGAGTCGAA |
126 | BC072457 |
| COMP | F: AGGGAGATCGTGCAGACAA R: AGCTGGAGCTGTCCTGGTAG |
154 | NM_000095 |
| LPL | F: AGAGCCAAAAGAAGCAG R: GGCAGAGTGAATGGGAT |
182 | NM_000237 |
| β-actin | F: CACACTGTGCCCATCTACGA R: TGAGGATCTTCATGAGGTAGTCAG |
106 | GZ549052 |
Differentiation potency of Klf2+ hBMSCs
In order to evaluate the multipotential differentiation abilities of hBMSCs after Klf2 overexpression, Klf2+ cells were subjected to differentiation under specific conditions to induce adipogenic, osteogenic, and chondrogenic lineages. Adipogenic differentiation was induced by culturing Klf2+ hBMSCs for 2 weeks in adipogenic differentiation medium (Gibco) and assessed using an Oil Red O stain. Osteogenic differentiation was induced by culturing Klf2+ hBMSCs for 3 weeks in osteogenic differentiation medium (Gibco) and examined for Alizarin red S staining. Chondrogenic differentiation was induced using the micromass technique. Briefly, 10 μl of a concentrated Klf2+ cell suspension (8 × 106 cells/ml) was seeded into the center of each well and cultured at 37 °C for 2 h. Chondrogenesis differentiation medium (Gibco) was gently put over the cell nodules which were cultured for 2 weeks. Chondrogenesis was confirmed using the histologic stain of Alician Blue.
Statistical analysis
Data are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to compare Klf gene expression in the three media. An unpaired Student’s t test was used to calculate the statistical significance of differences between the Klf2 overexpression group and control cultures. Significant differences in figures are marked with a single (p < 0.05), or double (p < 0.01) asterisk.
Results
Screening of Klf2 gene expression
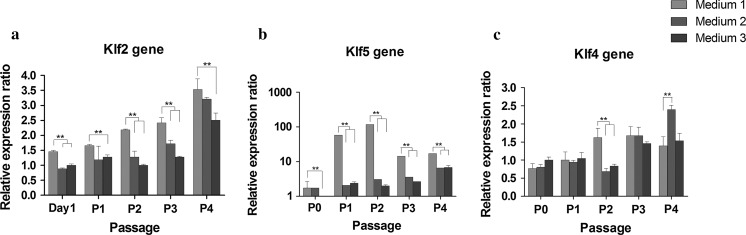
Previously, we demonstrated that culturing hBMSCs in medium M1 led to rapid proliferation without inducing differentiation, inhibited expression of apoptosis-related genes, and limited replicative senescence during repeated passaging, as compared to cells in media M2 and M3 (Zhu et al. 2010). To identify Klf genes related to proliferation and pluripotency of MSCs, we compared the Klf2, Klf4, and Klf5 expression of cultures in the three media by qPCR analysis. RNA was extracted from passages 0 to 4 of hBMSCs in each medium. Klf2 was expressed at a significantly higher level in M1 cultures than in the presence of M2 or M3 (p < 0.01). Expression increased gradually during passaging in all media, and there was no significant difference at passages 2 and 4 between Klf2 expression in M1 and M2 (p > 0.05; Fig. 1a). Klf5 expression revealed a profile similar to that of Klf2; both were highly expressed in M1, while Klf5 displayed a considerable increase in expression after passage 1 (~58-fold) and passage 2 (almost 120-fold) in M1, decreasing sharply during subsequent passages in the same medium (Fig. 1b). Expression of Klf4 in M1 was higher than in the other two media only at passage 2, and was significantly lower at passage 4 than that in M2 (p < 0.01; Fig. 1c). Hence, we hypothesized that the Klf2 gene may exert some positive effects on hBMSC proliferation and on the maintenance of stemness.
Fig. 1.
Relative gene expression ratios for three Krüppel-like factors in human bone marrow stromal cells (hBMSCs) cultured in M1, M2, and M3. a Klf2 expression was significantly increased in M1 over M2 and M3. **p < 0.01. b Klf5 expression was upregulated in M1 compared with the other two media. **p < 0.01. c Klf4 expression was higher only at passage 2 in M1 cultures. **p < 0.01
Efficient and stable Klf2 overexpression in human mesenchymal stem cells via lentivirus transduction
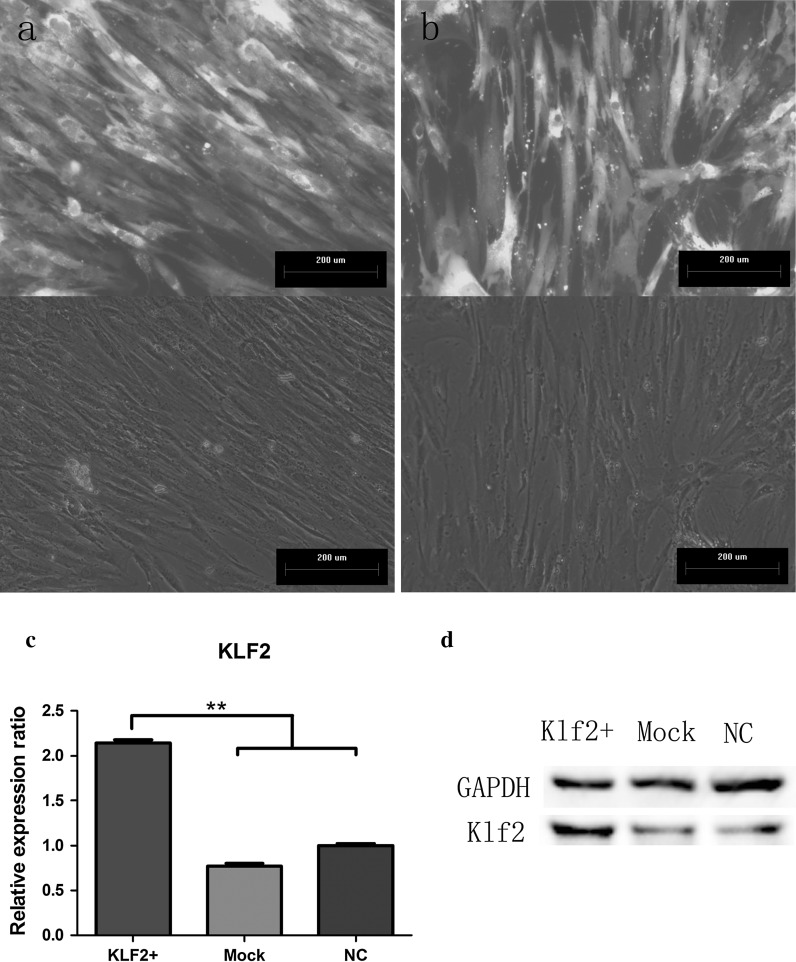
To test this hypothesis, we generated hBMSCs overexpressing Klf2 in traditional culture media using lentiviral vectors encoding human Klf2 and puromycin phosphotransferase. Control cells were modified with the same construct lacking the Klf2 coding sequence. Efficient overexpression was achieved after cells were subjected to puromycin selection, with fluorescence microscopy images indicating that almost all cells were transduced (up to 80 % GFP-positive hMSCs after selection; Fig. 2a, b). The stable overexpression of the Klf2 mRNA in hBMSCs was confirmed by real-time qPCR, showing a twofold (p < 0.001) induction over controls (Mock and NC; Fig. 2c). In addition, western blot analysis showed that Klf2 protein was highly expressed to well above the level in control cells (Fig. 2d).
Fig. 2.
Efficient overexpression of Klf2 in hBMSCs. Cells transduced with a lentivirus encoding Klf2 (LV-Klf2) and b lentivirus encoding GFP (LV-GFP) showed a strong fluorescent signal, present in almost all cells in the culture, with no morphological changes after puromycin selection. Stable expression of Klf2 was verified by, c qRT-PCR and d western blot
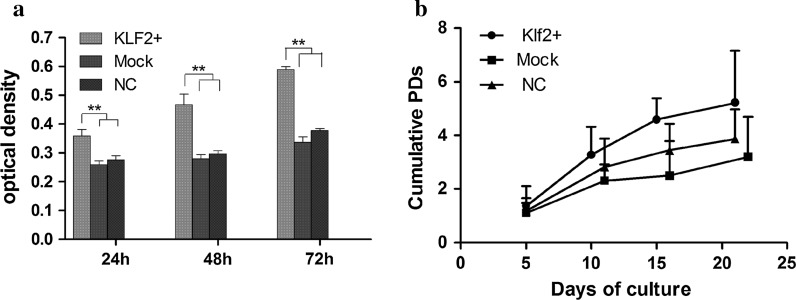
Overexpression of Klf2 increased proliferation of hBMSCs
Upregulation of the cell-cycle-related gene Klf2 in M1 cultures over those in M2 and M3 media suggested that Klf2 expression might be involved in the proliferation potential of hBMSCs. Indeed, experiments measuring cell proliferation rate supported this hypothesis. After Klf2 overexpression, we measured cell proliferation in hBMSCs using CCK-8 assay (Fig. 3a) and population doubling assay (Fig. 3b), and found that cell growth increased significantly compared to controls (Mock and NC). The proliferation rate in the mock transduction group was similar to that in the normal control (NC) group (Fig. 3).
Fig. 3.
Proliferation assay. a CCK-8 assay showed that proliferation of Klf2-overexpressing hBMSCs increased significantly after transduction, compared with mock-transduced and normal control MSCs. b Klf2 overexpression hMSCs possess the highest cumulative population doublings with time in culture as compared to the controls. **p < 0.01
Overexpression of Klf2 affect various functional genes in hBMSCs
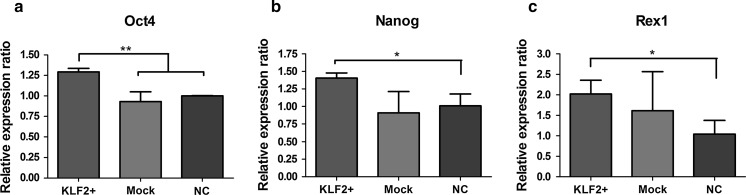
To further investigate the effect of Klf2 on self-renewal and stemness maintenance of MSCs, we used quantitative RT-PCR to determine the expression of various functional genes. We compared the mRNA level of pluripotency-associated genes, Nanog, Oct4, and Rex1, between Klf2-overexpressing (Klf2+) hBMSCs and control cells (Mock and NC). As shown in Fig. 4, Oct4 increased significantly (by ~1.3-fold) in Klf2+ hBMSCs as compared to controls (p < 0.01; Fig. 4a). The level of Nanog increased by more than 1.4-fold after Klf2 overexpression in hBMSCs; the difference between the Klf2+ group and the normal control group was statistically significant (p < 0.05; Fig. 4b). In addition, Rex1 expression was significantly higher in Klf2+ hBMSCs than in the normal controls (up to 2.0-fold, p < 0.05), but was not significantly higher than in the mock-transduction group (p > 0.05; Fig. 4c). These data were also confirmed by knockdown experiment using small intervering RNA (siRNA). The results indicated that OCT4, Nanog and Rex1 gene expression were all decreased after Klf2 knockdown (supplementary Figure 1).
Fig. 4.
Pluripotency-related gene expression changes in Klf2-overexpressing hBMSCs. a The expression of Oct4 was clearly increased in Klf2-overexpressing hBMSCs compared to controls (Mock and NC). **p < 0.01. b The expression of Nanog was significantly increased in Klf2-overexpressing hBMSCs relative to the normal control hBMSCs. *p < 0.05. c The expression of Rex1 was significantly increased in Klf2-overexpressing hBMSCs versus normal controls. *p < 0.05
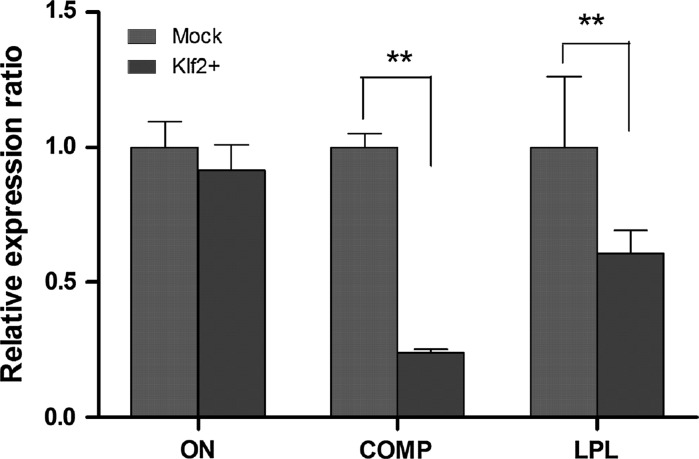
Three markers of ON, COMP, and LPL identified as essential to the MSC differentiation were also evaluated between Klf2+ group and Mock control. As shown in Fig. 5, ON expression was slightly decreased after klf2 overexpression compared to the Mock control cells, but the difference was not statistically significant; COMP exhibited a significantly lower rate of gene expression in Klf2+ cells than in Mock control cells. Similarly, LPL displayed an obvious decrease in Klf2+ group versus Mock group, and significant difference was observed. These results demonstrate that expression levels of pluripotency-associated genes are increased by Klf2 overexpression, while differentiation related genes are inhibited (Figs. 4, 5). Furthermore, the differentiation ability of Klf2+ hBMSCs was investigated. As the result showed, when Klf2 was stably overexpressed in hBMSCs, these cells can differentiate into adipocytes, chondrocytes, and osteocytes under specific induction (Fig. 6), indicating that Klf2 overexpressing hBMSCs are still primary cultured cells with multilineage differentiation potency.
Fig. 5.
Comparison of expression ratio of differentiation related genes between Klf2-overexpressing hBMSCs and Mock control cells. **p < 0.01
Fig. 6.
Differentiation potencies of hBMSCs after Klf2 overexpression. a adipogenesis stained with Oil Red O, and b chondrogenesis stained with Alcian Blue stain; c osteogenesis stained with Alzirin Red S. scale bar equals to 200 μm
Discussion
The use of mesenchymal stem cells (MSCs) derived from bone marrow, with an innate potential for multi-lineage differentiation, is a promising strategy for regenerative medicine and clinical cellular therapies. However, the use of MSCs in such settings is restrained by the low prevalence of the cells, which comprise ~0.001–0.01 % of cells in bone marrow (Friedenstein et al. 1982). Moreover, MSCs tend to undergo premature terminal differentiation during in vitro expansion, with the loss of proliferation and pluripotency (Banfi et al. 2000; Bonab et al. 2006). To overcome such limitations, it is critical to understand the physiology of these cells, and to determine the biological roles of the factors that regulate MSC self-renewal and stemness. Then the challenge may be addressed by maintaining the cells in an undifferentiated and self-renewing state by the application and/or stable expression of candidate pluripotency-related factors.
The Krüppel-like factors (Klfs) belong to the Sp1 family of transcription factors, comprising over 20 members (Dang et al. 2000; Kaczynski et al. 2003). Three members of this family (Klf2, Klf4, and Klf5) have been demonstrated to play a role in the control of embryonic stem cell pluripotency (Bruce et al. 2007; Jiang et al. 2008; Parisi et al. 2008; Chan et al. 2009; Hall et al. 2009). Jiang et al. proposed that Klf2, Klf4, and Klf5 had redundant functions in the control of pluripotency, because impairment in the ESC undifferentiated state was observed only following knockdown of all three. Hall et al. reported that post-implantation embryo-derived stem cells lack both Klf2 and Klf4, and that expression of either can reinstate naïve pluripotency, indicating that Klf2 and Klf4 are key markers of the naïve ES state. Others supported the notion that each Klf member (2, 4 and 5) plays a specific role in the maintenance of the pluripotent state (Bourillot and Savatier 2010). Here, we have demonstrated that Klf2 might be a novel transcriptional regulator of pluripotency and proliferation of hBMSCs.
In a previous study, we characterized the effects of culture media on the expansion of hBMSCs, and demonstrated that medium 1 (M1) promoted rapid proliferation without inducing differentiation. It also inhibited apoptosis and senescence of hBMSCs during repeated passaging when compared with media 2 and 3 (Zhu et al. 2010). Hence, we compared Klf gene expression by hBMSCs cultured in M1 with that seen in M2 and M3 by qPCR. Klf2 exhibited a significantly higher level of gene expression in M1 cultures than in M2 and M3, and increased from passage 0 to passage 4 (Fig. 1a). This increase is likely to be a result of the culture medium, as Bruce et al. reported Krüppel-like factors, including Klf2, to be highly expressed in undifferentiated ES cells and downregulated rapidly under differentiation conditions (Bruce et al. 2007). Hall et al. reported that Klf2 was included in a list of ESC-enriched genes, and conferred resistance to BMP-induced differentiation (Hall et al. 2009). Thus, the high expression of Klf2 and its generally increasing expression during passaging in M1 might depend on the characteristics of M1, which not only promoted rapid proliferation but also inhibited premature differentiation of human BMSCs (Zhu et al. 2010). Concurrently, Klf5 gene expression was also increased in M1; however, this increase occurred in passages 1 and 2, and decreased dramatically during subsequent passages in M1 (Fig. 1b). However, Klf4 expression was only highly expressed in the passage 2 of cells in M1 as compared to M2 and M3. This may be due to the interaction among the core Klf circuitry of Klf2, Klf4 and Klf5, for Klf5 gene expression was also very high in passage 2. On the other hand, we have evaluated the proliferation rate of MSC among the three different media in our previous work (Zhu et al. 2010). Figure 1d in the previous paper showed that the population doublings in M1in passage 2 increased sharply, while M2 and M3 displayed gradual increase of population doublings. From these observations, we may assume that high expression of Klf4 might have some correlation with the rapid growth rate in passage 2 although further experiments are required to confirm it. In conclusion, these results indicate that the transcription factor Klf2 is more likely to be associated with MSC proliferation and pluripotency.
To elucidate putative functions of Klf2 in undifferentiated hBMSCs, we overexpressed Klf2 via lentivirus transduction, and analyzed the downstream effects. Cell proliferation assays indicated that Klf2 overexpression resulted in significantly enhanced growth of hBMSCs, compared with control conditions (Fig. 3). Similar results were reported by Hall et al. indicating that Klf2 has the greatest effect of all Klfs in terms of enhancing ESC clonogenicity and self-renewal (Hall et al. 2009).
Previous work has shown that Oct4, Nanog, and Rex1, expressed in some types of hMSCs as well as in ESCs, are pluripotent marker genes (Liu et al. 2009; Bhandari et al. 2010; Jiang et al. 2002). Thus, a PCR assay for these three genes was performed using real-time RT-PCR to determine the effect of Klf2 on MSC stemness and self-renewal. Notably, Oct4 expression was significantly higher in the Klf2-overexpressing (Klf2+) hBMSCs relative to vector control-infected hBMSCs (Mock) and uninfected normal control hBMSCs (NC) (Fig. 4a). Several studies have reported a connection between Klf2 and Oct4 in terms of their effects on ESC pluripotency (Bruce et al. 2007; Parisi et al. 2008; Hall et al. 2009). Hall et al. demonstrated that Oct4 induces primarily Klf2 to sustain embryonic stem cell self-renewal (Hall et al. 2009). Conversely, knockdown of Oct3/4 significantly decreased Klf2 mRNA levels (Parisi et al. 2008). Others reported that Klf2 and Klf4 share an ability to co-operate with Oct4 in embryonic stem cells (Nakatake et al. 2006; Bruce et al. 2007), and are likely to regulate many stem cell genes, such as Pou5f1/Oct4, Nanog, Esrrb, Zfp42/Rex1, Lefty1 and Lefty2 (Bruce et al. 2007). These reports are in line with our present results, in terms of upregulation of Nanog and Rex1 expression in Klf2 + hBMSCs compared with control cells (Fig. 4b, c).
Conclusion
In this study, we determined that Klf2 overexpression in hBMSCs significantly affected their biological activity, resulting in increased proliferation and pluripotency in an ex vivo culture system. Taken together, these data lead us to speculate that Klf2 may exert positive effects on the stemness and self-renewal potential of hBMSCs. Therefore, the loss of proliferation and spontaneous differentiation of MSCs during in vitro expansion may be overcome by genetic modification with pluripotency-related factors to improve the stemness of hBMSCs. Future characterization of the partners of Klf2 and its direct targets should provide insight into the mechanisms by which Klf2 contributes to the pluripotency and self-renewal of MSCs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 Supplementary Figure 1. Pluripotency-related gene expression changes in hBMSCs infected with Klf2-siRNA (Klf2-) and Negative Control siRNA (NC). Transient knockdown was verified by real-time PCR and western blot. *p<0.05, **p<0.01. (TIFF 218 kb)
Acknowledgments
We thank Yu Xiaopeng, Yu Mengfei, and Wen Luxi for their superb technical assistance. This work was supported by Natural Science Foundation of Zhejiang Province, China [Grant Number: Y2100267], Medical Science Research Foundation of Zhejiang Province, China [Grant Number: 2013KYB085, 201352205], Medical and health Major science and technology plan of Zhejiang Province, China [Grant Number: 201471556].
References
- Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/S0301-472X(00)00160-0. [DOI] [PubMed] [Google Scholar]
- Bhandari DR, Seo KW, Roh KH, Jung JW, Kang SK, Kang KS. REX-1 expression and p38 MAPK activation status can determine proliferation/differentiation fates in human mesenchymal stem cells. PLoS One. 2010;5:e10493. doi: 10.1371/journal.pone.0010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieback K, Hecker A, Kocaomer A, Lannert H, Schallmoser K, Strunk D, Kluter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourillot PY, Savatier P. Kruppel-like transcription factors and control of pluripotency. BMC Biol. 2010;8:125. doi: 10.1186/1741-7007-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce SJ, Gardiner BB, Burke LJ, Gongora MM, Grimmond SM, Perkins AC. Dynamic transcription programs during ES cell differentiation towards mesoderm in serum versus serum-free BMP4 culture. BMC Genomics. 2007;8:365. doi: 10.1186/1471-2164-8-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KK, Zhang J, Chia NY, Chan YS, Sim HS, Tan KS, Oh SK, Ng HH, Choo AB. KLF4 and PBX1 directly regulate NANOG expression in human embryonic stem cells. Stem Cells. 2009;27:2114–2125. doi: 10.1002/stem.143. [DOI] [PubMed] [Google Scholar]
- Dang DT, Pevsner J, Yang VW. The biology of the mammalian Krüppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–1121. doi: 10.1016/S1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MT, Di Noto R, Mirabelli P, Gorrese M, Parisi S, Montanaro D, Del Vecchio L, Pastore L. Culture conditions allow selection of different mesenchymal progenitors from adult mouse bone marrow. Tissue Eng Part A. 2009;15:2525–2536. doi: 10.1089/ten.tea.2008.0509. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Latzinik NW, Grosheva AG, Gorskaya UF. Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges. Exp Hematol. 1982;10:217–227. [PubMed] [Google Scholar]
- Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, Morfopoulou S, Humphreys P, Mansfield W, Walker R, Tomlinson S, Smith A. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TM, Wu YN, Guo XM, Hui JH, Lee EH, Lim B. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 2009;18:1013–1022. doi: 10.1089/scd.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. BioEssays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatake Y, Fukui N, Iwamatsu Y, Masui S, Takahashi K, Yagi R, Yagi K, Miyazaki J, Matoba R, Ko MS, Niwa H. Klf4 cooperates with Oct3/4 and Sox2 to activate the Lefty1 core promoter in embryonic stem cells. Mol Cell Biol. 2006;26:7772–7782. doi: 10.1128/MCB.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi S, Passaro F, Aloia L, Manabe I, Nagai R, Pastore L, Russo T. Klf5 is involved in self-renewal of mouse embryonic stem cells. J Cell Sci. 2008;121:2629–2634. doi: 10.1242/jcs.027599. [DOI] [PubMed] [Google Scholar]
- Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Seo KW, Lee SR, Bhandari DR, Roh KH, Park SB, So AY, Jung JW, Seo MS, Kang SK, Lee YS, Kang KS. OCT4A contributes to the stemness and multi-potency of human umbilical cord blood-derived multipotent stem cells (hUCB-MSCs) Biochem Biophys Res Commun. 2009;384:120–125. doi: 10.1016/j.bbrc.2009.04.094. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- Zhu H, Miosge N, Schulz J, Schliephake H. Regulation of multilineage gene expression and apoptosis during in vitro expansion of human bone marrow stromal cells with different cell culture media. Cells Tissues Organs. 2010;192:211–220. doi: 10.1159/000313417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Supplementary Figure 1. Pluripotency-related gene expression changes in hBMSCs infected with Klf2-siRNA (Klf2-) and Negative Control siRNA (NC). Transient knockdown was verified by real-time PCR and western blot. *p<0.05, **p<0.01. (TIFF 218 kb)